Abstract
Growing evidence supports a role of the neuropeptide oxytocin in promoting social cognition and prosocial behavior, possibly via modulation of the salience of social information. The effect of intranasal oxytocin administration on the salience network, however, is not well understood, including in the aging brain. To address this research gap, 42 young (22.52 ± 3.02 years; 24 in the oxytocin group) and 43 older (71.12 ± 5.25 years; 21 in the oxytocin group) participants were randomized to either self-administer intranasal oxytocin or placebo prior to resting-state functional imaging. The salience network was identified using independent component analysis (ICA). Independent t-tests showed that individuals in the oxytocin compared to the placebo group had lower within-network resting-state functional connectivity, both for left amygdala (MNI coordinates: x = −18, y = 0, z = −15; corrected p < 0.05) within a more ventral salience network and for right insula (MNI coordinates: x = 39, y = 6, z = −6; corrected p < 0.05) within a more dorsal salience network. Age moderation analysis furthermore demonstrated that the oxytocin-reduced functional connectivity between the ventral salience network and the left amygdala was only present in older participants. These findings suggest a modulatory role of exogenous oxytocin on resting-state functional connectivity within the salience network and support age-differential effects of acute intranasal oxytocin administration on this network.
Keywords: Oxytocin, Aging, Salience network, Independent component analysis, Functional connectivity, Resting-state fMRI
1. Introduction
Originally known for its role in childbirth and lactation (Crowley and Armstrong, 1992; Erickson and Emeis, 2017), the neuropeptide oxytocin has attracted attention for its modulatory effects on social cognition (Bartz et al., 2011; Horta et al., 2019). Mainly synthesized in the hypothalamus, oxytocin travels along long axonal projections to different brain regions, such as the amygdala, the hippocampus, and the nucleus accumbens (Andreas et al., 2011), where it acts as neuromodulator (Baribeau and Anagnostou, 2015; Jurek and Neumann, 2018) on a diverse array of social capacities, including emotion identification (Horta et al., 2019), social memory (Tse et al., 2018), social perception (Yao et al., 2018a), and empathy (Geng et al., 2018; Hurlemann et al., 2010; Shamay-Tsoory, 2011). Oxytocin’s mechanisms of action in the human brain, however, are currently not well understood. Only more recently has the use of functional magnetic resonance imaging (fMRI) combined with pharmacological intervention via intranasal oxytocin administration—through which oxytocin can circumvent the blood–brain barrier (Quintana et al., 2018)—allowed the examination of oxytocin effects on the central nervous system in vivo in humans (Heinrichs and Domes, 2008). Studies utilizing this approach support oxytocin modulation in specific brain regions of interest (ROIs; Riem et al., 2011). For example, intranasal oxytocin compared to placebo resulted in lower amygdala activity during recognition of facial fear (Domes et al., 2007). Similarly, clinical research suggested that intranasal oxytocin compared to placebo attenuated brain activity in the anterior cingulate cortex and the amygdala to threatening social cues in generalized social anxiety disorder (Labuschagne et al., 2010; 2012).
In line with evidence that brain regions do not operate in isolation but in networks of interconnected nodes (van den Heuvel and Hulshoff Pol, 2010), previous research supported oxytocin-altered functional connectivity between brain regions (Dodhia et al., 2014; Ebner et al., 2016). In particular, using a task-related approach, intranasal oxytocin administration relative to placebo resulted in attenuated functional connectivity of the left globus pallidus with reward- and attachment-related regions in fathers in response to pictures of their own child (Wittfoth-Schardt et al., 2012). However, Riem et al. (2011, 2012) found enhanced functional connectivity between amygdala and orbitofrontal cortex, anterior cingulate cortex, hippocampus, precuneus, and the middle temporal sulcus in response to infant crying and laughing.
Similarly, the few extant studies on oxytocin modulation during resting-state functional connectivity generated mixed evidence. In particular, a literature synthesis suggests four broader findings supporting: (i) oxytocin-decreased coupling within the salience network; (ii) oxytocin-increased coupling within the salience network; (iii) no oxytocin modulation on within-salience-network coupling; and (iv) oxytocin-modulated coupling between the salience network and other large-scale networks (e.g., the default mode network; see Supplementary Materials Table S2 for overview). For example, in a study by Procyshyn et al. (2020), single-dose intranasal oxytocin (24 IUs; vs. placebo) decreased resting-state functional connectivity among social brain regions (amygdala, insula) in 23 healthy young adults (28.3 ± 8.8 years; see also Dodhia et al., 2014; Kumar et al., 2015 for similar findings). In contrast, Kovács and Kéri (2015) reported increased resting-state functional connectivity between the right amygdala and the dorsal anterior cingulate cortex after a single dose of intranasal oxytocin compared to placebo (35.2 ± 17.4 IUs) in 82 healthy young adults (25.55 ± 9.27 years); while, for example, Fan et al. (2014) reported no modulation by single-dose intranasal oxytocin admininstration (24 IUs; vs. placebo) on functional coupling among regions of the salience network in 18 healthy young men (27.8 ± 4.4 years) with high early life stress (see also Xin et al. (2018) for similar findings). Thus, the plurality of previous studies that directly examined regions within the salience network support oxytocin-decreased functional coupling at rest.
Of note, these previous studies examined oxytocin-modulation on functional coupling at rest limited to (mostly two) brain regions using a seed-based approach. Large-scale network approaches, in contrast, determine coupling between multiple brain regions within larger neural networks and are typically considered more robust to head motion and to variations in participants’ mental state during the resting-state scan (Buckner et al., 2013). Given oxytocin’s long projections and likely widespread impact on the brain (Meyer-Lindenberg et al., 2011), a large-scale network approach (e.g., independent component analysis; ICA) may be better suited to determine oxytocin brain modulation. Furthermore, unlike seed-based approaches that are based on a-priori assumptions, ICA is a data-driven approach that is not dependent on prior assumptions and seed-voxel choice (Marrelec and Perlbarg, 2008; Vincent et al., 2007), and offers a more stringent noise control by separating noise-related independent components (Bhaganagarapu et al., 2013; McKeown et al., 2003; Tohka et al., 2008).
In fact, so far, only four studies have examined intranasal oxytocin effects on large-scale brain networks Brodmann et al. (2017)., for example, observed reduced coupling of regions within the default mode network but increased coupling within regions of the cingulo-opercular network involved in salience processing at rest in young men after a single dose of 24 IUs intranasal oxytocin Bethlehem et al. (2017)., in contrast, reported that a single dose of 24 IUs intranasal oxytocin increased between-network resting-state functional connectivity in networks associated with social-communication, reward, and emotion processing in young women (see also Xin et al., 2018; Wu et al., 2018, for studies examining resting-state coupling between the salience network and other large-scale networks; Supplementary Materials Table S2).
In sum, research on oxytocin modulation of within-salience-network functional coupling is limited. That is particularly surprising as the salience network is implicated in enhancing detection, processing, and integration of social and emotional stimuli and associated behavior (Averbeck, 2010; Levy et al., 2016; Menon, 2015; Uddin, 2015) and regions of the salience network, including the amygdala and the insula as key nodes (Menon, 2015), emerge quite consistently as targets for oxytocin modulation (Ebner et al., 2016; Kovács and Kéri, 2015; Riem et al., 2011; Zhao et al., 2019). This combined with the growing literature that oxytocin modulates socioemotional processes (Clark-Elford et al., 2014; Kirsch, 2015; Labuschagne et al., 2012), including in aging (Horta et al., 2020) and evidence of age-related change in functional connectivity within the salience network (Horta et al., 2019), renders the examination of oxytocin modulation on salience network functional connectivity in aging particularly relevant.
