Abstract
Herein we report a novel palladium-catalyzed phosphorylation of arylsulfonium salts with P(O)H compounds via C–S bond cleavage under mild conditions. The protocol provides a pragmatic strategy applicable to the synthesis of diverse arylphosphonates.
Herein we report a novel palladium-catalyzed phosphorylation of arylsulfonium salts with P(O)H compounds via C–S bond cleavage under mild conditions.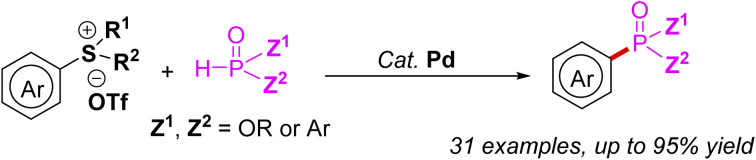
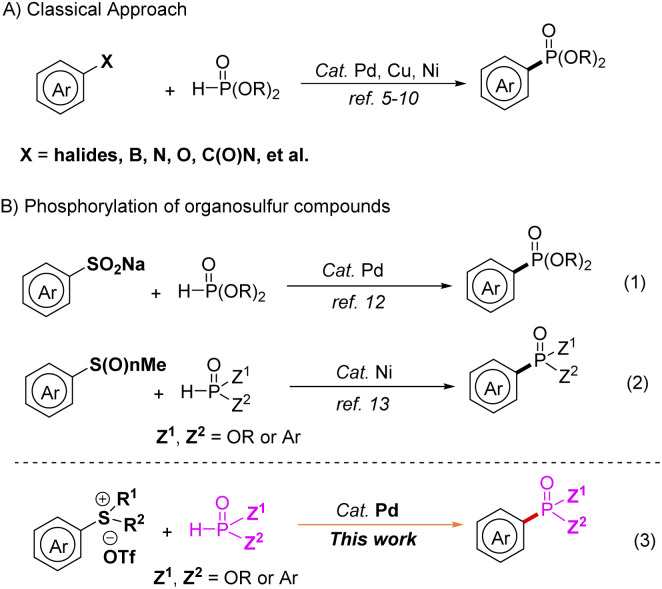
Arylphosphonates are ubiquitous constituents of numerous biologically active compounds, agrochemicals and functional materials,1 and also play an important role in organometallic catalysis2 and organocatalysis3 in organic synthesis. Ever since the pioneering work by Hirao and co-workers in the 1980s,4 various methods catalyzed or mediated by transition-metals for the synthesis of arylphosphonates have been developed. The cross-coupling reactions of P(O)H compounds with various aryl partners that have been reported so far include aryl halides,5 aryl boron reagents,6 aryl oxygen derivatives,7 aryl nitrogen reagents,8 and aryl amide derivatives,9etc. (Scheme 1A).10
Scheme 1. Transition metal-catalyzed phosphorylation reactions.
It is well known that organosulfur compounds have been of great importance in organic synthesis because of their accessibility and intriguing reactivity. As a valuable transformation of organosulfur compounds, transition-metal catalyzed C–S bond cleavage has attracted much attention.11 In this regard, Wang's group developed a novel palladium-catalyzed phosphonation of sodium arylsulfinates with H-phosphonate diesters in 2014 (Scheme 1B, eqn (1)).12 And then, Han's group developed the nickel-catalyzed phosphinylation of C–S bonds forming C–P bonds by using sulfides, sulfoxides and sulfones as substrates (Scheme 1B, eqn (2)).13 As a good choice for the aryl source, arylsulfonium salts are easily available, easy to handle, and reasonably reactive, they show great potential for transition metal-catalyzed transformation such as arylation, alkenylation, alkynylation, borylation, alkoxycarbonylation, and carboxylation,14 which can be viewed as an activated form of the inert C–S bond. As a continuation of our interest in the Ar–P bond construction,15 herein we wish to report palladium-catalyzed phosphorylation of arylsulfonium salts with P(O)H compounds (Scheme 1B, eqn (3)).
