Abstract
RTS,S/AS01 (Mosquirix®) is a vaccine against malaria caused by Plasmodium falciparum. In a phase 3 trial, RTS,S/AS01 showed vaccine efficacy against clinical malaria, severe malaria and malaria hospitalization, with an acceptable safety and tolerability profile, in children aged 6 weeks to 17 months; the vaccine efficacy was greater in children than in infants and waned over time. In another phase 3 trial, RTS,S/AS01 was noninferior to seasonal malaria chemoprevention in children. WHO recommends a 4-dose schedule of RTS,S/AS01 for the prevention of P. falciparum malaria in children from 5 months of age living in regions with moderate to high malaria transmission, with an optional 5-dose schedule for areas with highly seasonal malaria transmission. First results from large pilot implementation in Africa show that RTS,S/AS01 has a favourable safety profile, increases equity in access to malaria prevention, is highly cost effective, can be delivered through routine national immunization programmes and substantially reduces severe malaria burden.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40267-022-00937-3.
Plain Language Summary
Malaria is a life-threatening disease caused by Plasmodium parasites, which are spread to humans through bites of infected mosquitoes. RTS,S/AS01 (Mosquirix®) is a vaccine against malaria caused by P. falciparum. In phase 3 trials, RTS,S/AS01 showed vaccine efficacy against P. falciparum malaria and was at least as effective as seasonal malaria chemoprevention in children, with an acceptable safety and tolerability profile. Results of the first 2 years of a large scale pilot implementation of RTS,S/AS01 in Africa allowed WHO to recommend the vaccine for the prevention of P. falciparum malaria in children from 5 months of age living in regions with moderate to high malaria transmission, with an optional use for seasonal malaria.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40267-022-00937-3.
| Digital Features for this Adis Drug Q&A can be found at 10.6084/m9.figshare.19817521 |
Adis evaluation of RTS,S/AS01 in the management of malaria
| First malaria vaccine; targets the pre-erythrocytic stage of P. falciparum |
| Shows vaccine efficacy against P. falciparum malaria in children; can be used to prevent seasonal malaria |
| Acceptable safety and tolerability profile |
| Recommended by WHO; cost effective; provides equitable access to malaria prevention; can be incorporated in routine national immunization programmes |
What is the rationale for developing RTS,S/AS01 against malaria?
Malaria is a preventable and treatable, but deadly, disease caused by infection of red blood cells by Plasmodium parasites, transmitted to humans through the bites of infected female Anopheles mosquitoes [1–3]. Of the five Plasmodium species that infect humans, Plasmodium falciparum is one of the most prevalent and the most problematic. The initial symptoms of malaria (fever, headache and chills, typically appearing 8–14 days after the infective mosquito bite) may be mild and often indistinguishable from other undifferentiated febrile illnesses. If left untreated, P. falciparum malaria can progress to severe illness and death within 24 h. The manifestations of severe malaria include severe anaemia, coma, respiratory distress, circulatory shock, hypoglycaemia, kidney failure and pulmonary oedema [1–3]. In 2020, there were an estimated 241 million cases of malaria and 627,000 malaria-related deaths worldwide, with ≈ 95% of these occurring in sub-Saharan Africa [4]. P. falciparum malaria exacts the highest toll in African children aged < 5 years [4].
Malaria control interventions recommended by WHO include insecticide-treated bed nets, indoor residual spraying of insecticides, rapid diagnosis and treatment with highly effective artemisinin combination therapies and seasonal malaria chemoprevention (SMC) in children [3]. Thanks to these interventions, an estimated 1.7 billion cases of malaria and 10.6 million cases of malaria deaths have been averted globally in the past 20 years [4]. However, the trend line showing a decrease in the number of malaria cases/deaths has plateaued since 2015, with indeed a slight uptrend in 2020 which is likely related to disruptions of malaria programmes caused by the COVID-19 pandemic [4]. The addition of a vaccine against P. falciparum to the malaria control interventions represents an important and much needed development in the continued fight against malaria.
Vaccines targeting various life-cycle stages (pre-erythrocytic, blood and sexual stages) of the malaria parasite are being evaluated [5]. A pre-erythrocytic vaccine may be ideal as it prevents the first stage of parasite development, blocking the subsequent stages. RTS,S/AS01 (Mosquirix®) is a malaria vaccine that targets the pre-erythrocytic stage of P. falciparum [6]. GSK is the developer and the marketing authorization holder of the vaccine. RTS,S/AS01 received a positive scientific opinion from the European Medicines Agency (EMA) under ‘EU-Medicines for all’ (previously known as ‘article 58’) procedure [3]. This procedure allows EMA’s Committee for Medicinal Products for Human Use, in cooperation with WHO, to give scientific opinions on medicines and vaccines for human use that are intended exclusively for markets outside of the EU. RTS,S/AS01, the first malaria vaccine and also the first vaccine against a parasite in humans, is recommended by WHO.
For whom is RTS,S/AS01 indicated?
RTS,S/AS01 has EMA’s positive opinion for active immunisation of children aged 6 weeks to 17 months against P. falciparum malaria and against hepatitis B; however, the vaccine should be used based on official recommendations that consider the epidemiology of P. falciparum malaria in different geographical areas [6]. A summary of the EU prescribing information for RTS,S/AS01 is provided in Table 1.
Table 1.
