Abstract
Background & Aims:
Vacuole membrane protein 1 (VMP1) is an endoplasmic reticulum (ER) transmembrane protein that regulates the formation of autophagosomes and lipid droplets. Recent evidence suggests that VMP1 plays a critical role in lipoprotein secretion in zebra fish and cultured cells. However, the pathophysiological roles and mechanisms by which VMP1 regulates lipoprotein secretion and lipid accumulation in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) are unknown.
Methods:
Liver-specific and hepatocyte-specific Vmp1 knockout mice as well as Vmp1 knock-in mice were generated by crossing Vmp1flox or Vmp1KI mice with albumin-Cre mice or by injecting AAV8-TBG-cre, respectively. Lipid and energy metabolism in these mice were characterized by metabolomic and transcriptome analyses. Mice with hepatic overexpression of VMP1 who were fed a NASH diet were also characterized.
Results:
Hepatocyte-specific deletion of Vmp1 severely impaired VLDL secretion resulting in massive hepatic steatosis, hepatocyte death, inflammation and fibrosis, which are hallmarks of NASH. Mechanistically, loss of Vmp1 led to decreased hepatic levels of phosphatidylcholine and phosphatidylethanolamine as well as to changes in phospholipid composition. Deletion of Vmp1 in mouse liver also led to the accumulation of neutral lipids in the ER bilayer and impaired mitochondrial beta-oxidation. Overexpression of VMP1 ameliorated steatosis in diet-induced NASH by improving VLDL secretion. Importantly, we also showed that decreased liver VMP1 is associated with NAFLD/NASH in humans.
Conclusions:
Our results provide novel insights on the role of VMP1 in regulating hepatic phospholipid synthesis and lipoprotein secretion in the pathogenesis of NAFLD/NASH.
Lay summary:
Non-alcoholic fatty liver disease and its more severe form, non-alcoholic steatohepatitis, are associated with a build-up of fat in the liver (steatosis). However, the exact mechanisms that underly steatosis in patients are not completely understood. Herein, the authors identified that the lack of a protein called VMP1 impairs the secretion and metabolism of fats in the liver and could therefore contribute to the development and progression of non-alcoholic fatty liver disease.
Keywords: VLDL, endoplasmic reticulum, NAFLD, autophagy, liver injury
Graphical Abstract
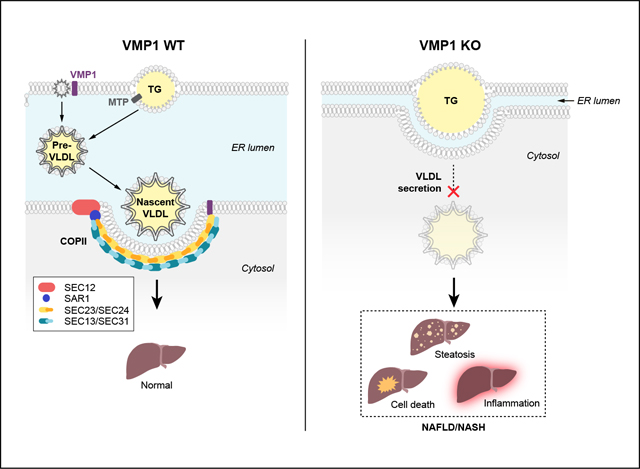
Introduction
Increased free fatty acid uptake and de novo lipogenesis account for the majority of the hepatic fat source in non-alcoholic fatty liver disease (NAFLD).1 Hepatic fat accumulation usually results in increased hepatic VLDL synthesis and secretion, which act as an adaptive mechanism to attenuate intrahepatic fat accumulation. Impaired hepatic VLDL secretion such as by microsomal triglyceride transfer protein (MTP) inhibition causes profound hepatic steatosis, and factors regulating hepatic VLDL synthesis and secretion are critical in modulating non-alcoholic steatohepatitis (NASH) progression and severity.2–4
Vacuole membrane protein 1 (VMP1) is an endoplasmic reticulum (ER) transmembrane protein which was originally identified in acute pancreatitis with vacuole formation.5 Subsequent studies revealed that VMP1 is crucial for autophagosome and lipid droplet (LD) formation.6,7 VMP1 is also known to regulate the secretion of soluble or specific proteins that are transported via the ER-to-Golgi trafficking pathway to maintain organelle homeostasis in Drosophila and Dictyostelium.8,9 A recent study revealed that loss of VMP1 causes lipoprotein accumulation in the intestine, liver cells of zebrafish and early embryos of mice, as well as in human hepatoma cells, suggesting VMP1 plays a critical role in lipoprotein secretion.10 VMP1 has phospholipid scramblase activity that regulates the cellular distribution of cholesterol and phosphatidylserine, which may be involved in LD biogenesis and SARS-CoV-2 (as well as other coronavirus) infections.11,12
However, the pathophysiological relevance of VMP1-regulated lipoprotein secretion in the context of NAFLD/NASH is unknown. Herein, we comprehensively characterized the function of VMP1 in regulating hepatic lipid homeostasis and VLDL secretion through physiological, biochemical, molecular and genetic rescue studies. Our findings provide novel insights and identify VMP1 as a target for NAFLD/NASH.
Materials and methods
Animals
Vmp1flox mice were purchased from the European Mouse Mutant Archive. Vmp1 conditional knock-in (KI) mice were generated in collaboration with Cyagen (for details see supplementary materials). Vmp1flox/Vmp1KI mice were generated by crossing Vmp1flox mice with Vmp1KI mice. To generate liver-specific Vmp1 (L-Vmp1) knockout (KO) mice, Vmp1flox mice were crossed with albumin-Cre mice. To generate hepatocyte-specific Vmp1 (H-Vmp1) KO or Vmp1 restoration mice (H-Vmp1 KO/KI), 8–10-week-old mice were injected intravenously with adeno-associated virus 8 (AAV8)-thyroxine binding globulin promoter (TBG)-null or AAV8-TBG-cre (1×1011 GC/mouse). All mice were fed with a chow diet unless otherwise indicated. To generate a model of diet-induced NASH, mice were fed a CDAHFD (choline-deficient, amino acid-defined high-fat diet (45% fat) containing 0.1% methionine) for 6 weeks. Mice were specific pathogen free and maintained in a barrier rodent facility under standard experimental conditions. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center.
Statistical analysis
Data were analyzed using SigmaPlot. All experimental data are expressed as mean ± SEM and subjected to unpaired Student’s t test (2 group comparisons) or one-way ANOVA with Holm-Sidak post hoc test (multigroup comparisons).
Additional reagents and experimental procedures are provided in the CTAT table and supplementary materials and methods. RNAseq data are accessible from GEO (GSE186642).
Results
Hepatocyte-specific deletion of Vmp1 causes accumulation of neutral lipids in mouse livers
To investigate the physiological functions of VMP1 in mice, Vmp1flox mice were injected with AAV8-TBG-null (wild-type, H-WT) or AAV8-TBG-cre (hepatocyte-specific Vmp1 KO, H-Vmp1 KO) for 1, 2 and 4 weeks. Compared to H-WT mice, H-Vmp1 KO mice had enlarged and yellowish livers (Fig. 1A), with a time-dependent increase in liver weight and liver-body weight ratio, but decreased body weight at 4 weeks post AAV injection (Fig. 1A,B). Contiguous patches of micro-steatosis, hepatocyte ballooning, lobular inflammation and accumulation of hepatic lipids were revealed by H&E and Oil Red O staining in H-Vmp1 KO mice (Fig. 1C). Levels of hepatic triglyceride (TG) and cholesterol markedly increased in H-Vmp1 KO mice compared with H-WT mice (Fig. 1D). In contrast, steady-state serum levels of TG and cholesterol significantly decreased in H-Vmp1 KO mice, although at 4 weeks serum levels of cholesterol in H-Vmp1 KO mice had recovered to almost the same levels as in H-WT mice (Fig. 1E). Hepatic LDLR levels decreased in H-Vmp1 KO mouse livers compared with WT mice (Fig. 1F), which may contribute to increased serum cholesterol at 4 weeks post AAV injection. H-Vmp1 KO mice at 4 weeks post AAV injection had similar energy expenditure (Fig. S1A) and respiratory exchange ratio (Fig. S1B) but significantly decreased activity and lean mass compared to H-WT mice (Fig. S1C,D), suggesting sickness of the mice may contribute to decreased bodyweight.
Fig. 1. Loss of hepatic VMP1 leads to steatosis in mice.