We commenced our investigation by studying the reaction conditions of arylsulfonium salts 1a with diethyl phosphonate 2a (Table 1). A series of Pd catalysts, including PdCl2, Pd2(dba)3, Pd(dppf)Cl2, Pd(PPh3)2Cl2, Pd(acac)2, and Pd(OAc)2 were examined in the presence of X-phos (5 mol%), K3PO4 (1.0 equiv.) in N,N-dimethylformamide (DMF) at 80 °C (Table 1, entries 1–6), which were found to be effective, thus affording the desired product 3a. Among these Pd catalysts, Pd(OAc)2 turned out to be the best catalyst for the reaction, affording the desired product in a yield of 58% (Table 1, entry 6). Subsequently, a survey of different phosphine ligands revealed that XPhos was the best choice (Table 1, entries 7–12). In order to improve the reaction yield, we opted to continue our optimization studies using different bases (Table 1, entries 13–16), but no better reactivity was achieved. Furthermore, the effect of the solvent was also probed (Table 1, entries 17–23). To our delight, iPrOH was found to be suitable for the reaction, affording the desired product 3a in 72% yield (Table 1, entry 22).
Optimization of the reaction conditionsa,b.
| |||||
|---|---|---|---|---|---|
| Entry | Cat. | P ligand | Base | Solvent | Yieldb (%) |
| 1 | PdCl2 | XPhos | K3PO4 | DMF | 40 |
| 2 | Pd2(dba)3 | XPhos | K3PO4 | DMF | 10 |
| 3 | Pd(dppf)Cl2 | XPhos | K3PO4 | DMF | 35 |
| 4 | Pd(PPh3)2Cl2 | XPhos | K3PO4 | DMF | 10 |
| 5 | Pd(acac)2 | XPhos | K3PO4 | DMF | 19 |
| 6 | Pd(OAc)2 | XPhos | K3PO4 | DMF | 58 |
| 7 | Pd(OAc)2 | dppe | K3PO4 | DMF | 5 |
| 8 | Pd(OAc)2 | PPh3 | K3PO4 | DMF | 6 |
| 9 | Pd(OAc)2 | dppp | K3PO4 | DMF | 15 |
| 10 | Pd(OAc)2 | JohnPhos | K3PO4 | DMF | 20 |
| 11 | Pd(OAc)2 | SPhos | K3PO4 | DMF | 32 |
| 12 | Pd(OAc)2 | Ruphos | K3PO4 | DMF | 30 |
| 13 | Pd(OAc)2 | XPhos | K2HPO4 | DMF | 50 |
| 14 | Pd(OAc)2 | XPhos | Cs2CO3 | DMF | 23 |
| 15 | Pd(OAc)2 | XPhos | NaHCO3 | DMF | 25 |
| 16 | Pd(OAc)2 | XPhos | Et3N | DMF | 21 |
| 17 | Pd(OAc)2 | XPhos | K3PO4 | DMSO | 43 |
| 18 | Pd(OAc)2 | XPhos | K3PO4 | DMA | 31 |
| 19 | Pd(OAc)2 | XPhos | K3PO4 | Dioxane | Trace |
| 20 | Pd(OAc)2 | XPhos | K3PO4 | MeCN | 58 |
| 21 | Pd(OAc)2 | XPhos | K3PO4 | EtOH | 46 |
| 22 | Pd(OAc)2 | XPhos | K3PO4 | iPrOH | 72 |
| 23 | Pd(OAc)2 | XPhos | K3PO4 | tAmOH | 63 |

| |||||
1a (0.36 mmol), 2a (0.30 mmol), cat. Pd (5 mo%), ligand (5 mo%), base (0.3 mmol), solvent (2.0 mL), 80 °C, 16 h, under N2.
Isolated yield.