EU prescribing summary of RTS,S/AS01 malaria vaccine (Mosquirix®) [6]. Consult local prescribing information for further details
| What is the approved indication? | |
| Active immunisation of children aged 6 weeks up to 17 months against Plasmodium falciparum malaria and hepatitis B (article 58 approval) | |
| Use should be based on official recommendations that consider the epidemiology of P. falciparum malaria in different geographical areas | |
| How is RTS,S/AS01 available? | |
| Powder (RTS,S antigen) and suspension (AS01 adjuvant), which must be reconstituted prior to administration | |
| What is the composition of RTS,S/AS01? | |
| After reconstitution, one 1 mL vial contains two 0.5 mL vaccine doses. One vaccine dose (0.5 mL) contains 25 µg of RTS,S, adjuvanted with AS01 | |
| RTS,S is a portion of P. falciparum circumsporozoite protein fused with hepatitis B surface antigen (RTS) and combined with hepatitis B surface antigen (S) in the form of non-infectious virus-like particles produced in yeast (Saccharomyces cerevisiae) cells by recombinant DNA technology | |
| AS01 is a liposome-based vaccine adjuvant that comprises 3-O-desacyl-4’- monophosphoryl lipid A (MPL) 25 µg and Quillaja saponaria Molina, fraction 21 (QS-21) 25 µg. MPL is manufactured by GSK while QA-21 is licensed by GSK from Antigenics LLC, a wholly owned subsidiary of Agenus Inc. | |
| How should RTS,S/AS01 be stored? | |
| Shelf-life is 3 years. Do not freeze the product. Store in the original package to protect it from light | |
| From a microbiological standpoint, reconstituted RTS,S/AS01 should be used immediately, although chemical and physical in-use stability has been demonstrated for 6 h at 25° C. If not used immediately, normal storage time is < 6 h in a refrigerator (2°–8° C) | |
| How should RTS,S/AS01 be administered? | |
| Intramuscular injection; the preferred injection site in children aged ≥ 5 months is the deltoid muscle | |
| What are the contraindications to the use of RTS,S/AS01? | |
| Hypersensitivity to the active substance or any of the excipients of RTS,S/AS01, previous doses of RTS,S/AS01 or hepatitis B vaccines | |
| What other special warnings/precautions pertain to the use of RTS,S/AS01? | |
| Protection against P. falciparum malaria | Does not provide complete protection; may delay the natural acquisition of immunity; efficacy wanes over time; efficacy data limited to children from sub-Saharan Africa; does not protect against malaria caused by pathogens other than P. falciparum |
| Protection against hepatitis B | Should not be used for the prevention of hepatitis B when prevention against P. falciparum malaria is not sought |
| Infants at risk of bleeding | Administer with caution in individuals with thrombocytopenia or any coagulation disorders, as bleeding may occur after intramuscular injection |
| Infants with immunodeficiency | No data other than for HIV infection for which data are limited |
| Preterm infants (born ≤ 28 weeks of gestation) | When administering the first three doses, consider the potential risk of apnoea and the need for respiratory monitoring for 48–72 h in infants who remain hospitalized at the time of vaccination, particularly those with a history of respiratory immaturity |
| Which vaccines can be administered concomitantly with RTS,S/AS01? | |
| Monovalent or combination vaccines including diphtheria, tetanus, whole cell pertussis, acellular pertussis, hepatitis B, Haemophilus influenzae type b, oral polio, measles, rubella, yellow fever, rotavirus and pneumococcal conjugate vaccines | |
| What are the potential clinically relevant drug interactions between RTS,S/AS01 and other drugs/vaccines? | |
| Pneumococcal conjugate vaccines | May increase the risk of fever within 7 days after vaccination |
| Rotavirus and pneumococcal conjugate vaccines | May reduce the antibody response to the circumsporozoite antigen of RTS,S/AS01 |
| Immunosuppressive therapy | No data, but decreased efficacy cannot be ruled out |
| Antipyretic prophylaxis | May decrease the immune response to the vaccine, and hence, not recommended |
What is WHO position on RTS,S/AS01?
WHO recommends RTS,S/AS01 in a 4-dose schedule for the prevention of P. falciparum malaria in children from 5 months of age living in regions with moderate to high malaria transmission, as defined by WHO [1, 3]. The schedule includes three primary doses with a minimum interval of 4 weeks between doses, followed by a booster dose approximately 12–18 months after the third dose. An optional 5-dose strategy may be considered in areas with high seasonal malaria transmission or areas with perennial malaria transmission with seasonal peaks. This strategy includes three primary doses administered at monthly intervals and two annual booster doses administered prior to peak malaria transmission season [1, 3].
How does RTS,S/AS01 work?
RTS,S/AS01 is a monovalent pre-erythrocytic recombinant protein vaccine that comprises P. falciparum circumsporozoite protein (CSP) regions known to induce humoral (R region) and cellular (T region) immune responses, covalently linked to the hepatitis B virus surface antigen (S) [3, 6, 7]. RTS is co-expressed in yeast (Saccharomyces cerevisiae) with free S, yielding RTS,S virus-like particles. The vaccine uses GSK’s proprietary AS01 adjuvant system. RTS,S/AS01 elicits humoral and cellular immunity against the circumsporozoite protein amply expressed on the sporozoite surface, thereby limiting the ability of P. falciparum to infect, mature and multiply in the liver [3, 6–8].
What is the vaccine efficacy of RTS,S/AS01 in malaria?
