Abstract
A previously-reported cadmium-based two-periodic metal-organic framework [Cd1.5(BTC)(H2O)4.5]n·nH2O (CP1) has been re-synthesized, where H3BTC = 1,3,5-benzenetricarboxylic acid. CP1 was characterized with single crystal X-ray diffraction (SCXRD), powder X-ray diffraction (PXRD) followed by various thermal analyses such as thermogravimetric analysis (TGA), hot stage microscopy (HSM) and differential scanning calorimetry (DSC). CP1 is composed of 2-periodic layers, which are interdigitated. Heating can effectively remove the uncoordinated and coordinated water molecules resulting in an amorphous product CP1′. The original framework can be regenerated by readsorption of water from the atmosphere, indicating that the dehydration is reversible.
Keywords: Metal-organic frameworks, 2-periodic, Water sorption
Graphical abstract
Metal-organic frameworks; 2-periodic; Water sorption.
1. Introduction
The design and synthesis of metal-organic frameworks (MOFs) have been extensively studied due to the attraction of their adjustable, structure-dependent properties which can be utilized in various applications such as gas sorption, catalysis, luminescence and molecular recognition [1, 2, 3]. The frameworks of MOFs, which fall in the category of coordination polymers, are constructed from metal ions that act as ‘nodes’ and organic bridging ligands that act as ‘spacers’ [4]. Water stability of these MOFs still remain an important challenge, as various industrial environments, where these materials may find applications, have atmospheric water present [5]. One such example, may be the removal of CO2 from flue gas mixtures (CO2/N2 = 85/15) which have a water-rich environment. In case the primary MOF for this purpose is not water-stable, a secondary MOF can be used to remove water from the atmosphere. In this work, the MOF [Cd1.5(BTC)(H2O)4.5]n·nH2O (CP1) (H3BTC = 1,3,5-benzenetricarboxylic acid) was synthesized by a hydrothermal method. The structure was first reported by Michaelides and Skoulika et al., and subsequently by Wu et al. (both at 293 K) as part of a fluorescence study [6, 7]. The current work adds to the studies on this MOF by reporting on the single crystal structure at 100 K and assessing the hydrogen bonding that involves coordinated and uncoordinated water molecules. In addition, the dehydration and rehydration properties of the MOF using infrared (IR), hot stage microscopy (HSM), thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), as well as in situ powder X-ray diffraction (PXRD) were assessed. The MOF's dehydration and subsequent loss of crystalline structure is reversible in the presence of water vapour, showing that the dehydrated MOF could possibly be used as a water ‘scavenger’ in applications where dry environments are required.
2. Materials and methods
2.1. Materials and instrumentation
All reagents and solvents were commercially available and used as received. 1,3,5-Benzenetricarboxylic acid (H3BDC), 2,3-di(4-pyridyl)-2,3-butanediol and CdBr2·4H2O were purchased from Sigma Aldrich. Solvents were not dried and may have contained some water. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed using a TA Instruments TGA Q500 and a DSC Q200, respectively, with samples heated at a heating rate of 10 °C min−1 in the temperature range 20–600 °C and 20–400 °C for TGA and DSC, respectively, under a dry nitrogen flow of 60 mL min−1. Approximately 2–5 mg of sample was placed in an open crucible for each analysis, except for dehydration and rehydration studies where sample masses were ∼10 mg. Fourier-transform infrared (FT-IR) spectroscopy was performed in the range 4000–500 cm−1 on a PerkinElmer Spectrum Two FT-IR spectrometer using attenuated total reflectance (ATR). Hot stage microscopy (HSM) was performed using a Linkam THMS600 hot stage and Linkam TP92 control unit fitted to a Nikon SMZ-10 stereoscopic microscope. Crystals were placed on a cover slip under silicon oil to visualize solvent release using HSM. Images of thermal events were monitored with a Sony Digital Hyper HAD colour video camera and visualized on the Soft Imaging System program analySIS. Powder X-ray diffraction (PXRD) measurements were performed on a Bruker D8 Advance X-ray diffractometer in the 4–40° 2θ range using a 0.02° s−1 step size rate and X-rays generated at 30 kV and 40 mA. Experimentally-obtained PXRD patterns were compared to the simulated PXRD patterns calculated from the single crystal structure coordinates using MERCURY [8].