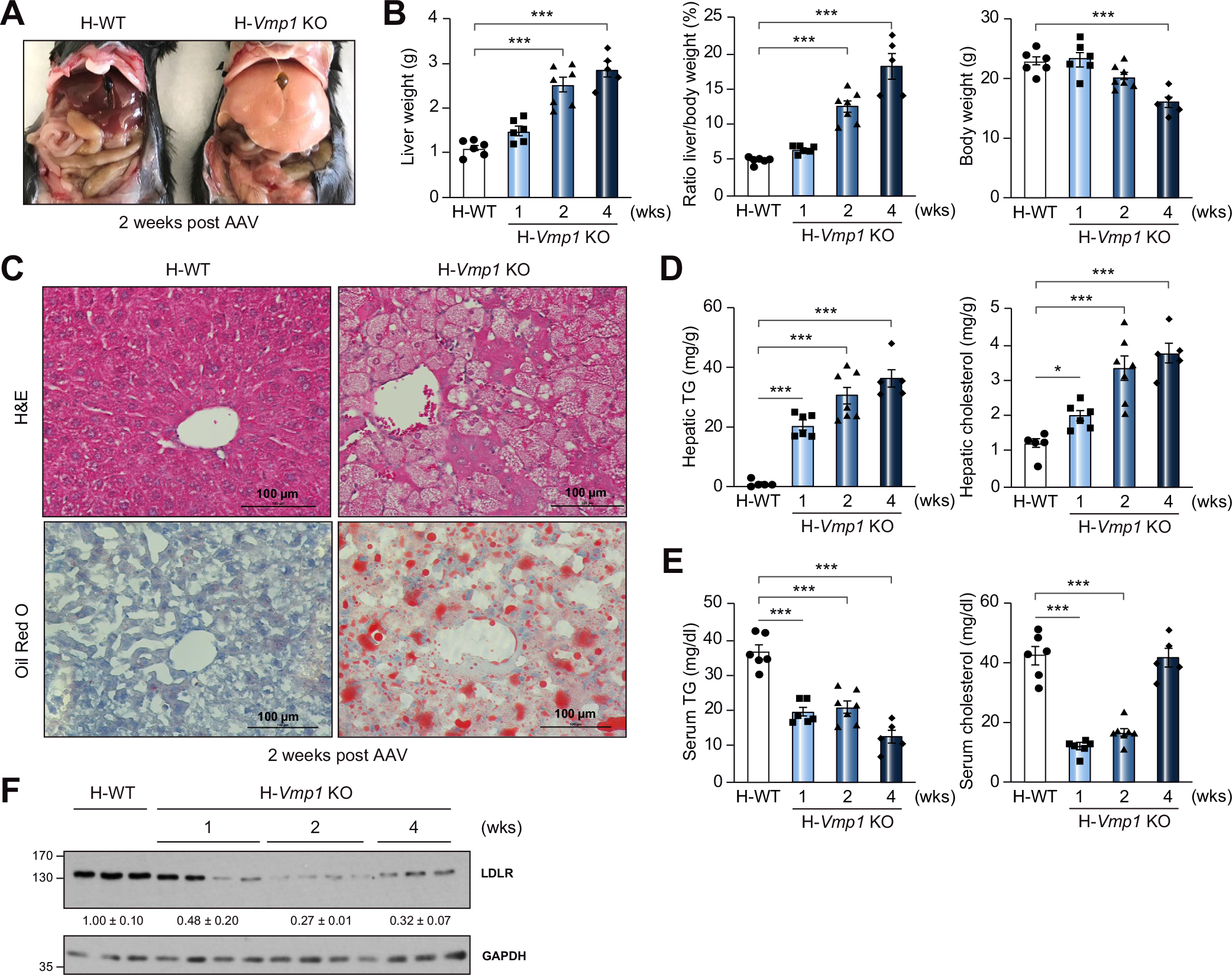
(A) Representative images of 8–10-week-old Vmp1flox mice at 2 weeks post AAV8-TBG-null (H-WT) or AAV8-TBG-cre (H-Vmp1 KO) injection. (B) Mouse liver, liver/body weight ratio and body weight. (C) H&E and Oil Red O staining of liver tissues from H-WT and H-Vmp1 KO mice. Hepatic (D) and serum (E) TG and cholesterol were quantified. (F) Total liver lysates were subjected to immunoblot analysis. Data represent mean ± SEM (n = 5–7). ***p <0.001 (One-way ANOVA with Holm-Sidak post hoc test). KO, knockout; TG, triglyceride; WT, wild-type.
Both male and female H-Vmp1 KO mice exhibited similar increases in liver-body weight ratio (Fig. S2A), levels of hepatic TG and cholesterol (Fig. S2B). However, female H-Vmp1 KO mice had lower serum levels of TG, but higher levels of cholesterol compared with male H-Vmp1 KO mice (Fig. S2C). Similar to H-Vmp1 KO mice, L-Vmp1 KO mice also showed decreased body weight, increased liver-body weight ratio but similar liver weight (Fig. S3A,B), severe hepatic steatosis (Fig. S3C), increased hepatic TG and cholesterol contents (Fig. S3D) and decreased serum TG and cholesterol (Fig. S3E). Hepatocytes isolated from L-Vmp1 KO mice or from Vmp1flox mice infected with adenovirus cre showed increased numbers of LD compared with WT hepatocytes (Fig. S3F–G). These data indicate that VMP1 plays a critical role in regulating hepatic lipid homeostasis in mice.
Deletion of hepatocyte Vmp1 impairs lipoprotein secretion in mice
Compared with matched WT mice, levels of serum TG and TG secretion rate significantly decreased in H-Vmp1 KO and L-Vmp1 KO mice following intravenous administration of tyloxapol to block lipolysis and uptake of circulating TG-rich lipoproteins (Fig. 2A,B). Immunoblot analysis showed decreased liver and serum levels of apolipoprotein (APO)B100 in L-Vmp1 KO mice and H-Vmp1 KO mice at 1, 2 but not 4 weeks post AAV injection (Fig. 2C,D). Radiolabeled APOB100 and APOB48 also decreased in H-Vmp1 KO mice with less effect on APOB48 (Fig. 2E). Interestingly, levels of serum APOB and hepatic VMP1 recovered in H-Vmp1 KO mice at 4 weeks post AAV injection (Fig. 2D,F) but TG secretion was only partially improved (Fig. 2G). Hepatic mRNA levels of Apob significantly decreased in both H-Vmp1 and L-Vmp1 KO mice (Fig. 2H), suggesting a possible downregulation of APOB by deletion of hepatic Vmp1. Intriguingly, some hepatocytes still had VMP1 expression with fewer LDs, and were PCNA (proliferating cell nuclear antigen)-positive at 4 weeks post AAV injection (Fig. S4A,B), suggesting increased compensatory hepatocyte proliferation. These data suggest that the recovery of VMP1 and APOB proteins in H-Vmp1 KO mice is likely due to compensatory proliferation from cells in which Vmp1 was not sufficiently deleted by cre.
Fig. 2. Reduced TG and lipoprotein secretion in hepatocyte-specific and liver-specific Vmp1 KO mice.
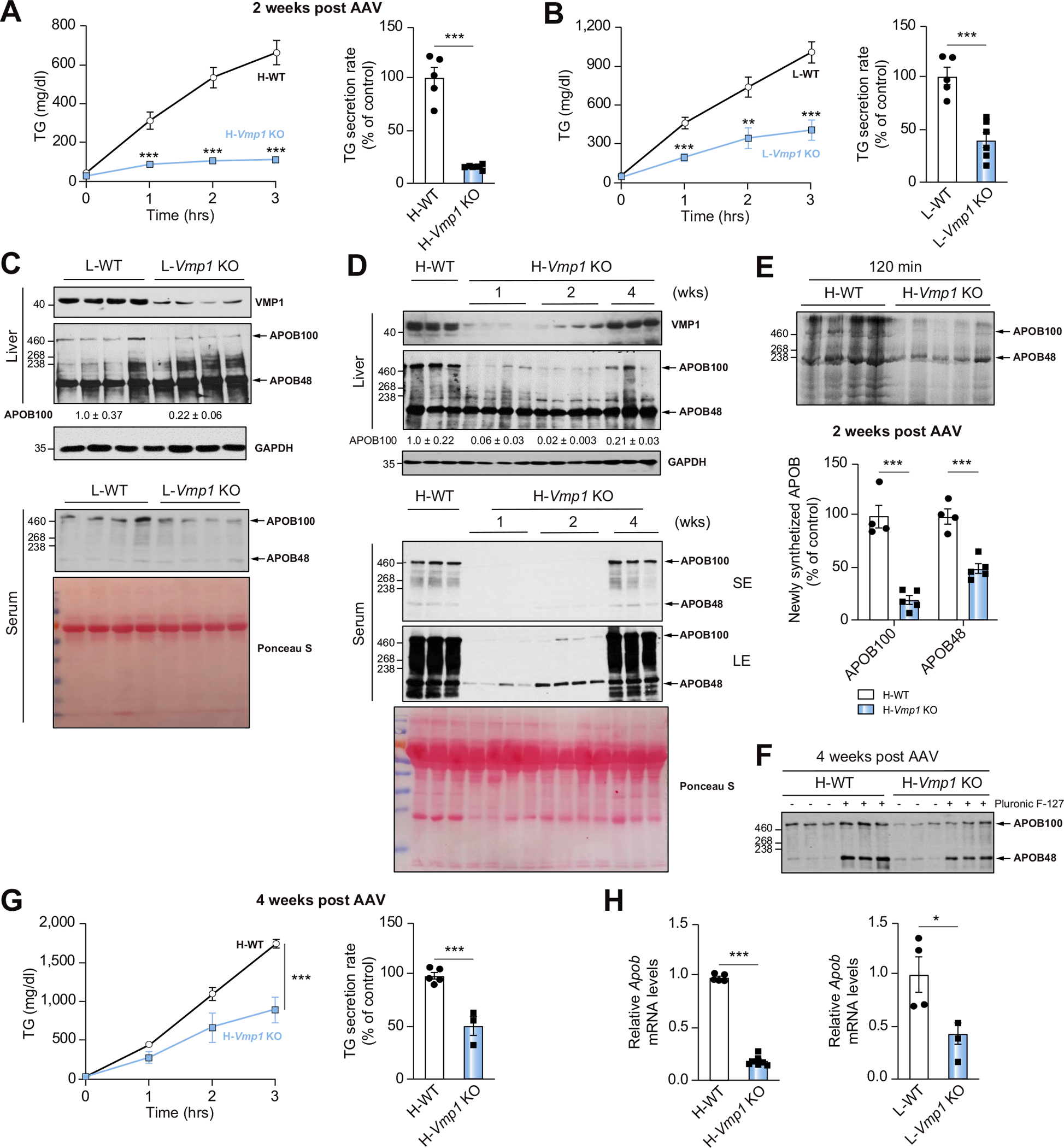
(A) H-WT and H-Vmp1 KO mice or (B) one-month-old L-WT and L-Vmp1 KO mice were injected with tyloxapol and serum TG concentrations and secretion rates were measured. Total liver lysates and serum from L-WT and L-Vmp1 KO mice (C) or H-WT and H-Vmp1 KO mice (D) were subjected to immunoblot analysis. (E) Mice were fasted for 4–5 hours followed by injection via tail vein with 1 mg/g Pluronic™ F-127 and 15 mCi/kg of 35S-methionine labeling mix. Newly synthesized APOB was quantified 120 minutes later in H-WT and H-Vmp1 KO mouse sera. (F) Immunoblot analysis of serum APOB. (G) TG concentrations and secretion rates were measured in H-WT and H-Vmp1 KO mice at 4 weeks post AAV. (H) qPCR analysis of hepatic Apob mRNA. Data represent mean ± SEM (n = 4–7). *p <0.05; **p <0.01; ***p <0.001 (Unpaired Student’s t test). APO, apolipoprotein; LE, long exposure; SE, short exposure.