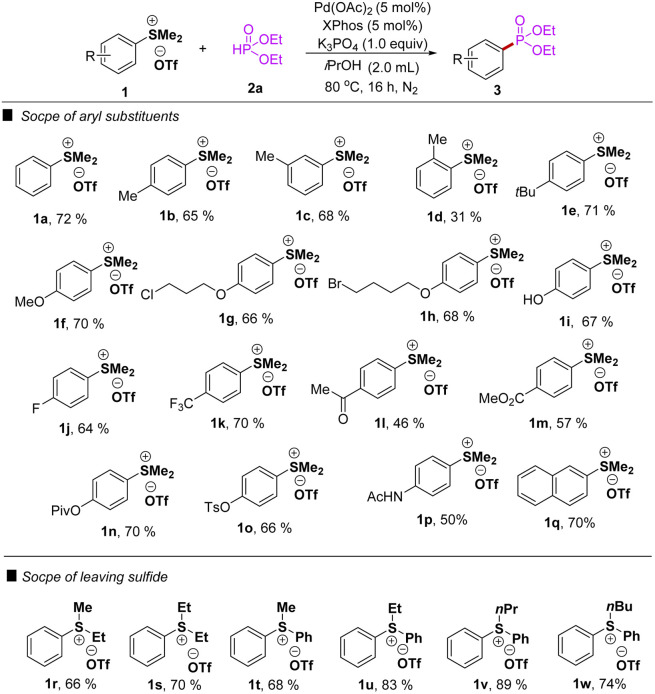
Having the optimized conditions established in hand, we then proceeded to explore the scope of the phosphorylation of various arylsulfonium salts with diethyl phosphonate 2a. The results were summarized in Table 2, in general moderate to good yields were obtained under the optimized reaction conditions. The phosphorylation reaction had shown excellent tolerance to both electron-withdrawing and electron-donating groups as aromatic substituents, including alkyl (1b–d), alkoxyl (1f–h), fluoro (1j), trifluoromethyl (1k), acetyl (1l), ester (1m–o), and acetamido groups (1p). For the substrate of arylsulfonium salts with the naphthalene group, the reaction gave the desired product in 70% yield. Surprisingly, the phosphorylation reaction could be conducted in the presence of unprotected active hydroxyl group (1i), affording 67% yield. The diminished yield in the case of 1d may be attributed to steric hindrance. The desired products were also obtained in good yields regardless of the alkyl substituents on the sulfonium moiety (1r–s). In addition, different substituents (1r–w) at the sulfur atom to evaluate the reactivity of leaving group on the Pd-catalyzed phosphorylation, several dialkyl-(phenyl) or alkyl(diphenyl)sulfonium triflates were tested under the optimized conditions. To our delight, the desired products were obtained in good to excellent yields (1t–v), in which ethyl- and propyl(diphenyl)-sulfonium triflates were more suitable, afforded 3a in 83%, and 89% yields, respectively.
Scope of the catalytic phosphorylation of arylsulfonium saltsa,b.
|
1 (0.36 mmol), 2a (0.30 mmol), Pd(OAc)2 (5 mo%), XPhos (5 mo%), K3PO4 (0.3 mmol), iPrOH (2.0 mL), 80 °C, 16 h, under N2.
Isolated yield.
For further evaluation of the substrate scope, different P(O)H compounds were used to test the reaction. As shown in Scheme 3, the H-phosphonate diesters with different alkyl groups can all react smoothly with arylsulfonium salt 1v to afford the corresponding phosphorylation product (4a–c) in moderate to good yields. Ethyl phenylphosphinate (4d) had good compatibility under the optimized conditions to give the corresponding products in 82% yield. Next, some di-p-tolylphosphine oxides were subjected to the reaction at 110 °C, diphenyl- and di-p-tolylphosphine oxides show reactivity to afford the corresponding products 4e and 4f in 23% and 64% yield, respectively. Unfortunately, bis(4-fluorophenyl)-phosphine oxide was incompatible to the current catalytic system (4g) (Table 3).
Scheme 3. One-pot phosphorylation of aryl sulfide.
Scope of the catalytic phosphorylation of P(O)H compoundsa,b.

|
1v (0.36 mmol), 2 (0.30 mmol), Pd(OAc)2 (5 mo%), XPhos (5 mo%), K3PO4 (0.3 mmol), iPrOH (2.0 mL), 80 °C, 16 h, under N2.
Isolated yield.
T = 110 °C.
Interestingly, the (4-bromophenyl)dimethylsulfonium 2w was used to test the reaction, unexpected diphosphorylation product 5 and phosphorylation product bearing 4-methylthio group 6 were obtained, respectively in 22% and 35% yield (Scheme 2).
Scheme 2. Phosphorylation of the (4-bromophenyl)dimethyl sulfonium.
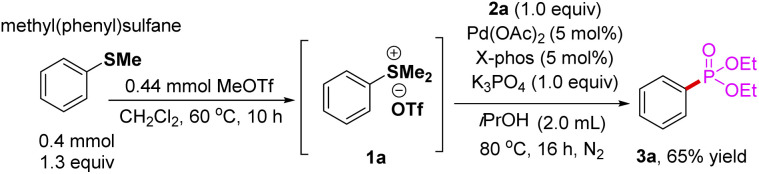
To prove the synthetic utility of the strategy, one-pot phosphorylation method was investigation. Treatment of methyl(phenyl)sulfane with MeOTf in 1,2-dichloroethane (DCE) afforded the corresponding aryl sulfonium salts 1a (confirmed with 1H NMR). After removal of all volatiles under a reduced pressure, one-pot phosphorylation was performed in the presence of Pd(OAc)2, XPhos, diethyl phosphonate 2a, K3PO4, and iPrOH. We are pleased to find that the reaction proceeded smoothly to provide the desired product 3a in 61% yield.