Importantly, current work on the effects of intranasal oxytocin on social cognition and underlying brain processes has been almost exclusively conducted with young adults (see Ebner et al., 2013; Riem et al., 2013; 2012). Only recently has the field started to examine age-related differences and has found age and age-by-sex variations in oxytocin modulation on brain and behavior (Campbell et al., 2014; Ebner et al., 2016, 2015; Frazier et al., 2021; Gao et al., 2016; Horta et al., 2019; Luo et al., 2017; but see Grainger et al., 2019). For example, intranasal oxytocin relative to placebo improved emotion recognition (Campbell et al., 2014) and attention to feelings (Ebner et al., 2015) in an age- (and sex-) differential fashion. In addition, intranasal oxytocin increased resting-state functional connectivity between the amygdala and the mPFC, specifically in older men and young women (Ebner et al., 2016), and age-differentially affected neural response patterns, including in main nodes of the salience network (i.e., amygdala and anterior insula), during facial expression identification (Horta et al., 2019). This evidence of age-related differences in effects of intranasal oxytocin on social cognition and oxytocin modulation of nodes within the salience network combined with evidence that age is associated with altered social cognition (Ebner and Fischer, 2014) as well as altered functional connectivity within the salience network (He et al., 2014) warrants thorough examination of intranasal oxytocin administration on the salience network, including in older adults, as addressed in the present research.
The primary goal of the present analysis was to determine effects of intranasal oxytocin on functional connectivity within the salience network during rest in a sample of generally healthy young and older men and women. The study furthermore explored age-related differences in oxytocin modulation on resting-state functional connectivity within the salience network. We used the same data set as Ebner et al. (2016) for the present analysis but significantly extended this previous analysis and prior work reported in the literature by applying a large-scale network data-driven approach (i.e., ICA) to determine oxytocin modulation in young and older adults on resting-state functional connectivity within the salience network, a likely, but currently understudied, target of oxytocin’s brain action.
Based on our literature synthesis, the plurality of previous studies that directly examined regions within the salience network support oxytocin-decreased functional coupling at rest (see Supplementary Materials Table S2 for details). Therefore, we predicted that intranasal oxytocin administration would decrease functional connectivity within the salience network (Hypothesis 1). Furthermore, based on emerging evidence of age-differential effects of intranasal oxytocin, including on the brain (Horta et al., 2019; see also Ebner et al., 2013; Horta et al., 2020 for overviews), we predicted that oxytocin modulation on functional connectivity within the salience network would vary by age (Hypothesis 2).
2. Materials and methods
2.1. Participants
Data for the present analysis was collected between August 2013 and October 2014 in the Department of Psychology, Institute on Aging, and McKnight Brain Institute at the University of Florida as part of a larger project (see Ebner et al., 2016, 2015, 2019; Frazier et al., 2021; Horta et al., 2019; Lin et al., 2018; Plasencia et al., 2019; for details and published data). Participants were recruited via university participant pools, registries, and recruitment services, through fliers across campus, town, and county, and via word-of-mouth. Volunteers were screened over the phone for study eligibility. Among exclusion criteria were non-native English speaking, pregnant or breastfeeding, psychological disorder, severe or progressive medical illness, non-normal neurological history, excessive smoking or drinking, and contraindications regarding oxytocin administration and MRI. All older women were postmenopausal. Older individuals were screened for cognitive status using the Telephone Interview of Cognitive Status (scores cut off < 30; Brandt et al., 1988).
The larger project comprised 105 participants. Of those, 20 did not complete the MRI visit or their resting-state data was not acquired (e.g., due to time limitations) or had to be discarded due to technical difficulties, image corruption, or a large extent of head motion (i.e., transfer in one of the directions x, y, or z was > 2 mm; or rotation around one of the axes was > 2°; Guo et al., 2015). The final sample for analysis therefore comprised 85 participants,1 including 42 young (M = 22.52 years, SD = 3.02, range = 18–31, 52.4% males) and 43 older (M = 71.12 years, SD = 5.25, range = 63–81, 44.2% males) participants. Of those, 24 young (M = 21.88 years, SD = 2.74, range = 19–31, 54.2% males) and 21 older (M = 71.05 years, SD = 5.88, range = 63–80, 47.6% males) participants were randomly assigned to the experimental group (oxytocin administration), while 18 young (M = 23.37 years, SD = 3.25, range = 18–30, 50% males) and 22 older (M = 71.18 years, SD = 4.72, range = 63–81, 40.9% males) participants were randomly assigned to the control group (placebo administration). Seven young women were on oral contraception.
As shown in Table 1, there were no significant age-group differences in height, weight, years of education, negative affect, or self-reported physical and mental health (all ps > 0.05). In line with the literature (Biss and Hasher, 2012; Briggs et al., 1999), older compared to young participants were slower in sensorimotor processing speed (F = 85.54, p < 0.001, partial η2 = 0.51), had poorer short-term verbal learning memory (F = 11.83, p = 0.001, partial η2 = 0.13), and reported higher positive affect (F = 28.21, p < 0.001, partial η2 = 0.26). The treatment groups (oxytocin vs. placebo) did not differ in any of the demographic, cognitive, affective, and health variables (all ps > 0.05) and there were no interaction effects between age and treatment for height, weight, years of education, sensorimotor processing speed, short-term verbal learning memory, positive affect, or mental health (all ps > 0.05). However, there were significant interaction effects of age and treatment for negative affect (F = 5.55, p = 0.02, partial η2 = 0.07) and physical health (F = 2.60, p = 0.11, partial η2 = 0.03). Any variables with significant age main or interaction effects were entered as covariates in the moderation models (see below).
Table 1.
Sample descriptive data (means and standard deviations) and inference statistics (age, treatment, and age × treatment effects) for demographic, cognitive, affective, and health measures (N = 85).
| Oxytocin Young M (SD) | Older M (SD) | Placebo Young M (SD) | Older M (SD) | Inference Statistics Age | Treatment | Age × Treatment | |
|---|---|---|---|---|---|---|---|
| Demographics Height (inches) | 69.06 (3.89) | 66.90 (4.83) | 67.50 (3.40) | 67.77 (5.12) | F = 0.97, p = 0.33, ηp2 = 0.01 | F = 0.13, p = 0.72, ηp2 = 0.002 | F = 1.61, p = 0.21, ηp2 = 0.02 |
| Weight (lbs) | 152.38 (26.34) | 159.52 (34.88) | 168.17 (46.25) | 174.14 (32.86) | F = 0.74, p = 0.40, ηp2 = 0.01 | F = 3.96, p = 0.05, ηp2 = 0.05 | F = 0.01, p = 0.94, ηp2 < 0.001 |
| Education (yrs) | 15.13 (2.07) | 16.95 (3.46) | 16.44 (2.83) | 16.14 (2.66) | F = 1.57, p = 0.22, ηp2 = 0.02 | F = 0.17, p = 0.68, ηp2 = 0.002 | F = 3.09, p = 0.08, ηp2 = 0.04 |
| Cognition DSST | 63.17 (8.16) | 46.00 (7.55) | 66.83 (12.86) | 46.36 (8.63) | F = 85.54, p < 0.001, ηp2 = 0.51 | F = 0.98, p = 0.33, ηp2 = 0.01 | F = 0.66, p = 0.42, ηp2 = 0.01 |
| RAVLT | 8.96 (2.12) | 7.05 (2.42) | 9.22 (1.93) | 7.73 (2.53) | F = 11.83, p = 0.001, ηp2 = 0.13 | F = 0.91, p = 0.34, ηp2 = 0.01 | F = 0.18, p = 0.68, ηp2 = 0.002 |
| Affect (PANAS) Positive | 27.29 (6.44) | 32.86 (5.27) | 26.83 (7.32) | 35.68 (5.84) | F = 28.21, p < 0.001, ηp2 = 0.26 | F = 0.76, p = 0.39, ηp2 = 0.01 | F = 1.46, p = 0.23, ηp2 = 0.02 |
| Negative | 11.33 (1.55) | 13.00 (3.83) | 12.78 (3.14) | 11.62 (2.01) | F = 0.18, p = 0.67, ηp2 = 0.002 | F = 0.003, p = 0.96, ηp2 < 0.001 | F = 5.55, p = 0.02, ηp2 = 0.07 |
| Health Physical | 8.71 (1.04) | 8.43 (1.08) | 8.17 (1.34) | 8.68 (1.09) | F = 0.23, p = 0.63, ηp2 = 0.003 | F = 0.34, p = 0.56, ηp2 = 0.004 | F = 2.60, p = 0.01, ηp2 = 0.03 |
| Mental | 8.50 (1.38) | 8.67 (1.53) | 8.39 (1.14) | 9.09 (0.92) | F = 2.46, p = 0.12, ηp2 = 0.03 | F = 0.32, p = 0.57, ηp2 = 0.004 | F = 0.93, p = 0.34, ηp2 = 0.01 |
| Plasma Oxytocin | 677.69 (255.00) | 647.99 (218.28) | 694.80 (174.02) | 629.87 (181.17) | F = 0.98, p = 0.33, ηp2 = 0.01 | F = 0.009, p = 0.93, ηp2 < 0.001 | F = 0.14, p = 0.71, ηp2 = 0.002 |
Note: Sensorimotor processing speed was measured by total items correct in the Digit Symbol Substitution Test (DSST; score range = 34 – 93; Wechsler, 1981). Short-term verbal learning memory was measured by total items correct in the Rey Auditory Verbal Learning Test (RAVLT; score range = 4 – 14; Rey, 1964). Present mood was measured by the Positive Affect Negative Affect Schedule (PANAS; score range of Positive Affect = 16 – 46, score range of Negative Affect = 10 – 20; Watson et al., 1988); Physical (score range = 5 – 10) and mental (score range = 3 – 10) health were assessed with single items on a scale from 1 = poor to 10 = excellent. M = Mean, SD = Standard deviation, ηp2 = partial eta-squared; Bold indicates significant effect at p < 0.05.
