Abstract
OBJECTIVE
: As known, older age and comorbidities are associated with poor clinical outcomes in patients with coronavirus disease 19. The aim of this study was to investigate the effect of the Charlson Comorbidity Index in predicting poor clinical outcomes in coronavirus disease 19 patients.
MATERIAL AND METHODS
Demographic characteristics and poor clinical outcomes (presence of pneumonia, respiratory failure, intensive care unit admission, and mortality) of the patients were evaluated retrospectively. Classical and modified Charlson Comorbidity Index was calculated and adjusted according to age.
Results
In this study, 106 women and 107 men were included. The comorbidity rate was 50.7% and the most common comorbidities were hypertension (21.6%) and diabetes mellitus (15%). The rates of respiratory failure, intensive care unit admission, and mortality were 15%, 2.3%, and 2.8%, respectively. Older age was a high risk for poor outcomes. Pneumonia (odds ratio: 6.6; 95% CI: 3.4-12.7), respiratory failure (odds ratio: 5.2; 95% CI: 2.03-13.2), and intensive care unit admission (odds ratio: 1.1; 95% CI: 1.01-1.1) were significantly higher in patients with comorbid diseases than patients without any comorbidity (P < .05). Both median-modified and classical Charlson Comorbidity Index and their age-adjusted scores were significantly higher in patients with poor outcomes.
Conclusions
It is suggested that evaluation of the Charlson Comorbidity Index might contribute to the management of the patients with coronavirus disease 19 by predicting risk group for poor clinical outcomes and mortality.
Keywords: COVID-19, comorbidity, Charlson Comorbidity Index, mortality, clinical outcome;
Main Points
Older age and comorbidities were associated with poor clinical outcomes in patients with coronavirus disease 19.
Charlson Comorbidity Index (CCI) is a tool for calculating comorbidity scores using a predefined list of medical conditions and also it can be adjusted for age and was associated with mortality.
It is thought that evaluating the CCI score during the initial admission will contribute to the identification of the risky group and the preparation of an early action plan.
Introduction
Severe acute respiratory syndrome coronavirus-2 is a novel virus that causes coronavirus disease 2019 (COVID-19) which has been responsible for the global pandemic since March 2020. According to World Health Organization (WHO) report, there have been 203 174 401 confirmed cases of COVID-19, including 4 304 114 deaths until August 08, 2021.1 From March 2020, when the first case was identified in our country, to August 2021, 5 895 841 cases were diagnosed and 52 088 people died.2
Coronavirus disease has a wide clinical spectrum from asymptomatic to severe disease including acute respiratory distress syndrome (ARDS) and death.3 Mortality is a major concern of all clinicians in all countries.4 The pathogenesis of COVID-19 infection remains unclear and there is no specific treatment protocol. Therefore, the management of COVID-19 and its outcomes are based on supportive therapy. Clinicians have been fighting against this new and unknown virus since the first day and are seeking to improve survival and outcomes in severe diseases.5 To know the risk factors of worse clinical outcomes and mortality can help us to define the patients with COVID-19 at higher risk and to plan specific approach for prevention of serious events.4,5
Older age and comorbidities are the main risk factors of severe COVID-19 disease.1 Most of the previous studies have identified comorbidities such as hypertension (HT), diabetes mellitus (DM), coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease (COPD), cerebrovascular disease (CVD), cancer, and chronic renal disease (CRD) as the high-risk factors.6,7
Charlson Comorbidity Index (CCI) is a validated index that calculates the comorbidities and predicts the risk of death within 1 year of hospitalization.8 It is found that CCI score above 0 was associated with an increased risk of severe disease and death when controlled for age and sex.9 The aim of this study was to investigate the effect of CCI in predicting poor clinical outcomes such as pneumonia, respiratory failure, intensive care unit (ICU) admission, and mortality in patients with COVID-19.
MATERIAL AND METHODS
Medical records of all hospitalized patients who were diagnosed with COVID-19 between March and June 2020 were evaluated retrospectively. The definitions of the COVID-19 disease were based on the WHO COVID-19 case definition sheet. While the presence of a positive nasopharyngeal polymerase chain reaction (PCR) test was defined as a definite case, cases with similar clinical and radiological findings (multiple ground-glass opacities, with peripheral and lower lung distribution) were defined as high probable COVID-19 despite the negative nasopharyngeal PCR test result.10 Thorax CT scans of all patients with COVID-19 (both definite and high probable) were examined by the same radiologist who is specialized in thoracic radiology.
Inclusion criteria were as follows:
to have definite COVID-19 infection (with positive nasopharyngeal PCR result),
to have high probable COVID-19 infection (clinical and radiological findings are compatible with COVID-19, but at least 2 PCR tests performed 24-48 hours apart are negative), and
hospitalization due to COVID-19 infection.
Exclusion criteria were as follows:
to be followed up in outpatient clinic with the diagnosis of definite or high probable COVID-19 infection and
hospitalization due to non-COVID-19 diseases.
