Abstract
Objectives
To study the associations between artificial sweeteners from all dietary sources (beverages, but also table top sweeteners, dairy products, etc), overall and by molecule (aspartame, acesulfame potassium, and sucralose), and risk of cardiovascular diseases (overall, coronary heart disease, and cerebrovascular disease).
Design
Population based prospective cohort study (2009-21).
Setting
France, primary prevention research.
Participants
103 388 participants of the web based NutriNet-Santé cohort (mean age 42.2±14.4, 79.8% female, 904 206 person years). Dietary intakes and consumption of artificial sweeteners were assessed by repeated 24 h dietary records, including brand names of industrial products.
Main outcomes measures
Associations between sweeteners (coded as a continuous variable, log10 transformed) and cardiovascular disease risk, assessed by multivariable adjusted Cox hazard models.
Results
Total artificial sweetener intake was associated with increased risk of cardiovascular diseases (1502 events, hazard ratio 1.09, 95% confidence interval 1.01 to 1.18, P=0.03); absolute incidence rate in higher consumers (above the sex specific median) and non-consumers was 346 and 314 per 100 000 person years, respectively. Artificial sweeteners were more particularly associated with cerebrovascular disease risk (777 events, 1.18, 1.06 to 1.31, P=0.002; incidence rates 195 and 150 per 100 000 person years in higher and non-consumers, respectively). Aspartame intake was associated with increased risk of cerebrovascular events (1.17, 1.03 to 1.33, P=0.02; incidence rates 186 and 151 per 100 000 person years in higher and non-consumers, respectively), and acesulfame potassium and sucralose were associated with increased coronary heart disease risk (730 events; acesulfame potassium: 1.40, 1.06 to 1.84, P=0.02; incidence rates 167 and 164; sucralose: 1.31, 1.00 to 1.71, P=0.05; incidence rates 271 and 161).
Conclusions
The findings from this large scale prospective cohort study suggest a potential direct association between higher artificial sweetener consumption (especially aspartame, acesulfame potassium, and sucralose) and increased cardiovascular disease risk. Artificial sweeteners are present in thousands of food and beverage brands worldwide, however they remain a controversial topic and are currently being re-evaluated by the European Food Safety Authority, the World Health Organization, and other health agencies.
Trial registration
ClinicalTrials.gov NCT03335644
Introduction
The harmful effects of added sugars on various health outcomes including cardiometabolic disorders have been extensively studied, meta-analysed1 2 and are currently recognised as major risk factors by public health authorities. In particular, the World Health Organization recommends that less than 5% daily energy intake should come from free sugar.3 Artificial sweeteners emerged as an alternative to added sugar that enabled the sweet taste to be reproduced without using sugar and therefore reduced calorie content from free sugar, which was highly appreciated by consumers.4 Artificial sweeteners currently represent a $7200m (£5900m; €7000m) market globally, with a 5% annual growth projected to attain $9700m by 2028.5 An extensive number of brands worldwide contain these food additives, especially ultra-processed foods such as artificially sweetened beverages, some snacks, and low calorie ready-to-go meals or dairy products; overall more than 23 000 products worldwide contain artificial sweeteners.6 Artificial sweeteners are also directly used by consumers as table top sweeteners instead of sugar. Acceptable daily intakes for each artificial sweetener have been set by the European Food Safety Authority (EFSA), the United States Food and Drug Administration, or the Joint Expert Committee on Food Additives. Nonetheless, they remain a topic of controversy and are currently undergoing a re-evaluation by several health authorities, including the EFSA7 and WHO.8
Some experimental in vivo and in vitro studies, observational studies, and human randomised controlled trials investigated early markers of cardiovascular health, for example, weight status,9 10 11 12 hypertension,13 inflammation,14 vascular dysfunction,15 16 or gut microbiota perturbation17 18 19 20 in association with consumption of artificial sweeteners or artificially sweetened beverages. Most of these studies suggested adverse effects,11 12 13 14 15 16 17 18 19 20 and few suggested neutral or beneficial properties.9 10 Although the results were mixed, this literature generally supports a potential involvement of artificial sweeteners in cardiovascular health, with plausible mechanisms.21 22 23
Cardiovascular diseases (CVDs) are the leading cause of death worldwide.24 Randomised controlled trials have not directly assessed the impact of artificial sweetener intake on hard endpoints such as CVD risk for ethical reasons. Similarly, observational prospective studies have not directly investigated the association between artificial sweetener intake (mg/day) and CVD risk, but several have used artificially sweetened beverage consumption (millilitres or servings/day) as a proxy to explore these associations with conflicting results.22 23 25 26 27 28 29 30 31 32 33 34 One of these studies was performed in the NutriNet-Santé cohort28 and found that sugary drinks and artificially sweetened beverages were associated with increased CVD risk. Systematic reviews and meta-analyses35 36 have suggested direct associations between artificially sweetened beverages and CVD risk. The WHO 2022 report on the health effects of artificial sweeteners notably observed associations between consumption of beverages with artificial sweeteners (used as a proxy) and some intermediate markers of CVD,8 including a modest increase in the unfavourable total cholesterol to HDL cholesterol ratio (meta-analysis of four randomised control trials), and an increased risk of hypertension (meta-analysis of four prospective studies). The international health authority also identified an increase in CVD mortality, and in the incidence of cardiovascular events and strokes associated with greater intake of soft drinks containing artificial sweeteners (meta-analysis of four randomised control trials). However, prospective studies remain limited and the level of evidence for these associations is still considered low by WHO.8 Additionally, because artificially sweetened beverages only represent part of the total artificial sweetener intake, it is crucial to consider all dietary sources in causal studies.
In this context, our objective was to conduct a large scale prospective study using quantitative data to investigate the associations between consumption of artificial sweeteners (mg/day) from all dietary sources (beverages but also table top sweeteners, dairy products, etc), overall and by type (aspartame, acesulfame potassium, and sucralose), and risk of CVD (overall, coronary, and cerebrovascular). Our study was performed within the population based NutriNet-Santé cohort, which includes detailed information on commercial names and brands of industrial food consumed.
Methods
Study population and data collection
This study was based on the prospective NutriNet-Santé e-cohort, launched in France in May 2009, with an open ongoing enrolment of volunteers. The main objective was to investigate the relations between nutrition and health.37 Participants are French adults, aged 18 years or older, with internet access, recruited from the general population by means of multimedia campaigns. They are followed through their personal account created at inclusion on the study website (https://etude-nutrinet-sante.fr/). Immediately after enrolment, each person completes five online questionnaires about diet (24 h dietary records, detailed below), health (eg, personal and familial history, prescription drug use), anthropometric data (height and weight38 39), lifestyle and sociodemographic data (eg, date of birth, sex, education level, professional occupation, smoking status, number of children40), and physical activity. Physical activity levels were defined based on the validated seven day assessment International Physical Activity Questionnaire (IPAQ).41 All activities declared by participants were converted into metabolic equivalent of task (MET) minutes per week according to the compendium of physical activities.42 Three levels of physical activity were defined: low (<600 MET-min/week), moderate (600-1500 MET-min/week), and high (>1500 MET-min/week) using standardised IPAQ processing guidelines.41 For instance, 600 MET-min/week is equivalent to 150 min/week of moderate intensity (4 METs) physical activity or 75 min/week of high intensity (8 METs) physical activity.
Each person included in the NutriNet-Santé cohort provides informed consent electronically. The study is registered at ClinicalTrials.gov (NCT03335644), conducted according to the Declaration of Helsinki guidelines, and approved by the Institutional Review Board of the French Institute for Health and Medical Research (IRB-Inserm) and the Commission Nationale de l’Informatique et des Libertés (CNIL No 908450/909216).
Dietary assessment
Three non-consecutive days of 24 h dietary records were randomly assigned over a two week period, at baseline, and every six months thereafter. During those recording days (two weekdays and one weekend day) participants indicated all foods and beverages consumed during the three main meals and any other eating occasions, and in what quantities, using validated photographs and standard serving containers43 or by directly entering the amount (in grams or millilitres). All 24 h dietary records provided during the first two years of each person’s follow-up were averaged to obtain baseline diet. This represents a reliable estimate of consumption habits, while respecting the prospective design and guaranteeing sufficient delay between consumption and CVD outcomes. Intakes of energy, alcohol, and nutrients were assessed using the NutriNet-Santé food composition table (≈3500 food/beverage items44). Nutritional contributions of mixed dishes were estimated by standard French recipes defined by nutrition professionals. Dietary assessment through these 24 h dietary records were validated against interviews by a trained dietitian45 and against blood and urinary biomarkers.46 47 The basal metabolic rate and the Goldberg cut-off method enabled any under reporting to be identified48 49 50; participants who under reported were excluded from the analyses. Supplementary method 1 gives details of methods used to identify under reporting.
