Abstract
Objective
The recently emerged coronavirus 2019 disease is an infectious disease that predominantly affects the respiratory system. In this study, we aimed to evaluate the persistent post-COVID symptoms and the related factors.
MATERIAL AND Methods
This study was conducted on 396 post-COVID patients. The demographic (age, gender, body mass index, smoking, location and duration of treatment, and date of post-COVID follow-up visit) and clinical (symptoms during and after the infection, comorbidities) data were evaluated by interview and a questionnaire.
Results
The mean age of the patients was 50.25 years (min-max: 19-85). There were equal numbers of males (n = 198) and females (n = 198) in the study. The mean body mass index was 27.94 (min-max: 17.90-44.92). The majority of patients (n = 222, 56.1%) had been treated at home, while the rates of patients admitted to ward and intensive care unit were 37.1% (n = 147) and 6.8% (n = 27), respectively. The number of patients with at least 1 persistent symptom during post-COVID follow-up visit was 348 (87.9%). The symptoms during the infection included fatigue (n = 339, 85.6%), cough (n = 373, 68.9%), joint pain (n = 267, 67.4%), appetite loss (n = 234, 59.1%), dyspnea (n = 231, 58.3%), while the persistent post-COVID symptoms were fatigue (n = 222, 56.1%), cough (n = 174, 43.9%), dyspnea (n = 171, 43.2%), and chest pain (n = 171, 43.2%). No significant relationships between post-COVID symptoms and age, body mass index, comorbidity, duration from diagnosis to a follow-up visit, and COVID-19 pneumonia during the infection were found, while a statistically significant relationship regarding gender was found.
Conclusion
There is still a lack of knowledge about the long-term consequences of coronavirus 2019 disease. Moreover, no standardized method exists for categorizing patients into post-COVID controls.
Keywords: COVID-19, pandemic, persistent symptoms, post-COVID symptoms
Main Points
In this study, we evaluated the frequency of symptoms and the related factors during coronavirus 2019 disease (COVID-19) and post-COVID follow-up visit. We found that 348 (87.9%) patients had at least 1 persistent symptom at the post-COVID follow-up visit.
As the clinicians and researchers have focused on the acute phase of COVID-19, there are no sufficient data on the long-term consequences and the quality of life the patients would have after the treatment period ends.
Introduction
The recently emerged coronavirus 2019 disease (COVID-19) is an infectious disease that predominantly affects the respiratory system. Due to its high contagiousness, COVID-19 rapidly spread to millions of people worldwide and caused hundreds of thousands of deaths. In a study by Wu and McGoogan,1 mild symptoms were observed in 81% of COVID-19 patients, while 14% suffered from severe respiratory failure and 5% had multi-organ dysfunction and septic shock in addition to respiratory failure.1
Most patients with COVID-19 were reported to recover based on negative polymerase chain reaction (PCR) results or symptom-free for a few days. Although the definition of recovery based on that criteria might be correct, it should be kept in mind that many patients continue to have the same or similar symptoms of COVID-19 after recovery.2 As most of the studies focused on the clinical features of patients during hospital admission, literature is lacking on the data regarding the sequel and long-term consequences of COVID-19.2,3 Recent research has demonstrated that 50-87% of the recovered COVID-19 patients had at least 1 persistent symptom.4,5
Literature shows that research about post-COVID symptoms is still in the early stages. In many studies, it was found that 50-70% of the patients discharged from the hospital within a 3-month period had persistent multi-systemic symptoms, including neurocognitive, autonomic, gastrointestinal, respiratory, musculoskeletal, and psychological dysfunctions.4,6-10 Thus, there is a need to describe, classify, and recognize the persistent post-COVID symptoms, especially by researchers and clinicians. However, even the first step of this process, to establish a standard terminology, still lacks a clear consensus.11
The health authorities focusing on the active COVID-19 disease should not underestimate the post-COVID symptoms. In our study, we aimed to evaluate the persistent post-COVID symptoms and the related factors.
Material and Methods
This study was conducted in a second-tier state hospital between January 1, 2021, and March 15, 2021. The 396 adult volunteer patients who were diagnosed with PCR-confirmed COVID-19 and under treatment at home or in the hospital were included in the study. Signed informed consent from all participants and institutional ethics committee approval (Ethics Committee of Harran University, HRU/21.07.17) was obtained for our study that was in compliance with the principles of the Helsinki Declaration.
The patients younger than 18 years, patients with more than 60 days of past COVID infection, and those who did not complete the 10-day treatment/quarantine period were excluded from the study.