Levels of LC3-II increased after starvation in WT hepatocytes, which further increased in the presence of chloroquine (CQ), suggesting increased autophagic flux. The basal levels of p62 and LC3-II were much higher in Vmp1 KO hepatocytes compared with WT hepatocytes, suggesting impaired autophagy in Vmp1 KO hepatocytes. The levels of LC3-II were also further increased by CQ treatment in Vmp1 KO hepatocytes, which is likely due to the incomplete deletion of VMP1 (Fig. S5A). Results of a RFP-GFP-LC3 puncta assay showed that starvation increased the number of red-only puncta in WT hepatocytes, which was markedly inhibited by CQ. Vmp1 KO hepatocytes had increased basal levels of yellow puncta with very few red-only puncta under starvation conditions (Fig. S5B). These data indicate that Vmp1 KO hepatocytes have impaired autophagy, which is consistent with previous findings.10 H-Atg5 KO mice developed hepatomegaly with elevated serum alanine aminotransferase (ALT) levels but had only slightly increased levels of hepatic TG and no change of cholesterol (Fig. S6A–D), which is consistent with our previous report that L-Atg5 KO mice do not exhibit impaired VLDL secretion and hepatic steatosis.13 Immunoblot analysis confirmed efficient deletion of VMP1 and ATG5 with increased SQSTM1/p62 in H-Vmp1 and H-Atg5 KO mouse livers. H-Atg5 KO mice had blunted LC3-II with increased LC3-I, whereas H-Vmp1 KO mice had increased LC3-I and LC3-II levels (Fig. S6E). These data indicate that loss of hepatic VMP1 impairs VLDL secretion and hepatic autophagy.
Deletion of Vmp1 in hepatocytes leads to lipid accumulation inside the ER bilayer
Lipid fractions from L-Vmp1 KO mouse livers were enriched with calnexin (ER marker) but had less perilipin-2 (cytosolic LD marker) and almost no detectable SEC24D (coat protein complex II [COPII]) and APOB100 (lipoprotein). In contrast, lipid fractions from WT mouse livers have abundant perilipin-2, APOB100 and SEC24D but had less calnexin (Fig. 3A), suggesting that the majority of lipid fractions are within the ER in Vmp1 KO mouse livers but are either cytosolic LDs or lipoproteins in WT mice. Oleic acid (OA) treatment markedly increased the number of perilipin-2-positive LDs in primary cultured WT hepatocytes. Increased numbers of LDs were readily observed in Vmp1 KO hepatocytes with or without OA treatment, and most of these LDs were perilipin-2-negative with larger size compared to WT hepatocytes (Fig. 3B,C). Most LDs in Vmp1 KO hepatocytes but not OA-treated WT hepatocytes were positive for APOB (Fig. 3D,F) and KDEL (an ER marker, Fig. 3E,F). EM analysis of Vmp1 KO hepatocytes revealed that “LDs” had membranes facing the cytosol (black arrows) with clear electron dense “edges” (likely representing the phospholipid monolayer) surrounding the lipid structure. The space (denoted by stars) between the ER membrane and the electron dense-edged lipid structures should be the ER lumen (Fig. 3G). Similar “LD” structures were also observed in L-Vmp1 KO mouse livers (Fig. 3H). Taken together, these data suggest that LDs in Vmp1 KO hepatocytes are not cytosolic LDs and are most likely within the ER bilayer, consistent with the findings reported by Morishita et al.10
Fig. 3. Loss of hepatic VMP1 causes accumulation of lipids in the endoplasmic reticulum bilayer.
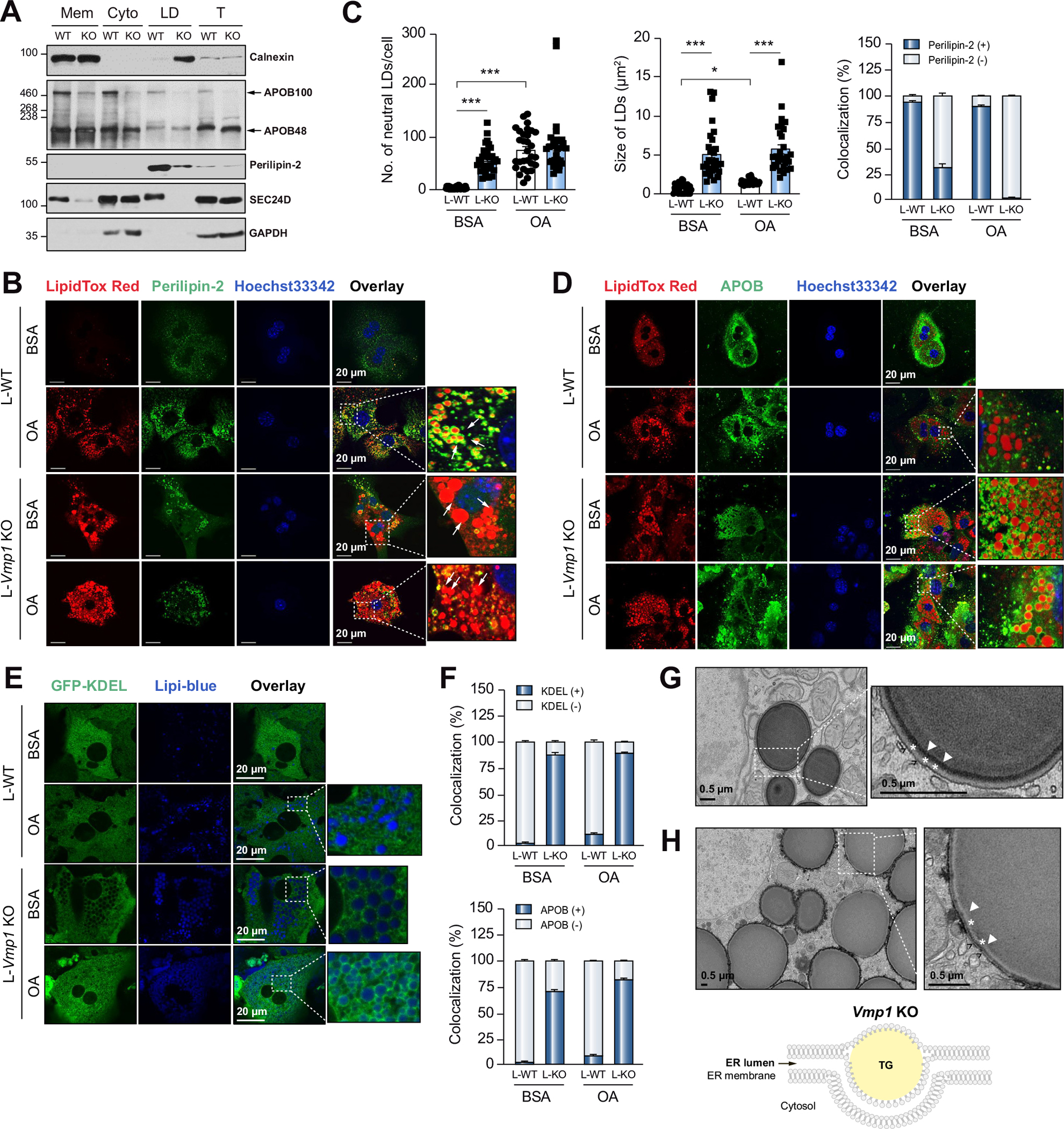
(A) Liver subcellular fractions were subjected to immunoblot analysis. (B) Primary hepatocytes were treated with BSA or OA (200 μM) for 6 hours and stained with perilipin-2 and LipidTOX Red followed by confocal microscopy. White arrows indicate LDs. (C) Total number, size of LDs, and colocalization of neutral lipids with perilipin-2 were quantified (≥30 cells of 3 independent experiments). (D) Primary hepatocytes were stained with APOB and LipidTOX Red followed by confocal microscopy. (E) Primary hepatocytes were infected with Ad-ssRFP-GFP-KDEL and stained with Lipi-blue followed by confocal microscopy. (F) Colocalization of LDs with APOB and KDEL were quantitated (≥20 cells of 2 independent experiments). Representative EM images of primary hepatocyte from L-Vmp1 KO mice (G) or H-Vmp1 KO mouse livers at 2 weeks post AAV (H). *p <0.05; ***p <0.001 (One-way ANOVA with Holm-Sidak post hoc test). BSA, bovine serum albumin; Cyto, cytosol; ER, endoplasmic reticulum; LD, lipid droplet; Mem, membrane; OA, oleic acid; T, total lysate.
