Abstract
Objective:
The effect of physical activity (PA) on the risk of developing knee osteoarthritis (OA) is unclear. Our aim was to examine the relationship between recreational PA and incident knee OA outcomes using comparable PA and OA definitions.
Methods:
Data were acquired from six global, community-based cohorts of participants with/without knee OA. Eligible participants had no evidence of knee OA and rheumatoid arthritis (RA) at baseline. Participants were followed for 5–12 years for incident outcomes including: i) radiographic knee OA (ROA) (Kellgren Lawrence (KL) ≥2), ii) painful radiographic knee OA (PROA) (ROA with knee pain) and iii) OA-related knee pain. Self-reported recreational PA included sport and walking/cycling activities was quantified at baseline as metabolic equivalents of tasks (METS) in days per week (days/wk). Risk ratios (RR) were calculated and pooled using Individual Participant Data (IPD) meta-analysis. Secondary analysis assessed the association between PA, defined as time (hrs/wk) spent in recreational PA and incident knee OA outcomes.
Results:
Based on a total of N=5065 participants, pooled risk ratio estimates for MET days/wk and PROA (1.02, 95% CI 0.93, 1.12), ROA (1.00, 95% CI 0.94, 1.07) and OA-related knee pain (1.00, 95% CI 0.96, 1.04) were non-significant, respectively. Similarly, analysis of hours per week spent in PA also showed no significant associations for all outcomes.
Conclusions:
Our findings suggest that whole-body, physiological energy expenditure during recreational activities and time spent in physical activity were not associated with incident knee OA outcomes.
INTRODUCTION
Osteoarthritis (OA) is a leading cause of global disability, and a major cause of reduced function and pain(1). As life expectancy is increasing, along with rising levels of obesity, the number of people living for prolonged periods with severe OA is expected to grow(2). Currently, there are a lack of disease-modifying treatments for OA and subsequently, attention has turned to identifying modifiable risk factors to help alleviate disease onset and burden.
Physical activity (PA) appears to have a positive, long-term influence on non-communicable diseases such as coronary heart disease and type 2 diabetes mellitus(3) and it is clear that efforts are needed to encourage increases in PA for health(4). In contrast, while there are some well-established risk factors for knee OA including joint injury(5), obesity(6) and female sex(7), the effect of PA on the risk of OA is unclear. A systematic review by Richmond et al. (8) reported that PA was a risk factor for OA in four studies and protective in another; with joint injury the potentially mediating factor. Further, in the same study cumulative PA and PA in midlife were not shown to be risk factors for incident knee OA; however, a borderline association was observed for exercise in early adult life. The systematic review, published in 2013, concluded that a meta-analysis exploring the relationship between PA and risk of OA was not possible using the current published literature due to heterogeneity in the definitions of PA and OA(8). Further, there is evidence to suggest that PA in the form of exercise improves clinical outcomes among those with OA(9). There is also evidence to suggest that some types of PA are a potential risk factor for the development of structural change at the knee(10–12). Despite this, ‘exercise’ is recommended as a core treatment for the non-surgical management of OA, with ‘low-impact aerobic exercise’ recommended by most treatment guidelines(13).
One of the likely explanations for the lack of consensus is due to the variable definitions of PA, differences in assessment of the PA constructs which include duration, severity and intensity and differences in PA domains (e.g. leisure, recreation, occupation). Further research is required to examine the components of PA and using different metrics of PA. This may help to advance our understanding of the biomechanical and pathophysiological changes that occur with PA which may ultimately help identify and explain the threshold between risk and protection.
It is important to identify the role of PA in disabling diseases such as OA and to inform prevention strategies targeted to reduce the global burden of OA and encourage, where appropriate, participation in PA for the benefits of overall health. To overcome the difficulties in synthesizing aggregate data, which use a variety of definitions for both PA-related exposures and OA outcomes, individual patient-level(14) meta-analysis provides a method to harmonise original raw data from cohorts and use standardised statistical methods to analyse and produce pooled estimates(14). This method also provides the opportunity to gain a better clinical understanding of the degree to which different components of knee OA (pain and/or structure) are affected by PA.
Therefore, our aim was to investigate the association between recreational PA and risk of incident knee OA outcomes in six prospective cohort studies of adults at risk of developing knee OA.
METHODS
The wider study is comprised of two parts. Firstly, due to the novel aspect of combining this type of data, three separate expert committees convened to; i) establish a common PA variable, ii) harmonise knee OA outcome variables, and iii) to establish a statistical strategy. The results of these consensus studies have been published previously(15, 16). The current study uses those previous decisions on outcome and exposure definitions to examine the relationship between recreational PA and incident knee OA outcomes (radiographic, painful radiographic (ROA plus symptoms) and OA-related knee pain).
1.1. Study Design
Cohort Selection and Participant Inclusion Criteria
We identified the appropriate cohorts by searching published literature for established longitudinal OA cohorts and by liaising with Principal Investigators and experts with knowledge of available data. Cohorts were included due to their availability of detailed PA, knee pain and knee radiographic data. Specifically, cohorts were selected based on the following inclusion criteria; 1) presence of self-reported PA sufficient to allow for the calculation of hrs/wk spent in recreational PA and MET days/wk at baseline, 2) OA-related knee pain and/or radiographic data at baseline and at follow-up, and 3) recruitment from the community (i.e. not identified through clinics, hospitals or healthcare professionals). Cohorts were not excluded based on whether or not they had already published data on the relationship between PA and OA.
Six cohorts were identified with appropriate data available for analysis: two US community-based cohorts (Framingham Osteoarthritis Study and Johnston County Osteoarthritis Project (JoCoOA))(17–20) and one US enhanced risk factor cohort (Multicentre Osteoarthritis Study (MOST))(21); two community-based cohorts from the United Kingdom (Chingford 1000 Women Study (Chingford) and Hertfordshire Cohort Study (HCS))(22, 23); and one Australian community-based cohort (The Tasmanian Older Adult Cohort (TasOAC))(24). See figure 1 for cohort selection.
Figure 1.