RTS,S/AS01 demonstrated vaccine efficacy in several phase 2 trials [9–12]. This section focuses on data from two randomized, double-blind, controlled phase 3 trials of RTS,S/AS01: a pivotal (NCT00866619) [13–16] and a seasonal malaria vaccination (NCT03143218) [17] trial.
Pivotal trial
This trial investigated the vaccine efficacy of RTS,S/AS01 given as a 3-dose primary series plus a booster dose schedule in more than 15,000 infants and children (aged 6–12 weeks and 5–17 months, respectively, at the time of enrolment) at 11 centres from seven sub-Saharan African countries, with malaria transmission intensities ranging from low to high [13–16]. Subjects were randomized to one of three groups: three doses of RTS,S/AS01 at months 0, 1, 2 and a booster dose at month 20; three doses of RTS,S/AS01 and a dose of control vaccine [meningococcal serogroup C conjugate vaccine (Menjugate®)] at month 20; three doses of control vaccines [Menjugate® for infants and rabies vaccine (VeroRab®) for children] at months 0, 1, 2, and a control vaccine (Menjugate® in both age categories) at month 20 [13–16]. Infants received RTS,S/AS01 concomitantly with the Expanded Programme on Immunization (EPI) vaccines [13]. Subjects had not received systematic malaria treatment before vaccination. Routine malaria control measures and treatment of malaria during the study were implemented in accordance with national guidelines. Patient baseline characteristics were generally similar across the treatment groups in each age category, with insecticide-treated bed nets used in > 75% of children and > 80% of infants at study end [13].
RTS,S/AS01 vaccine efficacy against the first and only episode of clinical malaria over 12 months after the third dose was 31.1% in infants (coprimary endpoint) [15] and 55.8% in children (coprimary endpoint) [16]. After 12 months, the vaccine efficacy remained lower in infants than in children. Based on the totality of evidence pointing to a lower of efficacy of RTS,S/AS01 in infants, WHO recommends the vaccine for children from 5 months of age [1, 3]. Accordingly, data for only children are discussed further in this article.
In children, in the 12 months after the third dose, RTS,S/AS01 vaccine efficacy was > 47 % against clinical malaria (all episodes), severe malaria and malaria-related hospitalization (Table 2) [16].Vaccine efficacy waned over time, although clinically relevant protection was still evident 18 months after the third dose (Table 2) [14]. Without a booster dose, vaccine efficacy against clinical malaria was 34% at 30 months, with no or minimal protection against severe malaria or malaria-related hospitalization (Table 2) [13]. Administration of a booster dose provided an incremental vaccine efficacy of 25.6% (95% CI 18.2–32.3) during the 12 months after the booster dose compared with the group that did not receive the booster dose, with the efficacy partially retained over up to 46 months after dose 3 (Table 2) [13]. The numbers of clinical malaria cases averted with RTS,S/AS01 were the highest in areas of high malaria incidence [e.g. Siaya (Kenya) and Nanoro (Burkina Faso)] [13]. In the trial site with the highest malaria incidence (Siaya), up to 6565 and 4443 cases of clinical malaria per 1000 children could be prevented with four and three doses of RTS,S/AS01, respectively [13].
Table 2.
Efficacy of RTS,S/AS01 (Mosquirix®) in African children aged 5–17 months
| Time point | No. of subjectsa (RTS,S/AS01 vs control) |
Vaccine efficacy (%) [95% CI] | ||
|---|---|---|---|---|
| Clinical malariab | Severe malariac | Malaria hospitalizationd | ||
| 12 months after dose 3 [16] | 2830 vs 1466 | 55.1 [50.5 to 59.3]** | 47.3 [22.4 to 64.2]** | 47.9 [34.6 to 58.5]**e |
| 18 months after dose 3 [14] | 4557 vs 2328 | 45.7 [41.7 to 49.5]** | 35.5 [14.6 to 51.1]** | 41.5 [29.1 to 51.7]** |
| 30 months after dose 3 [13] | ||||
| 3 doses | 2306 vs 2336 | 33.9 [28.9 to 38.6]** | 2.1 [− 27.5 to 24.8] | 18.1 [1.1 to 32.3]*e |
| 3 doses + 1 booster dose | 2276 vs 2336 | 46.1 [41.8 to 50.1]** | 32.4 [9.5 to 49.8] | 40.1 [26.2 to 51.5]*e* |
| 46 months after dose 3 [13] | ||||
| 3 doses | 2306 vs 2336 | 26.2 [20.8 to 31.2]** | − 5.8 [− 35.0 to 17.0] | 12 [− 5 to 26]e |
| 3 doses + 1 booster dose | 2276 vs 2336 | 39.0 [34.3 to 43.3]** | 28.5 [6.3 to 45.7]** | 37.2 [23.6 to 48.5]e |
| Over 6.8 years [18] | ||||
| 3 doses | 829 vs 839 | 19.1 [10.8 to 26.7]** | 10.1 [− 18.1 to 31.6] | Not reportedf |
| 3 doses + 1 booster dose | 844 vs 839 | 23.7 [15.9 to 30.7]** | 36.7 [14.6 to 53.1]** | Not reportedf |
*p < 0.05, **p ≤ 0.01 vs comparator
aModified intention-to-treat population for 6.8 years and per-protocol population for all other time points
bAll episodes. Primary case definition: temperature ≥ 37.5 °C and P. falciparum asexual parasitaemia (> 5000 parasites/mm3) or a case of malaria meeting the primary case definition of severe malaria
cPrimary case definition: P. falciparum asexual parasitaemia (> 5000 parasites/mm3) with ≥ 1 marker of disease severity and without comorbidity
dDefined as a medical hospitalization with confirmed P. falciparum asexual parasitaemia (> 5000 parasites/mm3)
eData from the EU assessment report [36] or summary of product characteristics[6]
fVaccine efficacy was similar to that for severe malaria, as most severe malaria cases also met the case definition for malaria hospitalisation
In an open-label extension study (NCT02207816) of the pivotal trial, subjects in three trial centres [Korogwe (Tanzania), Kombewa (Kenya) and Nanoro (Burkina Faso)] were followed for additional 3 years (7 years in total since the first vaccination in children aged 5–17 months) [18]. During the 3-year extension, the overall incidences of severe malaria were low in all three treatment groups: 0.004, 0.007 and 0.009 cases per person-years at risk in the RTS,S/AS01 4-dose, RTS,S/AS01 3-dose and control groups, respectively (primary outcome). RTS,S/AS01 vaccine efficacy for the 4-dose schedule against severe malaria was maintained over up to 7 years (Table 2) [18]. Rebound of clinical malaria (i.e. incidence higher in the RTS,S/AS01 group than in the control group) was seen in one of the study centre (Nanoro) with high malaria prevalence, although this did not result in a rebound of severe malaria [18].