Artificial sweetener intakes
Chazelas and colleagues described the quantitative evaluation of food additive consumption in participants of the NutriNet-Santé cohort.51 Briefly, food additive intakes, including artificial sweeteners, were assessed through the interactive online 24 h dietary record tool, in which commercial names and brands of industrial products consumed could be recorded. The presence of food additives was first determined for each food and beverage using ingredients lists available from three large scale food composition databases: Open Food Facts (https://world.openfoodfacts.org/)6; the French food safety agency database Oqali (https://www.oqali.fr/oqali_eng/)52; and Mintel’s Global New Products Database.53 Doses of additives were determined by around 2700 assays performed by accredited laboratories, requested by the Nutritional Epidemiology Research Team or by a consumer association (UFC Que Choisir). These quantitative data were completed by average doses per food group provided by EFSA and the Joint FAO/WHO Expert Committee on Food Additives.54 Food additive composition data were matched by date to account for possible industrial reformulations and changes in additive composition (date of consumption was considered to match the product to the closest consumption data). Supplementary method 2 gives additional information on food additive and artificial sweetener intake assessment.
For this study, we were able to estimate intakes of aspartame (European food additive identification number E951), acesulfame potassium (E950), sucralose (E955), cyclamates (E952), saccharin (E954), thaumatin (E957), neohesperidine dihydrochalcone (E959), steviol glycosides (E960), and salt of aspartame-acesulfame potassium (E962) and to create a sum variable labelled total artificial sweeteners.
Cardiovascular disease determination
Throughout follow-up, biannual health questionnaires and a permanently open personal health interface on the study account allowed participants to report any new health events, medical treatments, and examinations. For each incident CVD event declared, participants were contacted by a physician of the team and asked to provide any relevant medical records (eg, radiological reports, electrocardiogram, angioplasty). When necessary, the study physicians contacted the patient’s physician or any hospitals providing treatment to collect additional information. These medical data were reviewed by physician experts. An investigation was also conducted by the physicians of the NutriNet-Santé study by contacting the participant’s family or their physician when no connection to the study website was made for more than a year. Beyond this proactive health follow-up, data were paired with the medico-administrative databases of the national health insurance system database (SNIIRAM) and the national mortality registry (CépiDC), thereby limiting potential bias due to people with CVD not reporting their disease to the study investigators (further information available in supplementary method 3). International classification of diseases clinical modification, 10th revision, was used to classify CVD.55 For this study, first incidence of CVD, coronary heart disease (myocardial infarction, code I21; acute coronary syndrome, code I21.4; angioplasty, code Z95.8; angina pectoris, code I20.0), or cerebrovascular disease (stroke, code I64; transient ischaemic attack, codes G45.8 and G45.9) diagnosed between inclusion and 5 October 2021 were considered as events and investigated in the analyses.
Statistical analyses
Participants with at least two valid dietary records during the first two years of follow-up were included in the analysis. Those with prevalent CVD or pre-existing diabetes were excluded. To limit reverse causality bias (particularly sensitive when sugar was substituted by artificial sweeteners), participants with CVD diagnosed during the first two years of follow-up were also excluded. Supplementary figure 1 presents a flowchart showing detailed selection of the study population.
We classified participants into three categories of artificial sweetener consumption: non-consumers, lower consumers (participants with artificial sweetener intake below the sex specific median among consumers), and higher consumers. Baseline characteristics (sociodemographic, health, lifestyle, dietary intakes) were assessed for each category and compared using χ2 tests for categorical variables and analysis of variance tests for continuous variables (table 1).
Table 1.
Baseline characteristics of the study population, NutriNet-Santé cohort, France, 2009-21 (n=103 388)
| Characteristics | All participants | Categories of artificial sweetener intakes* | P value† | ||
|---|---|---|---|---|---|
| Non-consumers | Lower consumers | Higher consumers | |||
| No of participants (%) | 103 388 | 65 028 (62.90) | 19 221 (18.59) | 19 139 (18.51) | — |
| Age, years (mean (SD)) | 42.22 (14.41) | 42.96 (14.64) | 41.94 (14.42) | 39.97 (13.35) | <0.001 |
| Female sex | 82 485 (79.78) | 50 160 (77.14) | 16 200 (84.28) | 16 125 (84.25) | <0.001 |
| Body mass index (mean (SD)) | 23.59 (4.33) | 23.21 (4.06) | 23.68 (4.29) | 24.79 (4.99) | <0.001 |
| Family history of CVD | 28 053 (27.44) | 17 788 (27.69) | 5312 (27.84) | 4953 (26.2) | <0.001 |
| Prevalent hypercholesterolemia | 7696 (7.44) | 4525 (6.96) | 1676 (8.72) | 1495 (7.81) | <0.001 |
| Prevalent hypertriglyceridemia | 1321 (1.28) | 709 (1.09) | 280 (1.46) | 332 (1.73) | <0.001 |
| Prevalent hypertension | 7125 (6.89) | 4297 (6.61) | 1448 (7.53) | 1380 (7.21) | <0.001 |
| Education level | <0.001 | ||||
| No | 16 652 (16.24) | 10 814 (16.78) | 2935 (15.37) | 2903 (15.3) | — |
| Yes, <2 years | 16 282 (15.88) | 10 324 (16.02) | 2897 (15.18) | 3061 (16.13) | — |
| Yes, ≥2 years | 69 577 (67.87) | 43 309 (67.20) | 13 258 (69.45) | 13 010 (68.57) | — |
| Smoking status | <0.001 | ||||
| Current | 14 894 (14.42) | 9387 (14.45) | 2292 (11.93) | 3215 (16.81) | — |
| Former | 41 302 (39.98) | 25 533 (39.30) | 7895 (41.09) | 7874 (41.16) | — |
| Never | 47 121 (45.61) | 30 052 (46.25) | 9028 (46.98) | 8041 (42.03) | — |
| Physical activity level‡ | <0.001 | ||||
| Low | 21 823 (21.11) | 13 374 (20.57) | 4167 (21.68) | 4282 (22.37) | — |
| Moderate | 38 376 (37.12) | 23 937 (36.81) | 7420 (38.60) | 7019 (36.67) | — |
| High | 28 868 (27.92) | 18 807 (28.92) | 5070 (26.38) | 4991 (26.08) | — |
| Number of 24 h dietary records (mean (SD)) | 5.59 (3.05) | 5.31 (3.01) | 6.81 (3.10) | 5.28 (2.83) | <0.001 |
| Energy intake without alcohol, kcal/day (mean (SD)) | 1898.02 (469.80) | 1911.4 (477.34) | 1888.42 (432.76) | 1862.19 (477.55) | <0.001 |
| Alcohol intake, g/day (mean (SD)) | 7.71 (11.71) | 8.05 (12.19) | 7.51 (10.79) | 6.79 (10.88) | <0.001 |
| SFA intake, g/day (mean (SD)) | 33.18 (12.13) | 33.59 (12.29) | 33.16 (11.19) | 31.81 (12.39) | <0.001 |
| PUFA intake, g/day (mean (SD)) | 11.5 (4.94) | 11.63 (5.17) | 11.31 (4.30) | 11.25 (4.75) | <0.001 |
| Sodium intake, mg/day (mean (SD)) | 2705.38 (880.94) | 2698.58 (897.29) | 2711.43 (812.38) | 2722.43 (890.75) | 0.003 |
| Dietary fibre intake, g/day (mean (SD)) | 19.4 (7.19) | 19.75 (7.48) | 18.93 (6.26) | 18.69 (7.00) | <0.001 |
| Total sugar intake, g/day (mean (SD)) | 92.69 (33.11) | 92.11 (33.49) | 94.62 (30.56) | 92.73 (34.16) | <0.001 |
| Added sugar intake, g/day (mean (SD)) | 38.76 (23.73) | 38.5 (23.54) | 40.3 (22.46) | 38.12 (25.51) | <0.001 |
| Percentage of energy from added sugar (mean (SD)) | 8.20 (4.18) | 8.12 (4.16) | 8.58 (3.95) | 8.10 (4.45) | <0.001 |
| Sugary drinks, mL/day (mean (SD)) | 47.84 (106.70) | 42.77 (103.87) | 55.15 (97.73) | 57.72 (122.53) | <0.001 |
| Non-alcoholic beverages with no added sugars, mL/day (mean (SD)) | 1088.67 (536.6) | 1063.93 (529.25) | 1092.54 (517.02) | 1168.85 (571.66) | <0.001 |
| Fruit and vegetable intake, g/day (mean (SD)) | 404.83 (220.04) | 409.35 (222.68) | 397.73 (196.93) | 396.59 (232.24) | <0.001 |
| Red and processed meat intake, g/day (mean (SD)) | 76.41 (52.70) | 74.53 (53.23) | 76.10 (46.75) | 83.09 (55.89) | <0.001 |
| Whole-grain food intake, g/day (mean (SD)) | 34.16 (45.97) | 35.71 (49.16) | 31.42 (38.40) | 31.66 (41.29) | <0.001 |
| Ultra-processed foods intake, % of the diet in g/day (mean (SD)) | 17.40 (9.90) | 15.96 (9.12) | 17.42 (8.61) | 22.27 (11.92) | <0.001 |
| Weight loss diet during the first two years of follow-up | 17 585 (17.01) | 7729 (11.89) | 3654 (19.01) | 6202 (32.41) | <0.001 |
| Artificial sweetener intake, mg/day (mean (SD)) | 15.76 (48.57) | 0.00 (0.00) | 7.46 (4.95) | 77.62 (89.33) | <0.001 |
| Aspartame (E951) intake, mg/day (mean (SD)) | 9.13 (30.95) | 0.00 (0.00) | 3.16 (3.96) | 46.13 (58.91) | <0.001 |
| Acesulfame potassium (E950) intake, mg/day (mean (SD)) | 4.60 (14.90) | 0.00 (0.00) | 2.71 (2.79) | 22.14 (28.43) | <0.001 |
| Sucralose (E955) intake, mg/day (mean (SD)) | 1.59 (16.17) | 0.00 (0.00) | 1.08 (1.94) | 7.49 (36.95) | <0.001 |
CVD=cardiovascular disease; PUFA=polyunsaturated fatty acid; SD=standard deviation; SFA=saturated fatty acid.
