Abstract
Cochlear implant surgery in far-advanced otosclerosis can be challenging due to the degenerative process that affects the cochlea. We used OTOPLAN® to plan and define the details of surgery in a patient with such severe alteration of the cochlea that cochlear implant could be contraindicated. A 73-year-old man affected by bilateral far-advanced otosclerosis, previously treated by bilateral stapedotomy, presented 0% of speech discrimination using bilateral hearing aids. A unilateral cochlear implant was planned. The patient underwent radiologic investigation pre-surgery with temporal bone computer tomography, magnetic resonance imaging, and OTOPLAN. Radiology confirmed bilaterally advanced signs of fenestral and cochlear otosclerosis with large osteolytic cavities along the whole cochlea leading to the mixture of endolymph and perilymph. The OTOPLAN identified the alteration of the cochlea in detail. Based on the results of the software, we used a perimodiolar implant on the left ear. No intraoperative or post-operative surgical complications were observed. The patient was checked 6 months after surgery, he did not refer any problems and obtained 75% of speech discrimination at 65 dB. Our case suggests that OTOPLAN is a useful tool in far-advanced otosclerosis because careful planning of the surgery can positively affect the results. Despite the complexity of the anatomy, the software exactly described the real intrasurgical finding. We think that the use of OTOPLAN might improve the surgical indication.
Keywords: Cochlear implant, far-advanced otosclerosis, OTOPLAN, software, surgical indication
Introduction
Far-advanced otosclerosis (FAO) indicates a severe form of otosclerosis which has progressed ossification of the cochlea and sensorineural hearing loss (SNHL).1 Cochlear implant (CI) is the best option for treating severe SNHL,2 but in FAO could be challenging because of the alteration of cochlea anatomy and the presence of spongy bone.3,4 These factors limit the use of CI in FAO.
Other controversies about the use of the best surgical approach,5,6 electrodes to choose,7 and the side to implant8 are still open.
Recently, we showed that despite cochlea alterations due to bone remodeling (third ring), the good surgical plan based on computer tomography (CT) analysis allowed us to define the correct surgical approach guaranteeing to the patient, excellent auditory results.9 When the cochlea’s turns are destroyed or completely ossified,10 it is not easy to define the best approach.
Today a software (OTOPLAN®, MED-EL (Innsbruk, Austria)) is available that is able to analyze deeply the anatomy of the ear and to measure the length of the cochlea duct via the 3D reconstruction based on CT scan. Thanks to these analyses, it is possible to plan the best method for the electrode insertion and to identify the correct length of it.11
We present a case of FAO with the destruction of cochlear turns, in which we used the OTOPLAN to plan the surgery, identify the best electrode (perimodiolar vs. later wall), and check the correct position of the CI into the cochlea duct.
Case PRESENTATION
A 73-year-old man affected by bilateral FAO, previously treated by bilateral stapedotomy came to our clinic due to the worsening of his hearing (Figure 1); he presented 0% speech discrimination in the free field with bilateral hearing aids. For this reason, we proposed a single-side cochlear implant. Written informed consent was obtained from patients’ parents.
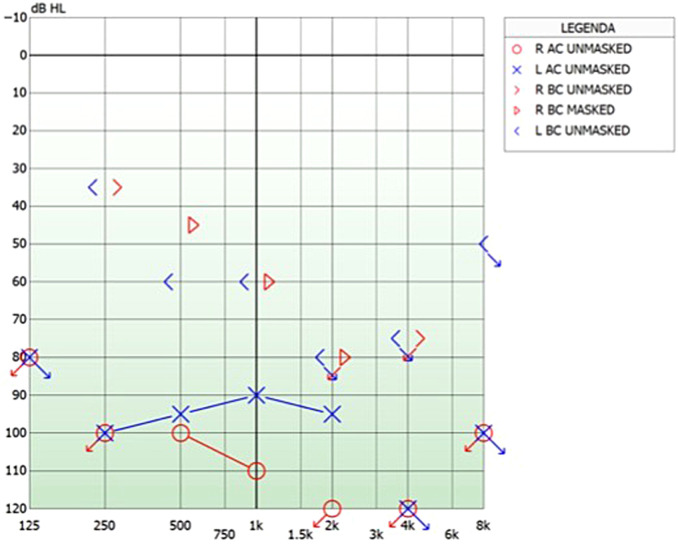
Figure 1.