2.2. Synthesis and crystallization: preparation of [Cd1.5(BTC) (H2O)4.5]n·nH2O (CP1)
Benzene-1,3,5-tricarboxylic acid (13 mg, 0.06 mmol) and 2,3-di(4-pyridyl)-2,3-butanediol (15 mg, 0.06 mmol) were dissolved together with cadmium bromide (49 mg, 0.14 mmol) in 3 mL N,N'-dimethylformamide (DMF), 2 mL water and 1 mL ethanol (EtOH) with continuous stirring. The mixture was heated in an oven at 90 °C for 48 h (Scheme 1). Flat, colourless crystals of CP1 were obtained upon slow cooling of the solution at a rate of 10 °C h−1. Interestingly, the pyridyl-based co-ligand was not present in the structure of CP1. Subsequently, the synthesis was attempted without its presence, however, in these cases, CP1 was co-crystallized with presence of other by-products, thus the presence of 2,3-di(4-pyridyl)-2,3-butanediol was retained for future preparations, even though it was not part of the crystallized product.
Scheme 1.
Synthesis of CP1.
2.3. Single crystal X-ray diffraction studies
A single crystal of suitable quality was selected and mounted using cryoloop in Paratone N oil. Data collections were carried out on Bruker DUO APEX II CCD diffractometer using graphite monochromated Mo Kα (λ = 0.71073 Å) radiation, generated at 50 kV and 30 mA, with the crystal cooled to 100 K using an Oxford Cryostream-700. Data reduction and unit cell refinement were performed using SAINT-Plus [9]. All intensity data were scaled and corrected for Lorentz-polarisation and absorption effects using SADABS [10]. Structure solution and refinement were implemented using the crystallographic suite OLEX2 [11]. The crystal structures were solved by SHELXT, with subsequent refinement proceeding using the full-matrix least square method, based on F2 values against all reflections, including anisotropic displacement parameters for all non-H atoms, as employed in SHELXL-2018/3 [12,13]. X-Seed and MERCURY were used for generating high-quality images using POV-RAY [8,14,15]. PLATON was used to identify intermolecular hydrogen-bonding interactions [16].
3. Results and discussion
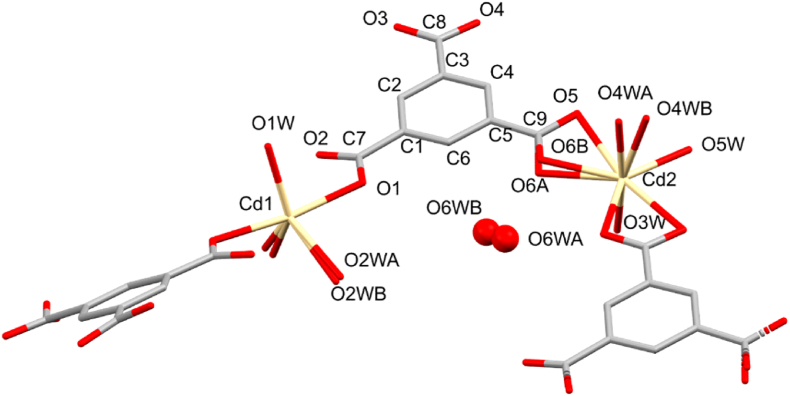
Single crystal X-ray diffraction analysis revealed that CP1 crystallizes in the monoclinic crystal system in the space group C2/c (Table 1). The asymmetric unit (ASU) comprises of one and a half cadmium(II) ions, one fully deprotonated, bridging benzene-1,3,5-tricarboxylate (BTC) anion, four and a half coordinated water molecules and one uncoordinated water molecule (Figure 1). The total 3+ charge on the one and half crystallographically unique Cd2+ ions is counter-balanced by the 3- charge of the fully deprotonated BTC ligand. Cd1 is located on a 2-fold axis and has a distorted trigonal bipyramidal coordination environment with coordination bonds that range from 2.251 to 2.296 Å, which agree well with the reported structures at 293 K (Figure 2a). The two axial positions are occupied by the O1 and O1_i (i: 1 − x, y, 3/2 − z) atoms of two different BTC anions (related by a 2-fold axis) that bind in a monodentate fashion, whilst three water molecules, O1W, O2W and O2W_i occupy the equatorial positions. Furthermore, O2W is disordered over two positions with site occupancy factors (sofs) of 0.44 and 0.56 for the A and B labelled positions, respectively (Figure 1). Cd2 has a pentagonal bipyramidal coordination environment; the axial positions being occupied by oxygen atoms of two water molecules (O3W and O4W), whilst the equatorial positions are occupied by O5W of a water molecule, two oxygen atoms (O5 and O6) from one carboxylate group of a BTC anion and two oxygen atoms O3_ii and O4_ii (ii: −½ + x, −½ + y, z) from the carboxylate group of a second BTC anion (Figure 2b). All the carboxylate groups are bonded to the Cd2 metal centres in bidentate coordination mode. In addition, the coordinated O4W water molecule is disordered over two positions with sofs of 0.77 and 0.23, for the A and B-labelled positions, respectively (Figure 1). The uncoordinated water molecule (O6W) is also disordered over two positions with sofs of 0.66 and 0.34, for the A and B-labelled positions, respectively (Figure 1). The reported structures at 293 K showed no signs of disorder for any of the atoms, except for uncoordinated water molecules, as indicated in the structure of Wu et al. [7].