Seasonal malaria vaccination trial
Taking advantage of the vaccine’s high initial efficacy, this trial investigated the efficacy of RTS,S/AS01 with or without SMC in children in the Sahel regions of Africa, where malaria transmission is high during a few months of the year [17]. In this double-blind trial, 6861 children aged 5–17 months from Burkina Faso and Mali received RTS,S/AS01 alone (three doses in April, May and June 2017, followed by fourth dose in June 2018 and fifth dose in June 2019), SMC alone (four courses of sulfadoxine–pyrimethamine and amodiaquine at monthly intervals each year) or RTS,S/AS01 plus SMC. In the SMC alone group, children also received three doses of rabies vaccine (Rabipur®) in 2017 and a dose of hepatitis A vaccine (Havrix®) in 2018 and 2019. In the RTS,S/AS01 alone group, children received four courses of placebo SMC each year. All children were given a long-lasting insecticide-treated bed net. Patient baseline characteristics and use of the bed nets were generally well balanced between treatment groups [17].
In the modified intention-to-treat population, RTS,S/AS01 was noninferior to SMC for the incidence of uncomplicated clinical malaria over the 3-year trial period (primary outcome; Table 3) [17]. The hazard ratio (HR) was 0.92, with 90%, 95% and 99% CIs for the HR all excluding the prespecified noninferiority margin of 1.20. Similar results were seen for the per-protocol population [17].
Table 3.
Efficacy of seasonal malaria vaccination with RTS,S/AS01 (Mosquirix®) over 3 years in African children aged 5–17 months in a randomized phase 3 trial [17]
| Outcomes/treatmentsa | No. of events/100 person-year at risk [95% CI] |
Protective efficacyb (%) [95% CI] | |
|---|---|---|---|
| Versus SMC alone | Versus RTS,S/AS01 alone | ||
| Uncomplicated clinical malariac | |||
| SMC alone | 304.8 [290.5 to 319.8] | ||
| RTS,S/AS01 alone | 278.2 [264.6 to 292.4] | 7.9 [− 1.0 to 16.0] | |
| RTS,S/AS01 + SMC | 113.3 [104.7 to 122.5] | 62.8 [58.4 to 66.8] | 59.6 [54.7 to 64.0] |
| Hospitalization for severe malariad | |||
| SMC alone | 6.8 [4.9 to 9.4] | ||
| RTS,S/AS01 alone | 6.7 [4.8 to 9.2] | − 0.4 [− 60.2 to 37.1] | |
| RTS,S/AS01 + SMC | 2.0 [1.1 to 3.6] | 70.5 [41.9 to 85.0] | 70.6 [42.3 to 85.0] |
| Death from malaria | |||
| SMC alone | 2.0 [1.1 to 3.6] | ||
| RTS,S/AS01 alone | 2.2 [1.2 to 3.8] | − 9.5 [− 148.3 to 51.7] | |
| RTS,S/AS01 + SMC | 0.5 [0.2 to 1.7] | 72.9 [2.9 to 92.4] | 75.3 [12.5 to 93.0] |
SMC seasonal malaria chemoprevention
aOutcomes were assessed in modified intention-treat population (n = 1965, 1988, 1967 in the SMC, RTS,S/AS01 and combination groups, respectively; see main text for treatment details)
bCalculated as (1−hazard ratio) × 100
cPrimary outcome: defined as body temperature ≥ 37.5 °C or a history of fever within the previous 48 h and P. falciparum parasitemia (parasite density ≥ 5000 per mm3) in children who presented to a clinical trial health facility
dClassified according to the WHO definition
RTS,S/AS01 plus SMC was superior to either treatment alone for the incidence of uncomplicated clinical malaria, with a protective efficacy of ≥ 59% over 3 years (Table 3) [17]. The protective efficacy of the combination decreased over time (71.7%, 63.2%, 58.6% at year 1, 2 and 3, respectively) and was similar in Burkina Faso and Mali. The combination also provided protection against several prespecified secondary outcomes, including hospitalization for severe malaria and death from malaria (Table 3). Weekly and end-of-season surveys showed that the prevalence of malaria parasitemia, anaemia (haemoglobin < 7 g/dL) and P. falciparum gametocytemia was consistently lower with the combination than with RTS,S/AS01 or SMC alone [17].