Demographic characteristics including body mass index (BMI), smoking history and comorbidities, clinical outcomes such as the presence of pneumonia, respiratory failure, ICU admission, and mortality were recorded. Respiratory failure was defined as oxygen saturation (SO2) less than 90% in room air at the time of admission or during follow-up.
The CCI is a simple, easy-to-perform, and valid method to estimate the risk of death related to comorbid diseases.8 Classical CCI includes 19 medical conditions weighted 1-6 with total scores ranging from 0 to 33. Furthermore, age-adjusted CCI score can be calculated by adding 1 point for each decade of age over 40 years. Modified CCI, on the other hand, is a simpler form that includes 12 comorbid conditions with a total score weighted from 0 to 24. Age-adjusted score can also be calculated by adding 1 point per decade over the age of 50.8,11-13 Definitions and items of classical and modified CCI are shown in Table 1.
Table 1.
Calculation of Classical and Modified Charlson Comorbidity Index Scores and Age-Adjusted Scores
| Comorbid Conditions | CCI Score | Modified CCI Score |
|---|---|---|
| Age | ||
| <40 | +0 | − |
| <50 | − | +0 |
| 41-50 | +1 | − |
| 50-59 | − | +1 |
| 51-60 | +2 | − |
| 60-69 | − | +2 |
| 61-70 | +3 | − |
| 70-79 | − | +3 |
| ≥71 | +4 | − |
| >80 | − | +4 |
| Myocardial infarction | 1 | 0 |
| Congestive heart failure | 1 | 2 |
| Peripheral vascular disease | 1 | 0 |
| Cerebrovascular disease | 1 | 0 |
| Dementia | 1 | 2 |
| Chronic pulmonary disease | 1 | 1 |
| Rheumatic disease | 1 | 1 |
| Peptic ulcer disease | 1 | 0 |
| Mild liver disease | 1 | 2 |
| Diabetes without chronic complication | 1 | 0 |
| Diabetes with chronic complication | 2 | 1 |
| Hemiplegia | 2 | 2 |
| Renal disease | 2 | 1 |
| Any malignancy without metastasis | 2 | 2 |
| Leukemia | 2 | |
| Lymphoma | 2 | |
| Moderate or severe liver disease* | 3 | 4 |
| Metastatic solid tumor | 6 | 6 |
| AIDS (exclude asymptomatic infection)** | 6 | 4 |
| Max. comorbidity score | 33 | 24 |
| Max. age-adjusted comorbidity score | 37 | 28 |
*Liver disease: mild, chronic hepatitis or cirrhosis without portal hypertension; moderate, cirrhosis, portal hypertension; severe, portal hypertension, history of variceal bleeding.
**AIDS: current CD4 count <200, opportunistic infection in the last 1 month, active AIDS such as Kaposi sarcoma.
AIDS, acquired immunodeficiency syndrome.
In this study, comorbidities were recorded from medical data. Classical and Modified CCI (cCCI and mCCI) were calculated for each patient. In addition, both scores of the cCCI and mCCI were adjusted and re-calculated according to age. Furthermore, CCI scores were classified according to the total score as follows: 0 point: no comorbidity, 1-2: mild, 3-4: moderate, and ≥5: severe.9
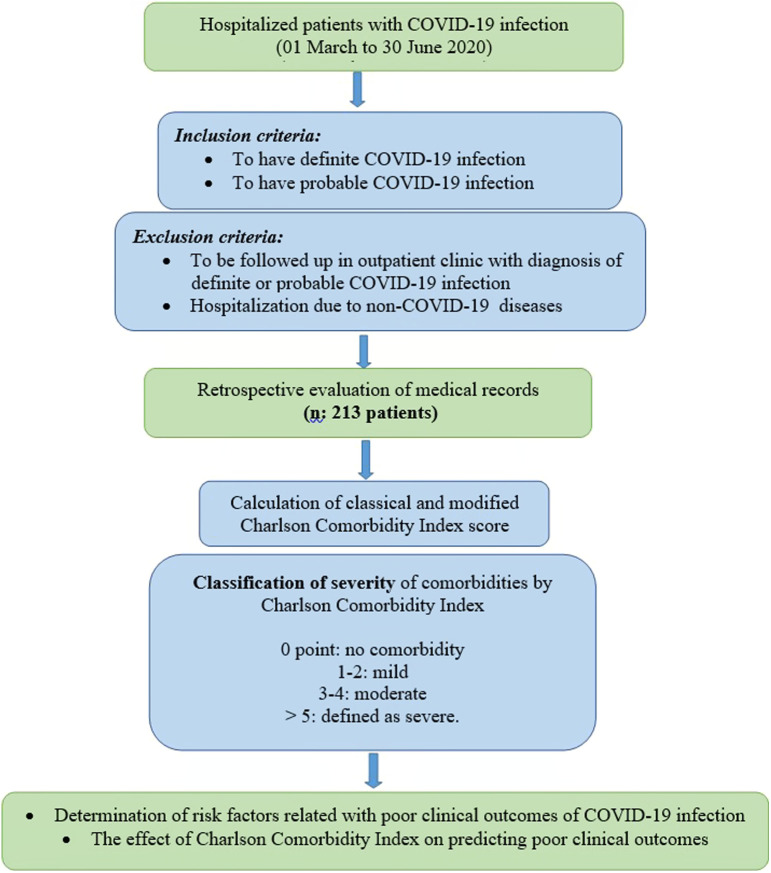
Our study was approved by the Ministry of Health Scientific Research Board and Kocaeli University Non-Interventional Research Ethics Committee with the number GOKAEK-2020/11.19. As informed consent from patients to review their medical records was not obtained, patient data were de-identified. The flowchart of the study is presented in Figure 1.