The demographic and clinical data of patients obtained using a questionnaire were recorded during the post-COVID follow-up visit. The demographic data consisted of age, gender, height, weight, smoking status, location and duration of treatment, and date of the post-COVID follow-up visit. The clinical data included the comorbidities and the symptoms that started with COVID-19 and continued after the treatment like fatigue, dyspnea, joint pain, chest pain, cough, anosmia, dry/red eye, rhinorrhea, dysgeusia, headache, sputum, loss of appetite, sore throat, dizziness, muscle pain, and diarrhea. The development of COVID-19 pneumonia in relevant cases was also recorded in the study forms.
The patients were grouped according to age, body mass index (BMI), smoking status, and the date of the follow-up visit. The age groups consisted of 18-30, 31-40, and >40 years. The BMI groups were defined as underweight, normal, overweight, obese stage 1, obese stage 2, and obese stage 3 when BMI was 18.5, 18.5-24.9, 25-29.9, 30-34.9, 35-39.9, and >40, respectively. The smoking status was grouped into 2 as smokers and non-smokers. The patients were grouped regarding the follow-up visit date as 11-20 days, 21-30 days, and >30 days that were defined as the duration between the end of the quarantine and the day of the follow-up visit.
The symptoms of patients during the active COVID-19 infection and the persisting symptoms at the follow-up visit were compared. The demographic and clinical data of patients were compared to the persistent symptoms.
Statistical Analysis
The Statistical Package for Social Sciences, version 15.0 software (SPSS Inc.; Chicago, IL, USA). The assumption of normal distribution of data was tested by the Kolmogorov–Smirnov test. Descriptive statistics of continuous variables were shown with mean and standard deviation (SD) values. The chi-square test was used to compare nominal variables, while Student’s t-test was used to compare the means values of scalar data between the 2 groups. Binary logistic regression analysis was used to evaluate the relationship between dependent and independent variables. The hypotheses are 2-sided, and a P value below .05 was considered statistically significant at a 95% CI.
Results
The study was completed with 396 patients. The mean age of the patients was 50.25 years (min-max: 19-85). The majority was over 40 years (n = 285, 72%), while the number of patients between 31-40 and 18-30 years was 72 (18.2%) and 39 (9.8%), respectively. There was an equal number of male (n = 198) and female (n = 198) patients in the study.
The number of patients with comorbidity was 189 (47.7%), and the most common comorbid disease was asthma in 102 patients (25.8%). COVID-19 pneumonia was detected in 204 of the patients (51.5%) during the active infection. The most common symptoms during COVID-19 were fatigue (n = 339, 85.6%), cough (n = 273, 68.9%), and joint pain (n = 234, 59.1%), while fatigue, cough, dyspnea, and chest pain were present in 56.1%, 43.9%, 43.2%, and 43.2% patients during the post-COVID follow-up visit, respectively. Clinical data of patients are presented in Table 1.
Table 1.
Clinical Data of Patients
| Present, n (%) | Absent, n (%) | |
|---|---|---|
| Comorbidity | 189 (47.7) | 207 (52.3) |
| Asthma | 102 (25.8) | 294 (74.2) |
| Diabetes mellitus | 48 (12.1) | 348 (87.9) |
| Hypertension | 75 (18.9) | 321 (81.1) |
| Chronic renal failure | 3 (0.8) | 393 (99.2) |
| Chronic heart failure | 33 (8.3) | 363 (91.7) |
| Chronic obstructive pulmonary disease | 15 (3.8) | 381 (96.2) |
| Cerebrovascular event | 3 (0.8) | 393 (99.2) |
| Thyroid disorder | 12 (3) | 384 (97) |
| Pneumonia | 204 (51.5) | 192 (48.5) |
The mean BMI of the patients was 27.94 (min-max: 17.90-44.92). The number of smokers in the study was 333 (84.1%). The majority of the patients (n = 222, 56.1%) were under treatment at home, while 147 (37.1%) and 37 (6.8%) patients were treated in the COVID-19 ward and intensive care unit (ICU), respectively. The mean duration of hospitalization was 4.39 days (min-max: 0-60). Most of the patients (n = 222, 56.1%) came for the post-COVID follow-up visit after 30 days (Table 2).
Table 2.