Hepatocyte deletion of Vmp1 reduces levels of phospholipids, alters the fatty acyl chain compositions of phospholipids and decreases fatty acid beta-oxidation
Unbiased lipidomics analysis revealed that levels of hepatic phospholipids significantly decreased, whereas sphingolipids and neutral lipids significantly increased in H-Vmp1 KO mouse livers (Fig. 4A). Levels of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) significantly decreased in H-Vmp1 KO mouse livers with striking reductions in abundance of 16:0, 18:2 and 16:0, 20:4 PC, two of the most abundant linoleoyl and arachidonoyl PC species (Fig. 4B,C). Several PE species containing linoleoyl and arachidonoyl chains were also markedly decreased in H-Vmp1 KO mouse livers (Fig. 4C).
Fig. 4. Loss of VMP1 in hepatocytes leads to reduced phospholipids and impaired fatty acid β-oxidation.
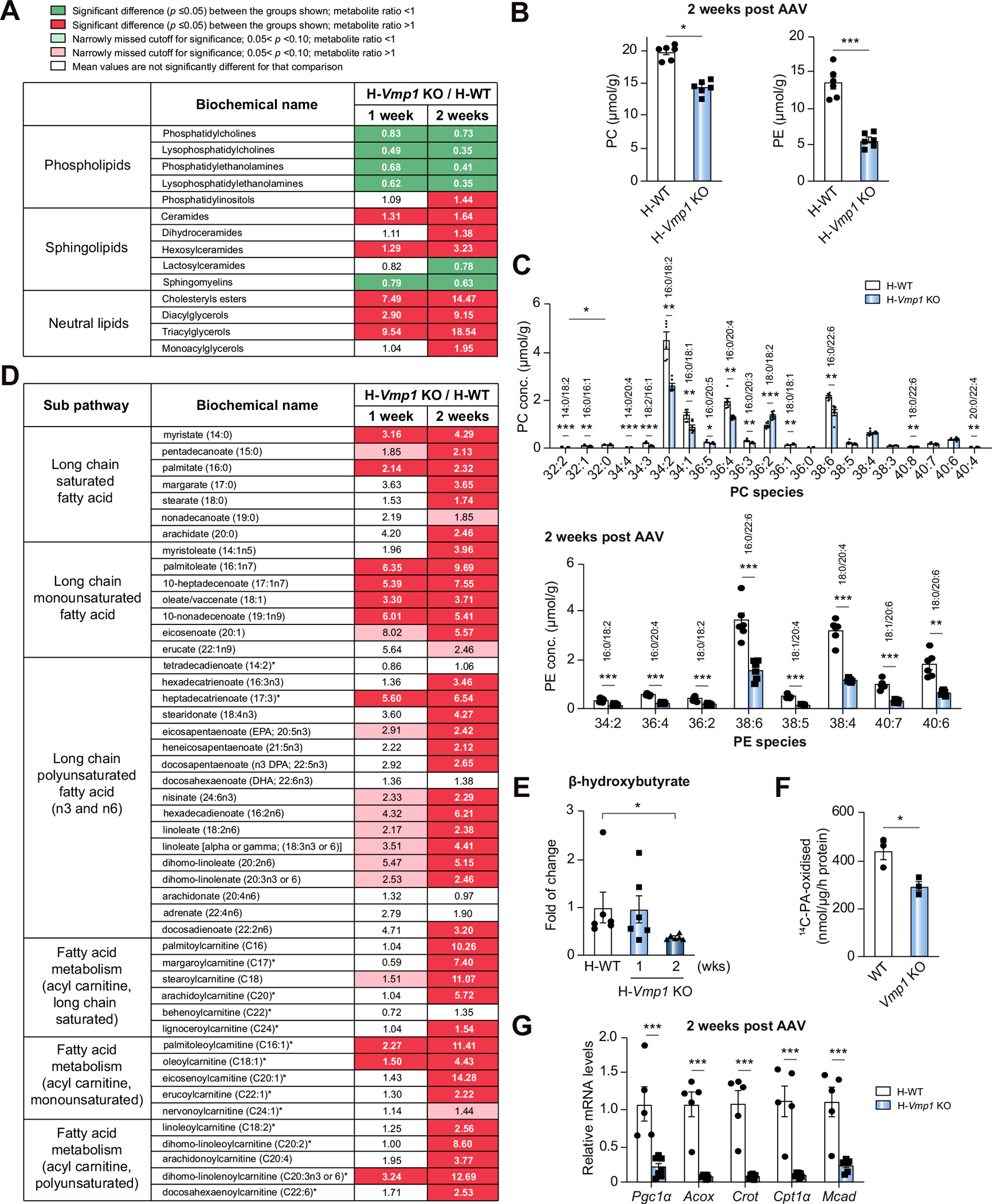
(A) Heatmaps of phospholipids, sphingolipids and neutral lipids, (B) total PC and PE, and (C) PC and PE species by metabolomics analysis of H-WT and H-Vmp1 KO mouse livers at 2 weeks. (D) Heatmaps of fatty acid metabolism and (E) β-hydroxybutyrate changes by metabolomics analysis. (n = 6). (F) FAO was measured in primary hepatocytes (n = 3 independent experiments). (G) qPCR analysis of hepatic FAO gene expression in mouse livers. Data represent mean ± SEM (n = 5–7). *p <0.05; **p <0.01; ***p <0.001 (Unpaired Student’s t test for 2 group comparison or one-way ANOVA with Holm-Sidak post hoc test for multigroup comparison). PC, phosphatidylcholine; PE, phosphatidylethanolamine.
Transcriptome analysis revealed significant gene expression changes in H-Vmp1 KO mouse livers, with 2,111 downregulated and 3,210 upregulated genes compared with H-WT mice (Fig. S7A). Pathway enrichment analysis showed that the top 20 downregulated pathways included metabolic pathways, complement and coagulation cascades, peroxisome and fatty acid (FA) degradation, whereas the top 20 upregulated pathways included cell adhesion molecules, hematopoietic cell lineage and cytokine-cytokine receptor interactions (Fig. S7B,C). Heatmaps of gene expression showed downregulation of several important genes involved in PC and PE synthesis, lipoprotein metabolism, FA, sterol biosynthesis and fatty acid oxidation (FAO) in H-Vmp1 KO mouse livers (Fig. S7D–H). Consistent with RNAseq data, qPCR analysis revealed the expression of lipogenesis and cholesterol metabolism genes significantly decreased in H-Vmp1 KO mice (Fig. S7I). The levels of lipin-1, a phosphatidate phosphatase that catalyzes diglyceride synthesis, were increased at 1 week but decreased at 2 and 4 weeks post AAV injection of H-Vmp1 KO mice (Fig. S7J). H-Vmp1 KO mice had increased levels of glycerol, glycerol-3-phosphate, total diglyceride and monoglyceride (Fig. S7K), consistent with accumulation of the intermediates of lipid synthesis due to a lack of new lipid synthesis.
Metabolomics analysis showed a pattern of increased FAs, such as palmitate (16:0), oleate/vaccentae (18:1), eicosapentaenoate (20:5n3), as well as increased acylcarnitine species including both monounsaturated and long-chain saturated acylcarnitine species in H-Vmp1 KO mouse livers (Fig. 4D). The increase in both FA and acylcarnitine in liver tissues is consistent with a decrease in overall β-oxidation, which was further supported by decreased levels of beta-hydroxybutyrate in H-Vmp1 KO mouse livers (Fig. 4E) and FA β-oxidation in Vmp1 KO hepatocytes (Fig. 4F). The expression of FA β-oxidation genes significantly decreased in H-Vmp1 KO mouse livers (Fig. 4G and Fig. S7H). These results indicate that impaired FA oxidation but not de novo lipogenesis may contribute to hepatic steatosis in H-Vmp1 KO mice.
Hepatocyte deletion of Vmp1 in mice leads to NASH
Serum levels of ALT and total bilirubin (Fig. 5A) as well as hepatic caspase-3 activity and cleaved caspase-3 increased in H-Vmp1 KO mice (Fig. 5B). The number of TUNEL- and F4/80-positive macrophages and infiltrating myeloperoxidase-positive neutrophils as well as hepatic mRNA levels of inflammatory genes markedly increased in H-Vmp1 KO livers (Fig. 5C,D). H-Vmp1 KO mice showed increased liver Sirius red staining, elevated hepatic α-smooth muscle actin and hydroxyproline levels as well as expression of fibrotic genes compared with H-WT mice (Fig. 5E–G). Increased inflammation and fibrosis were also consistent with our transcriptome analysis results, as both inflammation and fibrosis were among the top upregulated genes in H-Vmp1 KO mouse livers (Fig. S7C).
Fig. 5. H-Vmp1 KO mice develop NASH.