Figure 1.
Flowchart of the study.
Statistical Analysis
Statistical Package for the Social Sciences Statistics 20.0 (SPSS Inc.; Chicago, IL, USA) package program was used for statistical analyses of the study. Categorical variables were expressed as counts (percentage). Continuous variables were expressed as median or mean ± standard deviation. Mann–Whitney U test was used for the comparison of continuous variables. Comparisons of categorical variables between the groups were performed using the chi-square test. The risk was calculated by the chi-square test. A 2-sided P value <.05 was considered statistically significant.
Results
Totally 213 patients with COVID-19 were included in the study; 106 women (49.8%) and 107 men (50.2%). The median age and BMI of the study population were 46 years (min: 18, max: 93) and 25.5 kg/m2 (min: 17, max: 47.2), respectively. Among the study population, 46.7% of the patients had a smoking history, 20.7% was current smoker, and 26% was former smoker. Coronavirus disease19 PCR test was positive in 69.5% of the patients. At initial admission, 5.3% of the patients had room air SO2below 90%. Mean SO2 was 95.98 ± 3.3% during first admission, and the mean duration of hospitalization was 4.99 ± 3.2 days. During follow-up, 15% of the patients had respiratory failure. The need for ICU rate was 2.3% and the mortality rate was 2.8%. Demographic characteristics, CCI scores of the study population, and clinical outcomes of the disease are shown in Table 2.
Table 2.
Demographic Characteristics and CCI Scores of the Patients
| Variables | n(%) | |
|---|---|---|
| Gender, n (%) | ||
| Women | 106 (49.8) | |
| Men | 107 (50.2) | |
| Age category, years, n (%) | ||
| <65 | 156 (73.2) | |
| ≥65 | 57 (26.8) | |
| BMI category, kg/m2, n (%) | ||
| <18 | Cachexia | 2 (1.3) |
| 18-25 | Normal weight | 66 (41.5) |
| 25-30 | Over weight | 60 (37.7) |
| >30 | Obese | 24 (15.1) |
| >40 | Morbid obese | 7 (4.4) |
| Smoking history, n (%) | ||
| Non-smoker | 90 (53.3) | |
| Current smoker | 35 (20.7) | |
| Former smoker | 44 (26) | |
| Presence of comorbidity, n (%) | 108 (50.7) | |
| Presence of pneumonia, n (%) | 141 (66.2) | |
| Respiratory failure, n (%) | 32 (15) | |
| Need for ICU, n (%) | 5 (2.3) | |
| Mortality, n (%) | 6 (2.8) | |
| Charlson Comorbidity Index | ||
| Classical CCI | Median (25th-75th) | 0 (0-1) |
| Classical CCI—age | Median (25th-75th) | 1 (0-4) |
| Modified CCI | Median (25th-75th) | 0 (0-1) |
| Modified CCI—age | Median (25th-75th) | 1 (0-3.5) |
BMI, body mass index; PCR, polymerase chain reaction; ICU, intensive care unit; CCI, Charlson Comorbidity Index.
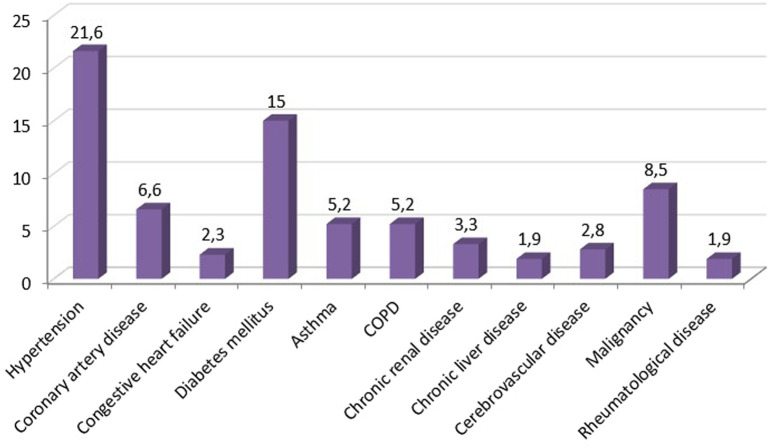
The comorbidity rate was 50.7% and the most common comorbidities were HT (21.6%), DM (15%), and malignancy (8.5%) (Figure 2). Although the presence of the comorbidity was significantly higher in men than women (57.9% vs. 43.4%, P = .03), clinical outcomes of the disease were similar between the gender.