Values are numbers (percentages) unless stated otherwise. 1 kcal=4.18 kJ=0.00418 MJ.
Categories of consumption were defined as non-consumers, lower consumers, and higher consumers, separated by the sex specific median among consumers, that is, 16.44 mg/day in men and 18.46 mg/day in women.
P values for crude comparison between the three categories of sweetener intake by analysis of variance or χ2 test when appropriate.
Available for 89 067 participants, categorised into high, moderate, and low categories according to International Physical Activity Questionnaire guidelines.
Associations between artificial sweeteners, overall and the most represented (aspartame, acesulfame potassium, and sucralose, consumed by more than 5% of participants), and CVD (overall, coronary heart disease, and cerebrovascular disease) were investigated using multivariable adjusted Cox proportional hazard models (table 2). Participants contributed person time from their inclusion in the cohort until the date of CVD, date of last follow-up, date of death, or 5 October 2021, whichever occurred first. We first tested dose-response analyses using the restricted cubic spline (RCS) functions with the SAS macro developed by Desquilbet and Mariotti.56 Given the logarithmic profile of the associations suggested by the RCS curves (supplementary fig 2) and to account for the large proportion of non-consumers (especially for each specific artificial sweetener), artificial sweetener intakes were log transformed (log10 of sweetener consumption in mg/g+1) to compute continuous models (+1 was uniformly added to all consumptions because log(0) is not allowed). The continuous model was used as the primary analyses to obtain hazard ratios and 95% confidence intervals. Supplementary tables 1 and 2 presentmodels using three categories (non-consumers, lower consumers, and higher consumers, separated by the sex specific median) and four categories (non-consumers and sex specific consumers in thirds) of sweetener consumption.
Table 2.
Associations between intake of total artificial sweeteners, aspartame, acesulfame potassium, and sucralose and overall cardiovascular diseases, coronary heart diseases and cerebrovascular diseases, NutriNet-Santé cohort, France, 2009-21 (n=103 388)
| Outcome and exposure | Hazard ratio (95% CI) | P value |
|---|---|---|
| Cardiovascular diseases (n=1502) | ||
| Total artificial sweeteners | 1.09 (1.01 to 1.18) | 0.03 |
| Aspartame | 1.03 (0.94 to 1.14) | 0.49 |
| Acesulfame potassium | 1.18 (0.98 to 1.41) | 0.08 |
| Sucralose | 1.11 (0.92 to 1.34) | 0.28 |
| Coronary heart diseases (n=730) | ||
| Total artificial sweeteners | 1.02 (0.91 to 1.14) | 0.79 |
| Aspartame | 0.91 (0.78 to 1.06) | 0.22 |
| Acesulfame potassium | 1.40 (1.06 to 1.84) | 0.02 |
| Sucralose | 1.31 (1.00 to 1.71) | 0.05 |
| Cerebrovascular diseases (n=777) | ||
| Total artificial sweeteners | 1.18 (1.06 to 1.31) | 0.002 |
| Aspartame | 1.17 (1.03 to 1.33) | 0.02 |
| Acesulfame potassium | 1.01 (0.79 to 1.29) | 0.93 |
| Sucralose | 0.99 (0.76 to 1.29) | 0.93 |
Median follow-up times for overall cardiovascular, coronary heart, and cerebrovascular diseases were all 9.0 years; person years were 904 205, 904 270, and 904 259, respectively. Exposure was coded as log10 of artificial sweetener intake in mg/day+1. Main models were adjusted for age (time scale), sex, physical activity (categorical: International Physical Activity Questionnaire variable: high, moderate, low, missing value), smoking status (categorical: never, former, current smoker), number of smoked cigarettes in pack years (continuous), higher educational level (categorical: less than high school degree, <2 years after high school degree, ≥2 years after high school degree), family history of cardiovascular disease (categorical: yes, no), energy intake without alcohol (continuous: kcal/day), daily intakes (continuous: g/day) of alcohol, sodium, saturated fatty acids, polyunsaturated fatty acids, fibre, sugar, fruit and vegetables, red and processed meat. Models were also mutually adjusted for sweetener intake other than the one studied (continuous).
The main models were adjusted for several variables suspected or known to be associated with diet and with CVD risk: sociodemographic (age, sex, educational level), lifestyle (smoking status, number of smoked cigarettes, physical activity), and health (family history of CVD) factors, and food groups and nutrients for which a role in CVD cause has been strongly suggested57 58 59 60 61 62 63 64 65: energy intake without alcohol, alcohol, sugar, sodium, saturated fatty acids, polyunsaturated fatty acids, fibre, fruit and vegetables, and red and processed meat. We added a table showing the rationale for selection of each covariate and information on how they were collected and measured (supplementary method 4). Analyses by specific artificial sweeteners (aspartame, acesulfame potassium, and sucralose) were additionally adjusted for other artificial sweetener intakes. Multiple imputation by chained equations66 was applied to handle any missing values for covariates (15 imputed datasets; supplementary method 5). Cox proportional hazard assumption was verified using the rescaled Schoenfeld type residual method (supplementary fig 3). Competing risks were accounted for in all analyses using cause specific Cox models,67 with death considered a competing risk for CVDs, coronary heart diseases, and cerebrovascular diseases. Additionally, cerebrovascular events were considered competing risks for coronary heart diseases and vice versa. Supplementary table 3 presents results from competing events. Cumulative incidence graphs were also plotted using the Fine and Gray model (presented in supplementary fig 4).
Associations were computed separately for each type of cerebrovascular or coronary disease event: myocardial infarction, acute coronary syndrome, angioplasty, angina pectoris, stroke and transient ischaemic event (supplementary table 4), and for all CVDs except transient ischaemic events. We also investigated associations between CVD risk and artificial sweeteners from beverages and from solid food (supplementary table 5). Substitution analyses were performed by entering added sugars and artificial sweeteners into the model. Hazard ratios and 95% confidence intervals for substituting artificial sweeteners for added sugars were estimated using the difference in coefficients obtained from this model. Supplementary method 6 presents these analyses. Formal interactions between body mass index (<25 or ≥25) and artificial sweeteners were tested for each outcome by entering the product of the two variables into Cox models.
We performed a sensitivity analysis in which we doubled the requested minimal number of 24 h dietary records (excluding participants with less than four records; supplementary table 6). Additionally, we computed models with artificial sweetener intakes coded as time dependent variables across the whole follow-up period (supplementary table 6). Other sensitivity analyses were also performed, with further adjustments for prevalent dyslipidaemia, for healthy and western dietary patterns (derived by principal components analysis) instead of food groups, added sugar intakes instead of sugar, proportion of ultra-processed foods in the diet, weight loss or calorie restricted diet, weight variation during follow-up, number of 24 h dietary records, body mass index, and social desirability score68; and analyses without excluding prevalent diabetes (details presented in supplementary table 6). All tests were two sided, and P<0.05 was considered statistically significant. We used the statistical analysis software SAS, version 9.4 for analyses.
Patient and public involvement
The research question developed in this article corresponds to a concern expressed by some participants involved in the NutriNet-Santé cohort, and by the public in general. Participants in the study are thanked in the Acknowledgments section.
Results
Descriptive characteristics
Overall, 103 388 participants were selected from the NutriNet-Santé cohort. Mean age at baseline was 42.2 years (standard deviation 14.4), 79.8% were women, and the mean number of 24 h dietary records during the first two years of follow-up was 5.6 (standard deviation 3.1). Supplementary figure 5 shows the distribution of the number of 24 h dietary records per person. Among the overall cohort, 0.94% (n=1639) participants have died since their inclusion (981 in the present population study) and 9.4% (n=16 306) dropped out because they did not want to receive any more questionnaires. A total of 37.1% of participants consumed artificial sweeteners. The average intake of artificial sweeteners was 15.76 mg/day among all participants and 42.46 mg/day among consumers only, which corresponds to approximately one individual packet of table top sweetener or 100 mL of diet soda.69 70 Among participants who consumed artificial sweeteners, mean intakes for lower and higher consumer categories were 7.46 and 77.62 mg/day, respectively. Compared with non-consumers, higher consumers (unadjusted comparisons) tended to be younger, have a higher body mass index, were more likely to smoke, be less physically active, and to follow a weight loss diet; they had lower total energy intake, and lower alcohol, lipid (saturated and polyunsaturated), fibre, carbohydrate, fruit and vegetable intakes, and higher intakes of sodium, red and processed meat, dairy products, and beverages with no added sugar (table 1). Aspartame, acesulfame potassium, and sucralose contributed to 58%, 29%, and 10% of total artificial sweetener intakes, respectively (fig 1). Soft drinks with no added sugar accounted for 53% of artificial sweeteners; table top sweeteners were also an important vector (30%), as well as artificially sweetened flavoured dairy products (eg, yoghurts, cottage cheese, 8%; fig 2). As shown in supplementary figure 6, food group contributions varied for each artificial sweetener; for example, table top sweeteners contributed to 48% of aspartame intake, followed by soft drinks with no added sugar (41%), whereas acesulfame potassium and sucralose were both mainly provided by the consumption of soft drinks with no added sugar (76% and 78%, respectively). Participants who consumed artificial sweeteners tended to consume more than one type of the main artificial sweeteners, and 7.23% of the total participants consumed all three of the main types (supplementary fig 7).