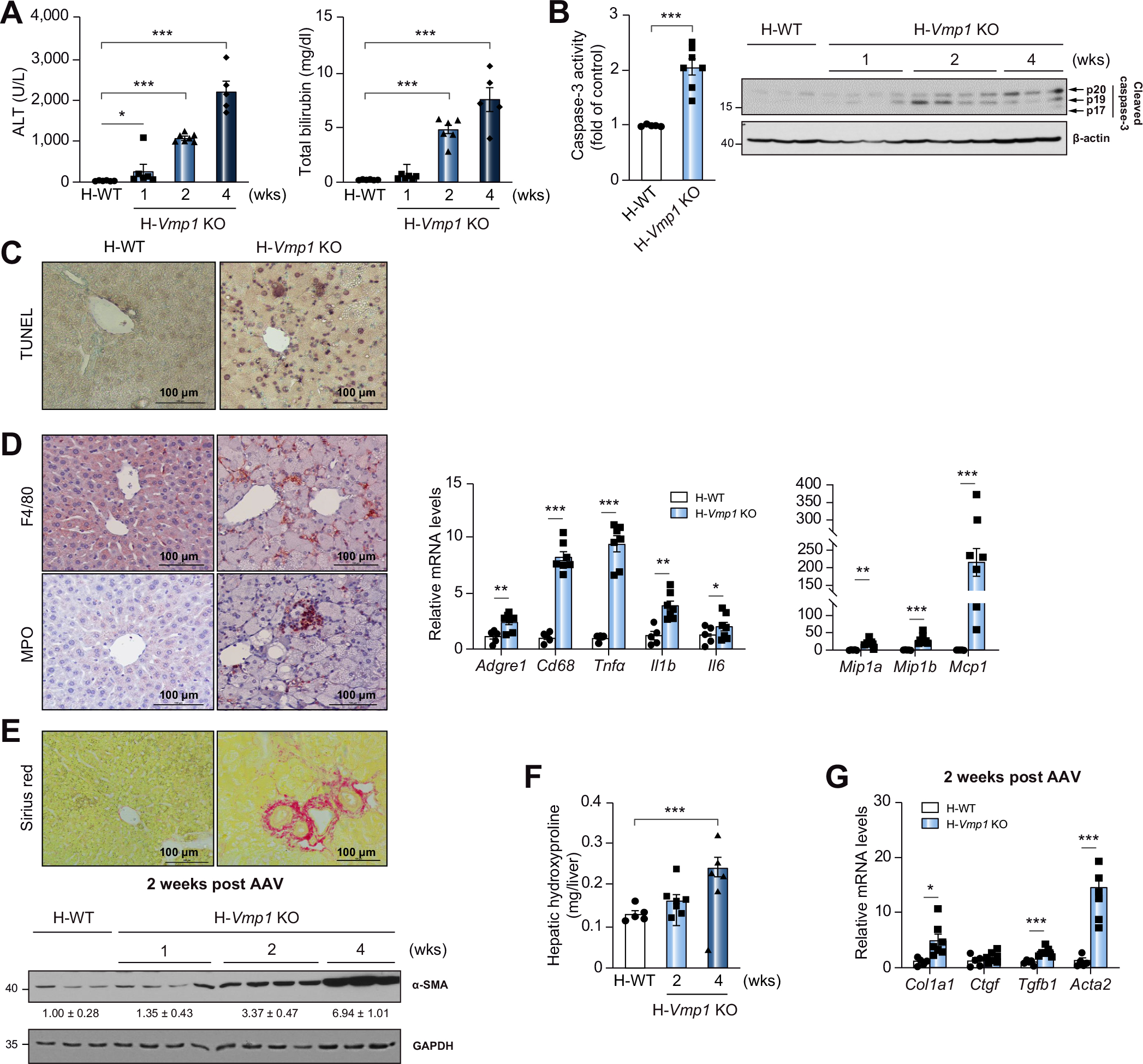
(A) Serum ALT and bilirubin of H-WT and H-Vmp1 KO mice were measured. (B) Caspase-3 activity and cleaved caspase-3 were analyzed using total liver lysates. (C) TUNEL staining and (D) immunohistochemistry staining for F4/80 and MPO in mouse livers as well as qPCR analysis of hepatic inflammatory genes. (E) Sirius red staining and immunoblot analysis of α-SMA in mouse livers. (F) Hepatic hydroxyproline and (G) qPCR analysis of fibrotic gene expression was quantified. Data represent mean ± SEM (n = 5–7). *p <0.05; **p <0.01; ***p <0.001 (Unpaired Student’s t test for 2 group comparison or one-way ANOVA with Holm-Sidak post hoc test for multigroup comparison). ALT, alanine aminotransferase.
L-Vmp1 KO mice also had increased serum ALT levels, elevated hepatic caspase-3 activity (Fig. S8A,B), increased number of liver F4/80-positive macrophages, myeloperoxidase- and LY6B-positive neutrophils (Fig. S8C), as well as increased Sirius red staining (Fig. S8D), compared with L-WT mice. The expression of Chop and the ratio of spliced and unspliced X box binding protein 1 (Xbp1s/Xbp1u) increased significantly in H-Vmp1 KO mouse livers (Fig. S9A,B). While levels of BIP decreased, levels of phosphorylated eIF-2α, ATF4 and CHOP all increased in H-Vmp1 KO mouse livers (Fig. S9C), indicating increased ER stress in H-Vmp1 KO mice. These results indicate that hepatocyte-specific deletion of Vmp1 leads to hepatic steatosis that progresses to NASH.
Hepatic deletion of Vmp1 leads to decreased COPII proteins that is rescued by hepatocyte-specific Vmp1 knock-in
MTP is required for the lipidation of APOB during the early assembly of VLDL, and protein disulfide isomerase (PDI) increases MTP activity for hepatic VLDL assembly.3,14 No changes in the protein levels of MTP and PDI were observed in H-Vmp1 KO mice. With the exception of SAR1A and SAR1B, levels of SEC23A, SEC24C and SEC24D, key components of COPII, decreased in H-Vmp1 KO mice compared to H-WT mice (Fig. 6A). Restoration of Vmp1 in Vmp1 KO mouse livers restored all COPII proteins (Fig. 6B,C) and impaired autophagic flux, as demonstrated by decreased hepatic SQSTM1/p62 and LC3-II levels in Vmp1KI mice compared with Vmp1flox mice (Fig. 6C). Immunoprecipitation analysis revealed that VMP1 interacted with SEC24D (Fig. 6D). Immunofluorescence staining also showed increased colocalization of SEC24D with VMP1-GFP in mouse livers (Fig. 6E). Deletion of VMP1 did not affect ERLIN1 and SURF4 but decreased TANGO1 and KLHL12, which were corrected by the restoration of VMP1 (Fig. S10A,C). These data indicate that VMP1 interacts with SEC24D and hepatic deletion of Vmp1 decreases levels of some COPII proteins. However, serum levels of albumin and α1-antitrypsin, two secretory proteins that are mediated by COPII, were comparable between H-Vmp1 KO and H-WT mice (Fig. S10A–B), suggesting that VMP1 may play a more important role in VLDL secretion than general secretion.
Fig. 6. Reduced COPII in hepatic Vmp1 KO mice.
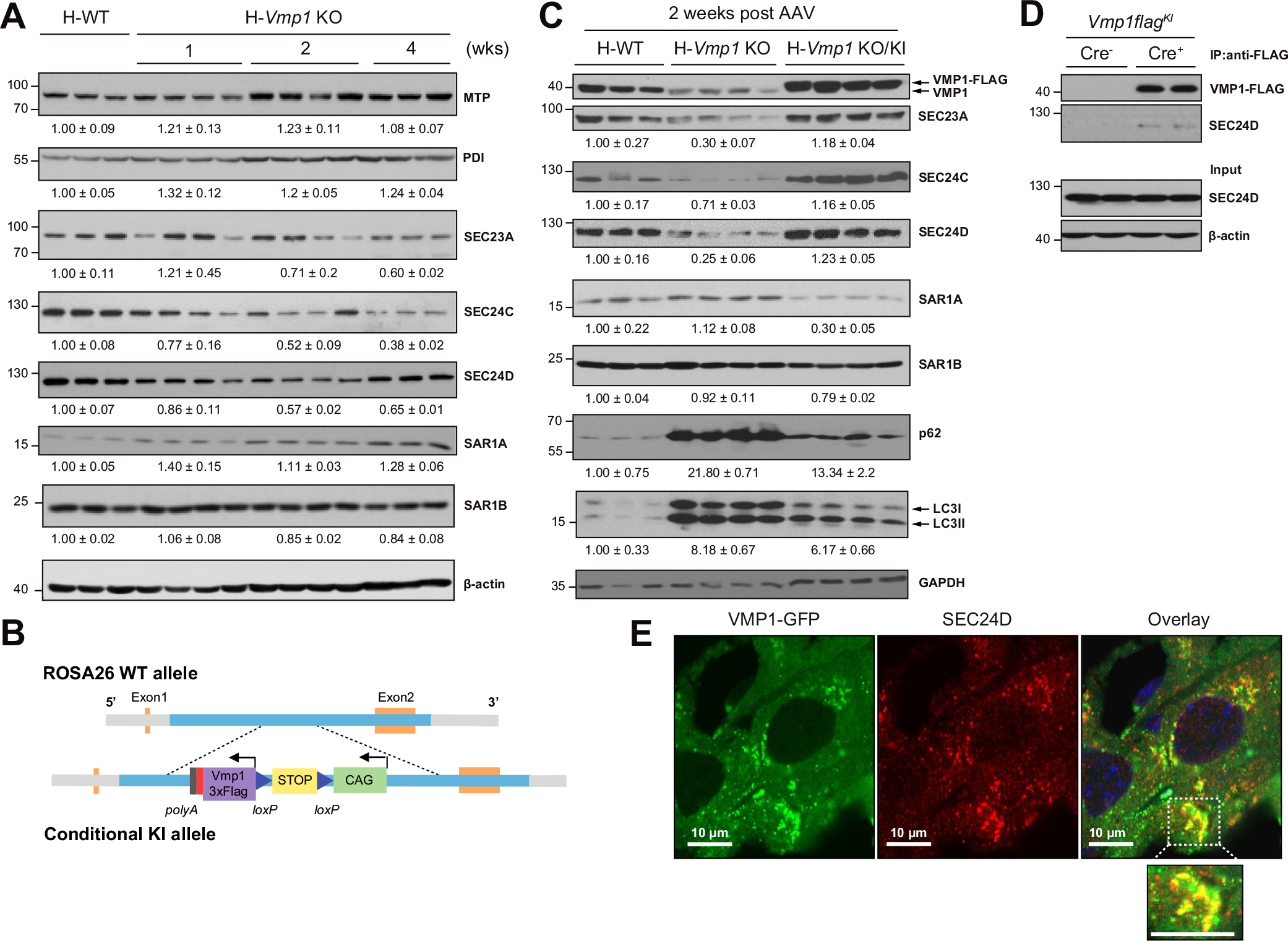
(A) Total liver lysates of indicated mice were subjected to immunoblot analysis. (B) Strategy for generating conditional Vmp1KI mice. (C) Total liver lysates of indicated mice were subjected to immunoblot analysis. (D) Immunoprecipitation assay for VMP1 and SEC24D in mouse livers. (E) Vmp1flox mice were injected with AAV8-TBG-cre for 1 week followed by injecting Ad-Vmp1-Gfp for another 2 weeks. Immunostaining for SEC24D was performed and the colocalization of VMP1-GFP and SEC24D was assessed by confocal microscopy. KI, knock-in.