2.2. Procedure
The larger clinical trial that this data analysis was embedded in was registered under NCT01823146. The Institutional Review Board at the University of Florida approved the study protocol and all participants provided written informed consent. The study adopted a randomized, double-blind, placebo-controlled, between-subject design and followed standardized procedures for intranasal oxytocin self-administration (Guastella et al., 2013). The placebo contained identical ingredients except the oxytocin. The principal investigator worked directly with the pharmacist on coordinating prescriptions and dispensing of the intranasal spray. The pharmacist assigned the treatment conditions. Neither the pharmacist nor the principal investigator directly interacted with any of the participants. All study personnel involved in recruitment and scheduling and who engaged directly with study participants, and the participants themselves, were blinded to the treatment condition. No consistent adverse side effects were reported.
In the following, only measures with direct relevance to the present data analysis are reported in detail (for more information about the larger project see Ebner et al., 2016, 2015, 2019; Frazier et al., 2021; Horta et al., 2019; Lin et al., 2018; Plasencia et al., 2019). In particular, the study included a phone prescreening to assure general study eligibility and safety, followed by a screening visit on campus to obtain consent and collect urine and blood (primarily for pre-screening purposes to assure acceptable sodium and osmolality levels before spray administration) as well as assess brief demographic, cognitive, and health measures (see Table 1). Two to ten days later, participants returned to campus to complete a brief measure on current affect (see Table 1) and to self-administer a single dose (24 IUs; only one puff per nostril was needed with the highly concentrated formulation used in this study (as per IND# 100,860) to administer the targeted dose of 24 IUs) of either oxytocin or placebo via nasal spray. Promptly after oxytocin (or placebo) administration, participants were transported to the imaging facility and settled into the MRI scanner and T1-weighted anatomical images were acquired. This was followed by task-evoked functional image acquisition (a social decision-making task and two face processing tasks; Frazier et al., 2021; Horta et al., 2019; Lin et al., 2018 under review), and the resting-state functional scan (see also Ebner et al., 2016).
During the resting-state scan, which took place 70–90 min after spray administration, participants were asked to relax and look at a white fixation cross on a black screen. The procedure was standardized for all participants and thus should not have influenced the group (treatment or age) differentially. Previous work also did multiple MRI scans before a resting-BOLD sequence (Sripada et al., 2013; Weng et al., 2010). Moreover, previous studies demonstrated that the effects of intranasal oxytocin could last for more than 90 min (Daughters et al., 2015; Gossen et al., 2012), thus within our assessment window. The session concluded with a test battery covering various socioemotional measures, followed by debriefing and reimbursement. For both campus visits, participants were instructed to stay hydrated as well as avoid food, exercise, and sexual activity for 2 h and smoking, caffeine, alcohol, and recreational drugs for 24 h before the visit. The visits began in the morning, usually around 9:00 a.m.
2.3. MRI acquisition
Brain images were acquired on a 3T Philips Achieva MR Scanner (Philips Medical Systems, Best, The Netherlands). A 32-channel head coil was used with foam padding to reduce head motion. High-resolution T1-weighted anatomical images were acquired using a magnetization-prepared rapid gradient echo (MP-RAGE) sequence (sagittal slice orientation, FOV = 240 mm × 240 mm × 240 mm, in plane resolution = 1 mm × 1 mm, slice thickness = 1 mm without skip). Resting-state functional images were acquired using a gradient-echo-planar imaging (EPI) sequence with a total of 38 interleaved slices (TR = 2 s, TE = 30 ms, FOV = 252 mm × 252 mm × 133 mm, flip angle = 90°, transverse slice orientation, in plane resolution = 3.15×3.15 mm, slice thickness = 3.5 mm without skip). Two-hundred and forty functional images were acquired during the 8-min resting-state scan.
2.4. MRI data preprocessing
Resting-state MRI images were preprocessed using CONN functional connectivity toolbox (v.18.b; Whitfield-Gabrieli and Nieto-Castanon, 2012) in conjunction with SPM12 software (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) on MATLAB R2018a (MathWorks Inc., Natick, MA, USA). All preprocessing steps were conducted using the default preprocessing pipeline for volume-based analysis (to MNI space; Montreal Neurological Institute, Canada). Functional image preprocessing included realignment, outlier detection (head motion > 0.5 mm), unwrapping, slice-timing correction (interleaved bottom-up), coregistration with structural data, spatial normalization into MNI space, and smoothing using a Gaussian kernel of 8 mm full-width at half-maximum (FWHM). Following the literature (Hoekzema et al., 2014; Meier et al., 2012), we did not remove the first few volumes of the resting-state data; we also did not band-pass filter or apply preprocessing on the time courses for each of the independent components (ICs), to avoid removing any information that the ICA approach may use to separate the ICs (Rachakonda et al., 2007).
2.5. Voxel-based morphometry (VBM)
As age has been associated with gray matter volume reduction (Good et al., 2001; Jernigan et al., 2001; Tisserand et al., 2002), we measured whole-brain gray matter volume in our sample. We preprocessed structural MRI images using SPM8 software (https://www.fil.ion.ucl.ac.uk/spm/) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) following the standard preprocessing pipeline (Liu and Feng, 2017). Whole-brain gray matter volume was calculated using the MATLAB script “get_totals” provided by Ridgway (http://www0.cs.ucl.ac.uk/staff/gridgway/vbm/get_totals.m). Young participants (M = 625.99 ml, SD = 60.28 ml) had greater whole-brain gray matter volume than older participants (M = 571.34 ml, SD = 61.11 ml; F = 16.98, p < 0.001, partial η2 = 0.17). Therefore, whole-brain gray matter volume was entered as covariate in the age moderation models (see below).
2.6. Independent component analysis (ICA)
We utilized the ICA protocol developed by Calhoun and colleagues (Calhoun et al., 2001, 2009) and widely used in the field (Assaf et al., 2010; Calhoun and Adali, 2012; De Luca et al., 2006). ICA has several methodological advantages over ROI-based or other functional connectivity methods. For example, it allows for control of motion artifacts by considering head motion in a separate component, and thus reduces data loss (Uddin et al., 2010). It also avoids bias from seed-voxel choice in traditional functional connectivity methods (Marrelec and Perlbarg, 2008).