Table 1.
Crystal data and refinement parameters of CP1.
| Empirical formula | C9H14Cd1.5O11.5 |
|---|---|
| Formula weight | 474.80 |
| Temperature/K | 100.0 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| a/Å | 19.0101 (16) |
| b/Å | 7.3447 (7) |
| c/Å | 20.4158 (17) |
| α/° | 90 |
| β/° | 97.281 (2) |
| γ/° | 90 |
| Volume/Å3 | 2827.5 (4) |
| Z | 8 |
| ρcalc/g cm−3 | 2.231 |
| μ/mm−1 | 2.336 |
| F(000) | 1856.0 |
| Crystal size/mm3 | 0.24 × 0.199 × 0.097 |
| 2Θ range for data collection/° | 4.022 to 61.348 |
| Index ranges | −27 ≤ h ≤ 27, −10 ≤ k ≤ 10, −29 ≤ l ≤ 29 |
| Reflections collected | 34428 |
| Independent reflections | 4388 [Rint = 0.0367, Rsigma = 0.0206] |
| Data/restraints/parameters | 4388/20/258 |
| Goodness-of-fit on F2 | 1.061 |
| Final R indexes [I>=2σ (I)] | R1 = 0.0164, wR2 = 0.0373 |
| Final R indexes [all data] | R1 = 0.0183, wR2 = 0.0380 |
| Largest diff. peak/hole/e Å−3 | 0.46/−0.37 |
Figure 1.
Coordination spheres of the two unique cadmium ions in CP1. Only ASU atoms are labelled and hydrogen atoms are omitted for clarity.
Figure 2.
Coordination geometry around the Cd1 and Cd2 ions in CP1. Only the major components of disordered positions are shown (labelled A or B).
CP1 is a coordination polymer where the coordination is extended infinitely in two co-planar directions by the bidentate coordination of two carboxylate groups (O3 and O4; O5 and O6) of the BTC anions to Cd2 ions. These co-planar 1D ‘strands’ make an angle of 33° with each other (Figure 3a). In turn these strands are connected via finite monodentate coordination of the third carboxylate group (O6) of the BTC anions to the Cd1 ions. The combination of the coordination modes results in 2-periodic frameworks that extend infinitely in the ab plane, whilst being interdigitated with each other along the c-axis direction (Figure 3b).
Figure 3.
Views along (a) the c-axis (oblique view of the ab plane) showing two co-planar directions of the bidentate coordination with an angle of 33° and (b) the b-axis (directly onto the ac plane) showing the interdigitation of neigbouring 2-periodic frameworks (each framework is colour coded). Hydrogen atoms have been omitted for clarity.