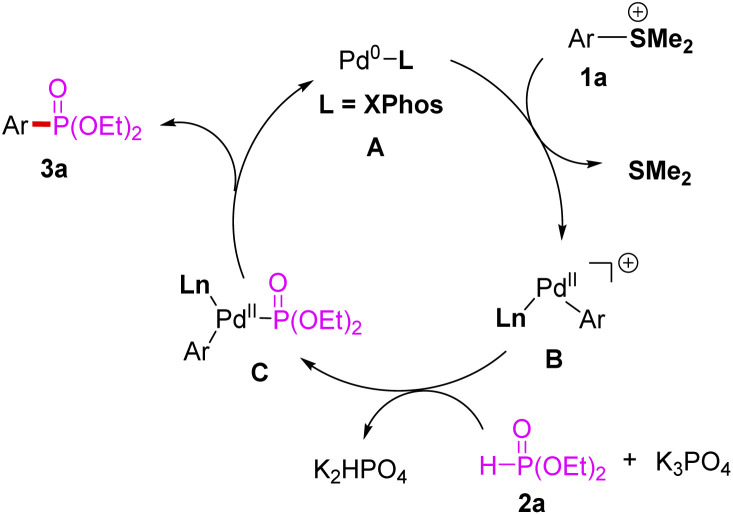
A plausible mechanism for the phosphorylation of arylsulfonium salts with P(O)H compounds was proposed as shown in Scheme 4. First, palladium(0) species A was generated from the reduction of palladium(ii) with phosphine ligand by the aid of K3PO4.16 Then, arylsulfonium 1a would undergo oxidative addition with A to form cationic arylpalladium(ii) B,17 followed by ligand exchange of diethyl phosphonate 2a with species B led to the generation of intermediate C in base condition.13 Subsequently, reductive elimination from C afforded arylphosphonate 3a and palladium (0) species A and the catalytic cycle was completed.
Scheme 4. Plausible reaction mechanism.
In summary, we have developed a palladium-catalyzed phosphorylation of arylsulfonium salts with P(O)H compounds. The transformation proceeded under mild reaction conditions and had advantages include good functional group tolerance, a wide scope of substrates, and easily available arylsulfonium salts. Mechanistically, this approach involves oxidative addition and reductive elimination processes as the two key steps to afford the desired product. The protocol provides pragmatic strategy applicable to the synthesis of diverse arylphosphonates.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 21762002).
Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra04297e
Notes and references
- (a) Tebby J. C. Appl. Organomet. Chem. 2000;14:514. [Google Scholar]; (b) Surry D. S. Buchwald S. L. Angew. Chem., Int. Ed. 2008;47:6338. doi: 10.1002/anie.200800497. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Queffélec C. Petit M. Janvier P. Knight D. A. Bujoli B. Chem. Rev. 2012;112:3777. doi: 10.1021/cr2004212. [DOI] [PubMed] [Google Scholar]
- For selected reviews, see: ; (a) McCarthy M. Guiry P. J. Tetrahedron. 2001;57:3809. [Google Scholar]; (b) Tang W. J. Zhang X. M. Chem. Rev. 2003;103:3029. doi: 10.1021/cr020049i. [DOI] [PubMed] [Google Scholar]; (c) Martin R. Buchwald S. L. Acc. Chem. Res. 2008;41:1461. doi: 10.1021/ar800036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected reviews, see: ; (a) Lu X. Zhang C. Xu Z. Acc. Chem. Res. 2001;34:535. doi: 10.1021/ar000253x. [DOI] [PubMed] [Google Scholar]; (b) Methot J. L. Roush W. R. Adv. Synth. Catal. 2004;346:1035. [Google Scholar]; (c) Wei Y. Shi M. Acc. Chem. Res. 2010;43:1005. doi: 10.1021/ar900271g. [DOI] [PubMed] [Google Scholar]
- Hirao T. Masunaga T. Ohshiro Y. Agawa T. Tetrahedron Lett. 1980;21:3595. [Google Scholar]