Figure 2.
Most common comorbidities of the patients with COVID-19. COVID-19, coronavirus disease 19.
The study population was categorized by age. Patients over 65 years old were defined as elderly and 26.8% of the study population were elderly. The frequency of comorbidities (37.2% vs. 87.7%, P = .001) and poor clinical outcomes such as pneumonia (58.3% vs. 87.7%, P = .001) and respiratory failure (9.7% vs. 29.8%, P = .001) were significantly higher in elderly than younger ones. The ICU admission rate was also higher in older patients, but the difference was not statistically significant (5.3% vs. 1.3% P = .09).
Approximately 20% of the patients were obese (BMI ≥ 30 kg/m2) in the study population. There was no significant difference in mortality rates between the obese and non-obese patients in this study. However, the rate of respiratory failure was significantly higher in obese patients (P = .02; odds ratio (OR): 3.1; 95% CI: 1.2-7.9).
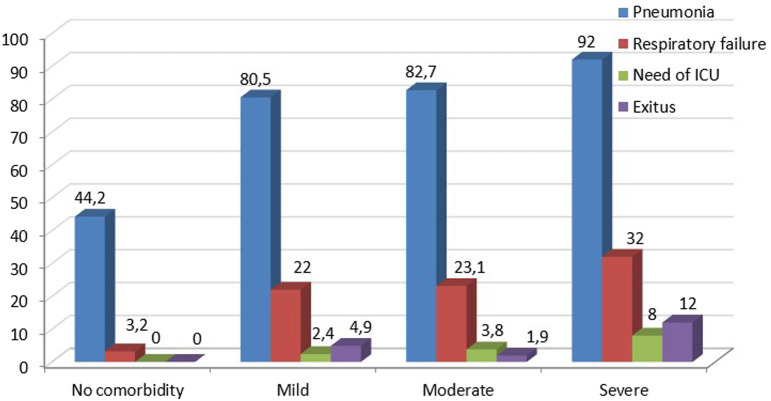
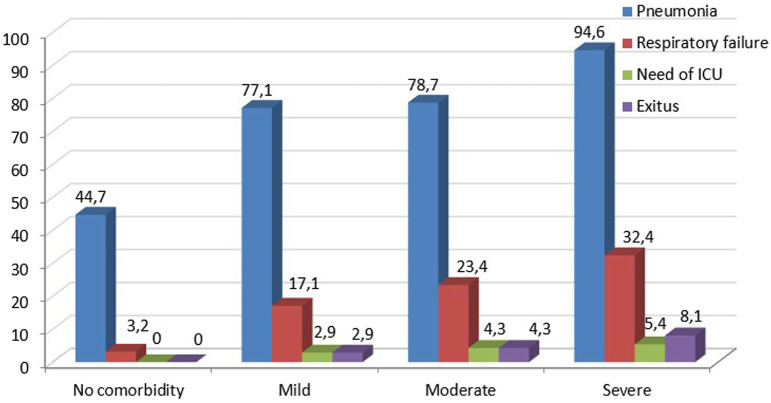
Both median-modified and classical CCI scores and their age-adjusted scores were significantly higher in patients with pneumonia, respiratory failure, who required ICU, and who died (Tables 3 and 4). Furthermore, as the severity of comorbidity increased according to the classification of CCI, clinical outcomes such as pneumonia, respiratory failure, and mortality were increased. Clinical outcomes and mortality rates according to age-adjusted modified and classical CCI scores are shown in Figures 3 and 4.
Table 4.