What is the safety and tolerability profile of RTS,S/AS01?
RTS,S/AS01 had acceptable safety and tolerability profiles in phase 3 trials [13, 17–23]. In a pooled analysis of > 11,000 children vaccinated with three doses of RTS,S/AS01, the very common (incidence ≥ 1/10) adverse reactions occurring within 7 days post-vaccination were fever (incidence 27%), irritability (14%) and injection site reactions, such as pain (16%) and swelling (7%) [6]. This section mainly focuses on data for children aged 5–17 months in the pivotal trial [13, 18, 20, 21].
Solicited local AEs occurring within 7 days post-vaccination in children receiving each of the first three doses of RTS,S/AS01 (n = 4321) versus control (n = 2128) vaccines were: pain (12.4% vs 5.8%), redness (3.1% vs 2.7%) and swelling (9.6% vs 7.6%) [20]. During this period, vaccination-related solicited general AEs in the respective groups included fever (16.9% vs 5.9%), irritability (5.9% vs 2.8%), loss of appetite (5.5% vs 3.1%) and drowsiness (3.4% vs 2.3%). Most solicited local and general AEs were of grade 1 or 2 severity [20]. In 4200 children who received a booster dose, decreased appetite as an adverse reaction to the vaccine was reported more frequently (very common) compared with the incidences seen after the first three doses [6]. The RTS,S/AS01 booster dose was also more reactogenic than the control vaccine, with grade 3 fever reported in 5.3% of children after the RTS,S/AS01 booster dose [13]. Fever occurred mainly on the day after vaccination and mostly resolved within a day [20]. Local and systemic reactogenicity are more likely in HIV-infected children than in children of unknown HIV status [6].
The incidences of unsolicited AEs occurring within 30 days post-vaccination were largely similar in children receiving four doses of RTS,S/AS01 and those receiving the control vaccine, with the exception of fever (20.4% vs 10.4%), reflecting the febrile reactions shortly after vaccination in children that were not part of the reactogenicity subset [13].
The incidence of serious AEs over the entire study period in children receiving RTS,S/AS01 with (24.2%) or without (25.3%) a booster dose was similar to that in the control group (28.4%) [20]. The most common of these were malaria, pneumonia, febrile convulsions, gastroenteritis and anaemia. Vaccination-related serious AEs occurred in 0–0.3% of subjects, with the most being fever-related. There were no vaccination-related deaths [20]. No vaccine-related severe AEs were reported during the 3-year extension study [18].
Among HIV-infected children, the incidence of serious AEs was similar between the RTS,S/AS01 and control groups in the pivotal trial [21]; this finding is further supported by data from a phase 3 trial (NCT01148459) in children with WHO stage 1 or 2 HIV disease [22]. The safety profile of RTS,S/AS01 in preterm infants and malnourished children (i.e. low weight-for-age) was generally similar to that in the overall study populations [20].
RTS,S/AS01 had acceptable reactogenicity and safety profiles when coadministered with EPI vaccines, such as measles, rubella and yellow fever vaccines [19] or pneumococcal conjugate and rotavirus vaccines [23]. There were no new safety signals when RTS,S/AS01 was coadministered with SMC [17].
Adverse events of special interest
RTS,S/AS01 was associated with an increased risk of febrile convulsions in children [20]. Following the fourth dose of RTS,S/AS01, the incidence of febrile convulsions within 7 days was 2.5 (vs 0.4 in the control group) per 1000 vaccine doses. Febrile convulsions occurred mainly within the first 2–3 days after dose 3 and 4. However, in children receiving four doses of RTS,S/AS01, the incidence of febrile convulsions reported as serious AEs was similar to that in the control group within 30 days post-vaccination (1.1% vs 1.1%) or from month 0 to study end (5.3% vs 5.5%) [20].
During the entire pivotal trial period, meningitis was reported in 21 of 5948 (0.35%) children receiving RTS,S/AS01 and 1 of 2974 (0.03%) children in the control group [20]. The relative risk was 11.0 (95% CI 1.4–85.1) for four doses of RTS,S/AS01 versus control. There was also an imbalance between the groups in the incidence of cerebral malaria, and all-cause mortality among girls. A causal relationship linking the vaccine to meningitis and cerebral malaria has not been established [20]. Furthermore, these safety signals were not seen in earlier phase 2 or subsequent phase 3 trials, or through prospective monitoring of large-scale pilot evaluations, indicating that these signals were chance findings [3].
What is the immunogenicity of RTS,S/AS01?
RTS,S/AS01 is highly immunogenic, inducing high levels of anti-CSP antibodies and CSP-specific CD4 T-cell responses in vaccinated individuals [7, 24–27]. The CD4 T-cell responses may provide additional protection, independent of the anti-CSP antibody response [27]. A definitive threshold level of anti-CSP antibody titres that can serve as a surrogate or correlate of protection against malaria has not been identified. However, based on a model, an anti-CSP antibody titre level of 121 EU/mL is expected to prevent 50% of malaria infections [28].