- (a) Keglevich G. Henyecz R. Mucsi Z. Kiss N. Z. Adv. Synth. Catal. 2017;359:4322. doi: 10.1002/adsc.201700895. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zeng H. Dou Q. Li C.-J. Org. Lett. 2019;21:1301. doi: 10.1021/acs.orglett.8b04081. [DOI] [PubMed] [Google Scholar]; (c) Zakirova G. Mladentsev D. Y. Borisova N. E. Synthesis. 2019;51:2379. [Google Scholar]; (d) Ghasemzadeh M. S. Akhlaghinia B. New J. Chem. 2019;43:5341. [Google Scholar]; (e) Bai Y. Liu N. Wang S. Wang S. Ning S. Shi L. Cui L. Zhang Z. Xiang J. Org. Lett. 2019;21:6835. doi: 10.1021/acs.orglett.9b02475. [DOI] [PubMed] [Google Scholar]; (f) Mungalpara M. N. Wang J. Coles M. P. Plieger P. G. Rowlands G. J. Tetrahedron. 2018;74:5519. [Google Scholar]; (g) Sengmany S. Ollivier A. Gall E. L. Léonel E. Org. Biomol. Chem. 2018;16:4495. doi: 10.1039/c8ob00500a. [DOI] [PubMed] [Google Scholar]; (h) Kuramshin A. I. Plotnikova A. V. Adel'shina M. V. Galkin V. I. Russ. J. Org. Chem. 2018;54:1746. [Google Scholar]; (i) Xuan J. Zeng T.-T. Chen J.-R. Lu L.-Q. Xiao W.-J. Chem.–Eur. J. 2015;21:4962. doi: 10.1002/chem.201500227. [DOI] [PubMed] [Google Scholar]; (j) Gelman D. Jiang L. Buchwald S. L. Org. Lett. 2003;5:2315. doi: 10.1021/ol0346640. [DOI] [PubMed] [Google Scholar]; (k) Kalek M. Jezowska M. Stawinski J. Adv. Synth. Catal. 2014;351:3207. [Google Scholar]
- (a) Zhuang R. Xu J. Cai Z. Tang G. Fang M. Zhao Y. Org. Lett. 2011;13:2110. doi: 10.1021/ol200465z. [DOI] [PubMed] [Google Scholar]; (b) Hu G. Chen W. Fu T. Peng Z. Qiao H. Gao Y. Zhao Y. Org. Lett. 2013;15:5362. doi: 10.1021/ol402672e. [DOI] [PubMed] [Google Scholar]; (c) Andaloussi M. Lindh J. Sävmarker J. Sjöberg P. J. R. Larhed M. Chem.–Eur. J. 2009;15:13069. doi: 10.1002/chem.200901473. [DOI] [PubMed] [Google Scholar]; (d) Fu T. Qiao H. Peng Z. Hu G. Wu X. Gao Y. Zhao Y. Org. Biomol. Chem. 2014;12:2895. doi: 10.1039/c3ob42470g. [DOI] [PubMed] [Google Scholar]; (e) Wan H. Zhao Y. Wang Q. Zhang Y. Li Y. Russ. J. Gen. Chem. 2016;86:150. [Google Scholar]; (f) Chen T.-H. Reddy D. M. Lee C.-F. RSC Adv. 2017;7:30214. [Google Scholar]
- (a) Yang G. Shen C. Zhang L. Zhang W. Tetrahedron Lett. 2011;52:5032. [Google Scholar]; (b) Yang J. Chen T. Han L.-B. J. Am. Chem. Soc. 2015;137:1782. doi: 10.1021/ja512498u. [DOI] [PubMed] [Google Scholar]; (c) Yang J. Xiao J. Chen T. Han L.-B. J. Org. Chem. 2016;81:3911. doi: 10.1021/acs.joc.6b00289. [DOI] [PubMed] [Google Scholar]; (d) Liao L.-L. Gui Y.-Y. Zhang X.-B. Shen G. Liu H.-D. Zhou W.-J. Li J. Yu D.-G. Org. Lett. 2017;19:3735. doi: 10.1021/acs.orglett.7b01561. [DOI] [PubMed] [Google Scholar]
- (a) Xu W. Hu G. Xu P. Gao Y. Yin Y. Zhao Y. Adv. Synth. Catal. 2014;356:2948. [Google Scholar]; (b) Berrino R. Cacchi S. Fabrizi G. Goggiamani A. Stabile P. Org. Biomol. Chem. 2010;8:4518. doi: 10.1039/c0ob00243g. [DOI] [PubMed] [Google Scholar]; (c) He Y. Wu H. Toste F. D. Chem. Sci. 2015;6:1194. doi: 10.1039/c4sc03092c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wang S. Qiu D. Mo F. Zhang Y. Wang J. J. Org. Chem. 2016;81:11603. doi: 10.1021/acs.joc.6b01820. [DOI] [PubMed] [Google Scholar]
