Abstract
A 73-year-old female was referred to our department for persistent left anterior benign paroxysmal positional vertigo refractory to multiple repositioning procedures and training physiotherapist exercises. The audiovestibular assessment and high resonance computed tomography of the petrosal bone confirmed the presence of a 5.4 mm large paucisymptomatic left anterior semicircular canal dehiscence. The connection between the 2 apparently distinct neurotological entities in the same patient was further sustained by additional imagery. T2-weighted and 3-dimensional labyrinthine sequences confirmed the presence of a partially “auto-plugged” superior semicircular canal dehiscence which progressively entrapped greater otolith particles proximal to the cupula of the superior semicircular canal.
Keywords: Benign paroxysmal positional vertigo, labyrinthine MRI, semicircular canal dehiscence
Introduction
The superior semicircular canal dehiscence (SSCD) is a bony defect responsible for a variable range of cochlear and vestibular symptoms. Diagnosis is based on specific audiovestibular tests and high-resolution computed tomography (HRCT) of the temporal bone, which measures the size of the bone defect. Sometimes its discovery can be fortuitous, and the correlation between dehiscence’s size and vestibulocochlear signs and symptoms along with audiovestibular findings is still debated.1 A self-limiting benign paroxysmal positional vertigo (BPPV) can become disabling when it recurs regularly or fails to respond to repositioning therapy, affecting the subject’s quality of life.2 Calcium crystals (otoliths) are usually easily repositioned when they are smaller than the diameter of the affected membranous canal, easily finding their way back to the utricle. Stagnation of larger debris could be responsible for persistent dizziness and recurrent vertigo. Here we report the case of a patient with disabling left superior semicircular canal BPPV, in whom an associated left SSCD was also found. Being partially self-sealing and therefore stretching the lumen of the SSC, it systematically prevented the performance of a fully effective repositioning maneuver.
Case Presentation
A 73-year-old female was referred to our department for recurrent BPPV lasting for over 1 year, triggered by neck extension and rolling over in bed on the right side. Repeated repositioning maneuvers by an experienced physical therapist did not completely alleviate the patient. Audiovestibular evaluation revealed normal hearing on the right side and a mixed hearing loss with a 40 dB Sound Pressure Level (SPL) air-bone gap limited to 250 Hz on the left side (Figure 1A). Middle ear reflexes were present bilaterally; cervical vestibular evoked myogenic potentials (cVEMPs) in air conduction were preserved with a lower than normal threshold (60 dB Hearing Level) on the left side (Figure 1B). Videonystagmography (VNG) (Ulmer System®, Synapsis, France) was normal. Head shaking test, fistula test, and Valsalva maneuver were normal. However, left-beating nystagmus was elicited by the skull vibration test.3 A written informed consent was obtained from the patient.
Figure 1. a,b.
(a) Left-sided mixed hearing loss with an air-bone gap of 40 dB at 250 Hz; (b) lower than normal cVEMPs at 60 dB. Left cVEMPs amplitudes were significantly greater when compared to the right cVEMPs. cVEMPs, cervical vestibular evoked myogenic potentials.
Benign paroxysmal positional vertigo of the left SSC was suspected when Dix–Hallpike maneuver4 elicited prominent leftward torsional down-beating nystagmus on lying supine from the sitting position (latency: 4 seconds, duration: 22 seconds, peak slow-phase velocity: 8°/seconds, Video – Supplementary material). Sitting up did not produce nystagmus reversal. Repetition of repositioning procedures including mastoid vibration5 during the maneuver to promote mobilization of the otolith and evacuate presumably attached particles was unsuccessful.