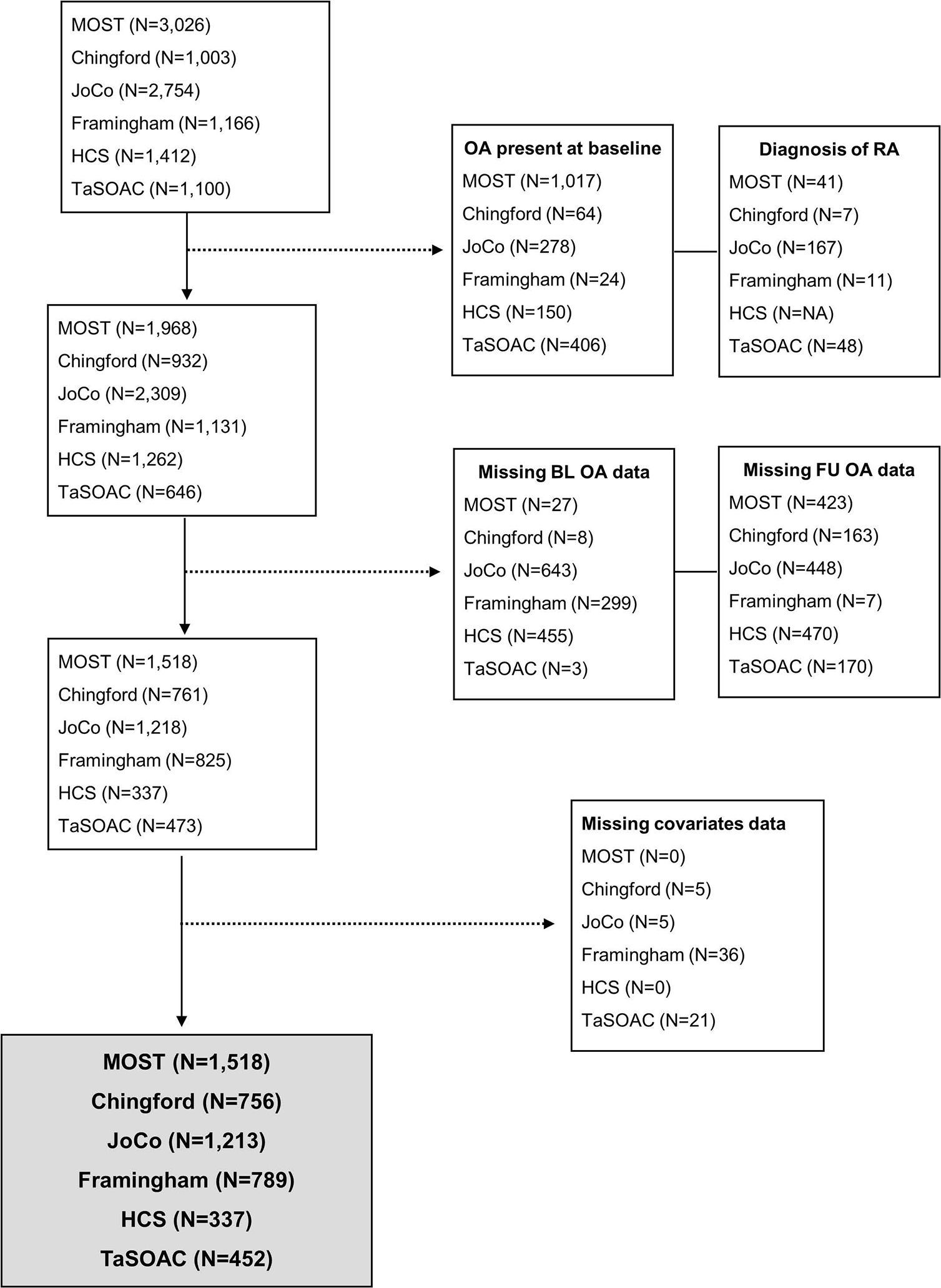
Flow chart of cohort selection process
Cohorts without radiographic follow-up data were only included in the OA-related knee pain analysis (TaSOAC). Cohorts without side specific knee pain at follow-up were only included in the OA-related knee pain and ROA only analyses (HCS). Across all analyses, participants were included if they were free of OA at baseline and did not have evidence of rheumatoid arthritis at baseline.
Primary Risk Factor – PA
A number of questions were used to assess PA in each of the respective cohorts resulting in variation in the type of responses. A more detailed description of the individual variables captured in each cohort can be seen in Appendix 1. To address these methodological differences, an international consensus study including experts in PA and clinical epidemiology was conducted to develop an approach to harmonise PA; key results from this consensus study have been described previously(15). In brief, agreement was met for the use of Metabolic Equivalent of Task (MET)(25) as a method for harmonising PA variables among cohorts. It was agreed that occupation is a less modifiable domain of PA, which may have a greater weighting over our findings above household and sport and leisure domains. Therefore, occupational PA was not included in the calculation of PA(15). Household activity was missing in a number of cohorts and was therefore also excluded. The exposure for all cohorts consisted of recreational PA except for Framingham and TaSOAC, for which we could not determine the type of activity as the question asked was “hours spent in sedentary/slight/moderate and heavy activity per day” and “days per week and minutes per day doing vigorous/moderate activities”, respectively.
The primary and secondary exposures included:
Primary exposure:
MET days/wk were calculated based on time spent in a given activity (sport and walking/cycling activities) multiplied by the MET value for that activity(25). Once MET days/wk were calculated, the original components of this physiological measure could not be distinguished.
Secondary exposure:
We included a second exposure based on the amount of time spent in recreational PA, this was based on hrs/wk spent in PA at baseline.
A lengthy process was undertaken to first assign a MET value to every activity recorded within each cohort according to the compendium of PA(25). For exposure 1 (MET days/wk), these MET values were multiplied by duration spent in the given activity. For exposure 2, each recreational activity was assigned to one of three intensity levels (light, moderate or vigorous) according to the classification of METs(26). Where PA questions were already based on low/moderate/vigorous PA (such as Framingham, MOST and TaSOAC) these cohort thresholds were used.
Incident Knee OA Outcomes
Comparing and pooling results between prospective cohorts is relatively rare in the disease area of OA. Therefore, a second expert consensus meeting was convened to determine how best to harmonise this variable between cohorts. Key results from this consensus study have been described previously(16). In brief, knee OA was defined using both self-reported pain and the presence of radiographic OA.
Incident Radiographic Osteoarthritis (ROA)
The presence of radiographic knee OA was defined using Kellgren and Lawrence (K/L) criteria in each cohort(27). Incident ROA was defined as the occurrence of ROA (KL score of ≥2) during follow-up in either/both knee(s) without ROA (KL 0–1) in both knees at baseline. Person-level OA was calculated by assessing the OA status for each knee joint and using the ‘highest’ level of OA based on this system. For example, if a participant had no evidence of OA (or data were not available) in their right knee and ROA in their left knee, their person-level knee OA status would be ROA. Total knee replacements (TKR) that occurred during follow-up were included as incident ROA cases if confirmed by radiograph.