Fig 1.
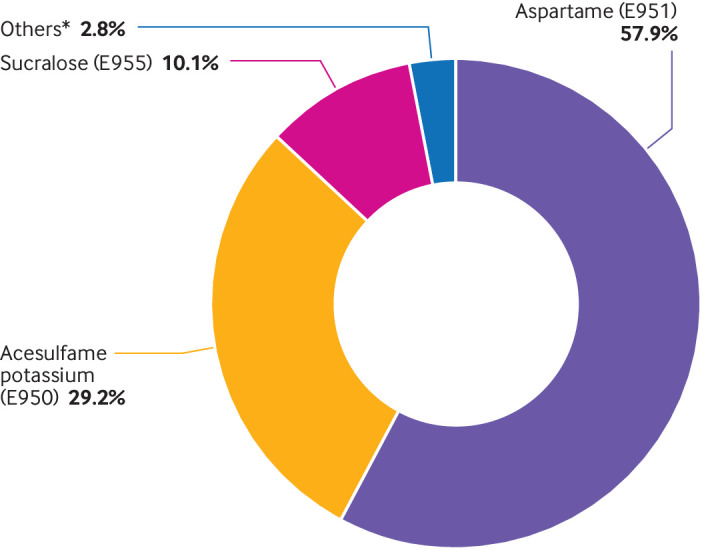
Relative contribution of each specific artificial sweetener to the total intake of artificial sweeteners (%), NutriNet-Santé cohort, France, 2009-21 (n=103 388). *Cyclamates (E952), saccharin (E954), thaumatin (E957), neohesperidine dihydrochalcone (E959), steviol glycoside (E960), aspartame-acesulfame salt (E962)
Fig 2.
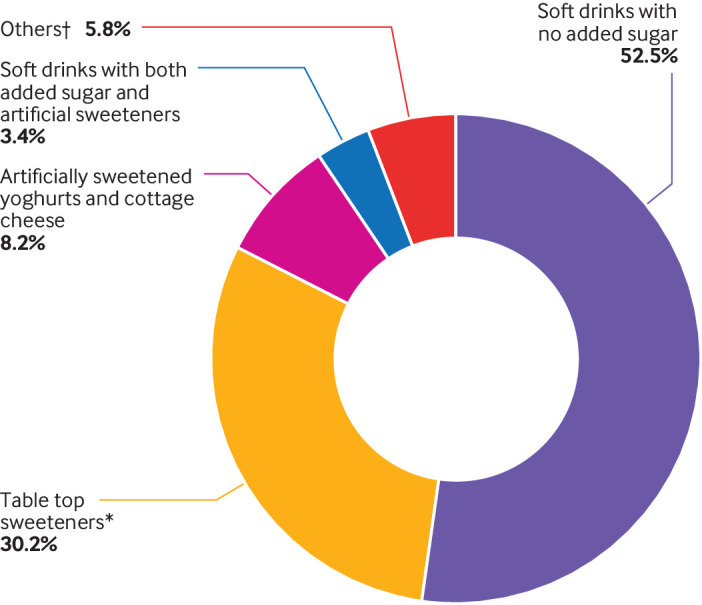
Relative contribution of each food group to the total intake of artificial sweeteners (%), NutriNet-Santé cohort, France, 2009-21 (n=103 388). *Used as tablets, liquid, or powder, added by the participants in yoghurts, hot drinks and so on, or for cooking. †High protein food substitutes, sugary foods, cookies, biscuits, cakes, pastries, breakfast cereals, sauces, savoury foods, ultra-processed fish products
Associations between artificial sweetener intakes and cardiovascular diseases
During follow-up (904 206 person years; median follow-up duration 9.0 years, interquartile range 7.5-10.1 years), 1502 incident cardiovascular events occurred, among which there were 730 coronary heart disease events (143 myocardial infarction, 75 acute coronary syndrome, 477 angioplasty, and 277 angina pectoris events) and 777 cerebrovascular disease events (203 strokes and 598 transient ischaemic events). Mean age at CVD event was 62.7 years (standard deviation 12.9). The RCS analyses suggested a log shaped association (increased risk followed by a plateau; P for non-linearity=0.067, 0.494, 0.016, and 0.021 for total artificial sweetener, aspartame, acesulfame potassium, and sucralose, respectively, for the overall CVD model; supplementary fig 2).
Total artificial sweetener intake was associated with increased risk of CVD (hazard ratio 1.09, 95% confidence interval 1.01 to 1.18, P=0.03; table 2); absolute incidence rate in higher consumers (above the sex specific median) was 346 per 100 000 person years, and in non-consumers it was 314 per 100 000 person years. Artificial sweeteners were more particularly associated with cerebrovascular disease risk (1.18, 1.06 to 1.31, P=0.002; incidence rates 195 and 150). Aspartame intake was associated with increased risk of cerebrovascular events (1.17, 1.03 to 1.33, P=0.02; incidence rates 186 and 151), and acesulfame potassium and sucralose were associated with increased coronary heart disease risk (acesulfame potassium: 1.40, 1.06 to 1.84, P=0.02; incidence rates 167 and 164; sucralose: 1.31, 1.00 to 1.71, P=0.05; incidence rates 271 and 161). Results were similar when artificial sweetener intakes were coded as time dependent variables (supplementary table 6). For each type of cerebrovascular disease or coronary heart disease, direct associations were observed between sucralose and risk of angioplasties (n=477; 1.60, 1.17 to 2.21, P=0.004) and between total artificial sweeteners and transient ischaemic events (n=598; 1.18, 1.05 to 1.33, P=0.006). For artificial sweeteners from beverages or solid food, associations were statistically significant between sweeteners from beverages and CVD risk (P=0.02) and between aspartame from beverages and coronary heart disease risk (P=0.03). Associations were borderline between acesulfame potassium and sucralose from beverages and coronary heart diseases (P=0.06 and P=0.08, respectively), and between aspartame, acesulfame potassium, and sucralose from solid food sources and cerebrovascular diseases (P=0.006, P=0.01, and P=0.002, respectively; supplementary table 5). Substitution analyses did not suggest a benefit for substituting artificial sweeteners for added sugars for CVD risk (hazard ratio 1.00, 95% confidence interval 0.99 to 1.01, P=0.28), cerebrovascular disease risk (1.00, 0.99 to 1.01, P=0.89), or coronary heart disease risk (1.00, 0.99 to 1.01, P=0.13; supplementary method 6). The results were stable across all sensitivity analyses tested (supplementary table 6). The artificial sweetener by body mass index variable was not statistically significant for overall cardiovascular disease, coronary heart disease, and cerebrovascular disease (all P>0.05), suggesting no interaction on the multiplicative scale.
Discussion
Principal findings
In the NutriNet-Santé cohort, total artificial sweetener intake was associated with increased risk of overall CVD and cerebrovascular disease. Aspartame intake was associated with increased risk of cerebrovascular events, and acesulfame potassium and sucralose were associated with increased coronary heart disease risk. Our results suggest no benefit from substituting artificial sweeteners for added sugar on CVD outcomes.
Strengths and limitations of this study
This study was based on a large sample size (n=103 388) and prospectively investigated the associations between artificial sweetener intake from all dietary sources and CVD risk. There is no perfect measure of dietary consumption, therefore classification bias cannot be ruled out. However, the assessment of artificial sweetener consumption performed in this study was a comprehensive assessment at the individual level in a large scale population based cohort. The NutriNet-Santé study is an epidemiological cohort with precise and high quality dietary data. Dietary records have previously been validated by interviews with a trained dietitian33 and against blood and urinary biomarkers for energy and nutrient intakes.34 35 Epidemiological studies worldwide generally use food frequency questionnaires (known to be less precise than repeated 24 h dietary records71) or a limited number of records or recalls at baseline.
The main vectors of artificial sweeteners are products that are generally consumed on a regular basis as part of daily dietary habits, including artificially sweetened beverages, table top sweeteners, and dairy products. Occasional artificial sweetener consumption is not likely to have a strong impact on CVD risk, and so even if some consumption might have been missed, it would probably have had a low impact on the study results. If there was a classification bias, it was non-differential due to the prospective design. Additionally, a sensitivity analysis was performed in the subgroup of participants with at least four records (mean 7.8, standard deviation 2.4, n=57 668), which doubled the minimal number of 24 h dietary records needed to be included in the analysis, and the results remained similar. Twenty four hour dietary recording days were decided in advance, which might have influenced the behaviour of participants on these days; however, adjustment for social desirability bias did not modify the findings, and the comprehensive recording enabled memory bias to be limited. In contrast to previous observational studies, artificially sweetened beverages were not used as a proxy to estimate artificial sweetener intakes. Detailed information on the brands of food or beverage consumed are collected as part of the NutriNet-Santé study. The dynamic date-to-date matching performed between the interactive web based 24 h dietary records and specific ingredient lists allowed the additive composition of industrial products to be identified, accounting for potential reformulations.51
Some limitations should be discussed. Residual confounding cannot be totally excluded and no causal relation can be established with results from a unique observational study. However, models were adjusted for a wide range of potential sociodemographic, anthropometric, dietary, and lifestyle confounders. Further adjustment for the proportion of ultra-processed food in the diet was conducted, ensuring that the associations observed were not entirely driven by following an ultra-processed diet in general.72 Additionally, reverse causality could lead to higher artificially sweetened food and beverage consumption among participants who were overweight or obese, and already had poorer cardiovascular health at baseline before CVD diagnosis.73 74 However, this factor probably does not entirely explain the observed associations because we excluded CVD events occurring during the first two years of follow-up and we also tested models adjusted for baseline body mass index, weight loss diet, and weight change during follow-up, which did not substantially change the results.