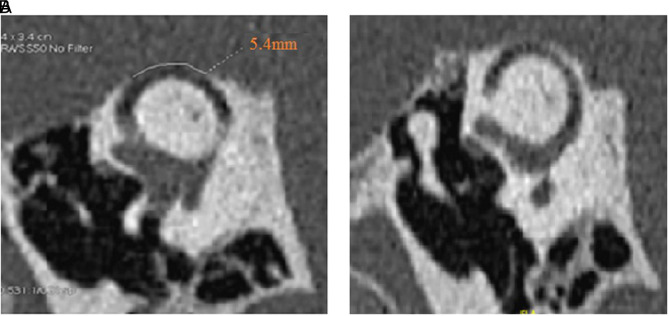
High-resolution computed tomography (HRCT) (GE GSI Revolution, GE healthcare, Chicago, Illinois, USA) of the petrous bone with reconstruction slices acquired in the Pöschl plane at nominal 0.625 mm slice thickness with a 50% overlap of 0.312 mm6 allowed to visualize a 5.4 mm left SSCD (Figure 2A). A thin bone covered the right SC (Figure 2B). Three-dimensional high-resolution magnetic resonance imagery (3D HR MRI) of the labyrinth (Figure 3A–B) supported this hypothesis by confirming the presence of a 0.5 mm endolymphatic membrane collapse over the left SSC membranous duct, not far from the cupula.
Figure 2. a,b.
High-resolution computed tomography (HRCT) scan of the petrous bone showing (a) a right-sided measured 3.8 mm “near dehiscence” of the SSC and (b) (left ear) a left-sided 5.4 mm large dehiscence of the SSC. SSC, superior semicircular canal.
Figure 3. a,b.
(a) Three-dimensional high-resolution MRI of the labyrinth. Narrowing and partial stenosis of the membranous duct of the left superior semicircular canal (right) matching the SSCD area; Fusion imaging between 3D T2 FLAIR and T2 Driven Equilibrium (DRIVE) confirming the narrowing left SSC (left); (b) (3D HR MRI) of the labyrinth confirming a narrowing at 0.5 mm of the SSC (for a normal diameter of about 1.2 mm)—multiaxial 3D plane view. SSCD, superior semicircular canal dehiscence; FLAIR, fluid-attenuated inversion recovery; 3D HR MRI, three-dimensional high-resolution magnetic resonance imaging.
Although the patient did not complain of autophony, sound/air sensitivity, or vertigo induced by loud sounds,6 the clinical presentation and audiovestibular findings were consistent with a third window abnormality combined with persistent BPPV on the same SSC.
Surgical treatment to complete spontaneous auto-plugging of the SSC to prevent further migration of trapped otoliths was therefore proposed. For the time being, the patient preferred daily Brandt–Darroff’s exercises—although partially effective—to surgery.
Discussion
The most intriguing finding in this patient was the persistence of a rare variant of BPPV, despite repeated repositioning maneuvers by experienced physical therapists, associated with the presence of large radiological dehiscence of 5.4 mm when one might have expected the absence of significant symptoms.1 Superior variant of BPPV is exceptional4 due to its high location, promoting otoconial self-clearance. Bell-shaped debris of calcium carbonate with a ranging size of 1-30 µm displaced from the utricular membrane float in the superior membranous SC ducts generating vertigo with head position movements.9 It arises generally as a complication of repositioning maneuvers or after prolonged hyperextension and its treatment consists of reverse Epley or similar maneuvers.4
Because both pathologies were located on the left side and the SSCD was sufficiently large, we hypothesized that the superior membrane of the SC could be progressively deformed and narrowed by a protrusion of the dura through the dehiscence to reach and compress the membranous SSC. Indeed, MRI showed significant narrowing of the SSC lumen, near the ampullary crus. Protrusion of the dura would result in partial plugging of SSCD, as previously hypothesized in some variants.1,7-8 Therefore, persistent and/or refractory BPPV to left SSC repositioning maneuvers may develop progressively, as larger otoliths may become trapped. Due to repeated repositioning maneuvers, a little more otoconia9 could be stacked between the SSC cup and the stretched area. Therefore, episodes of vertigo could frequently recur or become permanent, unresponsive to repositioning maneuvers since a progressively narrowed lumen would become a true barrier to otoconia passage. However, there are fewer explanations available for the absence of the classic auditory signs in this condition, except for conductive hearing loss. We hypothesize that an auto-plugging process develops slowly, perhaps concomitantly with the bone defect due to a local—as yet unknown—mechanism and that, as a result, no significant auditory signs have developed.