Incident OA-related knee pain
Current knee pain status was determined using the National Health and Nutrition Examination Survey (NHANES)-(28), for which a positive response to ‘have you had pain on most days in the last month in your joint’ would indicate the presence of pain. Alternatively, if the NHANES-type question was absent the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale(29) was used (see appendix 1 for cohort-specific pain questions). Due to known variations in the wording of pain questions(30), an analysis was previously undertaken to determine the most comparable wording of the variety of NHANES-type questions and to establish an equivalent threshold to use in the WOMAC pain subscale to create a binary pain variable (31).
In participants with and without ROA at baseline, incident OA-related knee pain was defined as the occurrence of knee pain during follow-up in participants with no evidence of knee pain at baseline.
Incident Painful radiographic OA (PROA)
In participants with no evidence of ROA with knee pain in the same knee at baseline (participant-level), incident PROA was defined as the occurrence of both knee symptoms and ROA in the same knee during follow-up. Side-specific radiographs and knee pain responses were available at baseline and follow-up in Framingham, JoCoOA, MOST and Chingford and therefore, PROA was calculated for these cohorts. In HCS only person-level pain was available at follow-up. In TaSOAC only person-level pain was available with no radiographs at follow-up. Therefore, these two cohorts were not included in the PROA analysis.
Confounders
Age, sex, race and body mass index (BMI) were considered as potential confounders. In all cohorts, age was defined as age at the time of the clinic visit, as was BMI (kg/m2), which was based on objective height and weight measurements. Chingford, HCS and Framingham comprised predominantly Caucasian participants; JoCoOA and MOST comprised both Caucasian and African American participants; and TaSOAC comprised a small percentage of Asian and Indigenous Australian participants.
Statistical Analysis
We conducted a complete case analysis. Descriptive statistics (percentages, means (standard deviations), medians (inter-quartile ranges) were calculated for baseline characteristics of all cohorts.
Modified Poisson regression analyses were conducted to assess the association between baseline recreational PA (hours/week spent in activity and MET days/wk) and incident ROA, PROA and OA-related knee pain respectively at 5–12 years follow-up. Models were adjusted for potential confounders. Sex and race were included in the fully adjusted models only when relevant to the specific cohort. When the study outcome is considered common, odds ratio overestimate the relative risk(32). Therefore, we used a modified Poisson approach to estimates the relative risk and 95% confidence interval (CI) by using robust variances as suggested by Zou(33).
IPD Meta-analysis
IPD meta-analysis involved estimating an appropriate summary statistic for each study and then calculating a weighted average of these statistics across studies(34). It allowed for cohorts to be compared using identical risk factors, outcomes and confounders. A two-stage IPD meta-analysis consisted of two distinct parts: first, each cohort was analysed individually using identical methodology. Risk ratios (RR) and 95% CI were produced for each individual cohort.
Second, the results of each individual analysis were pooled using standard meta-analysis statistical methods(35). Data was pooled using random-effects analysis. The Stata admetan command was used to produce the pooled estimates in addition to forest plots which graphically demonstrate the results(36). All analyses were conducted using Stata version 16.1 statistical software (StataCorp, College Station, Texas, USA).
Sensitivity Analysis
Occupational PA has been shown to be an important risk factor in the development of knee OA (38, 39). Within Framingham and TaSOAC it was not possible to isolate the contributions of occupational activity from recreational activity. Subsequently, results will be reported both with and without the inclusion of Framingham and TaSOAC data.
RESULTS
Five thousand and sixty-five participants (N = 5,065) were included in the IPD meta-analysis. Incidence of PROA at follow-up ranged from 6.1–20.3%, ROA from 9.2–33.8% and OA-related knee pain from 8.6–29.2%. Median PA in participants ranged from 0 MET days/wk in Chingford to 11.4 MET days/wk in Framingham (table 1).
Table 1.
Cohort characteristics for all subjects and stratified by baseline knee OA status
| Baseline Demographics | MOST | Chingford | JoCoOA | Framingham | HCS | TaSOAC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up period | 5 years | 9 years | 7 years | 7 years | 12 years | 5 years | ||||||||||||
| Original N | 3026 | 1003 | 2754 | 1166 | 1412 | 1100 | ||||||||||||
| ROA | OA-related Knee Pain | PROA | ROA | OA-related Knee Pain | PROA | ROA | OA-related Knee Pain | PROA | ROA | OA-related Knee Pain | PROA | ROA | OA-related Knee Pain | PROA | ROA | OA-related Knee Pain | PROA | |
| N | 1078 | 1102 | 1,518 | 681 | 556 | 756 | 997 | 795 | 1213 | 759 | 629 | 789 | 337 | 243 | - | - | 452 | - |
| Knee OA outcome at follow-up, n (%) | 262 (24.3) | 322 (29.2) | 308 (20.3) | 190 (27.9) | 65 (11.7) | 48 (6.4) | 255 (25.6) | 131 (16.5) | 132 (10.9) | 70 (9.2) | 116 (18.4) | 48 (6.1) | 114 (33.8) | 21 (8.6) | - | - | 60 (13.3) | - |
| MET days/week, median (IQR) | 1.2 (0.6–1.9) | 1.1 (0.6–1.9) | 1.1 (0.6–1.9) | 0 (0–0.5) | 0 (0–0.5) | 0 (0–0.5) | 0.3 (0.0–0.8) | 0.3 (0.1–0.8) | 0.3 (0.0–0.8) | 11.4 (8.7–14.4) | 11.4 (8.7–14.4) | 11.3 (8.4–14.3) | 0.2 (0–0.5) | 0.2 (0–0.5) | - | - | 1.4 (0.3–2.8) | - |
| Age (years), mean (48) | 60.5 (7.7) | 62.3 (7.9) | 61.6 (7.9) | 53.5 (5.8) | 53.8 (5.9) | 53.7 (5.9) | 60.7 (8.5) | 61.9 (9.3) | 61.7 (9.0) | 51.9 (8.7) | 51.9 (8.7) | 51.9 (8.7) | 64.7 (2.7) | 64.8 (2.8) | - | - | 62.0 (7.0) | - |
| Sex, n (% Female) | 638 (59.2) | 608 (55.2) | 890 (58.6) | 681 (100) | 556 (100) | 756 (100) | 673 (67.5) | 507 (63.8) | 802 (66.1) | 427 (56.3) | 348 (55.3) | 442 (56.0) | 170 (50.5) | 117 (48.2) | - | - | 219 (48.5) | - |
| Race, n (%) | ||||||||||||||||||
| Caucasian | 945 (87.7) | 969 (87.9) | 1325 (87.3) | 681 (100) | 556 (100) | 756 (100) | 731 (73.3) | 577 (72.6) | 879 (72.5) | 759 (100) | 629 (100) | 789 (100) | 337 (100) | 243 (100) | - | - | 446 (98.7) | - |
| African American | 117 (10.8) | 118 (10.7) | 169 (11.1) | - | - | - | 266 (26.7) | 218 (27.4) | 334 (27.5) | - | - | - | - | - | - | - | - | - |
| Chinese | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Other | 16 (1.5) | 15 (1.4) | 24 (1.6) | - | - | - | - | - | - | - | - | - | - | - | - | - | 6 (1.3) | - |
| BMI (kg/m2), mean (48) | 29.1 (4.7) | 29.6 (4.8) | 29.5 (5.0) | 25.1 (3.9) | 25.0 (3.9) | 25.3 (4.0) | 29.4 (5.5) | 29.5 (5.8) | 29.6 (5.8) | 27.0 (4.8) | 26.8 (4.6) | 27.1 (4.8) | 26.5 (4.0) | 26.3 (4.0) | - | - | 27.2 (4.2) | - |
Subjects not having knee osteoarthritis outcome at baseline, not having rheumatoid arthritis, not missing knee osteoarthritis data at baseline or follow-up, not missing PA, age, sex, race, BMI data
IPD Meta-analysis of PROA, ROA and OA-related Knee Pain
Analysis 1:
These analyses examined the association between PA, defined as MET days/wk, and incident knee OA as i) PROA ii) ROA and iii) OA-related knee pain at follow-up against participants who had no OA (pain and/or radiographic OA) at baseline. Multivariable meta-analyses adjusted for age, sex, BMI and race showed a non-significant pooled risk ratio (RR and 95% confidence interval) of 1.02 (0.93, 1.12) for PROA, 1.00 (0.94, 1.07) for ROA and 1.00 (0.96, 1.04) for OA-related knee pain (figure 2A). A non-significant pooled RR (95% CI) of 1.00 (0.75, 1.32) for PROA, 1.00 (0.90, 1.12) for ROA and 0.97 (0.85, 1.10) for OA-related knee pain when Framingham and TaSOAC cohorts were excluded from the analysis (Figure 2B).