Caution is needed to generalise these results to the whole French population. As generally observed in volunteer based cohorts, participants from the NutriNet-Santé study were more often women, with higher educational and socio-professional levels, and they were more likely to have a health conscious lifestyle and good dietary behaviours.75 Therefore, artificial sweetener intake among NutriNet-Santé participants could be lower compared with French adults in general. Mean intakes of aspartame and acesulfame potassium for consumers in the cohort were 0.49 and 0.22 mg/kg body weight/day, respectively versus 1.29 and 0.73 mg/kg body weight/day, respectively estimated in the French population.76 These intakes suggest that the associations found in our study between artificial sweetener consumption and risk of CVD might be underestimated. However, our assessment was more accurate than the one previously performed for the general French population,76 which was based on three days of dietary records by participants at most, and brand specific composition was not accounted for.
The order of magnitude obtained for the associations in this study is in line with the one traditionally observed in nutritional epidemiology studies for commonly consumed dietary factors,72 77 78 and with the findings of WHO in its recent report,8 which was based on meta-analyses of prospective cohort studies investigating intake of beverages containing artificial sweeteners.25 26 27 28 29 30 31 32 Furthermore, in terms of public health perspectives, the opportunity of preventing even a moderate proportion of CVD events through reduced artificial sweetener intake is of high interest given the extensive use of these substances in products on the global market. Associations were consistent across the many sensitivity analyses we performed; they were also consistent with previous epidemiological literature on proxies of sweetener intakes (eg, artificially sweetened beverages) and in line with mechanistic insights from experimental studies. All observed associations between sweetener intakes and CVD events went in the same (positive) direction, which is not in favour of random findings observed by chance.
The two complementary methods (self-reporting and medico-administrative databases) ensured good identification of CVD outcomes. However, the possibility of missing some events cannot be entirely ruled out. Additionally, despite efforts to identify transient ischaemic attacks as objectively as possible (based on medical or hospital reports, if possible a specialised neurological diagnosis, computed tomography or magnetic resonance imaging scan, or symptoms precisely described by the participant or a person close to them), these CVD events could not be diagnosed with the same certainty as for strokes or myocardial infarctions because they generally do not reveal sequelae on brain imaging. Finally, limited statistical power might have prevented us from detecting some associations for specific CVD pathologies.
Comparison with other studies
Observational prospective studies on the associations between artificial sweeteners, assessed from the whole diet (in mg/day), and CVD risk are lacking; therefore, no direct comparison was possible. However, several studies have been conducted25 26 27 28 29 30 31 32 33 34 and meta-analysed8 22 35 36 73 79 using artificially sweetened beverage consumption as a proxy (in mL or serving/day) and CVD risk. In line with recent results from the NutriNet-Santé study,28 multiple cohorts found associations between artificially sweetened beverages and CVD. Higher artificially sweetened beverage consumption was associated with increased risks of stroke and cardiovascular events in the Women’s Health Initiative,26 29 which is consistent with prospective investigations from the Nurses’ Health Study, the Health Professional Follow-up Study (HPFS),25 30 the Framingham Offspring cohort,31 and the Northern Manhattan Study.27 Similarly, meta-analyses reported increased risks of stroke, vascular events, coronary heart diseases, CVDs, and CVD mortality.35 36 73 79 Consistent with our findings, no association was observed for coronary heart diseases in the HPFS.33 These studies mostly took place in the United States35 and have not been as extensively explored in European populations. In line with our results, the recent WHO meta-analyses8 reported positive associations between the intake of beverages containing artificial sweeteners and cardiovascular events overall (hazard ratio 1.32, 95% confidence interval 1.17 to 1.50, three prospective studies26 27 28) and more specifically for the incidence of stroke (1.19, 1.09 to 1.29, five prospective studies25 27 29 31 32), but not for coronary heart disease (1.16, 0.97 to 1.39, four prospective studies27 29 33 80).
Meta-analyses performed by Azad and colleagues22 also suggested associations between high intake of drinks with non-nutritive sweeteners and higher risk of strokes25 and cardiovascular events,26 27 but no significant associations were found for coronary heart diseases.33 80 However, other studies suggested associations between artificially sweetened beverages and stroke but also coronary heart diseases.29 Differences between results for coronary heart and cerebrovascular diseases could be because these pathologies have different causes and therefore, although they might share common nutritional determinants, others might play a different role in the development of these diseases. Each type of artificial sweetener might not have the same metabolic effect.14 For instance, after ingestion, acesulfame potassium is absorbed from the small intestine and distributed to the blood and tissues through the systemic circulation and then excreted in urine. However, sucralose passes through digestion and is almost entirely excreted in the stools; only a small part is absorbed from the gastrointestinal tract. The aspartame molecule is broken down in different amino acids: aspartic acid and phenylalanine are sent to the systemic circulation while methanol is metabolised by the liver.14 Because this study quantified the intake of each specific sweetener and investigated the association with CVD risk, overall and by type, future epidemiological studies and experimental data will be needed to further investigate a potential differential effect of artificial sweeteners according to cerebrovascular or coronary CVD types.
Furthermore, according to WHO8 and as mentioned in the systematic reviews by Toews and colleagues and Zhang and colleagues,23 73 randomised controlled trials investigating the long term effects of artificial sweeteners on the risk of hard endpoints such as CVD are lacking. However, some have studied early markers of cardiovascular health, such as weight variations, hypertension, or blood glucose level.81 82 Most of these studies were conducted among participants with particular conditions (eg, people who were overweight or those with prevalent hypertension)22 and were of short duration (follow-up around six months), with a level of evidence ranging from very low to moderate.23 83 Additionally, it should be noted that many studies investigating the health effects of artificial sweeteners are funded by the industry, notably several randomised control trials included in reviews and meta-analyses. Azad and colleagues reported that industry sponsored randomised controlled trials suggest greater weight loss results compared with studies not financed by industry.22 For instance, a systematic review has specifically studied the issue of conflict of interest in this field84 and revealed that reviews sponsored by the artificial sweetener industry were more inclined to show beneficial weight loss effects. Therefore, no firm conclusion could be drawn from randomised controlled trials about the cardiometabolic impact of artificial sweeteners. However, several of these randomised controlled trials observed increased associations with several cardiometabolic outcomes, suggesting mechanistic plausibility for an impact of artificial sweeteners on cause of CVD.
Mechanistic plausibility from experimental studies
In some prospective cohort studies, associations have been reported between artificially sweetened beverage consumption and increased risk of obesity or weight gain.8 22 85 86 Low calorie sweeteners (from beverages, table top sweeteners, and foods) were associated with obesity in the National Health and Nutrition Examination Survey87 and abdominal obesity in the Baltimore Longitudinal Study of Ageing.88 A cross-sectional study also found that consumers of diet soft drinks had greater waist circumference.89 In the PREDIMED study (multicentre randomised trial) there was a positive association between artificially sweetened beverages and abdominal obesity.90 Therefore, part of the associations between artificial sweeteners and CVD risk observed in our study might be because of weight gain. However, the associations observed here are probably not entirely driven by increased body weight. The impact of artificial sweeteners on weight gain is debated.8 9 22 23 Some randomised controlled trials found no effect on body weight when replacing sugar sweetened beverages with artificially sweetened versions,91 and others suggested decreased body weight, body mass index, fat mass, and waist circumference.83 85 Adjustment for baseline body mass index and weight gain during follow-up did not modify the findings.