(A) The pure tone audiometry shows a bilateral profound mixed hearing loss, worse in the left ear. (B) The speech recognition test in the best-aided condition at 65 dB sound pressure level (SPL) presents 0% discrimination.
The CT on the temporal bone and the magnetic resonance imaging confirmed bilaterally advanced signs of fenestral and cochlear otosclerosis with large osteolytic cavities along the whole cochlea leading to the mixture of endolymph and perilymph. Due to the radiologic results, we decided to use OTOPLAN software to obtain precise details of the anatomy and for choosing the best ear for implantation and the type of CI.
OTOPLAN Results
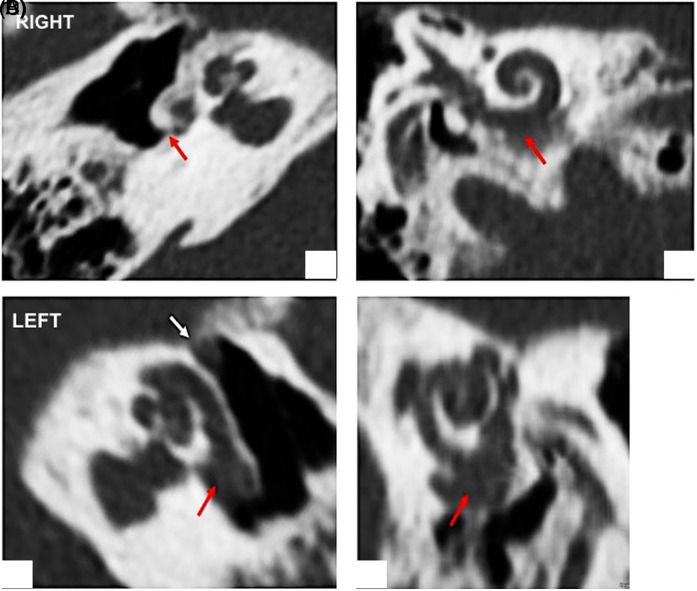
The right cochlea presented the following characteristics: diameter: 10.3 mm; height: 3.6 mm; width: 6.8 mm; estimated cochlear duct length (CDL): 37.6 mm. The reconstructed 3D images pointed out large areas of demineralization at the level of the distal part of the basal turn and an ossified round window, which made it impossible to access it (Figure 2A and B).
Figure 2.
OTOPLAN reconstructed study. Right ear: (A) Ossified round window (red arrow) (B) osteolytic area at the distal part of the basal turn (red arrow). Left ear: (A) Osteolytic enlargement between the round window and the basal turn (red arrow) in communication with (B) osteolytic cavity at the level of the proximal part of the basal turn (red arrow).
The left cochlea showed the following findings: diameter: 10.8 mm; height: 3.4 mm; width: 6.7 mm; estimated CDL: 37.9 mm. The reconstructed images showed large areas of demineralization greater than the ones observed on the right side, which were located in the basal, middle, and apical turn of the snail, and presented a wide connection with the vestibule. The normal anatomy of the cochlea was completely destroyed; in fact, there was a big hole located in the initial tract of the basal turn which put in communication this structure with the round window, creating a unique cavity. This finding could be a limitation to the correct insertion of the electrode (Figure 3C and D).
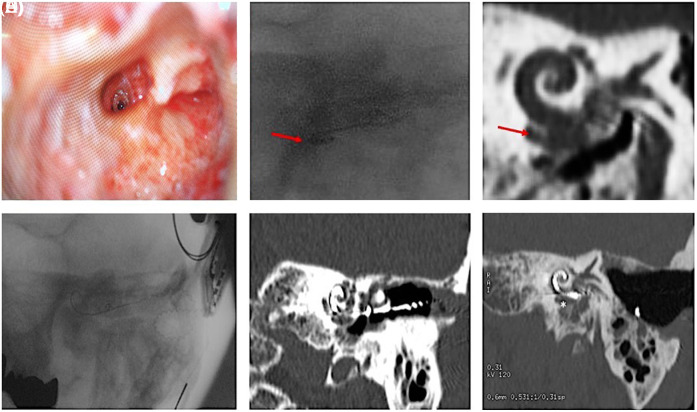
Figure 3.