The protocol comprises two phases: (i) component identification and (ii) component selection. These are described next as they applied to the present data analysis.
Component identification.
First, group spatial ICA was conducted across all 85 participants using Group ICA of the fMRI Toolbox (GIFT; http://icatb.sourcefttkorge.net/, version v4.0b; (Calhoun et al., 2009). After subject-wise data concatenation in time, ICA was performed in four steps: (1) Principal component analysis (PCA) was used to reduce the size or dimensionality of the fMRI data across all participants. (2) Maximally independent components were estimated using ICA infomax algorithm (Bell and Sejnowski, 1995) and the data were decomposed into 38 independent components (as determined by the minimum description length (MDL) criterion applied in the current data; Rissanen, 1983). (3) Back reconstruction was conducted for each individual participant’s data to generate time courses and spatial maps. Finally, (4) a spatial map for each IC across all participants was computed (Z-scores) and submitted to a one-sample T test using a threshold of p < 0.01, false discovery rate (FDR) corrected, to obtain the significant sample-specific spatial maps. The transformed Fisher’s Z-score value of each voxel in these IC spatial maps reflected the fit (i.e., degree of correlation) of the blood-oxygen-level-dependent (BOLD) signal time course from a given voxel with the average BOLD time course across all voxels within this IC (Greicius et al., 2007).
Component selection.
The selection of the salience network followed four steps: (1) Visual inspection of each of the 38 estimated ICs resulted in selection of a subset of ICs whose patterns of correlated signal change were largely constrained to gray matter (Naveau et al., 2012). We computed correlations (element-wise multiplication) between each component’s sample-specific IC map (converted to a binary mask) and a-priori binary mask maps of gray matter, white matter, and cerebrospinal fluid as provided by the WFU Pickatlas (Maldjian et al., 2003; http://fmri.wfubmgc.edu/cms/software). We dropped ICs with high spatial correlations to white matter or cerebrospinal fluid (top 10% of ICs were dropped). We also dropped ICs with low spatial correlations to gray matter masks (bottom 10% were dropped). This step left us with 30 ICs. (2) We repeated the Infomax ICA algorithm (Bell and Sejnowski, 1995) 20 times in ICASSO 3 to test the reliability of the signal decomposition (Himberg et al., 2004). The quality of IC clusters was quantified using the index Iq, which ranges from 0 to 1 and reflects the difference between intra-cluster and extra-cluster similarity (Himberg et al., 2004). All ICs (except IC #38) had a cluster quality index greater than 0.8, indicating stable ICA decomposition. (3) Of the remaining 29 ICs, 13 ICs were dropped based on visual inspection suggesting that they represented eye movements, head motion, or a cardiac-induced pulsatile artifact at the brain base. (4) Among the remaining 16 ICs (see Supplementary Materials, Fig. S1), we selected those representing the salience network using spatial correlation (r > 0.1, small effect size) to a set of a-priori defined network templates provided by GIFT (Chen et al., 2010; Li et al., 2017; Shi et al., 2018; Ye et al., 2014). In the present analysis, a threshold of r > 0.1 was used following Cohen’s convention that a correlation coefficient of 0.1 represents the minimum value of a small effect size (Chen et al., 2010). This same threshold convention was applied in other papers that used a comparable approach to ours (see Li et al., 2017; Shi et al., 2018; Ye et al., 2014).
This four-step component selection process resulted in two relevant ICs (IC #4, presented in Fig. 1, Panel A; and IC #11, presented in Fig. 1, Panel B). In line with the literature (Menon, 2015; Peters et al., 2016; Taylor et al., 2009), both ICs comprised the main nodes of the salience network, including the insula, anterior cingulate cortex, and inferior frontal gyrus; IC #4 additionally included the amygdala, hippocampus, superior temporal gyrus, and thalamus; IC #11 additionally included the supplementary motor area and supramarginal gyrus. Preclinical work has suggested a functional dissociation of the salience network into a more ventral sub-network and a more dorsal sub-network (Touroutoglou et al., 2012). The ventral salience network includes the right ventral anterior insula (MNI coordinates: x = 28, y = 17, z = −15) as key node and has been shown to be involved in processing of salience during intense affective experience (Touroutoglou et al., 2012). The dorsal salience network, in contrast, includes the right dorsal anterior insula (MNI coordinates: x = 36, y = 21, z = 1) as key node and has been shown to be involved in the processing of salience during tasks unrelated to emotion (Chand et al., 2017; Touroutoglou et al., 2012). Our data supported this functional differentiation of two salience sub-networks, in that we identified a more ventral salience network (IC #4) which included the ventral insula, the amygdala, and the anterior cingulate cortex; in addition to a more dorsal salience network (IC #11) which included the dorsal insula, the anterior cingulate cortex, and the supplementary motor area.
Fig. 1.
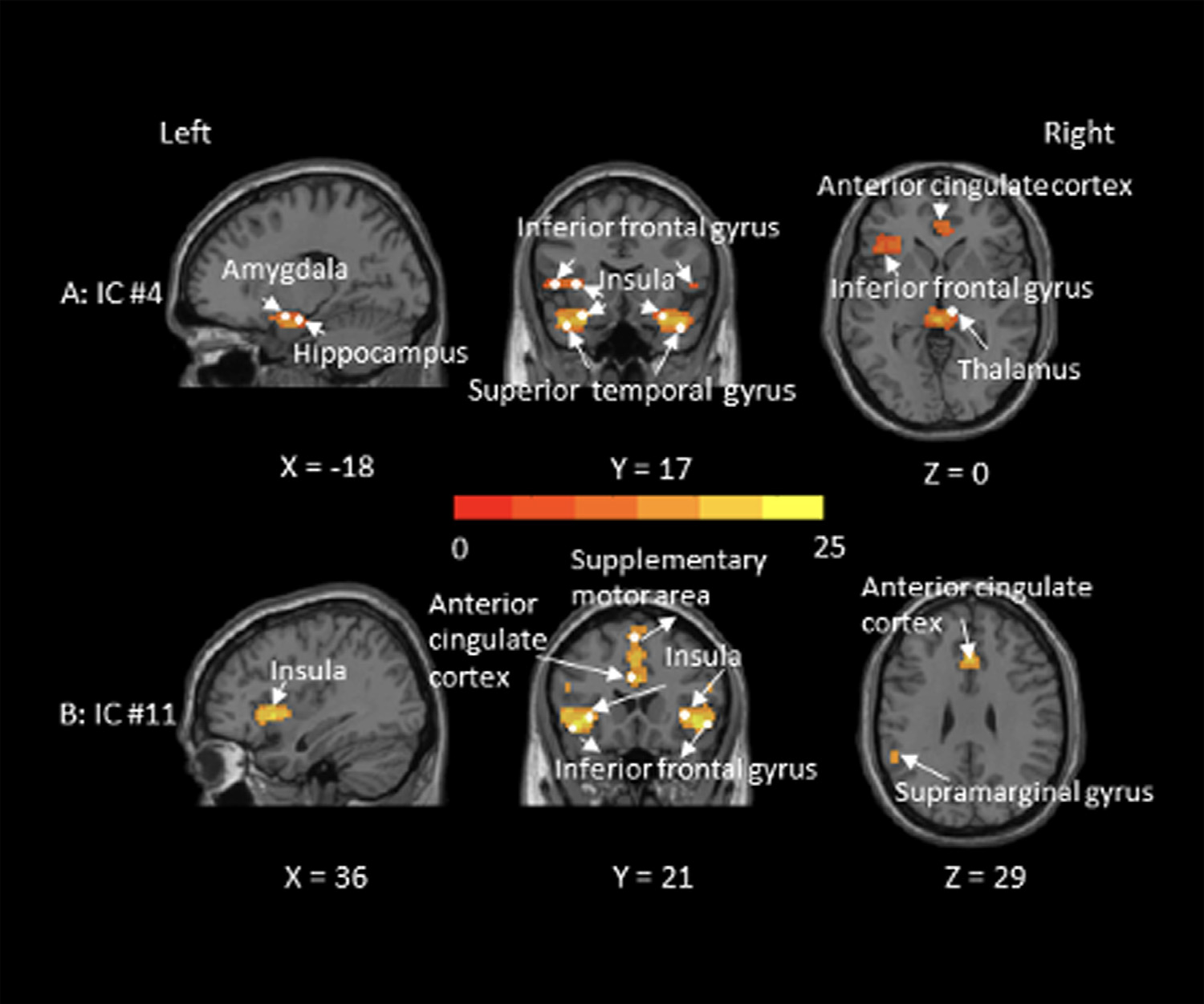
Graphical depiction of the salience network (IC #4 in Panel A and IC #11 in Panel B identified in the present sample (N = 85). Color bar represents t-values (one sample t-test that compares the functional connectivity of each voxel within the salience network against zero). MNI coordinates (x–z) are depicted.