RTS,S/AS01 induced a stronger immune response in children than in infants in the pivotal trial, where coadministered EPI vaccines differed between the age categories [6, 28]. The geometric mean titre (GMT) levels of anti-CSP antibodies in children versus infants were 621 versus 211 EU/mL at 1 month after the third dose, 34 versus 6 EU/mL before the booster dose and 318 versus 170 EU/mL at 1 month after the booster dose [6]. Anti-CSP titres waned in a biphasic exponential pattern [28], with rapid waning in the first 6.5 months and slow waning over 7 years [29]. The half-life of the short- and long-lived components of the antibody response was 45 and 591 days in children aged 5–17 months, respectively [28]. It is estimated that 12% of the response would be long-lived after primary vaccination in children, increasing to 30% after a booster dose. The decline in the antibody response predicted the duration of vaccine efficacy against clinical malaria, with the efficacy waning more rapidly in areas of higher transmission intensities [28].
RTS,S/AS01 was immunogenic in preterm infants and malnourished children [28], as well as in HIV-infected children [21, 22], including those with WHO stage 1 or 2 disease receiving high antiretroviral and co-trimoxazole treatment [22]. In the pivotal trial, anti-CSP antibody GMTs were lower in HIV-infected children than in children of unknown HIV status (193 vs 492 EU/mL) [21].
In children primed with three doses of RTS,S/AS01, subsequent annual booster doses given before the malaria transmission season induced strong antibody responses, albeit these responses tended to decreased over time (Fig. 1) [30].
Fig. 1.
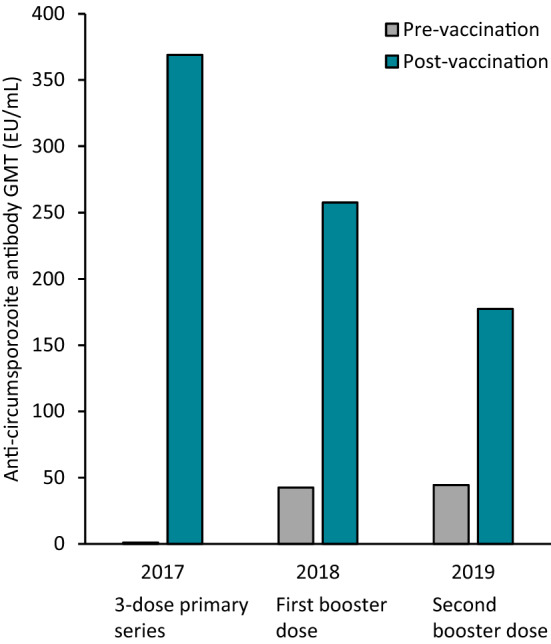
Immunogenicity of RTS,S/AS01 in children receiving three priming doses and annual booster doses in a phase 3 seasonal malaria vaccination trial [17]
Anti-CSP antibody response to RTS,S/AS01 was not impaired when coadministered with EPI vaccines, such as measles, rubella and yellow fever vaccines [19].
What are the public health and cost implications of RTS,S/AS01?
Four malaria transmission models developed by independent research groups using data from the pivotal trial predicted a significant positive public health impact and high cost-effectiveness of RTS,S/AS01 across a wide range of P. falciparum parasite prevalence settings in Africa [31].
An analysis based on one of these models (the one developed by GSK) predicted that RTS,S/AS01 vaccination would be more cost-effective in children than in infants [32]. Developed as a static Markov model, it followed a simulated 2017 birth cohort for 15 years in 41 African countries, comparing three strategies: vaccination in children at ages 6, 7.5, 9 and 27 months; vaccination in infants at ages 6, 10 and 14 weeks, and 21 months; and no vaccination. The base-case analysis was conducted from a healthcare system perspective, with an annual discount rate of 3% for both costs (2015 values) and disability-adjusted life-years (DALYs). Efficacy input was derived from the pivotal trial. The model estimated that 24.6 million children would be vaccinated across all countries. Compared with no vaccination, child vaccination was predicted to avert 16.8 million cases of clinical malaria, 359,962 cases of severe malaria, 192,213 malaria-related hospitalizations, 112,881 malaria deaths and 3.4 million DALYs over the 15-year period. At an assumed vaccine cost of $US 5 per dose, the incremental cost-effectiveness ratio per DALY averted was $US 200 (95% CI 141–314) for child vaccination. This represents 14% of the one-time gross domestic product per capita value. Across all countries, the estimated budget for child vaccination was $US 554 million in the first year, increasing up to $US 688 million in the third year. Infant vaccination was less cost effective, with a larger estimated budget impact, relative to child vaccination. Parasite prevalence, RTS,S/AS01 price and discount rate had the greatest effect on the cost effectiveness [32]. Similar findings have been reported in other analyses [33–35].
What is the current clinical position of RTS,S/AS01 in Malaria?
RTS,S/AS01, the first and currently only malaria vaccine, is borne out of 30 years of research and development through a unique public-private partnership [7]. RTS,S/AS01 prevents clinical malaria, with an acceptable safety and tolerability profile in children aged 5–17 months at the time of first vaccination. The efficacy is maximal in the first 12 months after a 3-dose primary series and wanes over time. A booster dose restores efficacy, albeit not to the extent seen after the primary series. In children who received four doses of RTS,S/AS01, vaccine efficacy remains positive over 7 years. Vaccine efficacy is lower in infants aged 6–12 weeks at the time of first vaccination than in older children. RTS,S/AS01 is also effective in a seasonal malaria setting and provides additional protection when added to SMC.