Restoration of Vmp1 promotes VLDL secretion and attenuates NASH in H-Vmp1 KO mice
Decreased serum TG and cholesterol levels in H-Vmp1 KO mice were completely recovered and serum cholesterol levels further increased when Vmp1 was restored (Fig. 7A). Fast protein liquid chromatography analysis demonstrated substantially decreased VLDL-TG levels in H-Vmp1 KO mice, which were not only corrected but further increased in H-Vmp1 KO mice following restoration of Vmp1 (Fig. 7B). H-Vmp1 KO mice had lower levels of APOB in VLDL fractions, which was completely recovered when Vmp1 was restored (Fig. 7B). TG secretion was higher in Vmp1-restored mice than in H-WT mice (Fig. 7C). Following restoration of Vmp1, H-Vmp1 KO mice had normal liver color and histology (Fig. 7D) as well as decreased hepatic TG contents (Fig. 7E). Restoration of Vmp1 in H-Vmp1 KO mice also corrected the loss of body weight, hepatomegaly and liver injury (Fig. 7F). These results indicate that restoration of Vmp1 ameliorates impaired VLDL secretion and improves NASH in H-Vmp1 KO mice.
Fig. 7. Restoration of VMP1 in Vmp1 KO mice improves VLDL secretion and ablates hepatic steatosis and liver injury.
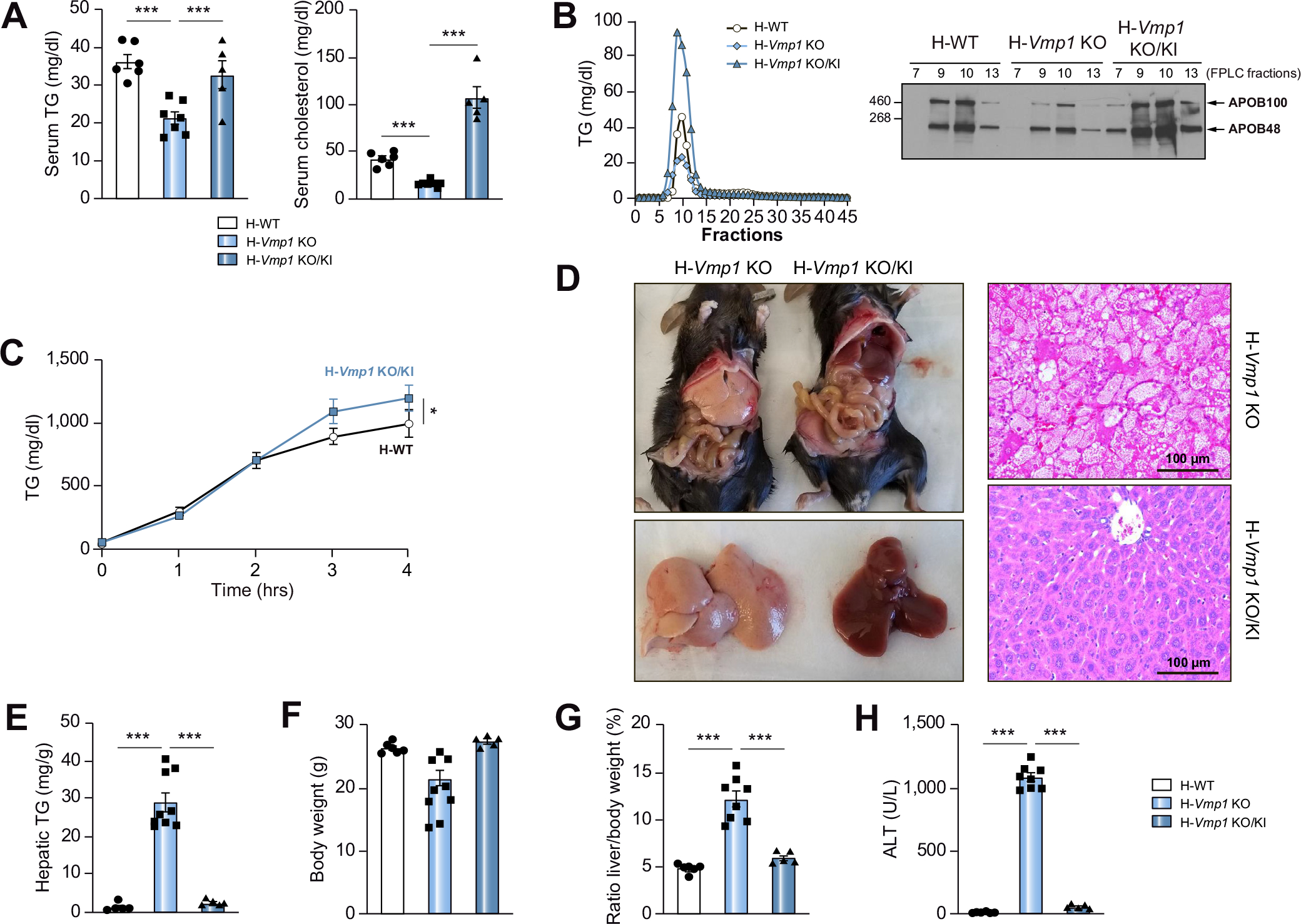
(A) Serum TG and cholesterol were measured in H-WT, H-Vmp1 KO and H-Vmp1 KO/KI mice at 2 weeks post AAV. (B) Mice were fasted for 4 hours followed by Pluronic™ F-127 injection for another 4 hours. Pooled serum samples were subjected to FPLC analysis of lipoproteins (n = 5), and each corresponding fraction was subjected to immunoblot analysis for APOB. (C) VLDL secretion was assessed in mice after VMP1 restoration. (D) Representative images of gross livers, H&E and Oil Red O staining of mouse livers. (E-H) Hepatic TG (E), body weight (F), liver/body weight ratio (G) and ALT (H) were quantified. Data represent mean ± SEM (n = 5–7). *p <0.05; ***p <0.001 (Unpaired Student’s t test for 2 group comparison or one-way ANOVA with Holm-Sidak post hoc test for multigroup comparison).
Decreased VMP1 is associated with human NAFLD livers and overexpression of VMP1 ameliorates diet-induced NASH in mice
Immunoblot and immunohistochemical analysis revealed that VMP1 decreased in human NAFLD and NASH livers (Fig. 8A,B), and NAFLD and NASH were confirmed by histology analysis (Fig. 8B). Consistent with previous reports,15 CDAHFD-fed mice developed typical NASH and had decreased hepatic protein and mRNA levels of VMP1 (Fig. 8C). Overexpression of VMP1 significantly alleviated CDAHFD-induced decreased hepatic PC and PE levels (Fig. 8D,E), steatosis and impaired VLDL secretion (Fig. 8F–H).
Fig. 8. Decreased VMP1 in human NAFLD and overexpression of VMP1 alleviates diet-induced steatosis in mice.