2.7. Statistical analysis
The individual spatial maps of IC #4 and IC #11 served as the two outcome variables, representing resting-state functional connectivity between each voxel within the salience network and the whole salience network. To test Hypothesis 1, we compared the treatment groups (oxytocin vs. placebo) on these two individual maps (IC #4 and IC #11) in two separate second-level General Linear Models (GLMs; independent t-tests) in SPM 12. Connectivity was computed in GIFT as the signal synchronization or coherency pattern of each voxel to the network this voxel belonged to (Calhoun et al., 2009). Two strategies were applied for multiple comparison correction: First, based on previous evidence that the amygdala and the insula constitute key targets of oxytocin effects (i.e., a-priori prediction of ROIs; Bethlehem et al., 2017; Ebner et al., 2016), we applied small volume correction based on a spherical mask with 6 mm radius (or cluster) surrounding the peak voxel (defined by the WFU PickAtlas; Gougelet et al., 2018; Schmitz and Johnson, 2006): for amygdala the peak MNI coordinates were x= ±20, y = 0, z = −8 (Kirsch, 2005) and for insula the peak MNI coordinates were x = ±30, y = 14, z = −2 (Rilling et al., 2014). Second, to reveal unpredicted effects in brain regions other than the amygdala and the insula, we applied p < 0.05 with family-wise error rate (FWE) correction across all voxels within the salience network. We used xjView (http://www.alivelearn.net/xjview) to visualize the results.
Hypothesis 2 predicted an age moderation in the effect of intranasal oxytocin on resting-state functional connectivity within the salience network. To test this hypothesis, we extracted the resting-state functional connectivity value from the spherical ROIs (radius = 6 mm) around the peak voxel of the cluster that showed significant treatment effects under Hypothesis 1 (oxytocin > placebo or oxytocin < placebo), for IC #4 and IC #11, respectively. These extracted parameter estimates represented resting-state functional connectivity within the given networks IC #4 and IC #11 respectively and served as outcome variables for the two separate moderation models; with treatment (oxytocin vs. placebo) as independent variable and age (young vs. older) as moderator (Preacher et al., 2007; model number = 1, confidence interval = 95%, number of bootstrap sample = 5000). The analyses reported here were secondary in nature and not pre-registered.
2.8. Data availability
The data and code can be found at GitHub and OpenNeuro (upon publication of the manuscript).
3. Results
3.1. Treatment effect on resting-state functional connectivity within the salience network
For IC #4, the oxytocin compared to the placebo group showed less functional coupling between the left amygdala (peak MNI coordinates: x = −18, y = 0, z = −15, peak t value = 3.2; corrected p = 0.02, number of voxels = 38) and the salience network; no region showed larger functional coupling in the oxytocin than the placebo group. Similarly, for IC #11, the oxytocin compared to the placebo group showed less functional coupling between the right insula (peak MNI coordinate: x = 33, y = 9, z = 0, peak t value = 3.5; corrected p = 0.003, number of voxels = 31) and the salience network; again, no region showed larger functional coupling in the oxytocin than the placebo group. The results2 supported Hypothesis 1 that intranasal oxytocin (relative to placebo) reduced resting-state functional connectivity between the salience network and the amygdala and the insula, respectively, as its key notes.
3.2. Age moderation of treatment effect on resting-state functional connectivity within the salience network
For IC #4, the age moderation was significant (t (81) = −2.01, p = 0.048, confidence interval = −2.38 – −0.01, Cohen’s f = 0.22, BFinclusion (treatment × age interaction) = 5.47; see Fig. 2, Panel A): older participants in the oxytocin group showed less resting-state functional connectivity between the salience network and the left amygdala than older participants in the placebo group (t (41) = −3.76, p = 0.0003, confidence interval = −2.39 – −0.74, Cohen’s d = 1.03), while there was no treatment group difference among young participants (t (40)= −0.87, p = 0.39, confidence interval = −1.22 – 0.48, Cohen’s d = 0.32). These results partially supported Hypothesis 2 that the effects of intranasal oxytocin on resting-state functional connectivity within the salience network (i.e., coupling between the salience network and the left amygdala) varied by age. For IC #11, however, age did not moderate the effect of treatment on resting-state functional connectivity of the salience network and the right insula (t (81) = 0.28, p = 0.78, confidence interval = −0.48 – 0.63, Cohen’s f = 0.03, BFinclusion (treatment × age interaction) = 0.69; see Fig. 2, Panel B): both young (t (41)= −2.19, p = 0.01, confidence interval = −0.82 – −0.04, Cohen’s d = 0.92) and older (t (40) = −2.55, p = 0.03, confidence interval = −0.90 – −0.11, Cohen’s d = 0.59) participants in the oxytocin group showed less resting-state functional connectivity between the salience network and the right insula than young and older participants in the placebo group.3 Further corroborating these findings, Bayesian ANOVA was conducted and the result supported these findings.4
Fig. 2.