Figure 2.
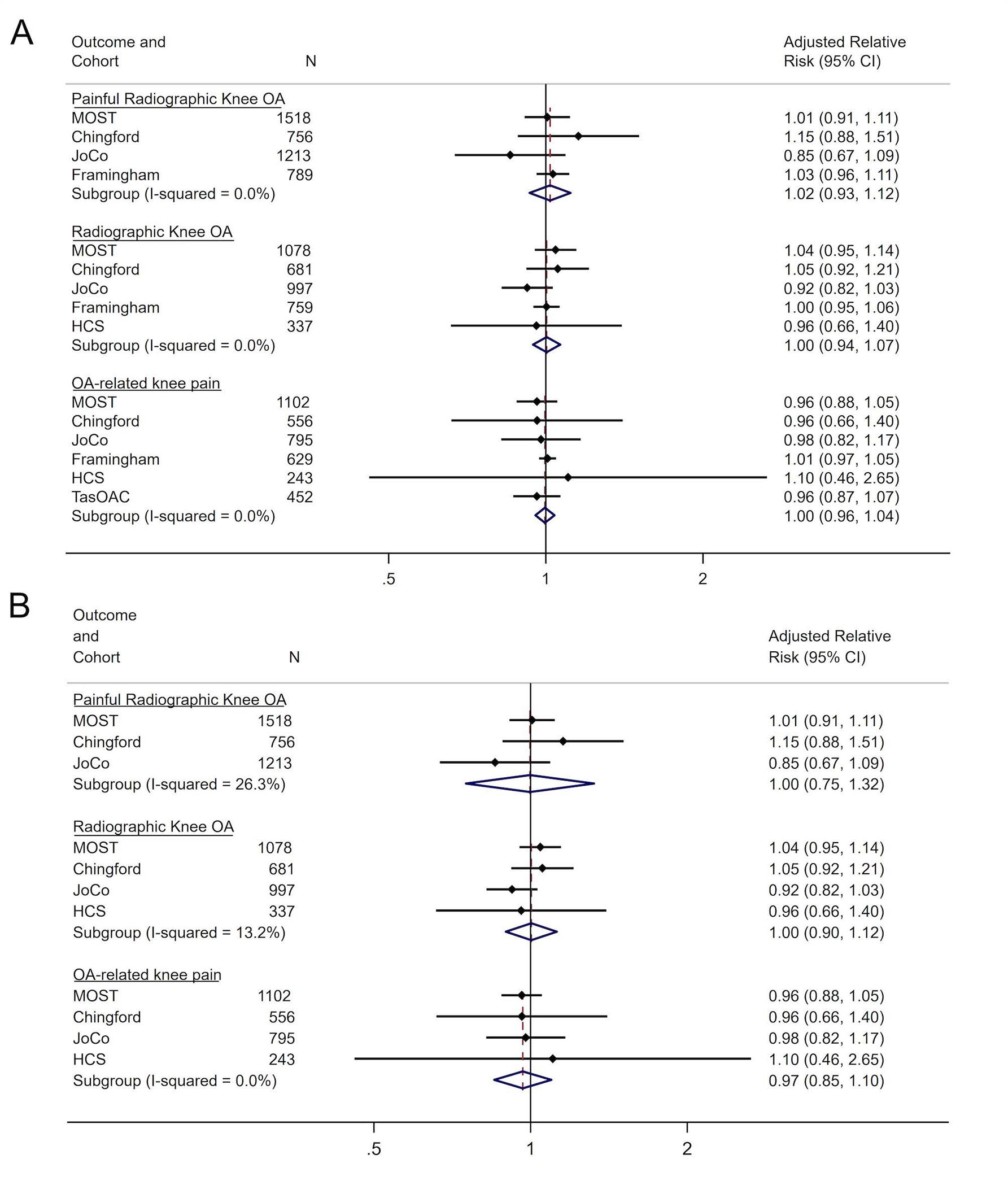
Forest Plots for fully adjusted models for METS days/week by OA Outcome and Cohort Study for (A) all cohort studies and (B) excluding Framingham and TasSOAC cohort studies.
Analysis 2:
These analyses compared the association between PA, defined as hrs/wk spent in PA, and participants who had incident i) PROA ii) ROA and iii) OA-related knee pain at follow-up against participants who had no OA (pain and/or radiographic OA). In the models adjusted for age, sex, BMI and race, meta-analyses for duration of PA on PROA, ROA and OA-related knee pain showed a non-significant pooled RR (95% CI) of 1.00 (0.98, 1.02), 1.00 (0.98,1.01) and 1.00 (0.98,1.01), respectively (figure 3A). A non-significant pooled RR (95% CI) of 1.01 (0.96, 1.05) for PROA, 1.01 (0.98, 1.04) for ROA and 0.97 (0.85, 1.10) for OA-related knee pain was also shown when Framingham and TaSOAC cohorts were excluded from the analysis (figure 3B).