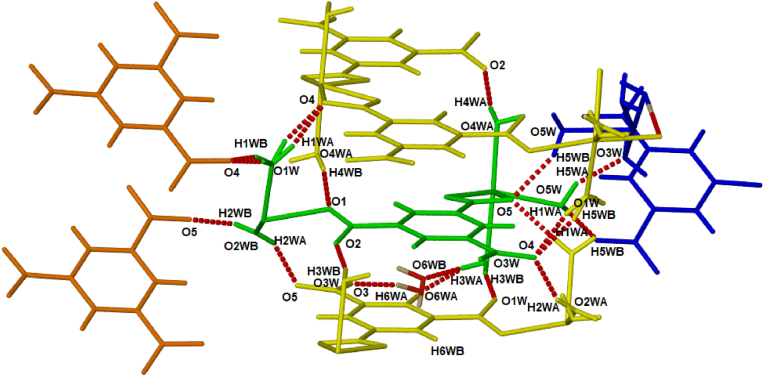
Intermolecular hydrogen bond analysis reveals that the water molecules are involved in several hydrogen bonds, with mostly coordinated water molecules acting as hydrogen bond donors to carboxylate oxygen acceptor atoms (Table 2). These hydrogen bonds show that neighbouring 2D frameworks are connected to each other and thus that the coordinated water molecules play an integral role in the stability of the 3D structure (Figure 4). In addition, the uncoordinated water molecule (O6WA position) acts as an indirect link between two neighbouring frameworks by acting as both a hydrogen bond donor (O6WA-H6WA⋅⋅⋅O3) and acceptor (O3W–H3WA⋅⋅⋅O6WA).
Table 2.
Hydrogen bond geometry for CP1 involving water molecules.
| D−H·····A | d(D−H)/Å | d(H···A)/Å | d(D···A)/Å | <D−H···A/° | Symmetry operation |
|---|---|---|---|---|---|
| O6WA-H6WA⋅⋅⋅O3 | 0.87 | 1.93 | 2.772 (3) | 164 | 1 − x,1 − y,1 − z |
| O3W–H3WA⋅⋅⋅O6WA | 0.87 | 1.69 | 2.718 (8) | 166 | x,y,z |
| O3W–H3WA⋅⋅⋅O6WB | 0.87 | 1.89 | 2.718 (2) | 159 | x,y,z |
| O3W–H3WB⋅⋅⋅O2 | 0.87 | 1.79 | 2.640 (2) | 166 | 1 − x,1 − y,1 − z |
| O5W–H5WA⋅⋅⋅O3W | 0.87 | 1.90 | 2.702 (2) | 153 | 1/2 − x,1/2 + y,1/2 − z |
| O5W–H5WB⋅⋅⋅O5 | 0.87 | 2.02 | 2.798 (2) | 147 | 1/2 − x,−1/2 + y,1/2 − z |
| O1W–H1WA⋅⋅⋅O4 | 0.87 | 1.93 | 2.752 (1) | 156 | 1 − x,2 − y,1 − z |
| O1W–H1WB⋅⋅⋅O4 | 0.87 | 1.91 | 2.752 (1) | 163 | x,2 − y,1/2 + z |
| O4WA–H4WA⋅⋅⋅O1 | 0.87 | 1.80 | 2.655 (2) | 165 | 1/2 − x,3/2 − y,1 − z |
| O2WB–H2WA⋅⋅⋅O4 | 0.87 | 1.89 | 2.71 (2) | 155 | 1 − x,1 − y,1 − z |
| O2WB–H2WB⋅⋅⋅O5 | 0.87 | 1.84 | 2.69 (2) | 166 | x,1 − y,1/2 + z |
Figure 4.
Hydrogen bonding in CP1 between the ASU (shown in green) and surrounding atoms. Colour-coded moieties belong to the same 2-periodic framework.
3.1. Thermal analysis
The TGA thermogram of CP1 indicates a two-step mass loss event, comprising of initial solvent loss around ∼50 °C, which significantly increases thereafter. The total mass loss occurring in the range of 50–400 °C is found to be 21.2 % (Calc. 20.85 %), fully accounting for the 5.5 water molecules per ASU. The DSC thermogram showed two overlapping endotherms between 50-100 °C, which probably corresponds to the concomitant release of uncoordinated and coordinated water molecules from the framework. Two other endothermic bands appear around 185 °C and 305 °C, which probably correspond to additional solvent loss as indicated by TGA (Figure 5). The framework decomposed after 400 °C.
Figure 5.
Overlay of DSC (green) and TGA (blue) thermograms of CP1.