(A) Promotorial cochleostomy; (B) Intraoperative radioscopy after the insertion of contour-advanced electrode template; (C) Hypothetical position indicated in the pre-operative CT; (D) Intraoperative radioscopy and post-surgery CT scan (E and F) images show the correct insertion of a slim modiolar CI632 electrode. CT, computer tomography.
Surgery Plan and Execution
In consideration of these morphological findings, the decision had to be a right implant with a 31.5-mm lateral wall-designed electrode (FLEXSOFT®). However, due to the patient’s refusal of having surgery on the right (he affirmed “having benefit thanks to the hearing aids”), in the end, we performed a left-side implant.
We choose a CI with a perimodiolar-designed electrode to bypass the problem of the widening communication between the round window, the basal turn, and the basal osteolytic cavitation.
Through posterior tympanotomy, we accessed the cochlea promontory and performed a cochleostomy far from the lateral wall (initial tract of the basal turn) to avoid a possible dislocation of the electrode in the accessorial cavity (Figure 3A). First, we did the insertion with Contour Advance® electrode template with the Advance Off-Stylet® technique; but, the intraoperative radioscopy showed the dislocation of the electrode outside the cochlea, at the same level of known basal cavitation (Figure 3B and C). The electrode was removed and we inserted a Slim Modiolar® array. This electrode was chosen because it presented the following advantages: (i) it could be reloaded into the inserter sheath to improve insertion if necessary, and (ii) thanks to its external sheath of 0.5 mm in length, combined with promontorial cochleostomy, it could allow overstepping the osteolytic enlargement of the proximal part of the basal turn.
After the radioscopical verification of successful insertion of the electrode template, Cochlear™ Nucleus® CI632 was implanted. Its correct allocation was confirmed first by intraoperative radioscopy and then by post-operative CT scan (Figure 3D-F). Also, the intraoperative telemetry confirmed the correct insertion of the array; in fact, we obtained good impedances for all electrodes and neural responses for all electrodes except for the basal 1-5 ones, probably due to the advanced osteolytic area.
No intraoperative or post-operative surgical complications were observed.
The patient was checked 6 months after surgery; he did not refer any problems and obtained 75% of speech discrimination at 65 dB.
Discussion
Thanks to the use of the OTOPLAN, we successfully implanted a severe case of FAO; our patient did not suffer from traditional otosclerosis problems (cochlea ossification1,4 and third ring9) he presented a cochlea with several perforations that were not clearly identifiable by traditional CT scan. The use of the software allowed the correct identification of these alterations, and we correctly placed the CI obtaining a very good recovery of the patient’s auditory functions.
Cochlear implant surgery although was considered a “problematic procedure” in FAO,6,11,12 today it is an option of treatment in these patients; thanks to the improvement of the radiologic technology, which permits to detect the abnormalities of the cochlea. The cochlear implant is used in those patients with very poor SPT score (<30%) and who had bone conduction thresholds indicative of progression of the disease.13 Although otosclerosis can affect the number of spiral ganglions depending on the site of inflammation,14 the number of these cells is quite preserved,15,16 so CI can be the perfect solution to recover the hearing function.1,3,4,9
Despite the improvement of CT, the technique still presents limitations,17,18 and the new methods, that is, cone-beam CT, which showed promising results for CI surgery,19,20 still lacks evidence in otosclerosis.21,22
The new software, as OTOPLAN, uses conventional CT imaging, reconstructing the cochlear lumen and can calculate the cochlear measurements (diameter, height, width, and length of the cochlear duct) and can facilitate cochlear implant surgery, and its validity has been confirmed by several studies.11,21,22
Thanks to it, we identified the best type of array, the surgical approach, and the most appropriate electrode to use for obtaining successful12,23 results although we implanted the most problematic cochlea because of patient’s refusal of CI on the right side.
The patient’s post-operative auditory recovery overlapped the results of other authors,3,4,9,11 confirming the usefulness of detailed studies of the cochlea and its alteration/defect.
The use of OTOPLAN could change the surgical indication for identifying the side of cochlear implant insertion. In fact, the choice of the ear could be done choosing the one with the worse auditory thresholds even in presence of the worse cochlea anatomy (compared with the contralateral side).