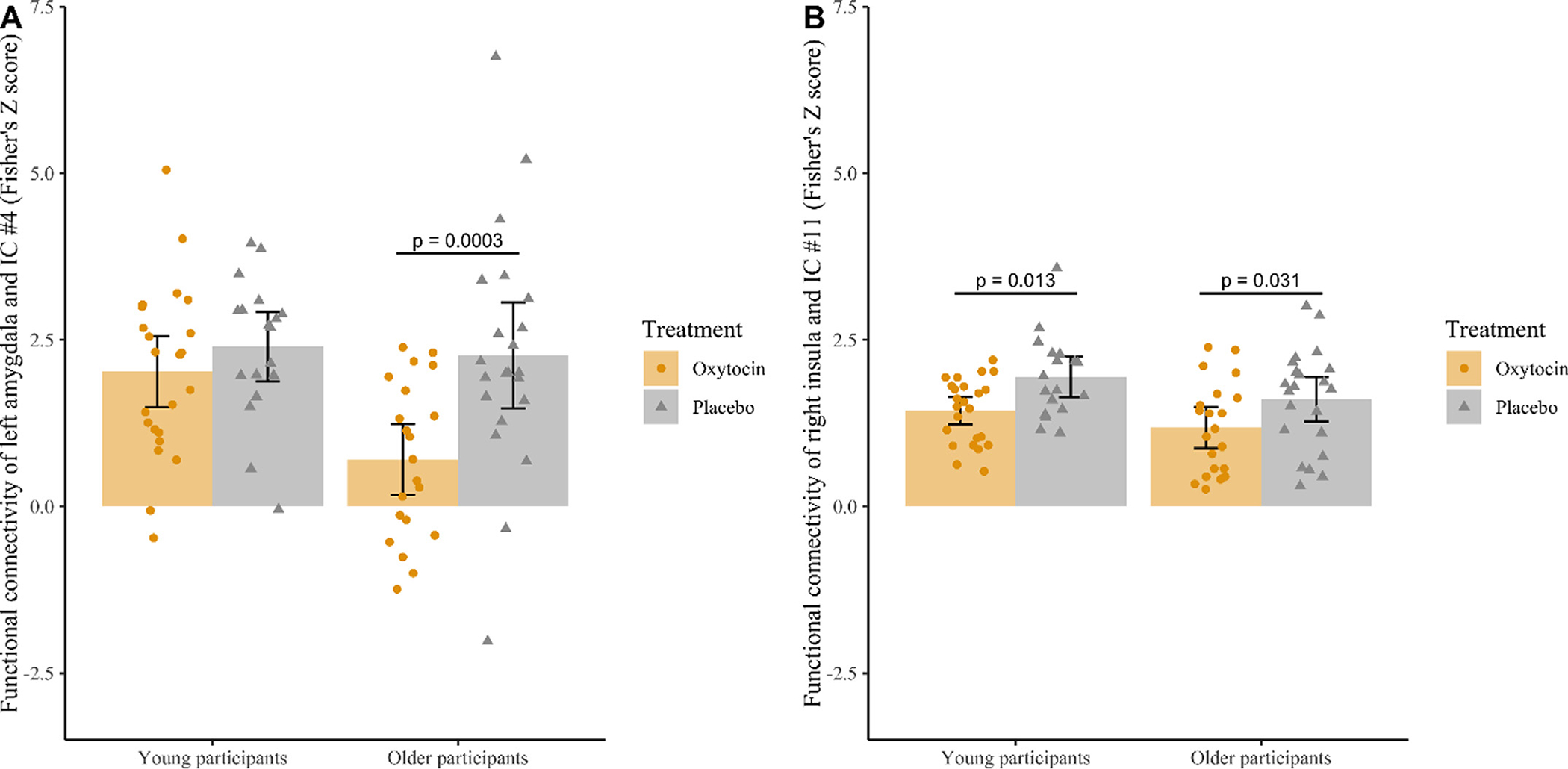
Age moderation of oxytocin’s effect on resting-state functional connectivity between IC #4 (Panel A) and the left amygdala and IC #11 (Panel B) and the right insula. Each circle/triangle represents a participant. Error bars represent the 95% confidence interval.
3.3. Exploratory analysis of treatment effect and age moderation of treatment effect on resting-state functional connectivity within the default mode network
The literature has identified the default mode network as another large-scale network relevant regarding oxytocin’s action in the brain (Brodmann et al., 2017; Wu et al., 2018; 2022). We therefore explored the treatment effect and its age modulation on resting-state functional connectivity within the default mode network by conducting parallel analyses to those described for the salience network. Results from these analyses showed oxytocin-reduced coupling between the right angular gyrus (MNI coordinates: x = 33, y = −66, z = 36; uncorrected p < 0.001) and the default mode network (IC #26); with the age modulation of this effect not significant (p = 0.29; see Fig. S4 in the Supplementary Materials for details). This finding is in line with work suggesting the temporoparietal junction (TPJ; Wu et al., 2018) and the superior temporal gyrus (STG; see Grace et al., 2018; for a meta-analysis) as target areas for oxytocin mechanisms of action in the brain.
4. Discussion
Utilizing ICA for a data-driven large-scale network approach, the present study, for the first time, demonstrated a modulatory role of intranasal oxytocin on resting-state functional connectivity within the salience network in a sample of healthy young and older adults. Additionally, we found an age moderation of these brain-modulatory effects of intranasal oxytocin on resting-state salience network coupling. These novel findings are discussed next.
4.1. Intranasal oxytocin administration reduced resting-state functional connectivity within the salience network
In support of Hypothesis 1, intranasal oxytocin (compared to placebo) resulted in reduced functional connectivity at rest within the salience network. In particular, we found lower functional connectivity for both the left amygdala (IC #4) and the right insula (IC #11) with the salience network in the oxytocin relative to the placebo group. Both the amygdala and the insula are involved in novel and emotional stimulus detection (Blackford et al., 2010; LeDoux, 1995) – processes essential for social-cognitive and affective tasks like empathy and interoception (Craig, 2009; Critchley et al., 2013). The present study’s result of attenuated crosstalk for both the amygdala and the insula with the rest of the salience network after intranasal oxytocin aligns with this previous evidence of amygdala and insula involvement in stimulus salience as well as oxytocin-reduced amygdala and insula response to (particularly negative) emotional information (Kumar et al., 2015; Labuschagne et al., 2010; I. 2012). These findings are also in accord with a two-level model regarding oxytocin modulation of social behavior and cognition put forth by Quintana et al. (2015). This model conceptualizes oxytocin as both an “anxiety reducer” (bottom-up level of processing) and a “salience information enhancer” (top-down level of processing) and builds on evidence of oxytocin effects on activation and functional connectivity of the amygdala, as a key region in anxiety and information salience (Bethlehem et al., 2013; Grace et al., 2018).
Quintana and Guastella (2020) furthermore recently proposed an allostatic theory of oxytocin. This theory describes the role of oxytocin as allostatic, in facilitating adaptation, consolidation, and stability in response to changing environments. In support of this account, intranasal oxytocin has for example been found to facilitate the rapid adaptation to fear signals (Eckstein et al., 2016). Our findings of oxytocin-reduced resting-state functional connectivity within the salience network could be reflective of this allostatic property of oxytocin and inform its function from a large-scale network perspective, highlighting oxytocin’s brain-modulatory potential for regulating stimulus sensitivity and/or allostatic approach/avoidance motivation.
Previous studies, including from our own group, suggested oxytocin-increased resting-state functional connectivity between the amygdala and the mPFC (Ebner et al., 2016; Sripada et al., 2013). The mPFC constitutes a key node in the default mode network (e.g., self-referential processing; D’Argembeau et al., 2007; Di Simplicio et al., 2012; Kurczek et al., 2015), while the amygdala and the insula constitute key nodes in the salience network (e.g., recognition and understanding of others’ emotions; Luo et al., 2014; Menon, 2015). Our previous finding in Ebner et al. (2016) of oxytocin-enhanced resting-state functional connectivity between the mPFC and the amygdala may reflect increased communication between these two large-scale (default and salience) networks, i.e., enhanced between-network connectivity. In contrast, the present paper’s finding reflects decreased within-network connectivity after intranasal oxytocin relative to placebo administration.
That is, oxytocin may modulate both coupling within the salience network as well as coupling between individual key salience mode nodes (i.e., amygdala and insula) with regions outside the salience network (e.g., the mPFC) involved in complex social and emotional processing (Etkin et al., 2011). It is possible that intranasal oxytocin exert its functions through two possible routes: (1) by reducing sensitivity to external negative social stimuli via decreasing functional connectivity within the salience network (a route supported by the present study’s findings); and/or (2) by enhancing “top-down” modulation of emotional processing via increasing functional connectivity between brain regions outside the salience network such as the mPFC (a route supported by findings from Ebner et al., 2016). Future work is warranted to follow up on these differential functional routes of oxytocin action in the brain beyond the data available in the present study and Ebner et al. (2016; see also Procyshyn et al. (2020), which cannot be directly compared given that different research questions were addressed and different methodology was applied).
In contrast to our findings, Xin et al. (2018; see also Fan et al., 2014) did not find an effect of exogenous oxytocin on functional coupling within the salience network. In their study, Xin et al. used a single dose of 40 IUs intranasal oxytocin in young adults. The dose in the current study was lower (24 IUs), and we examined an age-heterogeneous sample. Again, methodological differences across studies may have contributed to divergent findings.