Other underlying mechanisms could be causally involved.92 93 Meta-analyses suggest associations between artificially sweetened beverages and metabolic syndrome,94 95 a cardiometabolic risk factor defined by dyslipidaemia, abdominal obesity, high blood glucose, insulin resistance, and hypertension.96 Artificially sweetened beverages were associated with increased risk of metabolic syndrome89 90 97 in a cohort study,97 a cross-sectional study,89 and a multicentre randomised trial.90 More specifically, associations were observed with increased hypertension,8 98 99 type 2 diabetes,8 87 100 and hypertriglyceridemia.8 90 Another potential pathway could involve the interaction of artificial sweeteners with intestinal sweet taste receptors,92 which play a part in insulin secretion and glucose absorption. Experimental studies (rodent models) suggest that glucose and energy homoeostasis could be altered by artificial sweeteners.11 101 Ingestion of sugar by animals accustomed to artificial sweeteners could lead to low glucagon like peptide 1 levels (which normally stimulate insulin secretion) and induce hyperglycaemia,101 which could also be observed in humans.92 Additionally, the alteration of gut microbiota by some artificial sweeteners could increase glucose intolerance,17 but the results remain conflicting.20 Vascular dysfunction, which contributes to CVD onset and development, after the ingestion of artificial sweeteners, has been observed in experimental studies (rodent models)15 and in vitro (human cellular model),16 and could also play a part in the risk of CVD. Finally, Basson and colleagues14 suggest that artificial sweetener consumption might be associated with increased inflammation, a risk factor for CVD.96
Policy implications and conclusions
In conclusion, these findings suggest that higher artificial sweetener consumption might be associated with increased risk of CVDs. Further well designed, large scale prospective studies need to confirm these results and experimental studies should be conducted to clarify biological pathways. In the meantime, this study provides key insights into the context of artificial sweetener re-evaluation by the EFSA, WHO, and other health agencies worldwide. Our results indicate that these food additives, consumed daily by millions of people and present in thousands of foods and beverages, should not be considered a healthy and safe alternative to sugar, in line with the current position of several health agencies.23 102
What is already known on this topic
The harmful effects of added sugars have been established for several chronic diseases, leading food industries to use artificial sweeteners as alternatives in a wide range of foods and beverages
The safety of artificial sweeteners is debated and study findings remain divided about their role in the cause of various diseases
The negative influence of these food additives on cardiovascular disease has been suggested in experimental studies, but data from human studies remain limited and previous observational studies have focused solely on artificially sweetened beverages used as a proxy
What this study adds
In this large scale, prospective cohort of French adults, artificial sweeteners (especially aspartame, acesulfame potassium, and sucralose) were associated with increased risk of cardiovascular, cerebrovascular, and coronary heart diseases
The results suggest that artificial sweeteners might represent a modifiable risk factor for cardiovascular disease prevention
The findings indicate that these food additives, consumed daily by millions of people and present in thousands of foods and beverages, should not be considered a healthy and safe alternative to sugar, in line with the current position of several health agencies
Acknowledgments
We thank Thi Hong Van Duong, Régis Gatibelza, Jagatjit Mohinder, Aladi Timera, Rizvane Mougamadou (computer scientists), Julien Allegre, Nathalie Arnault, Laurent Bourhis, Nicolas Dechamp (data manager/statisticians), Merveille Kouam (health event validator), and Maria Gomes (Nutrinaute support) for their technical contribution to the NutriNet-Santé study. We sincerely thank all the volunteers of the NutriNet-Santé cohort.
Web extra.
Extra material supplied by authors
Web appendix: online data supplements
Contributors: CD, EC, MDT, BS, MT: designed the study; EC, NDP, YE, FSdE, CA, ADS, RL, SH: developed the additives composition database and matched consumption/composition data; CA: coordinated dietitian work; FSdE: data management work; NDP, YE: global technical work; NDP, YE, EC, MT: supervised this technical work; CD: performed statistical analysis; EC, MDT, BS, MT: supervised statistical analysis; CD: drafted the manuscript; MT: supervised the writing. All authors contributed to the data interpretation and revised each draft for important intellectual content. All authors read and approved the final manuscript. CD and MT had full access to all the data in the study, MT takes responsibility for the integrity of the data and the accuracy of the data analysis; she is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The NutriNet-Santé study was supported by the following public institutions: Ministère de la Santé, Santé Publique France, Institut National de la Santé et de la Recherche Médicale (INSERM), Institut national de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), Conservatoire National des Arts et Métiers (CNAM) and Université Sorbonne Paris Nord. CD was supported by a grant from the French National Cancer Institute (INCa, grant No 2019-158). EC was supported by a doctoral fellowship from Université Sorbonne Paris Nord to Galilée Doctoral School. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (consolidator grant agreement No 864219), the French National Cancer Institute (INCa_14059), the French Ministry of Health (arrêté 29.11.19) and the IdEx Université Paris Cité (ANR-18-IDEX-0001). This project was awarded the NACRe (French network for Nutrition and Cancer Research) Partnership Label and was awarded the Bettencourt Schueller foundation prize Coup d’élan pour la recherche française 2021. Researchers were independent from funders. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from Ministère de la Santé, Santé Publique France, Institut National de la Santé et de la Recherche Médicale (INSERM), Institut national de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), Conservatoire National des Arts et Métiers (CNAM) and Université Sorbonne Paris Nord, European Research Council, the French National Cancer Institute, the French Ministry of Health, and the IdEx Université Paris Cité for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: The results of the present study will be disseminated to the NutriNet-Santé participants through the cohort website, where lay summaries of all publications are posted (https://etude-nutrinet-sante.fr/link/zone/43-Publications). Additionally, results will be disseminated in public seminars and by a press release from the Journal or the French Medical Institute for Health and Medical Research, in association with Inrae, Cnam and Sorbonne Paris Nord communication and direction boards. This press release will be posted on their website and sent to their journalist contact book in France, Europe, and abroad (translated in English) as well as through their social medias Facebook and Twitter.
Provenance and peer review: Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Electronic informed consent is provided by each person included in the NutriNet-Santé cohort. The study is registered at https://clinicaltrials.gov/ct2/show/NCT03335644, conducted according to the Declaration of Helsinki guidelines and approved by the Institutional Review Board of the French Institute for Health and Medical Research (IRB-Inserm) and the Commission Nationale de l’Informatique et des Libertés (CNIL No 908450/909216).
Data availability statement
Researchers from public institutions can submit a collaboration request including information on the institution and a brief description of the project to collaboration@etude-nutrinet-sante.fr. All requests will be reviewed by the steering committee of the NutriNet-Santé study. If the collaboration is accepted, a data access agreement will be necessary and appropriate authorisations from the competent administrative authorities might be needed. In accordance with existing regulations, no personal data will be accessible.
References
- 1. Janzi S, Ramne S, González-Padilla E, Johnson L, Sonestedt E. Associations between added sugar intake and risk of four different cardiovascular diseases in a Swedish population-based prospective cohort study. Front Nutr 2020;7:603653. 10.3389/fnut.2020.603653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khan TA, Tayyiba M, Agarwal A, et al. Relation of total sugars, sucrose, fructose, and added sugars with the risk of cardiovascular disease: a systematic review and dose-response meta-analysis of prospective cohort studies. Mayo Clin Proc 2019;94:2399-414. 10.1016/j.mayocp.2019.05.034 [DOI] [PubMed] [Google Scholar]
- 3.WHO. Guideline: Sugars intake for adult and children. Geneva: World Health Organization; 2015. http://public.eblib.com/choice/publicfullrecord.aspx?p=2033879 [PubMed]
- 4. Bachmanov AA, Bosak NP, Floriano WB, et al. Genetics of sweet taste preferences. Flavour Fragr J 2011;26:286-94. 10.1002/ffj.2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Market Data Forecast. Global artificial sweetener market by type (aspartame, acesulfame-K, monosodium glutamate, saccharin, and sodium benzoate), by application (bakery items, dairy products, confectionery, beverages, and other), by distribution channel (supermarkets & hypermarkets, departmental stores, convenience stores, and others) and by regional analysis (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa) - global industry analysis, size, share, growth, trends, and forecast (2022-2027). 2022. https://www.marketdataforecast.com/
- 6.Open Food Facts 2020. https://world.openfoodfacts.org/discover
- 7. European Food Safety Authority (EFSA) . Outcome of the public consultation on a draft protocol for assessing exposure to sweeteners as part of their safety assessment under the food additives re-evaluation programme. EFSA Supporting Publications. 2020;17:1913E. [Google Scholar]
- 8.Rios-Leyvraz M, Montez J. Health effects of the use of non-sugar sweeteners: a systematic review and meta-analysis. Geneva: World Health Organization; 2022. https://www.who.int/publications/i/item/9789240046429