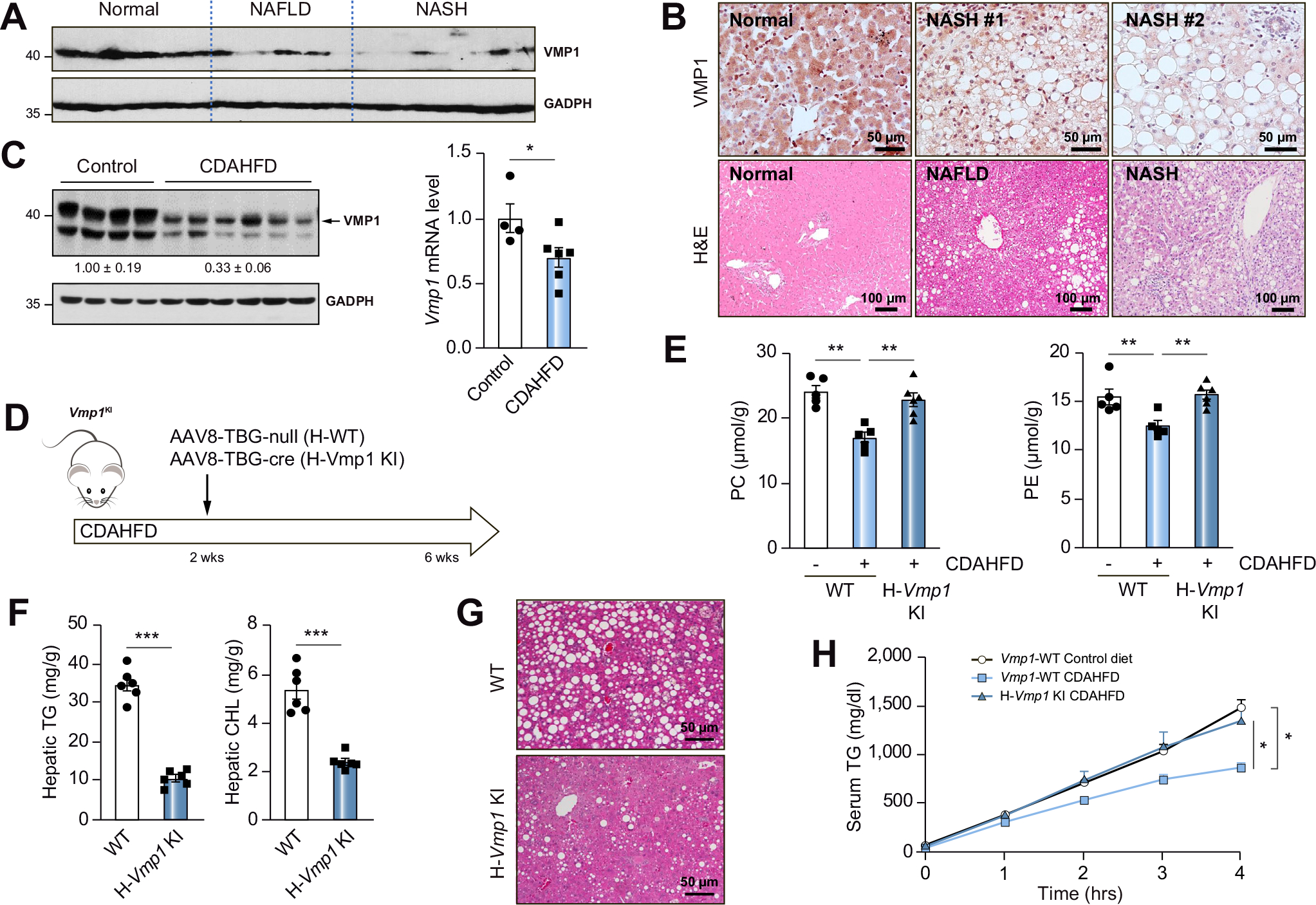
(A) Total lysates from human livers were subjected to immunoblot analysis. (B) Representative images of VMP1 IHC and H&E staining of normal and NAFLD/NASH patient livers. (C) H-WT mice were fed with CDAHFD for 6 weeks. Protein and mRNA levels of VMP1 in mouse livers were measured by immunoblot and qPCR analysis. (D) Scheme of CDAHFD-induced NASH in mice. (E) Hepatic concentrations of PC and PE, (F) TG and cholesterol and (G) H&E staining of liver tissues. (H) VLDL secretion was assessed in WT and VMP1 KI mice. Data represent mean ± SEM (n = 5–7). *p <0.05; ***p <0.001 (Unpaired Student’s t test for 2 group comparison or one-way ANOVA with Holm-Sidak post hoc test for multigroup comparison). NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
Discussion
We showed that H-Vmp1 KO mice had severely impaired VLDL secretion, resulting in hepatic steatosis that further progressed to NASH. Three processes that occur at the ER site are critical for VLDL secretion. These include the import of neutral lipids from the ER bilayer into the ER lumen, the assembly of pre-VLDL in the ER lumen, and the export of pre-VLDL from the ER lumen to the Golgi, where VLDL further undergoes a number of modifications before being transported to the plasma membrane for secretion. Results from our biochemical and morphological studies in VMP1 KO hepatocytes and mouse livers indicate that VMP1 is required for the release of lipoproteins from the ER membrane bilayer into the ER lumen, which is consistent with findings in the intestine and liver of VMP1-deficient zebrafish and early embryos of VMP1-deficient mice and HepG2 cells.10 However, some neutral lipids in our morphological studies seem wrapped with entire ER membrane. These structures may represent budded ER membranes with LDs, which separated from bulk ER and thus are unable to enter the secretory pathway. Moreover, as the electron microscopy sections only reflect one single section, some parts, which are not present in these sections, may not be covered by ER bilayers. 3D-electron microscopy may be necessary to further confirm these results in the future.
It is known that MTP is required for transferring the bulk of TGs into the ER lumen for VLDL assembly. MTP activity is enhanced by PDI, which is regulated by the IRE1α-XBP1s-PDI axis.14 No significant changes in either hepatic MTP or PDI were found in Vmp1 KO mice, suggesting that MTP and PDI are less likely to be involved in the retention of neutral lipids in the ER membrane bilayer of Vmp1 KO hepatocytes. The amount of PC and PE as well as the fatty acyl chain compositions of PC and PE, especially the arachidonyl chain of PC and PE, have been shown to regulate VLDL secretion.16,17 A recent report showed that VMP1 has scramblase activity,11 but whether loss of VMP1 may affect the equilibration of phospholipids in the ER membrane and contribute to impaired VLDL secretion remains to be investigated. However, we found that loss of VMP1 decreased hepatic PC and PE content, and altered the acyl chain composition of phospholipids. ER-mitochondria contact sites are enriched with phospholipid synthesis enzymes including phosphatidylserine synthase and phosphatidylethanolamine N-methyltransferase.18 Loss of VMP1 increases ER-mitochondria contact sites in cultured COS7 cells.7 Therefore, it is likely that VMP1 may affect biosynthesis of phospholipids by regulating ER-mitochondrial contact independent of its scramblase activity. Decreased PC and PE contents together with altered acyl chain composition of phospholipids may thus change the biophysical tension and curvature of the ER membrane that halts the import of neutral lipids from the ER membrane bilayer to the ER lumen. Future work is needed to investigate whether and how VMP1 could regulate the biosynthesis of PC and PE.
Hepatic mRNA and protein levels of APOB decreased in H-Vmp1 KO mice. However, this could be a secondary effect due to the lack of lipoproteins in Vmp1 KO hepatocytes, as it is known that APOB is degraded via the ER-associated degradation pathway when lipid availability is reduced.19 It has been reported that VMP1 directly interacted with APOB100.10 We found that VMP1 interacted with SEC24D, which raised the possibility that binding of VMP1 with APOB and SEC24D may increase the protein stability of APOB and COPII complex proteins. Future studies are needed to dissect how VMP1 might regulate hepatic APOB and COPII proteins either at the post-translational or transcriptional level or both. Nonetheless, as the majority of lipoproteins are already entrapped inside the ER membrane bilayer in Vmp1 KO hepatocytes, decreased hepatic APOB and COPII complex proteins may not be critical for impaired VLDL secretion in Vmp1 KO hepatocytes. Consistent with Morishita’s report,10 albumin and A1AT secretion did not seem to be impaired in H-Vmp1 KO mice, suggesting VMP1 may be specific for VLDL but not general secretion. Interestingly, hepatic deletion of VMP1 almost abolished APOB100 secretion with moderate reduction of APOB48. Newberry et al. 20 reported that liver-specific Tm6sf2 KO mice exhibited decreased VLDL secretion without changes in APOB100 and APOB48 secretion. Deletion of Tm6sf2 in Apobec1 KO (APOB100-only) mice led to a smaller decrease in VLDL secretion with increased APOB100 secretion compared to Tm6sf2 KO alone while Apobec1 KO mice had normal VLDL secretion. Those observations, coupled with our current findings suggest that the itinerary of a VLDL particle with APOB100 is distinct from that with APOB48.
Loss of hepatic VMP1 led to increased accumulation of acylcarnitines with decreased ketone bodies and 14C-palmitate oxidation, suggesting reduced FAO in Vmp1 KO mice. Decreased FAO was also found in Vps15, Atg7 and Atg5 KO mice.21,22 However, decreased FAO may not be the major cause of lipid accumulation in Vmp1 KO mouse livers, as L-Atg5 and Atg7 KO mice do not have obvious steatosis and L-Atg5 KO mice have normal VLDL secretion.13,22 Moreover, zebrafish lacking rb1cc1/fip200 or atg5 also do not affect VLDL secretion.10 Therefore, hepatic steatosis in Vmp1 KO mice is likely largely due to impaired VLDL secretion and less to reduced FAO and also likely independent of decrements in autophagy.
The NASH phenotypes in H-Vmp1 KO mice are distinct from other NASH models including L-Lpcat3 KO, Mea6 KO, Sar1b KO and Surf4 KO mice.16,23–25 While L-Lpcat3 KO, Mea6 KO, Sar1b KO and Surf4 KO mice all showed defective hepatic VLDL secretion and steatosis, none of them develop NASH. Notably, liver-specific Sar1b and Surf4 KO mice have near-total depletion of serum TG and cholesterol, but no obvious liver damage or inflammation. Liver-specific deletion of TMEM41B, another ER lipid scramblase and homologue of VMP1, also led to impaired VLDL secretion and NASH in mice.26 Unlike deletion of VMP1, deletion of TMEM41B does not affect levels of hepatic PC and only slightly decreased PE but increased hepatic lipogenesis without inducing ER stress. Deletion of either VMP1 or TMEM41B is sufficient to impair VLDL secretion resulting in NASH, suggesting that TMEM41B cannot compensate for the loss of VMP1 in H-Vmp1 KO mice and vice versa. Moreover, overexpression of VMP1 is able to correct the autophagy defect in TMEM41B-deficient cells but not vice versa,6 further supporting distinct functions of VMP1 and TMEM41B beyond lipid scramblase.