Demographic and Clinical Characteristics and CCI Scores of the Patients According to the Presence of ICU Stay and Mortality
| ICU (−) |
ICU (+) |
P | Mortality (−) | Mortality (+) | P | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Women, % | 50.5 | 20 | .2 | 49.8 | 50 | .9 |
| Men, % | 49.5 | 80 | 50.2 | 50 | ||
| Age, years, median | 46 | 67 | .06 | 46 | 65 | .04 |
| BMI, kg/m2, median | 25.5 | 28.1 | .6 | 25.4 | 26.6 | .6 |
| Smoking pack of years, median | 15 | 30 | .2 | 15 | 115 | .053 |
| Comorbidity, % | 49.5 | 100 | .03 | 49.8 | 83.3 | .12 |
| HT | 21.6 | 20 | .9 | 22.2 | 0 | .2 |
| CAD | 6.7 | 0 | .6 | 6.8 | 0 | .5 |
| CHF | 2.4 | 0 | .7 | 2.4 | 0 | .7 |
| DM | 15.4 | 0 | .3 | 15.5 | 0 | .3 |
| Asthma | 4.8 | 20 | .13 | 5.3 | 0 | .6 |
| COPD | 4.3 | 40 | .000 | 4.8 | 16.7 | .2 |
| CRD | 3.4 | 0 | .7 | 3.4 | 0 | .7 |
| CLD | 1.9 | 0 | .8 | 1.9 | 0 | .7 |
| CVD | 2.9 | 0 | .7 | 2.4 | 16.7 | .04 |
| Malignancy | 7.2 | 60 | .000 | 6.8 | 66.7 | .000 |
| Modified CCI | ||||||
| Median | 0.0 | 2.0 | .005 | 0.0 | 2.5 | .007 |
| Modified CCI—age | ||||||
| Median | 1.0 | 4.0 | .01 | 1.0 | 5.0 | .005 |
| Classical CCI | ||||||
| Median | 0.0 | 2.0 | .03 | 0.0 | 2.5 | .005 |
| Classical CCI—age | ||||||
| Median | 1.0 | 4.0 | .02 | 1.0 | 5.0 | .006 |
BMI, body mass index; HT, hypertension; CAD, coronary artery disease; CHF, chronic heart failure; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; CLD, chronic liver disease; CRD, chronic renal disease; CVD, cerebrovascular disease; CCI, Charlson Comorbidity Index; SD, standard deviation; ICU, intensive care unit.P value <.05 was considered statistically significant.
Figure 3.
Clinical outcomes and mortality rates according to the classification of age-adjusted, modified CCI scores. CCI, Charlson Comorbidity Index.
Figure 4.
Clinical outcomes and mortality rates according to the classification of age-adjusted classical CCI scores. CCI, Charlson Comorbidity Index.
Comparison of the patients according to the presence of pneumonia revealed that age (P = .0001), BMI (P = .005), and cigarette packs used in years (P = .001) were significantly higher in those with pneumonia. The rate of comorbidity was also higher in patients with pneumonia (65.2% vs. 22.2%, P = .000). Especially frequencies of HT, DM, COPD, and malignancy were found to be high in those with pneumonia, and these differences were statistically significant (Table 3). Coronavirus disease19 PCR test positivity was significantly lower in patients with pneumonia than cases without pneumonia (54.6% vs. 98.6%; P = .000).
Table 3.
Demographic and Clinical Characteristics and CCI Scores of the Patients According to the Presence of Pneumonia and Respiratory Failure
| Pneumonia (−) | Pneumonia (+) | P | Respiratory Failure (−) | Respiratory Failure (+) | P | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Women, % | 55.6 | 46.8 | .2 | 49.7 | 50 | .9 |
| Men, % | 44.4 | 53.2 | 50.3 | 50 | ||
| Age, years, median | 33.5 | 55 | .0001 | 41 | 65 | .0001 |
| BMI, kg/m2, median | 24.6 | 26.9 | .005 | 25.3 | 27.3 | .95 |
| Smoking pack of years, median | 7.0 | 20.0 | .001 | 11 | 30 | .002 |
| Comorbidity, % | 22.2 | 65.2 | .000 | 45.3 | 81.2 | .000 |
| HT | 8.3 | 28.4 | .001 | 18.8 | 37.5 | .02 |
| CAD | 2.8 | 8.5 | .1 | 7.7 | 0 | .1 |
| CHF | 0.0 | 3.5 | .1 | 1.7 | 6.2 | .1 |
| DM | 2.8 | 21.3 | .000 | 11.6 | 34.4 | .001 |
| Asthma | 1.4 | 7.1 | .08 | 4.4 | 9.4 | .2 |
| COPD | 0.0 | 7.8 | .02 | 5 | 6.2 | .8 |
| CRD | 0.0 | 5 | .06 | 2.8 | 6.2 | .3 |
| CLD | 2.8 | 1.4 | .5 | 2.2 | 0 | .4 |
| CVD | 0.0 | 4.3 | .08 | 2.2 | 6.2 | .2 |
| Malignancy | 2.8 | 11.3 | .03 | 5.5 | 25 | .000 |
| Modified CCI | ||||||
| Median | 0.0 | 0.0 | .001 | 0.0 | 0.5 | .0001 |
| Modified CCI—age | ||||||
| Median | 0.0 | 2.0 | .0001 | 0.0 | 3.5 | .0001 |
| Classical CCI | ||||||
| Median | 0.0 | 1.0 | .0001 | 0.0 | 1.0 | .001 |
| Classical CCI—age | ||||||
| Median | 0.0 | 3.0 | .0001 | 0.0 | 4.0 | .0001 |
BMI, body mass index; HT, hypertension; CAD, coronary artery disease; CHF, chronic heart failure; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; CLD, chronic liver disease; CRD, chronic renal disease; CVD, cerebrovascular disease; CCI, Charlson Comorbidity Index; SD, standard deviation.P value <.05 was considered statistically significant.