- 9. Pang MD, Goossens GH, Blaak EE. The impact of artificial sweeteners on body weight control and glucose homeostasis. Front Nutr 2021;7:598340. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7817779/. 10.3389/fnut.2020.598340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peters JC, Beck J, Cardel M, et al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance: a randomized clinical trial. Obesity (Silver Spring) 2016;24:297-304. 10.1002/oby.21327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davidson TL, Martin AA, Clark K, Swithers SE. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation. Q J Exp Psychol (Hove) 2011;64:1430-41. 10.1080/17470218.2011.552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feijó FM, Ballard CR, Foletto KC, et al. Saccharin and aspartame, compared with sucrose, induce greater weight gain in adult Wistar rats, at similar total caloric intake levels. Appetite 2013;60:203-7. 10.1016/j.appet.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 13. Kim Y, Je Y. Prospective association of sugar-sweetened and artificially sweetened beverage intake with risk of hypertension. Arch Cardiovasc Dis 2016;109:242-53. 10.1016/j.acvd.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 14. Basson AR, Rodriguez-Palacios A, Cominelli F. Artificial sweeteners: history and new concepts on inflammation. Front Nutr 2021;8:746247. 10.3389/fnut.2021.746247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Risdon S, Meyer G, Marziou A, Riva C, Roustit M, Walther G. Artificial sweeteners impair endothelial vascular reactivity: preliminary results in rodents. Nutr Metab Cardiovasc Dis 2020;30:843-6. 10.1016/j.numecd.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 16. Jang W, Jeoung NH, Cho KH. Modified apolipoprotein (apo) A-I by artificial sweetener causes severe premature cellular senescence and atherosclerosis with impairment of functional and structural properties of apoA-I in lipid-free and lipid-bound state. Mol Cells 2011;31:461-70. 10.1007/s10059-011-1009-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014;514:181-6. 10.1038/nature13793 [DOI] [PubMed] [Google Scholar]
- 18. Suez J, Korem T, Zilberman-Schapira G, Segal E, Elinav E. Non-caloric artificial sweeteners and the microbiome: findings and challenges. Gut Microbes 2015;6:149-55. 10.1080/19490976.2015.1017700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruiz-Ojeda FJ, Plaza-Díaz J, Sáez-Lara MJ, Gil A. Effects of sweeteners on the gut microbiota: a review of experimental studies and clinical trials. Adv Nutr 2019;10(suppl_1):S31-48. 10.1093/advances/nmy037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Plaza-Diaz J, Pastor-Villaescusa B, Rueda-Robles A, Abadia-Molina F, Ruiz-Ojeda FJ. Plausible biological interactions of low- and non-calorie sweeteners with the intestinal microbiota: an update of recent studies. Nutrients 2020;12:1153. 10.3390/nu12041153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bruyère O, Serge AH, Catherine A, et al. Review of the nutritional benefits and risks related to intense sweeteners. Arch Public Health 2015;73:41. 10.1186/s13690-015-0092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017;189:E929-39. 10.1503/cmaj.161390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ 2019;364:k4718. 10.1136/bmj.k4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joseph P, Leong D, McKee M, et al. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ Res 2017;121:677-94. 10.1161/CIRCRESAHA.117.308903 [DOI] [PubMed] [Google Scholar]
- 25. Bernstein AM, de Koning L, Flint AJ, Rexrode KM, Willett WC. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr 2012;95:1190-9. 10.3945/ajcn.111.030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vyas A, Rubenstein L, Robinson J, et al. Diet drink consumption and the risk of cardiovascular events: a report from the Women’s Health Initiative. J Gen Intern Med 2015;30:462-8. 10.1007/s11606-014-3098-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gardener H, Rundek T, Markert M, Wright CB, Elkind MSV, Sacco RL. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Intern Med 2012;27:1120-6. 10.1007/s11606-011-1968-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chazelas E, Debras C, Srour B, et al. Sugary drinks, artificially-sweetened beverages, and cardiovascular disease in the NutriNet-Santé Cohort. J Am Coll Cardiol 2020;76:2175-7. 10.1016/j.jacc.2020.08.075 [DOI] [PubMed] [Google Scholar]
- 29. Mossavar-Rahmani Y, Kamensky V, Manson JE, et al. Artificially sweetened beverages and stroke, coronary heart disease, and all-cause mortality in the Women’s Health Initiative. Stroke 2019;50:555-62. 10.1161/STROKEAHA.118.023100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation 2019;139:2113-25. 10.1161/CIRCULATIONAHA.118.037401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pase MP, Himali JJ, Beiser AS, et al. Sugar- and artificially sweetened beverages and the risks of incident stroke and dementia: a prospective cohort study. Stroke 2017;48:1139-46. 10.1161/STROKEAHA.116.016027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mullee A, Romaguera D, Pearson-Stuttard J, et al. Association between soft drink consumption and mortality in 10 European countries. JAMA Intern Med 2019;179:1479-90. 10.1001/jamainternmed.2019.2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012;125:1735-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hinkle SN, Rawal S, Bjerregaard AA, et al. A prospective study of artificially sweetened beverage intake and cardiometabolic health among women at high risk. Am J Clin Nutr 2019;110:221-32. 10.1093/ajcn/nqz094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meng Y, Li S, Khan J, et al. Sugar- and artificially sweetened beverages consumption linked to type 2 diabetes, cardiovascular diseases, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Nutrients 2021;13:2636. 10.3390/nu13082636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yin J, Zhu Y, Malik V, et al. Intake of sugar-sweetened and low-calorie sweetened beverages and risk of cardiovascular disease: a meta-analysis and systematic review. Adv Nutr 2021;12:89-101. 10.1093/advances/nmaa084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hercberg S, Castetbon K, Czernichow S, et al. The Nutrinet-Santé Study: a web-based prospective study on the relationship between nutrition and health and determinants of dietary patterns and nutritional status. BMC Public Health 2010;10:242. 10.1186/1471-2458-10-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lassale C, Peneau S, Touvier M, Julia C, Galan P, Hercberg S, et al. Validity of web-based self-reported weight and height: results of the Nutrinet-Sante study. J Med Internet Res 2013;15:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Touvier M, Mejean C, Kesse-Guyot E, Pollet C, Malon A, Castetbon K, et al. Comparison between web-based and paper versions of a self-administered anthropometric questionnaire. Eur J Epidemiol 2010;25:287-96. [DOI] [PubMed] [Google Scholar]
- 40. Vergnaud AC, Touvier M, Mejean C, Kesse-Guyot E, Pollet C, Malon A, et al. Agreement between web-based and paper versions of a socio-demographic questionnaire in the NutriNet-Sante study. Int J Public Health 2011;56:407-17. [DOI] [PubMed] [Google Scholar]
- 41. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 42. Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575-81. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 43. le Moullec N, Deheeger M, Preziosi P, et al. Validation du Manuel-photos utilisé pour l’enquête alimentaire de l’étude SU.VI.MAX. Cah Nutr Diét 1996;31:158-64. [Google Scholar]
- 44.Unité de recherche en épidémiologie nutritionnelle (Bobigny). Table de composition des aliments, Etude NutriNet-Santé. [Food composition table, NutriNet-Santé study] (in French). Paris: Les éditions INSERM/Economica; 2013.
- 45. Touvier M, Kesse-Guyot E, Mejean C, Pollet C, Malon A, Castetbon K, et al. Comparison between an interactive web-based self-administered 24 h dietary record and an interview by a dietitian for large-scale epidemiological studies. Br J Nutr 2011;105:1055-64. [DOI] [PubMed] [Google Scholar]
- 46. Lassale C, Castetbon K, Laporte F, Camilleri GM, Deschamps V, Vernay M, et al. Validation of a Web-based, self-administered, non-consecutive-day dietary record tool against urinary biomarkers. Br J Nutr 2015;113:953-62. [DOI] [PubMed] [Google Scholar]
- 47. Lassale C, Castetbon K, Laporte F, Deschamps V, Vernay M, Camilleri GM, et al. Correlations between fruit, vegetables, fish, vitamins, and fatty acids estimated by web-based nonconsecutive dietary records and respective biomarkers of nutritional status. J Acad Nutr Diet 2016;116:427-38. [DOI] [PubMed] [Google Scholar]
- 48. Black AE. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord. 2000;24:1119-30. [DOI] [PubMed] [Google Scholar]
- 49. Black AE. The sensitivity and specificity of the Goldberg cut-off for EI:BMR for identifying diet reports of poor validity. Eur J Clin Nutr 2000;54:395-404. 10.1038/sj.ejcn.1600971 [DOI] [PubMed] [Google Scholar]
- 50. Goldberg GR, Black AE, Jebb SA, et al. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 1991;45:569-81. [PubMed] [Google Scholar]
- 51. Chazelas E, Druesne-Pecollo N, Esseddik Y, et al. Exposure to food additive mixtures in 106,000 French adults from the NutriNet-Santé cohort. Sci Rep 2021;11:19680. 10.1038/s41598-021-98496-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Observatoire de l’alimentation (Oqali). OQALI - Home page. https://www.oqali.fr/oqali_eng/
- 53.Global New Products Database (GNPD). Banque de données mondiale de nouveaux produits, suivi des tendances nouveaux produits et innovations. 2022. https://www.gnpd.com/sinatra/anonymous_frontpage/
- 54.Food and Agriculture Organization/World Health Organization (FAO/WHO). Codex General Standard for Food Additives (GSFA, Codex STAN 192-1995). Codex Alimentarius Commission; 2019. https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B192-1995%252FCXS_192e.pdf
- 55. WHO . ICD-10: International statistical classification of diseases and related health problems. World Health Organization, 2011. [Google Scholar]
- 56. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037-57. 10.1002/sim.3841 [DOI] [PubMed] [Google Scholar]
- 57. Rodgers JLR, Jones J, Bolleddu SI, et al. Cardiovascular risks associated with gender and aging. journal of cardiovascular development and disease. 2019;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013-2020. 2013;55.