Increased cell death, inflammation and fibrosis have been observed in liver-specific Atg5 KO mice.27 Loss of autophagy can impair the removal of dysfunctional mitochondria resulting in decreased FAO, which can further exacerbate steatosis in Vmp1 KO mice. Therefore, the NASH phenotypes in Vmp1 and Tmem41b KO mice are likely due to combined defects in VLDL secretion and autophagy, which is unique to VMP1 and TEM41B but not for TANGO1, TALI, Mea6 and SAR1B.
In summary, our results indicate that lack of hepatic VMP1 impairs VLDL secretion and autophagy resulting in NASH. Intronic single-nucleotide polymorphism associations (rs11650106, rs2645492 and rs1292065) in the VMP1 gene have been identified and are associated with increased levels of circulating LDL cholesterol, total cholesterol, triglyceride or decreased level of lipoprotein-associated phospholipase A2 by human genome-wide association studies.28–31 The decreased circulating TG and cholesterol in Vmp1 KO mice may also provide novel insights into potential strategies for preventing cardiovascular disease and atherosclerosis by targeting VMP1.
Supplementary Material
Highlights.
VMP1 is critical in regulating the homeostasis of hepatic phospholipids and lipoprotein secretion.
Decreased hepatic VMP1 is associated with human NAFLD/NASH.
Overexpression of VMP1 improves diet-induced NAFLD.
Acknowledgements
The authors acknowledge the University of Kansas Medical Center (KUMC) Liver Cell Isolation Core at Department of Pharmacology, Toxicology and Therapeutic and Liver Center for primary hepatocyte isolation and human liver samples. The authors thank KUMC Electron Microscopy Research Lab facility for assistance with the transmission electron microscope. The authors also thank Drs. Bruno Hagenbuch, Jianming Qiu and Kang Ning for helping with the radioactive experiments.
Financial support
The research was supported in part by NIDDK DK129234, NIAAA AA026904 (to H-M. Ni), NIAAA AA020518, AA024733 (to W-X. Ding), DK119437, HL151328 and DDRCC P30 DK052574 (to N.O. Davidson), DK121970 (to F. Li), National Institute of General Medical Sciences of COBRE grant P30GM118247 (to H. Jaeschke) and NIGMS P20GM144269 (to J. Thyfault).
Abbreviations
- AAV
adeno-associated virus
- ALT
alanine aminotransferase
- APO
apolipoprotein
- BSA
bovine serum albumin
- COPII
coat protein complex II
- CQ
chloroquine
- ER
endoplasmic reticulum
- FA
fatty acid
- FAO
fatty acid oxidation
- KI
knock-in
- KO
knockout
- LD
lipid droplet
- MTP
microsomal triglyceride transfer protein
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- OA
oleic acid
- PC
phosphatidylcholine
- PDI
protein disulfide isomerase
- PE
phosphatidylethanolamine
- TBG
thyroxine binding globulin
- TG
triglyceride
- VMP1
vacuole membrane protein 1
- WT
wild-type
- XBP1
X box binding protein 1
Footnotes
Conflict of interest
The authors who have taken part in this study declare that they have nothing to disclose.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2022.04.010.
Data availability statement
RNAseq data associated with this study has been submitted to GEO database and can be accessed with the ID: GSE186642.
References
- [1].Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 2011;332:1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol 2017;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Davidson NO, Shelness GS. APOLIPOPROTEIN B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu Rev Nutr 2000;20:169–193. [DOI] [PubMed] [Google Scholar]
- [4].Samuel VT, Shulman GI. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab 2018;27:22–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dusetti NJ, Jiang Y, Vaccaro MI, Tomasini R, Azizi Samir A, Calvo EL, et al. Cloning and expression of the rat vacuole membrane protein 1 (VMP1), a new gene activated in pancreas with acute pancreatitis, which promotes vacuole formation. Biochem Biophys Res Commun 2002;290:641–649. [DOI] [PubMed] [Google Scholar]
- [6].Morita K, Hama Y, Izume T, Tamura N, Ueno T, Yamashita Y, et al. Genome-wide CRISPR screen identifies TMEM41B as a gene required for autophagosome formation. J Cell Biol 2018;217:3817–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhao YG, Chen Y, Miao G, Zhao H, Qu W, Li D, et al. The ER-localized transmembrane protein EPG-3/VMP1 regulates SERCA activity to control ER-isolation membrane contacts for autophagosome formation. Mol Cell 2017;67:974–989 e976. [DOI] [PubMed] [Google Scholar]
- [8].Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, et al. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature 2006;439:604–607. [DOI] [PubMed] [Google Scholar]
- [9].Calvo-Garrido J, Carilla-Latorre S, Lazaro-Dieguez F, Egea G, Escalante R. Vacuole membrane protein 1 is an endoplasmic reticulum protein required for organelle biogenesis, protein secretion, and development. Mol Biol Cell 2008;19:3442–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Morishita H, Zhao YG, Tamura N, Nishimura T, Kanda Y, Sakamaki Y, et al. A critical role of VMP1 in lipoprotein secretion. Elife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li YE, Wang Y, Du X, Zhang T, Mak HY, Hancock SE, et al. TMEM41B and VMP1 are scramblases and regulate the distribution of cholesterol and phosphatidylserine. J Cell Biol 2021:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schneider WM, Luna JM, Hoffmann HH, Sanchez-Rivera FJ, Leal AA, Ashbrook AW, et al. Genome-scale identification of SARS-CoV-2 and pancoronavirus host factor networks. Cell 2021;184:120–132 e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li Y, Chao X, Yang L, Lu Q, Li T, Ding WX, et al. Impaired fasting-induced adaptive lipid droplet biogenesis in liver-specific Atg5-deficient mouse liver is mediated by persistent nuclear factor-like 2 activation. Am J Pathol 2018;188:1833–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang S, Chen Z, Lam V, Han J, Hassler J, Finck BN, et al. IRE1alpha-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab 2012;16:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xiong X, Kuang H, Ansari S, Liu T, Gong J, Wang S, et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol Cell 2019;75:644–660 e645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rong X, Wang B, Dunham MM, Hedde PN, Wong JS, Gratton E, et al. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. Elife 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hashidate-Yoshida T, Harayama T, Hishikawa D, Morimoto R, Hamano F, Tokuoka SM, et al. Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. Elife 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stone SJ, Vance JE. Phosphatidylserine synthase-1 and −2 are localized to mitochondria-associated membranes. J Biol Chem 2000;275:34534–34540. [DOI] [PubMed] [Google Scholar]
- [19].Ginsberg HN, Fisher EA. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J Lipid Res 2009;50(Suppl):S162–S166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Newberry EP, Hall Z, Xie Y, Molitor EA, Bxayguinov PO, Strout GW, et al. Liver-specific deletion of mouse Tm6sf2 promotes steatosis, fibrosis, and hepatocellular cancer. Hepatology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Iershov A, Nemazanyy I, Alkhoury C, Girard M, Barth E, Cagnard N, et al. The class 3 PI3K coordinates autophagy and mitochondrial lipid catabolism by controlling nuclear receptor PPARalpha. Nat Commun 2019;10:1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Saito T, Kuma A, Sugiura Y, Ichimura Y, Obata M, Kitamura H, et al. Autophagy regulates lipid metabolism through selective turnover of NCoR1. Nat Commun 2019;10:1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang X, Wang H, Xu B, Huang D, Nie C, Pu L, et al. Receptor-mediated ER export of lipoproteins controls lipid homeostasis in mice and humans. Cell Metab 2020. [DOI] [PubMed] [Google Scholar]
- [24].Santos AJ, Nogueira C, Ortega-Bellido M, Malhotra V. TANGO1 and Mia2/cTAGE5 (TALI) cooperate to export bulky pre-chylomicrons/VLDLs from the endoplasmic reticulum. J Cell Biol 2016;213:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Y, Liu L, Zhang H, Fan J, Zhang F, Yu M, et al. Mea6 controls VLDL transport through the coordinated regulation of COPII assembly. Cell Res 2016;26:787–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang D, Xu B, Liu L, Wu L, Zhu Y, Ghanbarpour A, et al. TMEM41B acts as an ER scramblase required for lipoprotein biogenesis and lipid homeostasis. Cell Metab 2021;33:1655–1670 e1658. [DOI] [PubMed] [Google Scholar]
- [27].Ni HM, Woolbright BL, Williams J, Copple B, Cui W, Luyendyk JP, et al. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol 2014;61:617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chu AY, Guilianini F, Grallert H, Dupuis J, Ballantyne CM, Barratt BJ, et al. Genome-wide association study evaluating lipoprotein-associated phospholipase A2 mass and activity at baseline and after rosuvastatin therapy. Circ Cardiovasc Genet 2012;5:676–685. [DOI] [PubMed] [Google Scholar]
- [29].Hoffmann TJ, Theusch E, Haldar T, Ranatunga DK, Jorgenson E, Medina MW, et al. A large electronic-health-record-based genome-wide study of serum lipids. Nat Genet 2018;50:401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ripatti P, Ramo JT, Mars NJ, Fu Y, Lin J, Soderlund S, et al. Polygenic hyperlipidemias and coronary artery disease risk. Circ Genom Precis Med 2020;13:e002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Richardson TG, Sanderson E, Palmer TM, Ala-Korpela M, Ference BA, Davey Smith G, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Med 2020;17:e1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNAseq data associated with this study has been submitted to GEO database and can be accessed with the ID: GSE186642.