A clear effect of dehydration can be vizualized from HSM analysis where crystals of CP1 were placed under silicone oil and heated in a range of 25–450 °C (Figure 6). Although the first sign of solvent loss was noticed around 80 °C, the bubbles evidently came off at 120 °C (Figure 6). The clear crystal started to turn opaque at this stage, which at the very least indicates the loss of monocrystallinity due to loss of solvent molecules. Around 400 °C they became brown indicating decomposition of the framework.
Figure 6.
Hot stage microscope photographs of compound CP1 under silicone oil at various temperatures (bubbles indicate the release of solvent).
3.2. Powder X-ray diffraction studies
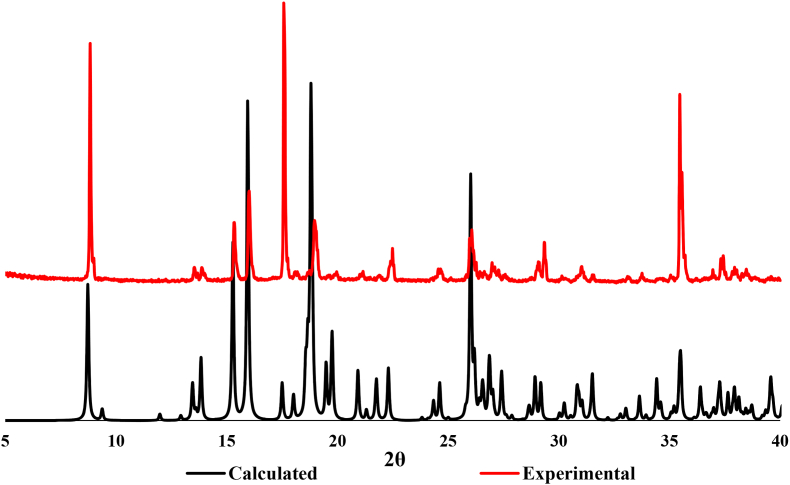
The PXRD pattern of compound CP1 recorded at room temperature and ambient pressure is in good agreement with its calculated pattern which proves the phase purity of the bulk material (Figure 7).
Figure 7.
Comparison of the experimental PXRD pattern of CP1 with the calculated PXRD pattern obtained from the single crystal structure.
3.3. Dehydration and rehydration studies
These studies were performed to investigate whether the dehydrated sample of CP1 (hereinafter CP1′) is able to transform to the original structure of CP1 by readsorbing moisture from the atmosphere (hereinafter CP1′R). TGA and PXRD experiments were used in conjunction to assess whether structural changes occurred when CP1 is dehydrated and rehydrated. A TGA thermogram of a fresh sample of CP1 was recorded first by heating the sample to 200 °C (Figure 8a). The sample was then cooled and immediately reheated (no exposure to air), after which no mass loss occurred on the reheating, confirming that all the solvent was released in the first run. This same sample was then exposed to water vapour by placing it in a smaller vial that was placed in a bigger vial with water. After two days, the TGA analysis revealed that the original amount of water was readsorbed. The corresponding PXRD patterns of the samples at different stages of the experiment show not only that the original amount of water is readsorbed but that the structure of CP1′R corresponds to the structure of CP1, thus showing that the dehydration is reversible in terms of both the loss of water and crystallinity of CP1 (Figure 8b).
Figure 8.
(a) TGA and (b) associated PXRD patterns of CP1, CP1′and CP1′R.