Based on its favourable risk–benefit profile in the pivotal trial, RTS,S/AS01 received a positive regulatory assessment from the EMA in July 2015 under article 58 for use in both infants and children [6]. In January 2016, WHO recommended further evaluation of RTS,S/AS01 in children aged 5–17 months in a large-scale pilot implementation before rolling it out at country level [4]. The evaluation began in 2019 in Ghana, Kenya and Malawi. In a little over 2 years, more than 830,000 children were vaccinated with over 2.4 million doses of RTS,S/AS01. In October 2021, WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Group (MPAG) jointly reviewed all available evidence. The key findings of this review are [4]:
RTS,S/AS01 reduces malaria episodes by 40%; it reduces hospital admission for severe malaria by ≈ 30% among children age-eligible for the vaccine (even in areas where insecticide-treated bed nets are widely used and there is good access to diagnosis and treatment); the vaccine saves one life for every 200 vaccinated children and has a favourable safety profile
RTS,S/AS01 could be delivered through the routine national immunization programmes, with effective and equitable coverage among target children; it has no negative impact on uptake of bed nets, other childhood vaccines or heath seeking behaviour for febrile illness
RTS,S/AS01 increases equity in access to malaria prevention: the vaccine reached more than two-thirds of children who were not sleeping under an insecticide-treated bed net; when layered, vaccination results in over 90% of children benefitting from at least one preventive intervention (insecticide-treated bed nets or the malaria vaccine)
RTS,S/AS01 is highly cost-effective in areas of moderate to high malaria transmission
Following advice from SAGE and MPAG based on the results of the pilot studies, WHO recommends RTS,S/AS01 for the prevention of P. falciparum malaria in children living in moderate to high transmission regions as defined by WHO [3, 4]. RTS,S/AS01 should be provided as part of a comprehensive malaria control strategy, with appropriate mixes of malaria control interventions identified for different subnational settings, based on local malaria epidemiology and contextual factors [3, 4]. Final full results of the pilot implementation are awaited with interest, as are additional studies assessing the efficacy and safety of RTS,S/AS01 in real-world settings.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The manuscript was reviewed by: F. J. Araujo, Department of Pharmacy, Virgen del Rocio Hospital, Seville, Spain; A. Al Hamid, Department of Pharmacy, University of Birmingham, Birmingham, UK. The author also requested that the manufacturer of RTS,S/AS01 vaccine (GSK group of companies) review this article during the peer review process. GSK’s review has been limited to the data related to GSK’s vaccine. The author retains sole responsibility for the scope and content of the article; changes resulting from comments were made by the author on the basis of scientific and editorial merit.
Declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
Yahiya Y. Syed is a salaried employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
References
- 1.World Health Organization. WHO guidelines for malaria (WHO/UCN/GMP/2022.01). 2022
- 2.World Health Organization. Malaria. 2021. https://www.who.int/. Accessed 25 Jul 2022.
- 3.World Health Organization Malaria vaccine: WHO position paper—March 2022. Wkly Epidemiol Rec. 2022;97(9):61–80. [Google Scholar]
- 4.World Health Organization. World malaria report 2021. https://www.who.int. Accessed 25 Jul 2022.
- 5.Laurens MB. Novel malaria vaccines. Hum Vaccin Immunother. 2021;17(11):4549–4552. doi: 10.1080/21645515.2021.1947762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GlaxoSmithKline. Mosquirix powder and suspension for suspension for injection: EU summary of product characteristics. 2020. https://www.ema.europa.eu. Accessed 25 Jul 2022.
- 7.Laurens MB. RTS, S/AS01 vaccine (MosquirixTM): an overview. Hum Vaccin Immunother. 2020;16(3):480–489. doi: 10.1080/21645515.2019.1669415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazmin D, Nakaya HI, Lee EK, et al. Systems analysis of protective immune responses to RTS, S malaria vaccination in humans. Proc Natl Acad Sci USA. 2017;114(9):2425–2430. doi: 10.1073/pnas.1621489114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olotu A, Fegan G, Wambua J, et al. Seven-year efficacy of RTS, S/AS01 malaria vaccine among young African childrens. N Engl J Med. 2016;374(26):2519–2529. doi: 10.1056/NEJMoa1515257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olotu A, Fegan G, Wambua J, et al. Four-year efficacy of RTS, S/AS01E and its interaction with malaria exposure. N Engl J Med. 2013;368(12):1111–1120. doi: 10.1056/NEJMoa1207564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asante KP, Abdulla S, Agnandji S, et al. Safety and efficacy of the RTS, S/AS01E candidate malaria vaccine given with expanded-programme-on-immunisation vaccines: 19 month follow-up of a randomised, open-label, phase 2 trial. Lancet Infect Dis. 2011;11(10):741–749. doi: 10.1016/S1473-3099(11)70100-1. [DOI] [PubMed] [Google Scholar]
- 12.Bejon P, Lusingu J, Olotu A, et al. Efficacy of RTS, S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359(24):2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386(9988):31–45. [DOI] [PMC free article] [PubMed]
- 14.RTS,S Clinical Trials Partnership. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014;11(7):e1001685. [DOI] [PMC free article] [PubMed]
- 15.RTS,S Clinical Trials Partnership. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367(24):2284–95. [DOI] [PMC free article] [PubMed]