Patients with respiratory failure were older (P = .0001), and they had higher comorbidity rates (P = .000). The presence of HT, DM, and malignancy was high in this group, and the differences were statistically significant (Table 3). The respiratory failure rate was significantly higher in patients with pneumonia (P = .000), and the presence of respiratory failure was significantly associated with mortality (P= .000).
Initial room air SO2 was significantly lower in patients with pneumonia, respiratory failure, and mortality (P = .0001, P = .0001, P = .03, respectively).
Compared to the patients who died and survived, comorbidity was higher among those who died, but the difference was not significant (83.8% vs. 49.8%, P = .12). However, according to the subgroup analysis of comorbidities, CRD (P = .04) and malignancy (P = .000) were significantly higher in patients with mortality (Table 4).
Discussion
Older age and comorbidities were found to be the risk factors for poor clinical outcomes in patients with COVID-19. There was a relationship between higher CCI scores and the rate of poor clinical outcomes and mortality.
In this study, there is a male predominance in COVID-19 patients.14-17 In a meta-analysis, 53% of all COVID-19 cases were reported to be male.14 In our study, 50.2% of the study population was male and there was no significant difference in gender distribution. Our study population was younger than reported in the previous studies and one-fifth of them were obese.16,17
Based on early data, it is estimated that in the USA and European Union, 80% of cases with COVID-19 result in mild illness, but 14% require hospitalization, and 6% require ICU admission.18 Recently, it was shown that more than one-third of patients with COVID-19 were admitted to ICU globally.19 In this study, the rate of the patients who were admitted to ICU was (2.3%) extremely lower than the literature.
Mortality data may vary by country and demographic features of the patients and range from 3.1% to 18%.6,9,15-17 While the rate is as high as 15%-16% in the USA and Sweden,15,16 it is 3.1% in China.6 The mortality rate of our study was 2.8% In this study, the need for ICU and mortality rates were found to be lower than in the literature. These differences may be related to the heterogeneity of our study population, as it was younger and all patients were hospitalized and isolated in the early stages of the pandemic, regardless of disease severity.
There are multiple risk factors associated with mortality and severity of disease in COVID-19 patients. Studies had shown that age, male gender, and comorbidities are predictors of mortality.15,19-23 Grasselli et al20 reported that male gender was an independent risk factor of mortality.20 Furthermore it was shown that there was an association between male gender and severity of the disease.14,21 Age ≥ 65 years were associated with a higher risk of death during the hospital stay for COVID-19.17 In contrast to these studies, poor clinical outcomes of the disease were similar between the genders in our study. However, older age was significantly a risk factor for pneumonia and respiratory failure.
The comorbidity rate of COVID-19 patients varies between 57.7% and 83%.15,16,19,20,24 This broad range may be due to demographic differences of the study population, such as age. Also, it may differ depending on whether the study population includes all COVID-19 patients or only severe patients. In a review, among the 22 753 COVID-19 patients, 57.7% had one or more comorbidity.24 There was a high rate of comorbidities (83%) at Pellaud et al’s study.16 The rate of comorbidity was 50.7% in our study. This rate, which is lower than the literature, may be related to the younger study population and not include intensive care patients.
Major comorbidities of COVID-19 patients are HT, DM, CVD, cancer, CRD, and COPD. Hypertension has the highest rate in all studies and its rate varies between 17% and 60%.4,5,15-17,24,25 As similar to the literature, the most common comorbidities were HT, DM, and malignancy in our study.
Previous studies reported a correlation between the presence of comorbidity and mortality in patients with COVID-19.4,5,19,21,26 The risk of death was increased in patients with DM, renal failure, and cardiovascular diseases such as heart failure and myocardial infarction.4,5,26 The presence of comorbidity in COVID-19 was also related to severe disease and poor outcomes.5,7,21 In a meta-analysis including 3994 COVID-19 patients reported that 13.16% of the patients had serious events (ICU admission, pneumonia, mechanical ventilation, ARDS, and death), and the presence of comorbidities increased the risk of developing serious events.5 A systematic review revealed that comorbidities including HT, DM, cardiovascular disease, CVD, COPD, CRD, and malignancy had strong associations with the severity and prognosis of COVID-19.21 Liang et al25 reported that patients with cancer had a higher risk of COVID-19 with a poorer prognosis than those without cancer.25
In this study, pneumonia, respiratory failure, and ICU admission rates were significantly higher in patients with comorbid diseases than patients without any comorbidity. The rate of HT and DM was significantly higher in patients with pneumonia and respiratory failure. Chronic obstructive pulmonary disease rate was significantly higher in patients with pneumonia and who needed to stay in ICU. Comparison of the patients according to mortality revealed that the presence of comorbidity was higher among those who died, but the difference was not significant (83.8% vs. 49.8%, P = .12). However, according to the subgroup analysis of comorbidities, CRD (P = .04) and malignancy (P = .000) were significantly higher in patients with mortality.