Conclusion
Our case suggests that OTOPLAN is an extremely useful tool in FAO. We think that large studies including patients with different severity of otosclerosis and auditory thresholds should be performed to confirm the usefulness of the software in changing the indication of the cochlear implant in these patients.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Informed Consent: Written informed consent was obtained from patients’ parents.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – X.X., X.X., X.X.X., X.X., X.X.; Design – X.X., X.X., X.X.X., X.X., X.X.; Supervision - X.X., X.X., X.X.X., X.X., X.X.; Resources – X.X.; Materials – X.X.; Data Collection and/or Processing – X.X., X.X., X.X.X., X.X.; Analysis and/or Interpretation - X.X., X.X., X.X.X.; Literature Search - X.X.; Writing Manuscript - X.X.; Critical Review - X.X., X.X.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. Eshraghi AA, Ila K, Ocak E, Telischi FF. Advanced otosclerosis: stapes surgery or cochlear implantation? Otolaryngol Clin North Am. 2018;51(2):429–440.. 10.1016/j.otc.2017.11.012) [DOI] [PubMed] [Google Scholar]
- 2. Calvino M, Sánchez-Cuadrado I, Gavilán J, Lassaletta L. Cochlear implant users with otosclerosis: are hearing and quality of life outcomes worse than in cochlear implant users without otosclerosis? Audiol Neurootol. 2018;23(6):345–355.. 10.1159/000496191) [DOI] [PubMed] [Google Scholar]
- 3. Dumas AR, Schwalje AT, Franco-Vidal V, Bébéar JP, Darrouzet V, Bonnard D. Cochlear implantation in far-advanced otosclerosis: hearing results and complications. Acta Otorhinolaryngol Ital. 2018;38(5):445–452.. 10.14639/0392-100X-1442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quaranta N, Bartoli R, Lopriore A, Fernandez-Vega S, Giagnotti F, Quaranta A. Cochlear implantation in otosclerosis. Otol Neurotol. 2005;26(5):983–987.. 10.1097/01.mao.0000185047.77017.31) [DOI] [PubMed] [Google Scholar]
- 5. Polo R, Del Mar Medina M, Arístegui M.et al. Subtotal petrosectomy for cochlear implantation: lessons learnt after 110 cases. Ann Otol Rhinol Laryngol. 2016;125(6):485–494.. 10.1177/0003489415620427) [DOI] [PubMed] [Google Scholar]
- 6. Vashishth A, Fulcheri A, Rossi G, Prasad SC, Caruso A, Sanna M. Cochlear implantation in otosclerosis: surgical and auditory outcomes with a brief on facial nerve stimulation. Otol Neurotol. 2017;38(9):e345–e353.. 10.1097/MAO.0000000000001552) [DOI] [PubMed] [Google Scholar]
- 7. Sainz M, Garcia-Valdecasas J, Ballesteros JM. Complications and pitfalls of cochlear implantation in otosclerosis: a 6-year follow-up cohort study. Otol Neurotol. 2009;30(8):1044–1048.. 10.1097/MAO.0b013e31819d34c9) [DOI] [PubMed] [Google Scholar]
- 8. Matterson AG, O’Leary S, Pinder D, Freidman L, Dowell R, Briggs R. Otosclerosis: selection of ear for cochlear implantation. Otol Neurotol. 2007;28(4):438–446.. 10.1097/MAO.0b013e31803115eb) [DOI] [PubMed] [Google Scholar]
- 9. Messineo D, Ralli M, Greco A, Di Stadio A. Double ring in cochlear otosclerosis: a limit to cochlear implantation? The solution is the surgical approach. Ear Nose Throat J. 2019;16:145561319895601. 10.1177/0145561319895601) [DOI] [PubMed] [Google Scholar]
- 10. Lee TC, Aviv RI, Chen JM, Nedzelski JM, Fox AJ, Symons SP. CT grading of otosclerosis. Am J Neuroradiol. 2009;30(7):1435–1439.. 10.3174/ajnr.A1558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lovato A, Marioni G, Gamberini L, Bonora C, Genovese E, de Filippis C. OTOPLAN in cochlear implantation for far-advanced otosclerosis. Otol Neurotol. 2020;41(8):e1024-e1028. 10.1097/MAO.0000000000002722) [DOI] [PubMed] [Google Scholar]