- (a) Liu C. Szostak M. Angew. Chem., Int. Ed. 2017;56:12718. doi: 10.1002/anie.201707102. [DOI] [PubMed] [Google Scholar]; (b) Dong J. Liu L. Ji X. Shang Q. Liu L. Su L. Chen B. Kan R. Zhou Y. Yin S.-F. Han L.-B. Org. Lett. 2019;21:3198. doi: 10.1021/acs.orglett.9b00922. [DOI] [PubMed] [Google Scholar]
- For selected examples, see: ; (a) Yang G. Shen C. Quan M. Zhang W. Tetrahedron. 2016;72:333. [Google Scholar]; (b) Dhokale R. A. Mhaske S. B. Org. Lett. 2013;15:2218. doi: 10.1021/ol400780f. [DOI] [PubMed] [Google Scholar]; (c) Shen C. Yang G. Zhang W. Org. Lett. 2013;15:5722. doi: 10.1021/ol402748u. [DOI] [PubMed] [Google Scholar]; (d) Shen C. Yang G. Zhang W. Org. Biomol. Chem. 2012;10:3500. doi: 10.1039/c2ob25225b. [DOI] [PubMed] [Google Scholar]; (e) Feng C. Ye M. Xiao K. J. Li S. Yu J. Q. J. Am. Chem. Soc. 2013;135:9322. doi: 10.1021/ja404526x. [DOI] [PubMed] [Google Scholar]; (f) Kagayama T. Nakano A. Sakaguchi S. Ishii Y. Org. Lett. 2006;8:407. doi: 10.1021/ol052406s. [DOI] [PubMed] [Google Scholar]; (g) Wang S. Guo R. Wang G. Chen S.-Y. Yu X.-Q. Chem. Commun. 2014;50:12718. doi: 10.1039/c4cc06246a. [DOI] [PubMed] [Google Scholar]; (h) Li C. Yano T. Ishida N. Murakami M. Angew. Chem., Int. Ed. 2013;52:9801. doi: 10.1002/anie.201305202. [DOI] [PubMed] [Google Scholar]; (i) Mao X. Ma X. Zhang S. Hu H. Zhu C. Cheng Y. Eur. J. Org. Chem. 2013:4245. [Google Scholar]; (j) Zhang J. S. Chen T. Yang J. Han L. B. Chem. Commun. 2015;51:7540. doi: 10.1039/c5cc01182e. [DOI] [PubMed] [Google Scholar]; (k) Wang S. Xue Q. Guan Z. Ye Y. Lei A. ACS Catal. 2021;11(7):4295. [Google Scholar]; (l) Liu C. Ji C.-L. Zhou T. Hong X. Szostak M. Org. Lett. 2019;21:9256. doi: 10.1021/acs.orglett.9b03678. [DOI] [PubMed] [Google Scholar]; (m) Zhang J.-S. Chen T. Yang J. Han L.-B. Chem. Commun. 2015;51:7540. doi: 10.1039/c5cc01182e. [DOI] [PubMed] [Google Scholar]; (n) Chen L. Zhu Y. Chen T. Liu L. Zhang J.-S. Han L.-B. Org. Biomol. Chem. 2018;16:5090. doi: 10.1039/c8ob01299g. [DOI] [PubMed] [Google Scholar]; (o) Xu K. Liu L. Li Z. Huang T. Xiang K. Chen T. J. Org. Chem. 2020;85:14653. doi: 10.1021/acs.joc.0c01557. [DOI] [PubMed] [Google Scholar]; (p) McErlain H. Riley L. M. Sutherland A. J. Org. Chem. 2021;86:17036. doi: 10.1021/acs.joc.1c02172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected reviews, see: ; (a) Dubbaka S. R. Vogel P. Angew. Chem., Int. Ed. 2005;44:7674. doi: 10.1002/anie.200463007. [DOI] [PubMed] [Google Scholar]; (b) Wang L. He W. Yu Z. Chem. Soc. Rev. 2013;42:599. doi: 10.1039/c2cs35323g. [DOI] [PubMed] [Google Scholar]; (c) Modha S. G. Mehta V. P. Van der Eycken E. V. Chem. Soc. Rev. 2013;42:5042. doi: 10.1039/c3cs60041f. [DOI] [PubMed] [Google Scholar]; (d) Pan F. Shi Z.-J. ACS Catal. 2014;4:280. [Google Scholar]