Charlson Comorbidity Index is a tool for calculating comorbidity scores using a predefined list of medical conditions and also it can be adjusted for age. It is an easy-to-perform and validated index estimating the risk of mortality related to comorbid disease and has been studied in many diseases.8,11-13 Coronavirus disease 19 is also one of these diseases, and studies evaluating the effect of CCI on mortality in COVID-19 patients have been increased in the literature.9,27-29 A recent systematic review and meta-analysis revealed that a higher CCI score was associated with increased mortality and severity of the disease in patients with COVID-19. There was a 16% increase in mortality for each increase in CCI score.28 The CCI score >3 (OR: 2.71; 95% CI: 1.85-3.97) was found to be associated with mortality.15 Gregoriano et al30 reported that patients had a high burden of comorbidities, with a median CCI of 3 points.30 It is found that CCI score above 0 was associated with an increased risk of severe COVID-19 and death when controlled for age and sex.9 There was also an increasing trend in the length of stay in the hospital related to increasing CCI scores.29
In some studies, categorized CCI score9,27 was used, while in others, both the median CCI score and the categorized CCI were evaluated together.28,29 In our study, both median scores and categorized CCI were evaluated to improve the power of the study. The median scores of CCI and age-adjusted CCI were 0.00 and 1.00, respectively, in all study populations. These scores were relatively lower than the literature, but it is thought that it might be explained by the younger study population. Despite the relatively low CCI score in all patients with COVID-19, both median-modified and classical CCI scores and their age-adjusted scores were significantly higher in patients with poor clinical outcomes including pneumonia, respiratory failure, the need for a stay in ICU, and mortality. In addition, it was observed that as the severity of comorbidity increased according to the classification of CCI scores, poor clinical outcomes were also increased. These results were similar to the literature.
Modified CCI is a shorter and simpler form of CCI.13 Unlike CCI, comorbidities do not include myocardial infarction, peripheral vascular disease, CVD, peptic ulcer, and uncomplicated diabetes. However, thromboembolic complications are frequent in patients with COVID-19. In addition, DM is one of the most common comorbidities. In order not to ignore the effect of these comorbidities, both classical CCI and modified CCI were evaluated in our study. However, the effects of both classical and modified CCI scores on poor clinical course and mortality were similar.
There are some limitations of this study. At first, it was a retrospective study and it was carried out in a single center (a university hospital). Second, the study population included a limited number of patients, with a median age of 46 years. Approximately one-quarter of the population is 65 years old or older. Especially, the relatively small number of the elderly population, where comorbidities are common, may not reflect the real effect of the severity of comorbidity. Third, retrospective screening of comorbidities from the database and calculating CCI seems problematic. However, since the first day of the pandemic in our clinic, medical information and comorbidities of inpatients have been recorded in detail in order to reduce unknowns about COVID-19. Therefore, we believe that this issue can be ignored.
In conclusion, older age and comorbidities were associated with poor clinical outcomes in patients with COVID-19. Charlson Comorbidity Index was significantly higher in patients with poor clinical outcomes including pneumonia, respiratory failure, intensive care, and death. There was a relationship between higher CCI scores and the rate of poor clinical outcomes and mortality. It is thought that evaluating the CCI score during the initial admission will contribute to the identification of the risky group and the preparation of an early action plan.
Footnotes
Ethics Committee Approval: This study was approved by Ethics committee of Kocaeli University, (Approval No: GOKAEK-2020/11.19).
Informed Consent: As informed consent from patients to review their medical records was not obtained, patient data were de-identified.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – S.A.B., H.B., S.A., B.M., M.D., İ.B.; Design – S.A.B., H.B., S.A., B.M., M.D., İ.B.; Supervision – S.A.B., H.B., M.D., İ.B.; Resources – S.A.B., H.B., İ.B.; Materials – S.A.B., H.B., S.A., B.M., M.D., İ.B.; Data Collection and/or Processing – S.A.B., M.D., İ.B.; ; Analysis and/or Interpretation – S.A.B., M.D., İ.B.; Literature Search – S.A.B., M.D.; Writing Manuscript – S.A.B., M.D., İ.B.; Critical Review – S.A.B., H.B., İ.B.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. World Health Organization (WHO). Coronavirus Disease (COVID-19) Pandemic. Geneva: World Health Organization; 2020. [Google Scholar]
- 2. Ministry of Health Republic of Turkey. COVID-19 web page of the Republic of Turkey. Coronavirus Disease (COVID-19) Pandemic. https://covid19.saglik.gov.tr Ministry of Health [Internet]. 2021 [accessed 2021 August 01]. [Google Scholar]