- 12. Marfatia H, Shah K, Pareek A, Chatterjee C, Goyal P. Case study: cochlear implantation in cochlear otospongiosis. Cochlear Implants Int. 2020;21(2):121–125.. 10.1080/14670100.2019.1678894) [DOI] [PubMed] [Google Scholar]
- 13. Merkus P, van Loon MC, Smit CF, Smits C, de Cock AF, Hensen EF. Decision making in advanced otosclerosis: an evidence-based strategy. Laryngoscope. 2011;121(9):1935–1941.. 10.1002/lary.21904) [DOI] [PubMed] [Google Scholar]
- 14. Sato T, Morita N, Cureoglu S.et al. Cochlear otosclerosis adjacent to round window and oval window: a histopathological temporal bone study. Otol Neurotol. 2010;31(4):574–579.. 10.1097/MAO.0b013e3181d8d73b) [DOI] [PubMed] [Google Scholar]
- 15. Kwok OT, Nadol JB Jr. Correlation of otosclerotic foci and degenerative changes in the organ of Corti and spiral ganglion. Am J Otolaryngol. 1989;10(1):1–12.. 10.1016/0196-0709(89)90086-0) [DOI] [PubMed] [Google Scholar]
- 16. Di Stadio A, Volpe AD, Ralli M, Korsch F, Greco A, Ricci G. Spiral ganglions and speech perception in the elderly. Which turn of the cochlea is the more relevant? A preliminary study on human temporal bones. J Int Adv Otol. 2020;16(3):318–322.. 10.5152/iao.2020.8481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castillo F, Polo R, Gutiérrez A, Reyes P, Royuela A, Alonso A. Cochlear implantation outcomes in advanced otosclerosis. Am J Otolaryngol. 2014;35(5):558–564.. 10.1016/j.amjoto.2014.03.011) [DOI] [PubMed] [Google Scholar]
- 18. Rotteveel LJ, Snik AF, Cooper H, Mawman DJ, van Olphen AF, Mylanus EA. Speech perception after cochlear implantation in 53 patients with otosclerosis: multicentre results. Audiol Neurootol. 2010;15(2):128–136.. 10.1159/000235578) [DOI] [PubMed] [Google Scholar]
- 19. Nateghifard K, Low D, Awofala L.et al. Cone beam CT for perioperative imaging in hearing preservation cochlear implantation - a human cadaveric study. J Otolaryngol Head Neck Surg. 2019;48(1):65. 10.1186/s40463-019-0388-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abd El Aziz TT, El Fiky L, Shalaby MH, Essam A. Radiological evaluation of inner ear trauma after cochlear implant surgery by cone beam CT(CBCT). Eur Arch Otorhinolaryngol. 2019;276(10):2697–2703.. 10.1007/s00405-019-05507-4) [DOI] [PubMed] [Google Scholar]
- 21. Redfors YD, Gröndahl HG, Hellgren J, Lindfors N, Nilsson I, Möller C. Otosclerosis: anatomy and pathology in the temporal bone assessed by multi-slice and cone-beam CT. Otol Neurotol. 2012;33(6):922–927.. 10.1097/MAO.0b013e318259b38c) [DOI] [PubMed] [Google Scholar]
- 22. Liktor B, Révész P, Csomor P, Gerlinger I, Sziklai I, Karosi T. Diagnostic value of cone-beam CT in histologically confirmed otosclerosis. Eur Arch Otorhinolaryngol. 2014;271(8):2131–2138.. 10.1007/s00405-013-2702-y) [DOI] [PubMed] [Google Scholar]
- 23. Ramsden R, Bance M, Giles E, Mawman D. Cochlear implantation in otosclerosis: a unique positioning and programming problem. J Laryngol Otol. 1997;111(3):262–265.. 10.1017/s0022215100137028) [DOI] [PubMed] [Google Scholar]
- 24. Matterson AG, O’Leary S, Pinder D, Freidman L, Dowell R, Briggs R. Otosclerosis: selection of ear for cochlear implantation. Otol Neurotol. 2007;28(4):438–446.. 10.1097/MAO.0b013e31803115eb) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a