Figure 3.
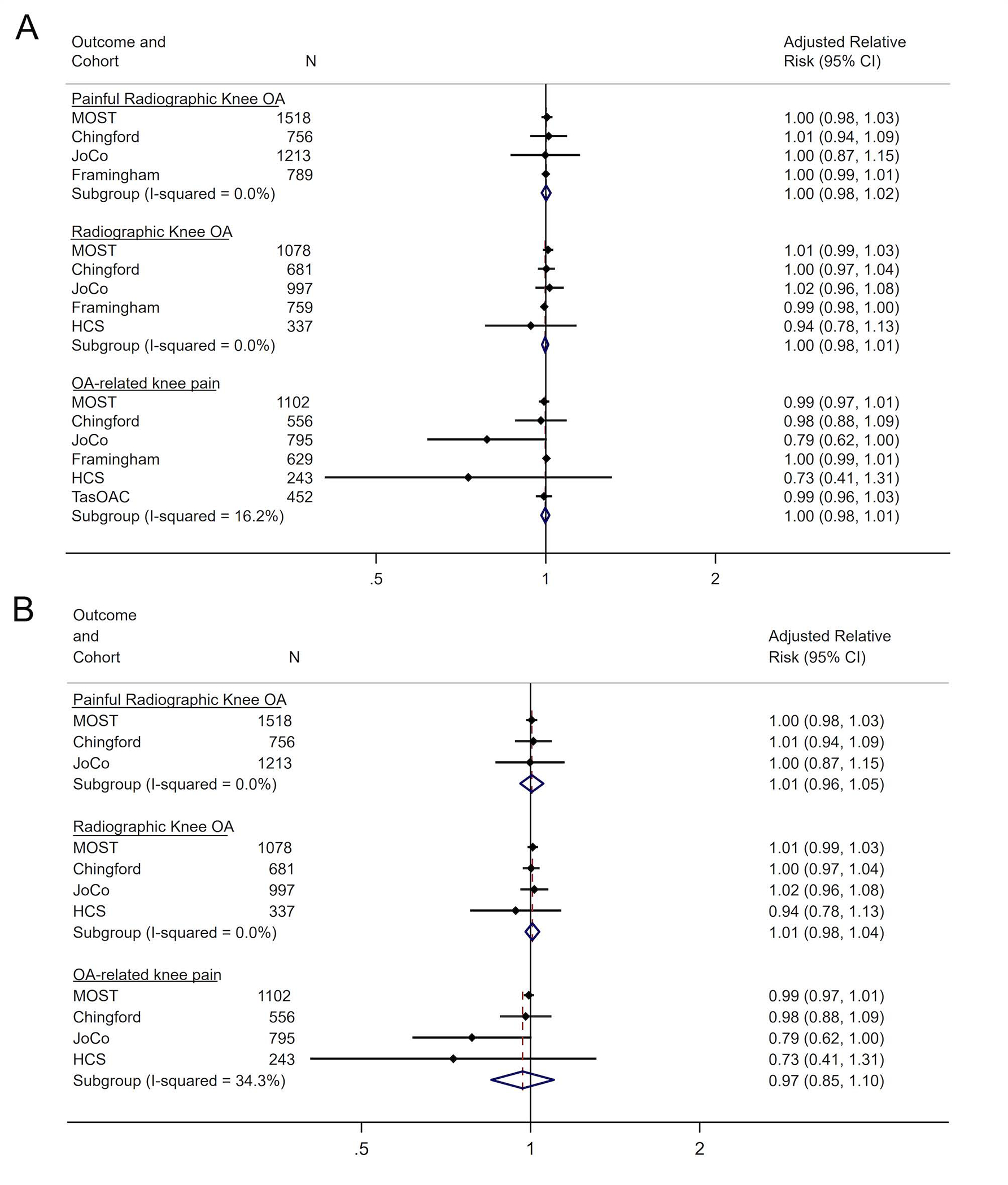
Forest Plots for fully adjusted models for duration (hours/week) of PA by OA Outcome and Cohort Study for (A) all cohort studies and (B) excluding Framingham and TasSOAC cohort studies.
DISCUSSION
This multi-cohort study, utilising data from six national and international OA cohorts, examined the relationship between recreational PA and incident knee OA outcomes. Our exposure of recreational PA, a composite of leisure-sports and walking/cycling activity, was assessed using MET days/wk as a means of estimating whole-body energy expenditure. Whilst this over-all physiological measure of recreational PA is useful for the interpretation of the effect between recreational PA on incident knee OA, further information is required to provide a clearer public health message. Therefore, to consider the role that duration of PA may play we also investigated the effect of time spent in PA.
No association was observed between PA defined as total energy expenditure (MET days/wk) and incident knee ROA, PROA and OA-related knee pain, respectively. There was also no association observed between time spent in PA (hrs/wk) on incident ROA, PROA and OA-related knee pain.
The role of PA in knee OA remains questionable, as shown from the findings from a comprehensive literature review(8). As the first study to harmonise and analyse original individual-level OA and PA data from multiple cohorts, our findings suggest that recreational PA, as defined by physiological energy expenditure and time spent in PA, was not associated with incident knee OA outcomes.
The variation in both PA and OA definitions and follow-up times makes true comparison of both previous and the current findings difficult. For instance Felson et al.(37) found PA increased the risk of OA using data from the Framingham study. PA was not limited to recreational activity but defined as activity over 24 hours and OA based on a radiographic definition. McAlindon et al(10) also found an association in the Framingham study, only with vigorous activity. In the current study PA levels within Framingham and TaSOAC were markedly higher than all other cohorts. This is likely due to the inability to differentiate between particular activities (question based on time spent in slight, moderate and heavy activities), which meant, unlike all other cohorts, we were unable to exclude household, gardening or occupation-related activities. Also, participants self-reporting vigorous activities are perhaps more likely to consider hours spent in heavy occupations as part of ‘vigorous’ activity. Previous evidence suggests that occupation, particularly manual jobs, are associated with radiographic and symptomatic knee OA(38–41).
Hootman et al(42) found participation in PA as an adult does not increase the risk of knee OA. In their study PA was based on calculation and quantification of PA-related joint stress and knee OA was based on self-reported, physician-diagnosed OA. The current study aimed to overcome these variations by harmonising measures across cohorts prior to analysis. An early case control study by Imeokparia et al(43) combined the PA components of occupation, sport leisure-recreational, and home-based activities to derive four activity categories in METs (very hard, hard, moderate, light activities). They demonstrated gender differences in high levels of cumulative PA as a risk factor in the development of OA of the knee, with females aged 55 to 64, but not males, being at increased risk of knee OA. Occupation is a well-known risk factor for knee OA(38–41) and the combination of occupation with leisure and home-based activities in Imeokparias’ case-control study means it is possible that these effects were being driven by occupation.