- 3. Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: an overview. J Chin Med Assoc. 2020;83(3):217 220. 10.1097/JCMA.0000000000000270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim DW, Byeon KH, Kim J, Cho KD, Lee N. The correlation of comorbidities on the mortality in patients with COVID-19: an observational study based on the Korean national health insurance big data. J Korean Med Sci. 2020;35(26):e243. 10.3346/jkms.2020.35.e243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nandy K, Salunke A, Pathak SK, et al. Coronavirus disease (COVID-19): a systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes Metab Syndr. 2020;14(5):1017 1025. 10.1016/j.dsx.2020.06.064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5). 10.1183/13993003.00547-2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zaki N, Alashwal H, Ibrahim S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: a systematic review. Diabetes Metab Syndr. 2020;14(5):1133 1142. 10.1016/j.dsx.2020.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373 383. 10.1016/0021-9681(87)90171-8) [DOI] [PubMed] [Google Scholar]
- 9. Christensen DM, Strange JE, Gislason G, et al. Charlson comorbidity index score and risk of severe outcome and death in Danish COVID-19 patients. J Gen Intern Med. 2020;35(9):1 3. 10.1007/s11606-020-05991-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization (WHO)COVID-19 Case definition. Updated in Public health surveillance for COVID-19. 16 December 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2. [Google Scholar]
- 11. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245 1251. 10.1016/0895-4356(94)90129-5) [DOI] [PubMed] [Google Scholar]
- 12. Hall WH, Ramachandran R, Narayan S, Jani AB, Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4:94. 10.1186/1471-2407-4-94). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ternavasio-de la Vega HG, Castaño-Romero F, Ragozzino S, et al. The updated Charlson comorbidity index is a useful predictor of mortality in patients with Staphylococcus aureus bacteraemia. Epidemiol Infect. 2018;146(16):2122 2130. 10.1017/S0950268818002480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galbadage T, Peterson BM, Awada J, et al. Systematic review and meta-analysis of sex-specific COVID-19 clinical outcomes. Front Med. 2020;7:348. 10.3389/fmed.2020.00348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imam Z, Odish F, Gill I, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288(4):469 476. 10.1111/joim.13119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pellaud C, Grandmaison G, Thien HP, et al. Characteristics, comorbidities, 30-day outcome and in-hospital mortality of patients hospitalised with COVID-19 in a Swiss area - a retrospective cohort study. Swiss Med Wkly. 2020;150:w20314. 10.4414/smw.2020.20314) [DOI] [PubMed] [Google Scholar]
- 17. Pérez FM, Pino JLD, García NJ, et al. Comorbidity and prognostic factors on admission in a COVID-19 cohort of a general hospital. Rev Clin Esp. 2020:S0014-2565(20)30179-X. 10.1016/j.rce.2020.05.017) [DOI] [Google Scholar]
- 18. Kinross P, Suetens C, Dias JG, et al. Rapidly increasing cumulative incidence of coronavirus disease (COVID-19) in the European Union/European Economic Area and the United Kingdom, 1 January to 15 March 2020. Euro Surveill. 2020;25(11). 10.2807/1560-7917.ES.2020.25.11.2000285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abate SM, Ali SA, Mantfardo B, Basu B. Rate of Intensive Care Unit admission and outcomes among patients with coronavirus: a systematic review and meta-analysis. PLoS One. 2020;15(7):e0235653. 10.1371/journal.pone.0235653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345 1355. 10.1001/jamainternmed.2020.3539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging. 2020;12(13):12493 12503. 10.18632/aging.103579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hussain A, Mahawar K, Xia Z, Yang W, El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract. 2020;14(4):295 300. 10.1016/j.orcp.2020.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Güner R, Hasanoğlu İ, Kayaaslan B, et al. COVID-19 experience of the major pandemic response center in the capital: results of the pandemic’s first month in Turkey. Turk J Med Sci. 2020;50(8):1801 1809. 10.3906/sag-2006-164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bajgain KT, Badal S, Bajgain BB, Santana MJ. Rate of comorbidities among individuals with COVID-19: a rapid review of current literature. Am J Infect Control. 2021;49(2):238 2 4 6. 10.1016/j.ajic.2020.06.213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335 337. 10.1016/S1470-2045(20)30096-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu ZH, Tang Y, Cheng Q. Diabetes increases the mortality of patients with COVID-19: a meta-analysis. Acta Diabetol. 2021;58(2):1 39 144. 10.1007/s00592-020-01546-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17(9):e1003321. 10.1371/journal.pmed.1003321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuswardhani RAT, Henrina J, Pranata R, Lim MA, Lawrensia S, Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(6):2103 2109. 10.1016/j.dsx.2020.10.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou W, Qin X, Hu X, Lu Y, Pan J. Prognosis models for severe and critical COVID-19 based on the Charlson and Elixhauser comorbidity indices. Int J Med Sci. 2020;17(15):2257 2263. 10.7150/ijms.50007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gregoriano C, Koch D, Haubitz S, et al. Characteristics, predictors and outcomes among 99 patients hospitalised with COVID-19 in a tertiary care centre in Switzerland: an observational analysis. Swiss Med Wkly. 2020;150:w20316. 10.4414/smw.2020.20316) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a