Young et al10 described the case of a 55-year-old patient with SSCD, in whom the clinical examination revealed a positional torsional and down-beating nystagmus suggestive of ipsilateral SC BPPV.10 Repositioning maneuvers were ineffective. The authors hypothesized "auto-plugged" dehiscence by intracranial contents, with "excitatory" nystagmus in the plane of the SC caused by the positional unblocking of the dehiscent canal in the supine position, producing an ampullofugal flow. In our opinion, our case report should be considered as either the natural evolution or a different clinical variant of the abovementioned case report. However, we describe a potential link between 2 neurological conditions, a true left anterior BPPV and left partial auto-plugged SSCD, strongly supported by appropriate labyrinthine imagery.
Conclusion
Furthermore, in cases of unexplained persistent BPPV, we support the previous opinion11 that 3D HR MRI should be performed to exclude membrane deformations, which would promote otolithic blockage of the SC, including the cases of large and possibly auto-plugged SSCD.
Footnotes
Informed Consent: A written informed consent was obtained from the patient.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – E.I., S.I., A.L-B., P.R.; Design – P.R., E.I., S.I.; Supervision – H.T-V, E.I., P.R.; Resources – P.R., S.I.; Materials – A.L-B.; Data Collection and/or Processing – S.I., A.L-B., P.R.; Analysis and/or Interpretation – S.I., A.L-B., P.R.; Literature Search – P.R., S.I.; Writing Manuscript – S.I., P.R.; Critical Review – H.T-V, E.I.
Acknowledgments: The authors would like to thank Mrs. Ruxandra-Cecilia Ionescu for proofreading the manuscript.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. Chien WW, Janky K, Minor LB, Carey JP. Superior canal dehiscence size: multivariate assessment of clinical impact. Otol Neurotol. 2012;33(5):810–815.. 10.1097/MAO.0b013e318248eac4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Handa PR, Kuhn AMB, Cunha F, Schaffleln R, Ganança FF. Quality of life in patients with benign paroxysmal positional vertigo and/or Menière’s disease. Braz J Otorhinolaryngol. 2005;71(6):776–782.. 10.1016/S1808-8694(15)31248-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dumas G, Curthoys IS, Lion A, Perrin P, Schmerber S. The skull vibration-induced nystagmus test of vestibular function: a review. Front Neurol. 2017;8:41. 10.3389/fneur.2017.00041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anagnostou E, Kouzi I, Spengos K. Diagnosis and treatment of anterior-canal benign paroxysmal positional vertigo: a systematic review. J Clin Neurol. 2015;11(3):262–267.. 10.3988/jcn.2015.11.3.262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li JC. Mastoid oscillation: a critical factor for success in canalith repositioning procedure. Otolaryngol Head Neck Surg. 1995;112(6):670–675.. 10.1016/s0194-5998(95)70174-5) [DOI] [PubMed] [Google Scholar]
- 6. Ward BK, Carey JP, Minor LB. Superior canal dehiscence syndrome: lessons from the first 20 years. Front Neurol. 2017;8:177. 10.3389/fneur.2017.00177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cremer PD, Minor LB, Carey JP, Della Santina CC. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology. 2000;55(12):1833–1841.. 10.1212/wnl.55.12.1833) [DOI] [PubMed] [Google Scholar]
- 8. Brandolini C, Modugno GC. Do signs of natural plugging of superior semicircular canal dehiscence exist? Am J Otolaryngol. 2012;33(2):268–271.. 10.1016/j.amjoto.2011.06.005) [DOI] [PubMed] [Google Scholar]
- 9. Jang YS, Hwang CH, Shin JY, Bae WY, Kim LS. Age-related changes on the morphology of the otoconia. Laryngoscope. 2006;116(6):996–1001.. 10.1097/01.mlg.0000217238.84401.03) [DOI] [PubMed] [Google Scholar]
- 10. Young AS, McMonagle B, Pohl DV, Magnussen J, Welgampola MS. Superior semicircular canal dehiscence persenting with recurrent positional vertigo. Neurology. 2019;93(24):1070–1072.. 10.1212/WNL.0000000000008624) [DOI] [PubMed] [Google Scholar]
- 11. Schratzenstaller B, Wagner-Manslau C, Alexiou C, Arnold W. High-resolution three-dimensional magnetic resonance imaging of the vestibular labyrinth in patients with atypical and intractable benign positional vertigo. ORL J Otorhinolaryngol Relat Spec. 2001;63(3):165–177.. 10.1159/000055734) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a