- Miao T. Wang L. Adv. Synth. Catal. 2014;356:967. [Google Scholar]
- Yang J. Xiao J. Chen T. Yin S.-F. Han L.-B. Chem. Commun. 2016;52:12233. doi: 10.1039/c6cc06048j. [DOI] [PubMed] [Google Scholar]
- (a) Srogl J. Allred G. D. Liebeskind L. S. J. Am. Chem. Soc. 1997;119:12376. [Google Scholar]; (b) Ma N.-N. Ren J.-A. Liu X. Chu X.-Q. Rao W. Shen Z.-L. Org. Lett. 2022;24:1953. doi: 10.1021/acs.orglett.2c00357. [DOI] [PubMed] [Google Scholar]; (c) Vasu D. Yorimitsu H. Osuka A. Angew. Chem., Int. Ed. 2015;54:7162. doi: 10.1002/anie.201501992. [DOI] [PubMed] [Google Scholar]; (d) Cowper P. Jin Y. Turton M. D. Kociok-Kohn G. Lewis S. E. Angew. Chem., Int. Ed. 2016;55:2564. doi: 10.1002/anie.201510666. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kawashima H. Yanagi T. Wu C.-C. Nogi K. Yorimitsu H. Org. Lett. 2017;19:4552. doi: 10.1021/acs.orglett.7b02147. [DOI] [PubMed] [Google Scholar]; (f) Zhao J.-N. Kayumov M. Wang D.-Y. Zhang A. Org. Lett. 2019;21:7303. doi: 10.1021/acs.orglett.9b02584. [DOI] [PubMed] [Google Scholar]; (g) Wang S.-M. Song H.-X. Wang X.-Y. Liu N. Qin H.-L. Zhang C.-P. Chem. Commun. 2016;52:11893. doi: 10.1039/c6cc06089g. [DOI] [PubMed] [Google Scholar]; (h) Tian Z.-Y. Wang S.-M. Jia S.-J. Song H.-X. Zhang C.-P. Org. Lett. 2017;19:5454. doi: 10.1021/acs.orglett.7b02764. [DOI] [PubMed] [Google Scholar]; (i) Minami H. Otsuka S. Nogi K. Yorimitsu H. ACS Catal. 2018;8:579. [Google Scholar]; (j) Minami H. Nogi K. Yorimitsu H. Org. Lett. 2019;21:2518. doi: 10.1021/acs.orglett.9b00067. [DOI] [PubMed] [Google Scholar]; (k) Berger F. Plutschack M. B. Riegger J. Yu W. Speicher S. Ho M. Frank N. Ritter T. Nature. 2019;567:223. doi: 10.1038/s41586-019-0982-0. [DOI] [PubMed] [Google Scholar]; (l) Yanagi T. Somerville R. J. Nogi K. Martin R. Yorimitsu H. ACS Catal. 2020;10:2117. [Google Scholar]
- (a) Luo H. Liu H. Chen X. Wang K. Luo X. Wang K. Chem. Commun. 2017;53:956. doi: 10.1039/c6cc08408g. [DOI] [PubMed] [Google Scholar]; (b) Luo H. Sun K. Xie Q. Li X. Zhang X. Luo X. Asian J. Org. Chem. 2020;9:2083. [Google Scholar]; (c) Sun K. Liu H. Xie Q. Luo H. Chin. J. Org. Chem. 2020;40:2275. [Google Scholar]
- (a) Martin R. Buchwald S. L. Acc. Chem. Res. 2008;41:1461. doi: 10.1021/ar800036s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) DeBergh J. R. Niljianskul N. Buchwal S. L. J. Am. Chem. Soc. 2013;135:10638. doi: 10.1021/ja405949a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami H. Nogi K. Yorimitsu H. Org. Lett. 2019;21:2518. doi: 10.1021/acs.orglett.9b00067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.