PA is a complex behaviour with numerous components to consider. For example, the ability to measure a specific type of activity (such as running, swimming, gardening) over a specific volume (such as duration per day over the prolonged period until incident disease occurs), whilst capturing all relevant covariates (such as injury, lifestyle factors) would be the ideal method, however this would be timely, costly, invasive and unrealistic.
There are several potential limitations to this study. Firstly, the six cohorts were all drawn from Western, and largely Caucasian populations whose demographics, diet, anthropometry, and types of PA may not be applicable to all societies. The cohorts included were designed as independent studies, and were not designed for direct comparison. Therefore, recreational PA, and knee OA were assessed differently between cohorts. It is known that self-reported PA is susceptible to reporting bias, including recall and social desirability bias, which may lead to over-estimation of PA(44), moreover and importantly for this study, there are indications that social desirability bias is larger in lower educated individuals(45). It is also known that even small variations in the way a pain question is worded, or x-rays are graded, can result in differences in OA prevalence (16, 46). In order to minimise this variation, we made every effort to harmonise PA, pain and ROA variables between cohorts by conducting two international expert consensus studies (15, 16).
Self-reported PA provides its own challenges in terms of potential recall bias. In this instance, whilst there was an arguably appropriate temporal proximity between the measure of exposure and incident outcome, we cannot be certain that PA, which is mostly based on relatively current activity, and all other covariates for that matter, remained continuous throughout the study period. Also, the absence of particular variables such as previous injury and knee surgery across cohorts meant that these variables could not be adjusted for within the analysis. In addition weight change may play a role in incident knee outcomes, unfortunately capturing BMI over multiple time points throughout the study period was not possible.
In an attempt to identify a global, whole-body physiological risk factor of knee OA, we used physiological energy expenditure (MET days/wk) as our exposure. To our knowledge, this is the first time METs have been used to describe the relationship between recreational PA and incident knee OA. The use of METs could be considered a limitation as we could not extrapolate the individual contributions of type, frequency (or intensity) and duration of each respective activity on risk of developing knee OA; all of which are likely to contribute differently to the development of knee OA.
To overcome the potential limitations of using an exposure representative of whole-body energy expenditure, we undertook a secondary analysis in which we created an exposure based on time spent in PA (hrs/wk). This in itself was also limited as it does not show duration of time spent in particular activities or activity intensities. We were unable to categorise duration according to intensity level (light/moderate/vigorous) as a number of cohorts did not capture activities representative of light intensity.
As well as variation in variable definitions (i.e. OA definitions, PA), the key differences between cohorts were year of baseline visit, length of follow-up, the age of participants at baseline, and the lack of side-specific pain and radiographic follow-up data in one cohort. Differences in PA observed between cohorts is also likely due to differences in self-reported questions asked. It is suggested that self-reported PA measures are likely to overestimate or underestimate activity levels compared to directly measured levels of PA (47). Also, for cohorts where duration or frequency of the activity was not reported, a mean value was produced from a cohort where this information was present and was applied to the missing values, this may also contribute to over or under estimation of activity levels.
Individual types of activities (i.e. hockey, swimming) would be a useful exposure to consider in order to provide a clearer public health message. We were unable to explore this further given the limitations in the self-reported PA measures available, however it would be valuable to understand which specific activities are associated with knee OA, and ideally via prospective objective measures of PA.
Conclusion
This is the first study to assess the relationship between PA defined as MET days/wk and knee OA. It is a comprehensive analysis of six, well-described observational studies of knee OA, pooling approximately 5000 study participants over 45 years old. These findings suggest that PA as defined by whole-body, physiological energy expenditure during sport/walking/cycling activities is not associated with knee OA. Likewise, time spent in recreational PA is not associated with incident knee OA. Further investigation with clear disaggregation between all components of PA (including type of activity, intensity, frequency and duration) over a lifetime would be of most use, however incredibly difficult to obtain such robust data. Given what we also know about the effects of manual occupation on knee OA it would be useful to understand the association between activities according to loading, along with relative lifetime volume (intensity and duration) on knee OA using prospective investigation.
Acknowledgements:
We gratefully acknowledge Dr Julia Newton for her insight into physical activity, Dr Kirsten Leyland for her early input and contributions to data harmonisation, James Van Santen and Sally Sheard for their help in acquiring the data and all members of the early Methodology Consensus Group for their methodological expertise, including Professor Andrew Judge, Professor Karel Moons, Assistant Professor Thomas Debray, Professor Richard Riley, Associate Professor Joel Gagnier, Professor Gary Collins and the late Professor Doug Altman. We thank Professor Tim Spector, Dr Deborah Hart and Mr Jem Lawson, for their dedication to the Chingford Womens Study. We also acknowledge all the participants involved in each study.
Role of Funding Source:
This study was funded by the Centre for Sport Exercise and Osteoarthritis Research Versus Arthritis (grant 21595). The Framingham Study is supported by the National Institutes of Health (NIH) AR072571. The Johnston County Osteoarthritis Project was supported by the Centers for Disease Control and Prevention/Association of Schools of Public Health grants (S043, S1734, S3486, U01DP003206, U01DP006266) and NIAMS (P60AR30701, P60AR049465, P60AR064166, and P30AR072580). The Hertfordshire Cohort Study was supported by the Medical Research Council of the UK; Wellcome Trust; Versus Arthritis; Dunhill Trust; British Heart Foundation and NIHR. The Multicenter Osteoarthritis Study (MOST) was funded by the National Institutes of Health, grant numbers: Felson – UO1 AG18820; Torner – UO1 AG18832; Lewis – UO1 AG18947; Nevitt – UO1 AG19069. The Tasmanian Older Adult Cohort study is supported by the National Health and Medical Research Council of Australia (302,204), the Tasmanian Community Fund, Masonic Centenary Medical Research Foundation, Royal Hobart Hospital Research Foundation and Arthritis Foundation of Australia. The Chingford Womens Study was supported by Versus Arthritis UK and the Oxford NIHR Musculoskeletal Biomedical Research Unit.