The Relationship Between Post-COVID Symptoms and Demographic/Clinical Data of Patients
| Post-COVID Symptoms | Post-COVID Symptoms | P | |
|---|---|---|---|
| None, n (%) | Present, n (%) | ||
| Age (years) | |||
| 18-30 | 3 (7.6) | 36 (92.7) | .600 |
| 30-40 | 6 (8.3) | 66 (92.7) | |
| >40 | 39 (13.6) | 246 (86.4) | |
| Gender | |||
| Female | 12 (6.06) | 186 (93.04) | .033 |
| Male | 36 (18.18) | 162 (81.82) | |
| Comorbidity | |||
| None | 33 (15.9) | 174 (84.1) | .150 |
| Present | 15 (7.9) | 174 (92.1) | |
| BMI | |||
| Underweight | 0 (0) | 3 (100) | .300 |
| Normal | 21 (24.1) | 66 (75.9) | |
| Overweight | 21 (10.2) | 183 (89.8) | |
| Stage 1 obese | 6 (8.6) | 63 (91.4) | |
| Stage 2 obese | 0 (0) | 21 (100) | |
| Stage 3 obese | 0 (0) | 12 (100) | |
| Post-COVID follow-up visit (days) | |||
| 11-20 | 0 (0) | 75 (100) | .100 |
| 21-30 | 12 (12.1) | 87 (87.9) | |
| >30 | 36 (16.2) | 186 (83.8) | |
| COVID-19 pneumonia | |||
| Present | 33 (16.1) | 171 (83.9) | .100 |
| Absent | 15 (7.8) | 187 (92.2) |
BMI, body mass index; COVID-19, coronavirus disease 2019.
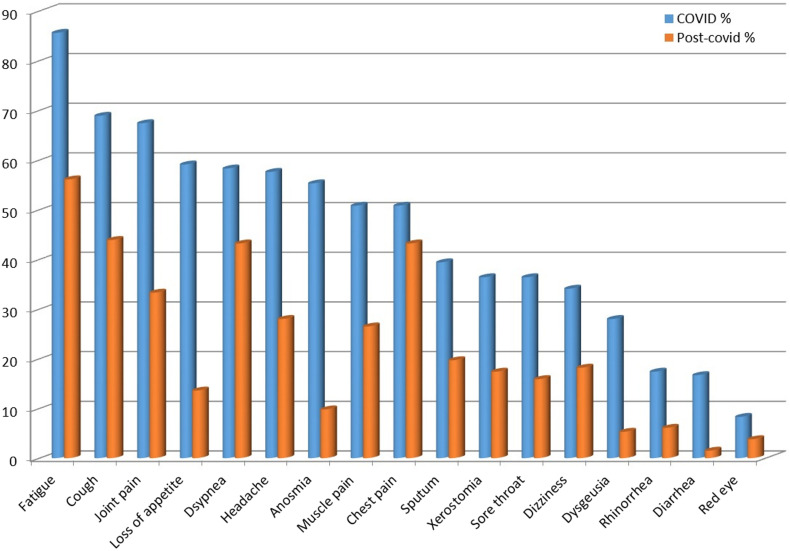
The number of patients with at least 1 symptom at the follow-up visit was 348 (87.9%). The number of female patients (n = 186, 93.04%) with at least 1 symptom at the post-COVID follow-up visit was significantly more than that of the male patients (n = 162, 81.82%) (P = .033). No such significant relationships between post-COVID symptoms and age, comorbidity, BMI, COVID-19 pneumonia, and date of the follow-up visit were found (P > .05). The distribution of symptoms during COVID-19 and at the post-COVID visit was shown in Figure 1.
Figure 1.
The distribution of symptoms during COVID-19 and at post-COVID visit. COVID-19, coronavirus disease 2019.
The regression analysis showed that asthma patients had persistent post-COVID symptoms 28.9 times more than the ones without it (P = .041). The patients with fatigue, joint pain, chest pain, and dysgeusia without those had significantly 5.7 (P = .05), 5 115 (P = .048), 15.57 (P = .021), and 16 245 (P = .029) times more persistent post-COVID symptoms compared to the patients without those (Table 3).
Table 3.
Regression Analysis of Clinical Data During COVID-19 Infection
| B | SE | Wald | df | Sig. | Exp(B) | 95% CI for EXP (B) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Comorbidity | -1.865 | 1.103 | 2.86 | 1 | .091 | 0.155 | 0.018 | 1.345 |
| Hypertension | 3.075 | 1.699 | 3.275 | 1 | .07 | 21.649 | 0.775 | 605.007 |
| Asthma | 3.364 | 1.644 | 4.191 | 1 | .041 | 28.918 | 1.154 | 724.68 |
| Fatigue | 1.740 | 0.889 | 3.832 | 1 | .050 | 5.7 | 0.998 | 32.563 |
| Joint pain | 1.632 | 0.825 | 3.914 | 1 | .048 | 5.115 | 1.015 | 25.77 |
| Chest pain | 2.745 | 1.19 | 5.319 | 1 | .021 | 15.57 | 1.51 | 160.538 |
| Cough | 1.266 | 0.703 | 3.243 | 1 | .072 | 3.546 | 0.894 | 14.062 |
| Dysgeusia | 2.788 | 1.274 | 4.788 | 1 | .029 | 16.245 | 1.337 | 197.353 |
| Muscle pain | -1.649 | 0.939 | 3.084 | 1 | .079 | 0.192 | 0.031 | 1.211 |
| Constant | -1.463 | 0.875 | 2.793 | 1 | .095 | 0.232 | ||
Sig, significant.