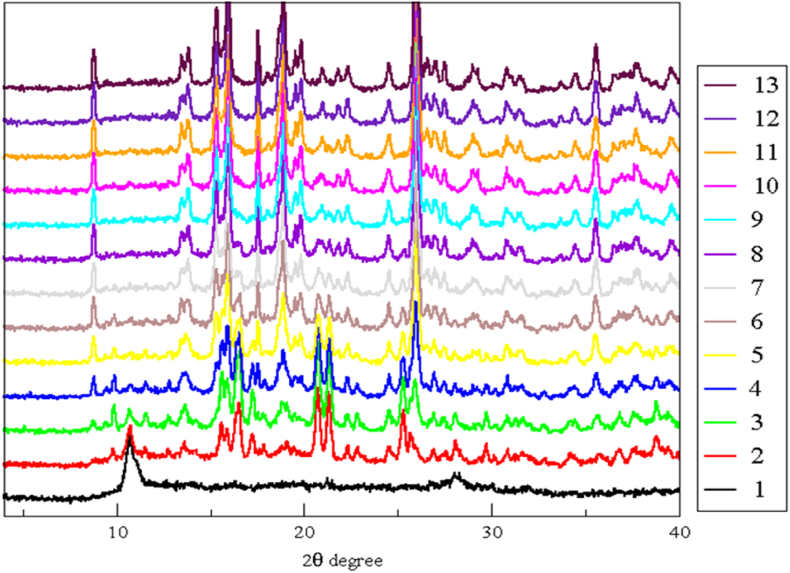
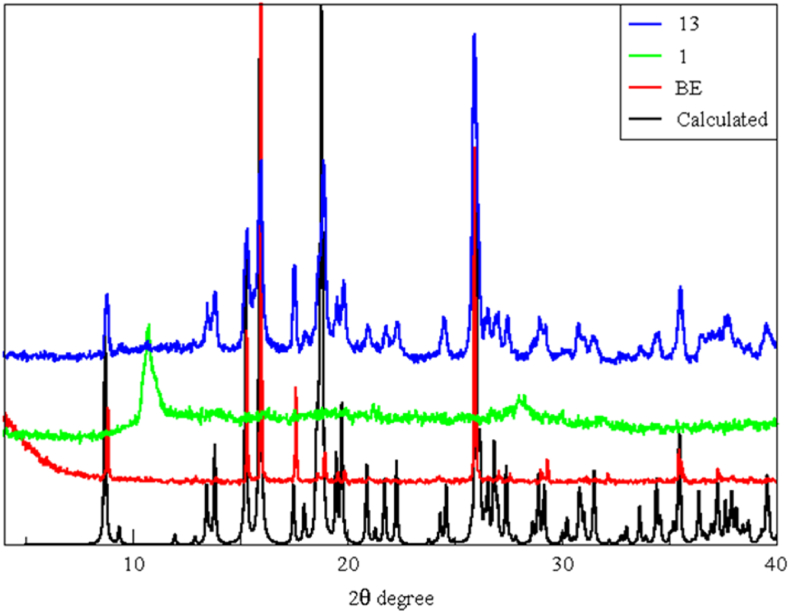
In situ PXRD rehydration studies were also performed by using a modified sample stage which consisted of water wells next to the sample. The sample was heated to 300 °C (maintained for 30 min) and cooled back to room temperature (∼25 °C) before commencement of the in situ experiment. Diffractograms were recorded over a period of 14 h but after 3 h and 15 min no further changes in the patterns were observed. Each diffractograms was recorded over ∼15 min in the scan range of 4–40° 2θ (Figure 9). Initially, the PXRD pattern indicates that the sample is amorphous but even after 15 min the sample regains crystallinity. Eventually after ∼3 h the PXRD pattern matched that of the as-synthesized CP1 sample (Figures 9 and 10).
Figure 9.
Evolution of PXRD diffractograms of CP1′ during water vapour exposure. Each step corresponds to 15 min. No patterns recorded after 3 h and 15 min are shown as those are similar to pattern 13.
Figure 10.
Comparison between different PXRD patterns of CP1, from bottom: calculated (black), recorded before in situ rehydration study (red) and the first (light green) and the last (blue) significant diffractograms recorded during the in situ experiment. BE = before experiment.
3.4. Infrared spectroscopy
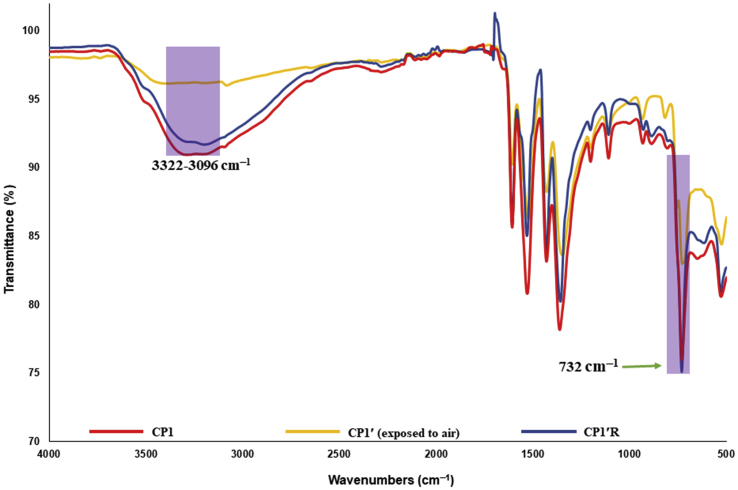
The infrared spectroscopy data further supports the reversibility of the dehydration of CP1 when CP1′ adsorbs moisture from the atmosphere. The IR spectrum of CP1 (Figure 11) showed a broad band at the region of 3322–3096 cm−1 that can be assigned to the asymmetric and symmetric OH streching vibration modes of water molecules [17, 18, 19]. This band was not observed in the IR spectrum of CP1′ indicating the absence of the water molecules in the cavity. The presence of the metal coordinated water molecules in CP1 was indicated by a strong band at 732 cm−1 and was further proved by the shortening of that band upon dehydration in CP1′ [20].