Appendix 1. Harmonisation of outcome, main risk factor and confounders
| Knee Joint pain | Radiographic Knee OA | Baseline PA | Sex | Race | |
|---|---|---|---|---|---|
| Final harmonised variable | Symptomatic Radiographic OA based on radiographic OA and self-reported pain | 1) MET days/week 2) Hrs/wk spent in PA | Male/female | 1. Caucasian 2. African American 3. Japanese 4. Asian 5. Indigenous Australian 6. Hispanic 7. Other |
|
| Chingford | Baseline: Participants were classified as having current joint if they reported positively to: current pain (yes) and duration was more than 1 month. Follow up: pain for more than 15 days in the last month. How many days in the last month have you had knee pain? |
Baseline and follow up K&L knee grade 0–4 | Length walked in a week? Time spent in sport over a week? Ever engage in regular activity long enough to work up a sweat? Type of activity engaged in for long enough to work up a sweat? Times per week engaged in the activity which gives you a sweat? Name of activity(1) which you regularly participate in? Minutes spent per week engaging in activity(1)? Continues for activities 1–5. |
Female | Caucasian |
| HCS | Baseline: Pain on most days in the last month (Right/left) Follow up: person level WOMAC (not side specific) |
Baseline and follow up: K&L knee grade 0–4 | Which of the following activities do you do at least once a month on average or at least 12 times per year? Bowls Cycling Swimming Golf Fishing Dancing Other active sport Duration of activity based on averages from Herts West participants |
Male & female | Caucasian |
| JoCoOA 1 | Baseline: In the PAST MONTH on MOST DAYS, have you had pain, aching, or stiffness in your KNEES? Follow up: side level WOMAC |
Baseline and follow up: K&L knee grade 0–4 | In the last year did you perform this activity? Which months? Average number of times per month Time per occasion (list of over 50 leisure time activities) |
Male & female | -Caucasian -African American |
| Framingham | Baseline and follow-up: On most days do you have pain, aching or stiffness in either of your knees? Is the pain aching or stiffness in your right knee, left knee, or both knees? |
Baseline and follow up K&L knee grade 0–4 | Hours spent on slight, sedentary, moderate and heavy PA per day | Male & female | -Caucasian -African American -Asian -other |
| MOST | Baseline: i) “During the past 12 months, have you had any pain, aching, or stiffness in your knee?” ii) “During the past 30 days, have you had any pain, aching, or stiffness in your knee?” iii) “During the past 30 days, have you had pain, aching, or stiffness in your knee on most days?”. Participants who reported a positive response to all three questions were classified as having current knee symptoms. (same for both baseline and follow-up) follow-up: Knee pain lasting most days in the last month? |
Baseline and follow up: K&L knee grade 0–4 | Hours/day - Light rec activities/Moderate recreational activities/Strenuous recreational activities/Exercise for muscle strength PAST 7 Days | Male & female | -Caucasian -Black or African American -other |
| TASOAC | Baseline and follow-up: Person level WOMAC (not side specific) | Days per week and minutes per day doing vigorous activities Days per week and minutes per day doing moderate activities |
|||
Footnotes
Conflicts of interest:
LSG and MTSS received funding from the Centre for Sport, Exercise & Osteoarthritis Research Versus Arthritis. TAP has no related interests to declare. YMG, AEN and LFC received institutional grants from Association of Schools of Public Health/Centers for Disease Control and Prevention (CDC) and National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). YMG also received grants from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) outside of the submitted work. YMG is on the NIAMS Data Safety Monitoring Board, TeMPO trial (treatment of meniscal tears in osteoarthritis). AEN also received honorarium from Lilly. DF has no related interests to declare. MN received funding from NIH to his institution. GJ received grants from Covance, personal fees from BMS, Roche, Abbvie, Amgen, Lilly, Novartis, and Jannsen, outside the submitted work. CC has received lecture fees and honoraria from Amgen, Danone, Eli Lilly, GSK, Kyowa Kirin, Medtronic, Merck, Nestlé, Novartis, Pfizer, Roche, Servier, Shire, Takeda and UCB outside of the submitted work. NKA received funding from Centre for Sport, Exercise & Osteoarthritis Research Versus Arthritis and consulting fees from Pfizer/Lilly and Bristows LLP.
Availability of data:
Requests for access to individual cohort level data used within this report should be submitted to the cohort principal investigators.
Patient and Public Involvement (PPI):
We did not include PPI in this study.
References
- 1.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–59. [DOI] [PubMed] [Google Scholar]
- 2.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Annals of the Rheumatic Diseases. 2014;73(7):1323. [DOI] [PubMed] [Google Scholar]
- 3.Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity--a systematic review of longitudinal studies. BMC Public Health. 2013;13:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haider S, Grabovac I, Dorner TE. Fulfillment of physical activity guidelines in the general population and frailty status in the elderly population : Acorrelation study of data from 11European countries. Wien Klin Wochenschr. 2019;131(11–12):288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poulsen E, Goncalves GH, Bricca A, Roos EM, Thorlund JB, Juhl CB. Knee osteoarthritis risk is increased 4–6 fold after knee injury - a systematic review and meta-analysis. British journal of sports medicine. 2019;53(23):1454–63. [DOI] [PubMed] [Google Scholar]
- 6.Zheng H, Chen C. Body mass index and risk of knee osteoarthritis: systematic review and meta-analysis of prospective studies. BMJ Open. 2015;5(12):e007568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38(10):1500–5. [DOI] [PubMed] [Google Scholar]
- 8.Richmond SA, Fukuchi RK, Ezzat A, Schneider K, Schneider G, Emery CA. Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. The Journal of orthopaedic and sports physical therapy. 2013;43(8):515–B19. [DOI] [PubMed] [Google Scholar]
- 9.Maly MR, Marriott KA, Chopp-Hurley JN. Osteoarthritis year in review 2019: rehabilitation and outcomes. Osteoarthritis Cartilage. 2020;28(3):249–66. [DOI] [PubMed] [Google Scholar]
- 10.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med. 1999;106(2):151–7. [DOI] [PubMed] [Google Scholar]
- 11.Lin W, Alizai H, Joseph GB, Srikhum W, Nevitt MC, Lynch JA, et al. Physical activity in relation to knee cartilage T2 progression measured with 3TMRI over a period of 4 years: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2013;21(10):1558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dore DA, Winzenberg TM, Ding C, Otahal P, Pelletier J-P, Martel-Pelletier J, et al. The association between objectively measured physical activity and knee structural change using MRI. Ann Rheum Dis. 2013;72(7):1170–5. [DOI] [PubMed] [Google Scholar]