- 59. Kubota Y, Heiss G, MacLehose RF, Roetker NS, Folsom AR. Association of educational attainment with lifetime risk of cardiovascular disease: the Atherosclerosis Risk in Communities Study. JAMA Intern Med 2017;177:1165-72. 10.1001/jamainternmed.2017.1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lloyd-Jones DM, Nam BH, D’Agostino RB, Sr, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA 2004;291:2204-11. 10.1001/jama.291.18.2204 [DOI] [PubMed] [Google Scholar]
- 61. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(Suppl):1220S-8S, discussion 1229S-31S. 10.1093/ajcn/65.4.1220S [DOI] [PubMed] [Google Scholar]
- 62. Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med 2014;174:516-24. 10.1001/jamainternmed.2013.13563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Guasch-Ferré M, Babio N, Martínez-González MA, et al. PREDIMED Study Investigators . Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr 2015;102:1563-73. 10.3945/ajcn.115.116046 [DOI] [PubMed] [Google Scholar]
- 64. Threapleton DE, Greenwood DC, Evans CEL, Cleghorn CL, Nykjaer C, Woodhead C, et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 2013;347:f6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Papier K, Knuppel A, Syam N, Jebb SA, Key TJ. Meat consumption and risk of ischemic heart disease: a systematic review and meta-analysis. Crit Rev Food Sci Nutr 2021;0:1-12. 10.1080/10408398.2021.1949575 [DOI] [PubMed] [Google Scholar]
- 66. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012;41:861-70. 10.1093/ije/dyr213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tournois J, Mesnil F, Kop JL. Autoduperie et héteroduperie: Un instrument de mesure de la désirabilité sociale. [Self-deception and other-deception: a social desirability questionnaire]. European Review of Applied Psychology / Revue Européenne de Psychologie Appliquée 2000;50:219-33. 22253319 [Google Scholar]
- 69.Franz M. Amounts of sweeteners in popular diet sodas. Diabetes Self-Management. 2010. https://static.diabetesselfmanagement.com/pdfs/DSM0310_012.pdf
- 70.US Food and Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. 2018. https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states
- 71. Willett WC. Nutritional Epidemiology. Vol. 2. Oxford University Press, 1998. 10.1093/acprof:oso/9780195122978.001.0001. [DOI] [Google Scholar]
- 72. Srour B, Fezeu LK, Kesse-Guyot E, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ 2019;365:l1451. 10.1136/bmj.l1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang YB, Jiang YW, Chen JX, Xia PF, Pan A. Association of consumption of sugar-sweetened beverages or artificially sweetened beverages with mortality: a systematic review and dose–response meta-analysis of prospective cohort studies. Adv Nutr. 2021. [https://academic.oup.com/advances/advance-article/doi/10.1093/advances/nmaa110/5909627 [DOI] [PMC free article] [PubMed]
- 74. Pereira MA. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk. Adv Nutr 2014;5:797-808. 10.3945/an.114.007062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Andreeva VA, Salanave B, Castetbon K, Deschamps V, Vernay M, Kesse-Guyot E, et al. Comparison of the sociodemographic characteristics of the large NutriNet-Sante e-cohort with French Census data: the issue of volunteer bias revisited. J Epidemiol Community Health 2015;69:893-8. [DOI] [PubMed] [Google Scholar]
- 76. Vin K, Connolly A, McCaffrey T, et al. Estimation of the dietary intake of 13 priority additives in France, Italy, the UK and Ireland as part of the FACET project. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2013;30:2050-80. 10.1080/19440049.2013.851417 [DOI] [PubMed] [Google Scholar]
- 77. Debras C, Chazelas E, Srour B, et al. Artificial sweeteners and cancer risk: results from the NutriNet-Santé population-based cohort study. PLoS Med 2022;19:e1003950. 10.1371/journal.pmed.1003950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ho FK, Gray SR, Welsh P, et al. Associations of fat and carbohydrate intake with cardiovascular disease and mortality: prospective cohort study of UK Biobank participants. BMJ 2020;368:m688. 10.1136/bmj.m688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Narain A, Kwok CS, Mamas MA. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Clin Pract 2016;70:791-805. 10.1111/ijcp.12841 [DOI] [PubMed] [Google Scholar]
- 80. Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 2009;89:1037-42. 10.3945/ajcn.2008.27140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McGlynn ND, Khan TA, Wang L, et al. Association of low- and no-calorie sweetened beverages as a replacement for sugar-sweetened beverages with body weight and cardiometabolic risk: a systematic review and meta-analysis. JAMA Netw Open 2022;5:e222092. 10.1001/jamanetworkopen.2022.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wiebe N, Padwal R, Field C, Marks S, Jacobs R, Tonelli M. A systematic review on the effect of sweeteners on glycemic response and clinically relevant outcomes. BMC Med 2011;9:123. 10.1186/1741-7015-9-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Laviada-Molina H, Molina-Segui F, Pérez-Gaxiola G, et al. Effects of nonnutritive sweeteners on body weight and BMI in diverse clinical contexts: systematic review and meta-analysis. Obes Rev 2020;21:e13020. 10.1111/obr.13020 [DOI] [PubMed] [Google Scholar]
- 84. Mandrioli D, Kearns CE, Bero LA. Relationship between research outcomes and risk of bias, study sponsorship, and author financial conflicts of interest in reviews of the effects of artificially sweetened beverages on weight outcomes: a systematic review of reviews. PLoS One 2016;11:e0162198. 10.1371/journal.pone.0162198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr 2014;100:765-77. 10.3945/ajcn.113.082826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Qin P, Li Q, Zhao Y, et al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol 2020;35:655-71. 10.1007/s10654-020-00655-y [DOI] [PubMed] [Google Scholar]
- 87. Drewnowski A, Rehm CD. The use of low-calorie sweeteners is associated with self-reported prior intent to lose weight in a representative sample of US adults. Nutr Diabetes 2016;6:e202. 10.1038/nutd.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chia CW, Shardell M, Tanaka T, et al. Chronic low-calorie sweetener use and risk of abdominal obesity among older adults: a cohort study. PLoS One 2016;11:e0167241. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5120853/. 10.1371/journal.pone.0167241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Crichton G, Alkerwi A, Elias M. Diet soft drink consumption is associated with the metabolic syndrome: a two sample comparison. Nutrients 2015;7:3569-86. 10.3390/nu7053569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ferreira-Pêgo C, Babio N, Bes-Rastrollo M, et al. PREDIMED Investigators . Frequent consumption of sugar- and artificially sweetened beverages and natural and bottled fruit juices is associated with an increased risk of metabolic syndrome in a Mediterranean population at high cardiovascular disease risk. J Nutr 2016;146:1528-36. 10.3945/jn.116.230367 [DOI] [PubMed] [Google Scholar]
- 91. Ebbeling CB, Feldman HA, Steltz SK, Quinn NL, Robinson LM, Ludwig DS. Effects of sugar-sweetened, artificially sweetened, and unsweetened beverages on cardiometabolic risk factors, body composition, and sweet taste preference: a randomized controlled trial. J Am Heart Assoc 2020;9:e015668. 10.1161/JAHA.119.015668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pepino MY. Metabolic effects of non-nutritive sweeteners. Physiol Behav 2015;152:450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schiano C, Grimaldi V, Scognamiglio M, et al. Soft drinks and sweeteners intake: possible contribution to the development of metabolic syndrome and cardiovascular diseases. Beneficial or detrimental action of alternative sweeteners? Food Res Int 2021;142:110220. 10.1016/j.foodres.2021.110220 [DOI] [PubMed] [Google Scholar]
- 94. Narain A, Kwok CS, Mamas MA. Soft drink intake and the risk of metabolic syndrome: a systematic review and meta-analysis. Int J Clin Pract 2017;71. 10.1111/ijcp.12927 [DOI] [PubMed] [Google Scholar]
- 95. Zhang X, Li X, Liu L, et al. Dose-response association between sugar- and artificially sweetened beverage consumption and the risk of metabolic syndrome: a meta-analysis of population-based epidemiological studies. Public Health Nutr 2021;24:3892-904. 10.1017/S1368980020003614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cannon CP. Cardiovascular disease and modifiable cardiometabolic risk factors. Clin Cornerstone 2007;8:11-28. 10.1016/S1098-3597(07)80025-1 [DOI] [PubMed] [Google Scholar]
- 97. Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116:480-8. 10.1161/CIRCULATIONAHA.107.689935 [DOI] [PubMed] [Google Scholar]
- 98. Cohen L, Curhan G, Forman J. Association of sweetened beverage intake with incident hypertension. J Gen Intern Med 2012;27:1127-34. 10.1007/s11606-012-2069-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Winkelmayer WC, Stampfer MJ, Willett WC, Curhan GC. Habitual caffeine intake and the risk of hypertension in women. JAMA 2005;294:2330-5. 10.1001/jama.294.18.2330 [DOI] [PubMed] [Google Scholar]
- 100. Fagherazzi G, Gusto G, Affret A, et al. Chronic consumption of artificial sweetener in packets or tablets and type 2 diabetes risk: evidence from the E3N-European Prospective Investigation into Cancer and Nutrition Study. Ann Nutr Metab 2017;70:51-8. 10.1159/000458769 [DOI] [PubMed] [Google Scholar]
- 101. Swithers SE, Laboy AF, Clark K, Cooper S, Davidson TL. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav Brain Res 2012;233:1-14. 10.1016/j.bbr.2012.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anses. Evaluation des bénéfices et des risques nutritionnels des édulcorants intenses. Maisons-Alfort: Anses; 2015. https://www.anses.fr/fr/system/files/NUT2011sa0161Ra.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: online data supplements
Data Availability Statement
Researchers from public institutions can submit a collaboration request including information on the institution and a brief description of the project to collaboration@etude-nutrinet-sante.fr. All requests will be reviewed by the steering committee of the NutriNet-Santé study. If the collaboration is accepted, a data access agreement will be necessary and appropriate authorisations from the competent administrative authorities might be needed. In accordance with existing regulations, no personal data will be accessible.