Discussion
In this study, we evaluated the frequency of symptoms and the related factors during COVID-19 and post-COVID follow-up visit. We found that 348 (87.9%) patients had at least 1 persistent symptom at the post-COVID follow-up visit. Among the most common post-COVID symptoms in this study, fatigue, cough, dyspnea, and chest pain were present in 222 (56.1%), 174 (43.9%), 171 (43.2%), and 171 (43.2%), respectively. We found a significant relationship between being female and the persistence of symptoms during the post-COVID follow-up visit. In the regression analysis, asthma, fatigue, joint pain, chest pain, cough, and dysgeusia were observed to be statistically related to post-COVID persistent symptoms.
In our study, 116 (87.9%) of the patients had at least 1 persistent symptom at the post-COVID follow-up visit. Similar to our study results, Carfì et al4 reported the persistence of at least 1 symptom in 87.4% of the patients who recovered from COVID-19.
In previous studies, the most commonly reported symptoms during COVID-19 included cough, fever, dyspnea, musculoskeletal symptoms (myalgia, joint pain, fatigue), gastrointestinal symptoms, and anosmia/dysgeusia.12-14 Similarly, the most common symptoms during COVID-19 in this study were fatigue (85.6%), cough (68.9%), joint pain (67.4%), loss of appetite (59.1%), and dyspnea (58.3%).
Previously, Garrigues et al6 reported fatigue (55%), dyspnea (42%), memory loss (34%), and disruptions of concentration and sleep in 120 patients examined on a mean of 110.9 (± 11.1) day after COVID-19 diagnosis. In another study from the United Kingdom, the most common post-COVID symptoms were fatigue (72% in ICU and 60.3% in ward patients), followed by dyspnea (65.6% in ICU and 42.6% in ward patients).15 In 35% of the symptomatic COVID-19 patients who were under treatment as an outpatient mainly complained of fatigue, cough, and headache and stated that they did not reach their pre-COVID performances.16 Carfì et al4 evaluated COVID-19 patients who were discharged after treatment in the hospital for 60 days and reported that 87% had at least 1 symptom, and the life quality of 44% of the patients had worsened due to persistent symptoms.4 In the current study, the most common symptoms at the post-COVID follow-up visit observed in 74 (56.1%), 58 (43.9%), 57 (43.2%), and 57 (43.2%) patients were fatigue, cough, dyspnea, respectively.
There are studies that indicated the age and comorbidities in patients were related to the severity of COVID-19.17 Halpin et al18 suggested that a severe course of COVID-19 had been observed in old patients who had comorbidity and treated in the hospital. Moreover, there are studies that suggested a link between the severity and the duration of symptoms, that is, a 2-week continuation of symptoms in mild cases, 3-6 weeks in severe cases.13 On the contrary to those studies, we observed a statistically significant relationship only between the persistency of symptoms at post-COVID follow-up visit and being female; no such relationships were found regarding the BMI, duration between diagnosis and visit, and COVID-19 pneumonia in the current patients. Additionally, no significant relationships between symptom persistency and age and comorbidity were found, although 72% of the patients were over 40 years and 47.7% had at least 1 comorbid condition. The lack of a standardized COVID-19 follow-up algorithm that could allow the assessment of patients from multiple aspects might be responsible for the varied results among studies. The persistency of at least 1 symptom in 83.8% of the patients on the follow-up visit, which was 30 days after the diagnosis, is a noteworthy result of our study. One of the important problems of the post-COVID period is the selection of time intervals for describing the symptoms as persistent. Baig et al19 suggested that symptoms present in the 3rd week after COVID-19 should be considered as long-term or persistent. Symptoms that lasted over 3 weeks were suggested to be classified as post-acute COVID, while symptoms that lasted more than 12 weeks were suggested to be classified as persistent chronic post-COVID in another study.18
Although it has been more than a year since the onset of the pandemic, there are still no international standardized guidelines for COVID-19 follow-up control. The functional limitations in COVID-19 sufferers should be assessed to predict the long-term load of this disease.20,21 The attention on the management of post-COVID symptoms and psychology of the patients should be more increased. Future longitudinal studies that investigate objective parameters using tests for pulmonary function, 6-min walk, and the quality of life, depression, anxiety, and post-traumatic stress disorder would provide more information to understand the long-term consequences of COVID-19 in general.