- 13.Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Seminars in Arthritis and Rheumatism. 2014;43(6):701–12. [DOI] [PubMed] [Google Scholar]
- 14.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. Bmj. 2010;340:c221. [DOI] [PubMed] [Google Scholar]
- 15.Gates LS, Leyland KM, Sheard S, Jackson K, Kelly P, Callahan LF, et al. Physical activity and osteoarthritis: a consensus study to harmonise self-reporting methods of physical activity across international cohorts. Rheumatology International. 2017;37(4):469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyland KM, Gates LS, Nevitt M, Felson D, Bierma-Zeinstra SM, Conaghan PG, et al. Harmonising measures of knee and hip osteoarthritis in population-based cohort studies: an international study. Osteoarthritis Cartilage. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. the framingham osteoarthritis study. Arthritis & Rheumatism. 1987;30(8):914–8. [DOI] [PubMed] [Google Scholar]
- 18.Jordan JM, Linder GF, Renner JB, Fryer JG. The impact of arthritis in rural populations. Arthritis Care Res. 1995;8(4):242–50. [DOI] [PubMed] [Google Scholar]
- 19.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Preventative Medicine. 1975;4:518–25. [DOI] [PubMed] [Google Scholar]
- 20.Jordan J M, Linder G F, Renner J B, Fryer J G The Impact of Arthritis in Rural Populations. Arthritis Care and Research. 1995;8(4):242–50. [DOI] [PubMed] [Google Scholar]
- 21.Segal NA, Nevitt MC, Gross KD, Hietpas J, Glass NA, Lewis CE, et al. The Multicenter Osteoarthritis Study: opportunities for rehabilitation research. PM & R : the journal of injury, function, and rehabilitation. 2013;5(8):647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart D, Spector T, Egger P, Coggon D, Cooper C. Defining osteoarthritis of the hand for epidemiological studies: the Chingford Study. Ann Rheum Dis. 1994;53(4):220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syddall HE, Simmonds SJ, Carter SA, Robinson SM, Dennison EM, Cooper C, et al. The Hertfordshire Cohort Study: an overview. F1000Res. 2019;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding C, Parameswaran V, Cicuttini F, Burgess J, Zhai G, Quinn S, et al. Association between leptin, body composition, sex and knee cartilage morphology in older adults: the Tasmanian older adult cohort (TASOAC) study. Annals of the Rheumatic Diseases. 2008;67(9):1256–61. [DOI] [PubMed] [Google Scholar]
- 25.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, et al. Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;2011. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Noncommunicable diseases and their risk factors. 2020.
- 27.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson JJ, Felson DT. Factors associatd with osteoarthritis of the knee in the first national health and nutrition examination survey (HANES I): Evidence for an association with overweight, race, and physical demands of work American Journal of Epidemiology. 1988;128(1):179–89. [DOI] [PubMed] [Google Scholar]
- 29.Bellamy N, Campbell J, Stevens J, Pilch L, Stewart C, Mahmood Z. Validation study of a computerized version of the Western Ontario and McMaster Universities VA3.0 Osteoarthritis Index. Journal of Rheumatology. 1997;24(12):2413–5. [PubMed] [Google Scholar]
- 30.O’Reilly SC, Muir KR, Doherty M. Screening for pain in knee osteoarthritis: which question? Annals of the Rheumatic Diseases. 1996;55(12):931–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leyland KM, Gates LS, Nevitt M, Felson D, Bierma-Zeinstra SM, Conaghan PG, et al. Harmonising measures of knee and hip osteoarthritis in population-based cohort studies: an international study. Osteoarthritis Cartilage. 2018;26(7):872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–3. [DOI] [PubMed] [Google Scholar]
- 33.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 34.Bradburn MJ, Deeks JJ, Altman DG. Metan – an alternative meta-analysis command. Stata Technical Bulletin Reprints. 1998;44:4–15. [Google Scholar]
- 35.Thomas D, Radji S, Benedetti A. Systematic review of methods for individual patient data meta- analysis with binary outcomes. BMC Medical Research Methodology. 2014;14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris R, Bradburn M, Deeks J, Harbord R, Altman D, Sterne J. metan: fixed- and random-effects meta-analysis. Stata Journal. 2008;8(1):3–28. [Google Scholar]
- 37.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly. The framingham study. Arthritis & Rheumatism. 1997;40(4):728–33. [DOI] [PubMed] [Google Scholar]
- 38.Parsons CM, Gates LS, Perry T, Nevitt M, Felson D, Sanchez-Santos MT, et al. Predominant lifetime occupation and associations with painful and structural knee osteoarthritis: An international participant-level cohort collaboration. Osteoarthritis and Cartilage Open. 2020:100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry TA, Wang X, Gates L, Parsons CM, Sanchez-Santos MT, Garriga C, et al. Occupation and risk of knee osteoarthritis and knee replacement: A longitudinal, multiple-cohort study. Semin Arthritis Rheum. 2020;50(5):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Perry TA, Arden N, Chen L, Parsons CM, Cooper C, et al. Occupational Risk in Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Observational Studies. Arthritis Care & Research. 2020;72(9):1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McWilliams DF, Leeb BF, Muthuri SG, Doherty M, Zhang W. Occupational risk factors for osteoarthritis of the knee: a meta-analysis. Osteoarthritis and Cartilage. 2011;19(7):829–39. [DOI] [PubMed] [Google Scholar]
- 42.Hootman JM, Macera CA, Helmick CG, Blair SN. Influence of physical activity-related joint stress on the risk of self-reported hip/knee osteoarthritis: a new method to quantify physical activity. Preventive Medicine. 2003;36(5):636–44. [DOI] [PubMed] [Google Scholar]
- 43.Imeokparia RL, Barrett JP, Arrieta MI, Leaverton PE, Wilson AA, Hall BJ, et al. Physical activity as a risk factor for osteoarthritis of the knee. Annals of Epidemiology. 1994;4(3):221–30. [DOI] [PubMed] [Google Scholar]
- 44.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Research quarterly for exercise and sport. 2000;71 Suppl 2:1–14. [DOI] [PubMed] [Google Scholar]
- 45.Winckers ANE, Mackenbach JD, Compernolle S, Nicolaou M, van der Ploeg HP, De Bourdeaudhuij I, et al. Educational differences in the validity of self-reported physical activity. BMC Public Health. 2015;15(1):1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiphof D, Boers M, Bierma-Zeinstra SM. Differences in descriptions of Kellgren and Lawrence grades of knee osteoarthritis. Ann Rheum Dis. 2008;67(7):1034–6. [DOI] [PubMed] [Google Scholar]
- 47.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. The international journal of behavioral nutrition and physical activity. 2008;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerkhof HJM, Meulenbelt I, Akune T, Arden NK, Aromaa A, Bierma-Zeinstra SMA, et al. Recommendations for standardization and phenotype definitions in genetic studies of osteoarthritis: the TREAT-OA consortium. Osteoarthritis Cartilage. 2011;19(3):254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