Our data support the notion of a division between two major neural systems within the salience network (IC #4: ventral salience network; IC #11: dorsal salience network) in line with preclinical work (Touroutoglou et al., 2012): a ventral system relevant for “hot” emotion-laden processing and a dorsal system relevant for “cold” executive control processing (Iordan et al., 2013). Our finding of oxytocin-reduced functional connectivity within both the ventral and the dorsal salience sub-networks suggests that oxytocin may impact stimulus salience both during “hot” and “cold” processing, in line with previous work of oxytocin’s effects on stimulus salience for both emotional and non-emotional information (Domes et al., 2007; Labuschagne et al., 2010). However, age-differential oxytocin modulation in our study varied for the two salience sub-networks, as discussed next.
4.2. Age moderated intranasal oxytocin effect on resting-state functional connectivity within the salience network
In support of Hypothesis 2, age moderated the oxytocin-modulation on functional connectivity between the more ventral salience network (IC #4) and the amygdala, while no age moderation was observed for oxytocin-modulation on functional connectivity between the more dorsal salience network (IC #11) and the insula. Given evidence that the ventral salience network is recruited during emotional salience processing, while the dorsal salience network is recruited during non-emotional salience processing (Touroutoglou et al., 2012), our finding supports the idea that aging may differentially influence oxytocin’s impact on affective vs. non-affective salience processing. This pattern of findings also generally aligns with the literature suggesting that age-related differences in information processing are more pronounced for emotional than neutral stimuli, possibly due to older adults’ greater focus on, and their reduced attentional shifting away from, emotional (and specifically negatively valenced) cues (Carstensen and DeLiema, 2018; Comblain et al., 2004; Mather and Carstensen, 2005; Reed et al., 2014; Yao et al., 2018b). The differences between young and older adults in oxytocin’s large-scale brain-modulation observed in the present study are also in line with previous results reported by our group of age-differential recruitment of amygdala networks during dynamic face expression identification (Horta et al., 2019).
There are various possible explanations for the observed age-differential pattern. For example, research suggests that the estrogen/androgen ratio interacts with the oxytocin system by increasing oxytocin receptor expressivity (Bale and Dorsa, 1997; Vasudevan et al., 2001; see also Ebner et al., 2016, 2015 for a discussion). This coupled with age-related effects on hormone concentrations (Gooren, 2003; Sherwin, 2006; e.g., relatively lower levels of androgens among older than young adults affecting the estrogen/androgen ratio) may underlie age variations in oxytocin’s brain-modulatory effects. Future research will benefit from systematic examination of the processes in age-differential functions of oxytocin, including the role of gonadal hormone levels.
In addition, compared to young adults, older adults may respond more strongly to intranasal oxytocin given their generally lower levels of androgens (Gooren, 2003; Sherwin, 2006), which may be associated with increased oxytocin receptor expressivity (Bale and Dorsa, 1997; Li et al., 2018; Vasudevan et al., 2001). It is also possible that, similar to older adults’ reduced facial muscle activity that may limit their ability to regulate facial muscle reactivity using expressive suppression (Labuschagne et al., 2020), oxytocin-reduced within resting-state functional connectivity of the ventral salience network in older adults is a reflection of accommodation to an already reduced neurobiological response in aging.
Furthermore, the observed age-related differences of oxytocin effects on functional connectivity within the salience network can be interpreted in the context of Quintana and Guastella’s (2020) allostatic theory of oxytocin, which emphasizes oxytocin’s social effects as serving an allostatic function, adjusting cognition in reference to homeostatic demands. In particular, oxytocin-reduced resting-state functional coupling between the amygdala and the salience network in older (but not young) adults may be reflective of an age-related shift in primary motivations and emotion processing strategies, robustly demonstrated in the literature, that renders older adults particularly sensitive to social/emotional information (Carstensen and DeLiema, 2018).
4.3. Limitations
The present study had some limitations. First, three task-evoked functional scans preceded the resting-state scan in this study (see Frazier et al., 2021; Horta et al., 2019; Lin et al., under review). This same standardized sequence was applied to all participants and previous oxytocin studies used comparable protocols with multiple MRI scans before the resting-BOLD sequence (e.g., Brodmann et al., 2017; Dodhia et al., 2014). Moreover, it has been previously demonstrated (in young adults) that intranasal oxytocin effects could last for more than 90 min (Daughters et al., 2015; Gossen et al., 2012), thus within our assessment window. However, we acknowledge that engagement in prior scans could have impacted brain activity during the resting-state scan and future work is warranted in which the resting-state scan is administered at first and more in line with the peak of oxytocin’s effects on the hemodynamic response (see Striepens et al., 2013) to exclude this possibility. Future work will also need to specifically determine age-related differences in the pharmacodynamics of oxytocin action. For example, it is possible that the time course of oxytocin action differs with age and the age-group differences observed in our study could be due to differences in rate of effect decay or due to differences in peak effect.
Furthermore, given the growing literature about sex-dimorphic effects of oxytocin on brain activity (Domes et al., 2010; Ebner et al., 2016; Rilling et al., 2014) and social function (Campbell et al., 2014; Grainger et al., 2018; Luo et al., 2017; Reed et al., 2019), future studies will benefit from larger sample sizes that allow for systematic comparison between females and males among young and older adults (see Leppanen et al., 2017, for sample size guidelines in this field of investigation). Our sample size was based on sample sizes used in comparable studies in the field of aging neuroscience (e.g., Bach et al., 2021; Chaby et al., 2015; Leppanen et al., 2017; Noh and Isaacowitz, 2013) and intranasal oxytocin research (Barraza et al., 2013; Fan et al., 2014; Riem et al., 2014) at the time when this study was conducted (between 2013 and 2014). After completion of our data collection, a meta-analysis was published supporting a sample size of 64 participants in each group for between-subjects designs for a power of 0.80 to detect significant effects of oxytocin administration on social cognition (Leppanen et al., 2017). Future research with larger sample sizes will also be able to address key issues regarding low statistical power and unsuccessful replication in research on the effects of intranasal oxytocin (Mierop et al., 2020; Walum et al., 2016).
Finally, moving forward, direct assessment of brain–behavior relationships pertaining to intranasal oxytocin in young and older adults will be fruitful such as by adding a social task to the study design. This extension of the current work will increase knowledge about oxytocin’s impact on the interplay between brain and behavior in social contexts in adults of different ages.
5. Conclusion
The present study advances understanding of oxytocin effects on the salience network and, for the first time, delineates these brain-modulatory effects in young and older adults. We found that a single-dose of intranasal oxytocin decreased resting-state functional connectivity within the salience network. More specifically, oxytocin altered coupling for left amygdala with a more ventral salience network and coupling for right insula with a more dorsal salience network, in line with a ventral–dorsal functional dissociation of the salience network. Furthermore, the effects of intranasal oxytocin on functional coupling between the left amygdala and the ventral salience network differed by age, with within-salience-network connectivity reduced for older but not young adults after oxytocin self-administration.
The observed modulatory role of exogenous oxytocin on resting-state salience network connectivity may constitute a neural mechanism underlying oxytocin’s effect on prosocial behavior (Singer et al., 2008; Theodoridou et al., 2009). Age-related differences in this large-scale brain-modulation via oxytocin could contribute to age-related differences of oxytocin’s effects on social-cognitive and socioemotional information processing and related behaviors (e.g., empathy, trust; Bailey et al., 2015; Sutter and Kocher, 2007; Sze et al., 2012). Moving forward, it will be informative to determine behavioral impact as well as delineate the role of intranasal oxytocin on resting-state functional connectivity in other large-scale brain networks (e.g., default mode network, executive control network) and to examine effects on coupling between large-scale brain network (e.g., salience network–default mode network coupling; Dixon et al., 2017) in aging.
Supplementary Material
Acknowledgements
This work was supported by the University of Florida Clinical and Translational Science pilot award (NIH/NCATS, UL1 TR000064); the Scientific Research Network on Decision Neuroscience and Aging pilot award (NIH/NIA, R24 AG039350); the NIH/NIA R01AG059809; the University of Florida Psychology Department; the National Science Foundation Division of Materials Research (DMR-1157490); the Center for Cognitive Aging and Memory; and the Claude D. Pepper Older Americans Independence Center. A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and the State of Florida. The authors thank Gaby Maura and Tammy Nicholson for data collection.