Among the limitations of the current study is the lack of information about the history of complaints before acute COVID-19 and the details of the severity of the symptoms. The size of the study group with a limited number of ICU patients, the lack of a control group discharged for other causes, and the results from a single healthcare facility can be considered as other limitations of our study. On the other hand, the post-COVID symptoms mentioned in the study are not specific to COVID-19, as persistent symptoms might occur after community-acquired pneumonia.
As the clinicians and researchers have focused on the acute phase of COVID-19, there are no sufficient data on the long-term consequences. Moreover, the lack of a standardized method to group patients as post-COVID controls leads to confusion of information. Thus, a structured follow-up algorithm should be established for the post-COVID period. Also, a need for rehabilitation centers may potentially arise for patients in that period. Future and larger studies from multiple healthcare centers are needed to confirm and expand the results of the current study.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Ethics Committee Approval: This study was approved by Ethical thics committee of the Harran University (Approval No: HRU/21.02.27).
Informed Consent: Verbal informed consent was obtained from the patients who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – B.Ç., M.K.; Design – B.Ç., M.K.; Supervision – B.Ç., M.K.; Resources – B.Ç., M.K.; Materials – B.Ç., M.K.; Data Collection and/or Processing – B.Ç., M.K.; Analysis and/or Interpretation – B.Ç., M.K.; Literature Search – B.Ç., M.K.; Writing Manuscript – B.Ç., M.K.; Critical Review – B.Ç., M.K.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239 1242. 10.1001/jama.2020.2648) [DOI] [PubMed] [Google Scholar]
- 2. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574 1581. 10.1001/jama.2020.5394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727 733. 10.1056/NEJMoa2001017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carfì A, Bernabei R, Landi F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603 605. 10.1001/jama.2020.12603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murray A, Gerada C, Greenhalgh T. We need a Nightingale model for rehab after COVID-19 | Comment. Health Serv J. 2020. Available at: https://www.hsj.co.uk/commissioning/we-need-a-nightingale-model-for-rehab-after-covid-19-/7027335.article. [Google Scholar]
- 6. Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4 e6. 10.1016/j.jinf.2020.08.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carvalho-Schneider C, Laurent E, Lemaignen A, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258 263. 10.1016/j.cmi.2020.09.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76(4):399 401. 10.1136/thoraxjnl-2020-216086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mandal S, Barnett J, Brill SE, et al. “Long-COVID”: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalization for COVID-19. Thorax. 2021;76(4):396-398. 10.1136/thoraxjnl-2020-215818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nehme M, Braillard O, Alcoba G, et al. COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med. 2021;174(5):723 725. 10.7326/M20-5926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Editorial. Long COVID: let patients help define long-lasting COVID symptoms. Nature. 2020;586(7828):170. 10.1038/d41586-020-02796-2) [DOI] [PubMed] [Google Scholar]
- 12. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. 10.1136/bmj.m1985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061 1069. 10.1001/jama.2020.1585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landi F, Barillaro C, Bellieni A, et al. The new challenge of geriatrics: saving frail older people from the SARS-COV-2 pandemic infection. J Nutr Health Aging. 2020;24(5):466 470. 10.1007/s12603-020-1356-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013 1022. 10.1002/jmv.26368). [DOI] [PubMed] [Google Scholar]
- 16. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network — United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993 998. 10.15585/mmwr.mm6930e1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343 346. 10.15585/mmwr.mm6912e2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halpin S, O’Connor R, Sivan M. Long COVID and chronic COVID syndromes. J Med Virol. 2021;93(3):1242 1243. 10.1002/jmv.26587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baig AM.Chronic COVID syndrome: need for an appropriate medical terminology for long-COVID and COVID long-haulers. J Med Virol. 2021;93(5):2555 2556. 10.1002/jmv.26624) [DOI] [PubMed] [Google Scholar]
- 20. Belli S, Balbi B, Prince I, et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur Respir J. 2020;56(4). 10.1183/13993003.02096-2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCue C, Cowan R, Quasim T, Puxty K, McPeake J. Long term outcomes of critically ill COVID-19 pneumonia patients: early learning. Intensive Care Med. 2021;47(2):240 241. 10.1007/s00134-020-06313-x) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a