Figure 11.
IR spectra of CP1, CP1′ (exposed to air) and CP1′R.
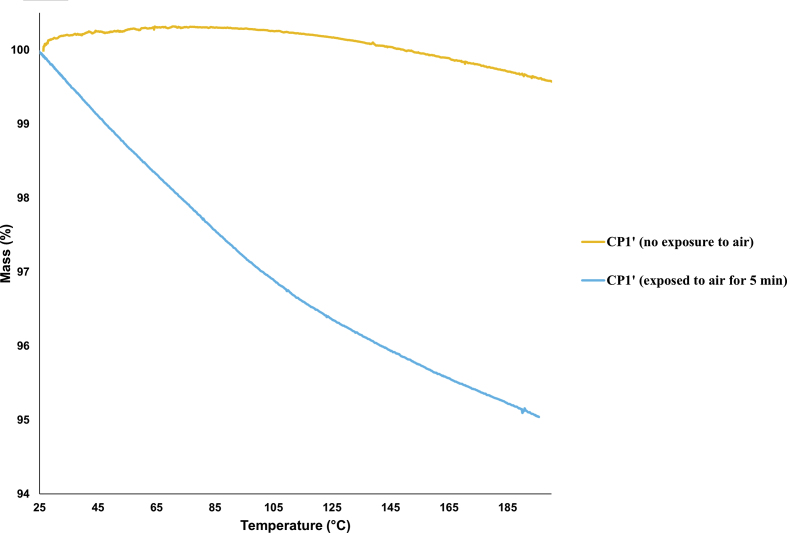
These bands do not completely disappear as the dehydrated sample readily absorbs moisture from air, which was proved by the TGA of the dehydrated sample exposed to moisture for 5 min only, which was the typical time required to perform the IR analysis (Figure 12). Those specific bands reappeared at the same position with same transmittance when CP1′ was exposed to water vapour for two days.
Figure 12.
TGA thermograms of CP1′not exposed to air and exposed to air for 5 min.
4. Conclusion
In conclusion, a 2-periodic MOF has been resynthesized and subjected to single crystal X-ray diffraction reporting additional information on the hydrogen bonding present in the structure, as well as on the thermal properties, dehydration and rehydration behaviour, which were not reported in the previous studies on this compound. We have shown that this MOF can be rapidly rehydrated, after dehydration, upon exposure to water vapour which leads to regaining of the MOFs original structure. Thus, it could prove useful as water scavenger in applications where dry environments are desired.
Declarations
Author contribution statement
Nabanita Chatterjee, Marcello Mutti: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Paolo Pelagatti: Analyzed and interpreted the data; Wrote the paper.
Clive L. Oliver: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the South African National Research Foundation (Grant No: 120849), and the University of Cape Town.