- 16.RTS,S Clinical Trials Partnership. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365(20):1863–75. [DOI] [PubMed]
- 17.Chandramohan D, Zongo I, Sagara I, et al. Seasonal malaria vaccination with or without seasonal malaria chemoprevention. N Engl J Med. 2021;385(11):1005–1017. doi: 10.1056/NEJMoa2026330. [DOI] [PubMed] [Google Scholar]
- 18.Tinto H, Otieno W, Gesase S, et al. Long-term incidence of severe malaria following RTS, S/AS01 vaccination in children and infants in Africa: an open-label 3-year extension study of a phase 3 randomised controlled trial. Lancet Infect Dis. 2019;19(8):821–832. doi: 10.1016/S1473-3099(19)30300-7. [DOI] [PubMed] [Google Scholar]
- 19.Asante KP, Ansong D, Kaali S, et al. Immunogenicity and safety of the RTS, S/AS01 malaria vaccine co-administered with measles, rubella and yellow fever vaccines in Ghanaian children: a phase IIIb, multi-center, non-inferiority, randomized, open, controlled trial. Vaccine. 2020;38(18):3411–3421. doi: 10.1016/j.vaccine.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerra Mendoza Y, Garric E, Leach A, et al. Safety profile of the RTS, S/AS01 malaria vaccine in infants and children: additional data from a phase III randomized controlled trial in sub-Saharan Africa. Hum Vaccin Immunother. 2019;15(10):2386–2398. doi: 10.1080/21645515.2019.1586040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otieno L, Guerra Mendoza Y, Adjei S, et al. Safety and immunogenicity of the RTS, S/AS01 malaria vaccine in infants and children identified as HIV-infected during a randomized trial in sub-Saharan Africa. Vaccine. 2020;38(4):897–906. doi: 10.1016/j.vaccine.2019.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otieno L, Oneko M, Otieno W, et al. Safety and immunogenicity of RTS, S/AS01 malaria vaccine in infants and children with WHO stage 1 or 2 HIV disease: a randomised, double-blind, controlled trial. Lancet Infect Dis. 2016;16(10):1134–1144. doi: 10.1016/S1473-3099(16)30161-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valéa I, Adjei S, Usuf E, et al. Immune response to the hepatitis B antigen in the RTS, S/AS01 malaria vaccine, and co-administration with pneumococcal conjugate and rotavirus vaccines in African children: a randomized controlled trial. Hum Vaccin Immunother. 2018;14(6):1489–1500. doi: 10.1080/21645515.2018.1442996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS, S: a review of the available data. Malar J. 2009;8:312. doi: 10.1186/1475-2875-8-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moncunill G, De Rosa SC, Ayestaran A, et al. RTS, S/AS01E malaria vaccine induces memory and polyfunctional T cell responses in a pediatric African phase III trial. Front Immunol. 2017;8:1008. doi: 10.3389/fimmu.2017.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moncunill G, Mpina M, Nhabomba AJ, et al. Distinct helper T cell type 1 and 2 responses associated with malaria protection and risk in RTS, S/AS01E vaccinees. Clin Infect Dis. 2017;65(5):746–755. doi: 10.1093/cid/cix429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White MT, Bejon P, Olotu A, et al. The relationship between RTS, S vaccine-induced antibodies, CD4+ T cell responses and protection against Plasmodium falciparum infection. PLoS ONE. 2013;8(4):e61395. doi: 10.1371/journal.pone.0061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White MT, Verity R, Griffin JT, et al. Immunogenicity of the RTS, S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis. 2015;15(12):1450–1458. doi: 10.1016/S1473-3099(15)00239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mugo RM, Mwai K, Mwacharo J, et al. Seven-year kinetics of RTS, S/AS01-induced anti-CSP antibodies in young Kenyan children. Malar J. 2021;20(1):452. doi: 10.1186/s12936-021-03961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sagara I, Zongo I, Cairns M, et al. The anti-circumsporozoite antibody response of children to seasonal vaccination with the RTS, S/AS01E malaria vaccine. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penny MA, Verity R, Bever CA, et al. Public health impact and cost-effectiveness of the RTS, S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet. 2016;387(10016):367–375. doi: 10.1016/S0140-6736(15)00725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauboin C, Van Bellinghen LA, Van De Velde N, et al. Economic impact of introducing the RTS, S malaria vaccine: cost-effectiveness and budget impact analysis in 41 countries. MDM Policy Pract. 2019;4(2):2381468319873324. doi: 10.1177/2381468319873324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ndeketa L, Mategula D, Terlouw DJ, et al. Cost-effectiveness and public health impact of RTS, S/AS01E malaria vaccine in Malawi, using a Markov static model. Wellcome Open Res. 2021;5:260. doi: 10.12688/wellcomeopenres.16224.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galactionova K, Bertram M, Lauer J, et al. Costing RTS, S introduction in Burkina Faso, Ghana, Kenya, Senegal, Tanzania, and Uganda: A generalizable approach drawing on publicly available data. Vaccine. 2015;33(48):6710–6718. doi: 10.1016/j.vaccine.2015.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bell GJ, Loop M, Topazian HM, et al. Case reduction and cost-effectiveness of the RTS, S/AS01 malaria vaccine alongside bed nets in Lilongwe, Malawi. Vaccine. 2020;38(25):4079–4087. doi: 10.1016/j.vaccine.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.European Medicines Agency. Mosquirix™: assessment report. 2015. https://www.ema.europa.eu. Accessed 25 Jul 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