Data availability statement
Data associated with this study has been deposited at Cambridge Crystallographic Data Centre.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Footnotes
This article is a part of the "Coordination compounds" Special issue
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Guo C., Duan F., Zhang S., He L., Wang M., Chen J., Zhang J., Jia Q., Zhang Z., Du M. Heterostructured hybrids of metal–organic frameworks (MOFs) and covalent–organic frameworks (COFs) J. Mater. Chem. 2022;10:475–507. [Google Scholar]
- 2.Kreno L.E., Leong K., Farha O.K., Allendorf M., Van Duyne R.P., Hupp J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012;112 2:1105–1125. doi: 10.1021/cr200324t. [DOI] [PubMed] [Google Scholar]
- 3.Gupta M., Chatterjee N., De D., Saha R., Chattaraj P.K., Oliver C.L., Bharadwaj P.K. Metal−Organic frameworks of Cu(II) constructed from functionalized ligands for high capacity H2 and CO2 gas adsorption and catalytic studies. Inorg. Chem. 2020;59:1810–1822. doi: 10.1021/acs.inorgchem.9b03012. [DOI] [PubMed] [Google Scholar]
- 4.Zhou H., Long J.R., Yaghi O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012;112:673–674. doi: 10.1021/cr300014x. [DOI] [PubMed] [Google Scholar]
- 5.Wang C., Liu X., Demir N.K., Chen J.P., Li K. Applications of water stable metal–organic frameworks. Chem. Soc. Rev. 2016;45:5107–5134. doi: 10.1039/c6cs00362a. [DOI] [PubMed] [Google Scholar]
- 6.Dimos A., Michaelides A., Skoulika S. A molecular bilayer motif constructed from a three-connected organic ligand and Cd2+ cations: crystal structure of [Cd3(trimesate)2(H2O)9].2H2O. Chem. Mater. 2000;12:3256–3258. [Google Scholar]
- 7.Dai J.-C., Wu X.-T., Fu Z.-Y., Cui C.-P., Hu S.-M., Du W.-X., Wu L.-M., Zhang H.-H., Sun R.-Q. Synthesis, structure, and fluorescence of the novel cadmium(II)-Trimesate coordination polymers with different coordination architectures. Inorg. Chem. 2002;41:1391–1396. doi: 10.1021/ic010794y. [DOI] [PubMed] [Google Scholar]
- 8.Macrae C.F., Sovago I., Cottrell S.J., Galek P.T.A., McCabe P., Pidcock E., Platings M., Shields G.P., Stevens J.S., Towler M., Wood P.A. Mercury 4.0: from visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020;53:226–235. doi: 10.1107/S1600576719014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruker Apex3 v2019.3, SAINT V8.40A. Bruker AXS Inc.; Madison, WI, USA: 2019. [Google Scholar]
- 10.Sheldrick G.M. University of Göttingen; Germany: 2007. SADABS, Version 2.05. [Google Scholar]
- 11.Dolomanov O.V., Bourhis L.J., Gildea R.J., Howard J.A.K., Puschmann H. OLEX2: a complete structure solution, refinement and analysis program (2009) J. Appl. Crystallogr. 2009;42(42):339–341. [Google Scholar]
- 12.Sheldrick G.M. SHELXT - integrated space-group and crystal-structure determination. Acta Crystallogr. A: Found. Adv. 2015;71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Section C-Struct. Chem. 2015;71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbour L.J. X-Seed 4: updates to a program for small molecule supramolecular crystallography. J. Appl. Crystallogr. 2020;53:1141–1146. [Google Scholar]
- 15.POV-Ray for Windows, Ver. 3.6. Persistence of Vision Raytracer, Pty. Ltd.; 2004. [Google Scholar]
- 16.Spek A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009;65:148–155. doi: 10.1107/S090744490804362X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manna S.C., Mistri S., Jana A.D. A rare supramolecular assembly involving ion pairs of coordination complexes with a host–guest relationship: synthesis, crystal structure, photoluminescence and thermal study. Cryst. Eng. Comm. 2012;14:7415–7422. [Google Scholar]
- 18.Hakimi M., Moeini K., Mardani Z., Mohr F. Microwave-assisted template synthesis of diazacyclam-based macrocyclic copper complex and forming octahedral, square planar and square pyramidal geometries by ion exchanging and introducing a novel 2D square-grid copper–mercury coordination polymer. Polyhedron. 2014;70:92–100. [Google Scholar]
- 19.Nakamoto K. Wiley; New York: 1986. Infrared and Raman Spectra of Inorganic and Coordination Compounds. [Google Scholar]
- 20.Fujita J., Nakamoto K., Kobayashi M. Infrared spectra of metallic complexes. II. The absorption bands of coordinated water in aquo complexes. J. Am. Chem. Soc. 1956;78:3963–3965. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study has been deposited at Cambridge Crystallographic Data Centre.