Footnotes
Declaration of Competing Interest
The authors declare no competing financial interests.
Credit authorship contribution statement
Peiwei Liu: Conceptualization, Methodology, Software, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Visualization, Funding acquisition. Tian Lin: Conceptualization, Methodology, Writing – review & editing. David Feifel: Writing – review & editing. Natalie C. Ebner: Conceptualization, Methodology, Investigation, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.neuroimage.2022.119045.
Note that both this paper and Ebner et al. (2016) were based on the same original dataset. However, the present paper applied slightly different criteria for image quality assurance and removal of images with excessive head motion than the Ebner et al. (2016) paper. This was the case because in the present paper we used ICA, which offers a more stringent noise control by separating noise-related independent components and thus allowed use of more liberal criteria during preprocessing (Bhaganagarapu et al., 2013; McKeown et al., 2003; Tohka et al., 2008).
These results survived Bonferroni correction for two separate GLMs (IC #4 and IC #11; corrected ps < 0.025).
Results were comparable after (i) removing outliers, i.e., values above/below 2.5 SDs of the grand mean of the whole dataset; (ii) removing the seven young women on oral contraception; and (iii) controlling for sensorimotor processing speed, short-term verbal learning memory, positive affect, negative affect, sex, menstrual cycle, whole-brain gray matter volume, physical health in individual covariate analyses.
Bayesian ANOVA showed substantial support in favor of including the treatment × age interaction in the model on functional connectivity between the salience network and the amygdala, compared to models without inclusion of this interaction term (BFinclusion = 3.69; Jeffreys, 1998). In contrast, there was only very weak evidence for the treatment × age interaction in the model on functional connectivity between the salience network and the insula (BFinclusion = 0.53; Held & Ott, 2016).
Data availability
The data and code can be found at GitHub and OpenNeuro (upon publication of the manuscript).
References
- Andreas M, Gregor D, Peter K, Markus H, 2011. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 12 (9), 524–538. [DOI] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, …, Pearlson GD, 2010. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, 2010. Oxytocin and the salience of social cues. Proc. Natl. Acad. Sci. 107 (20), 9033–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P, Koopmann A, Bumb JM, Zimmermann S, Bühler S, Reinhard I, …, Kiefer F, 2021. Oxytocin attenuates neural response to emotional faces in social drinkers: an fMRI study. Eur. Arch. Psychiatry Clin. Neurosci. 271 (5), 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey PE, Rieger M, Moustafa AA, Slessor G, Rendell PG, Ruffman T, 2015. Trust and trustworthiness in young and older adults. Psychol. Aging doi: 10.1037/a0039736. [DOI] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM, 1997. Cloning, novel promoter sequence, and estrogen regulation of a rat oxytocin receptor gene. Endocrinology doi: 10.1210/endo.138.3.4998. [DOI] [PubMed] [Google Scholar]
- Baribeau DA, Anagnostou E, 2015. Oxytocin and vasopressin: linking pituitary neuropeptides and their receptors to social neurocircuits. Front. Neurosci. doi: 10.3389/fnins.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraza JA, Grewal NS, Ropacki S, Perez P, Gonzalez A, Zak PJ, 2013. Effects of a 10-day oxytocin trial in older adults on health and well-being. Exp. Clin. Psychopharmacol. doi: 10.1037/a0031581. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN, 2011. Social effects of oxytocin in humans: context and person matter. Trends Cognit. Sci. (Regul.Ed.) doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ, 1995. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Bethlehem RAI, Lombardo MV, Lai MC, Auyeung B, Crockford SK, Deakin J, …, Baron-Cohen S, 2017. Intranasal oxytocin enhances intrinsic corticostriatal functional connectivity in women. Transl. Psychiatry doi: 10.1038/tp.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem, Richard AI, van Honk J, Auyeung B, Baron-Cohen S, 2013. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Bhaganagarapu K, Jackson GD, Abbott DF, 2013. An automated method for identifying artifact in independent component analysis of resting-state fMRI. Front. Hum. Neurosci. 7, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biss RK, Hasher L, 2012. Happy as a lark: morning-type younger and older adults are higher in positive affect. Emotion doi: 10.1037/a0027071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, Zald DH, 2010. A unique role for the human amygdala in novelty detection. Neuroimage doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein M, 1988. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol. Behav. Neurol. 1 (2), 111–117. [Google Scholar]
- Briggs SD, Raz N, Marks W, 1999. Age-related deficits in generation and manipulation of mental images: I. The role of sensorimotor speed and working memory. Psychol. Aging doi: 10.1037/0882-7974.14.3.427. [DOI] [PubMed] [Google Scholar]
- Brodmann K, Gruber O, Goya-Maldonado R, 2017. Intranasal oxytocin selectively modulates large-scale brain networks in humans. Brain Connect. doi: 10.1089/brain.2017.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BTT, 2013. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ, 2001. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Hum. Brain Mapp. doi: 10.1002/hbm.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, 2012. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev. Biomed. Eng. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T, 2009. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A, Ruffman T, Murray JE, Glue P, 2014. Oxytocin improves emotion recognition for older males. Neurobiol. Aging 35 (10), 2246–2248. doi: 10.1016/j.neurobiolaging.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, DeLiema M, 2018. The positivity effect: a negativity bias in youth fades with age. Curr. Opin. Behav. Sci. doi: 10.1016/j.cobeha.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaby L, Boullay VL, Chetouani M, Plaza M, 2015. Compensating for age limits through emotional crossmodal integration. Front. Psychol. 6, 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand GB, Wu J, Hajjar I, Qiu D, 2017. Interactions of the salience network and its subsystems with the default-mode and the central-executive networks in normal aging and mild cognitive impairment. Brain Connect. doi: 10.1089/brain.2017.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Cohen P, Chen S, 2010. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun. Stat. Simul. Comput. doi: 10.1080/03610911003650383. [DOI] [Google Scholar]
- Clark-Elford R, Nathan PJ, Auyeung B, Voon V, Sule A, Müller U, …, Baron-Cohen S, 2014. The effects of oxytocin on social reward learning in humans. Int. J. Neuropsychopharmacol. doi: 10.1017/S1461145713001120. [DOI] [PubMed] [Google Scholar]
- Comblain C, D’Argembeau A, Van der Linden M, Aldenhoff L, 2004. The effect of ageing on the recollection of emotional and neutral pictures. Memory doi: 10.1080/09658210344000477. [DOI] [PubMed] [Google Scholar]
- Craig AD, 2009. How do you feel - now? The anterior insula and human awareness. Nat. Rev. Neurosci. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Eccles J, Garfinkel SN, 2013. Interaction between cognition, emotion, and the autonomic nervous system. Handb. Clin. Neurol. doi: 10.1016/B978-0-444-53491-0.00006-7. [DOI] [PubMed] [Google Scholar]
- Crowley WR, Armstrong WE, 1992. Neurochemical regulation of oxytocin secretion in lactation*. Endocr. Rev. doi: 10.1210/edrv-13-1-33. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, …, Salmon E, 2007. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J. Cognit. Neurosci. 19 (6), 935–944. [DOI] [PubMed] [Google Scholar]
- Daughters K, Manstead ASR, Hubble K, Rees A, Thapar A, van Goozen SHM, 2015. Salivary oxytocin concentrations in males following intranasal administration of oxytocin: a double-blind, cross-over study. PLoS One 10 (12), e0145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM, 2006. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Di Simplicio M, Norbury R, Harmer CJ, 2012. Short-term antidepressant administration reduces negative self-referential processing in the medial prefrontal cortex in subjects at risk for depression. Mol. Psychiatry 17 (5), 503–510. [DOI] [PubMed] [Google Scholar]
- Dixon ML, Thiruchselvam R, Todd R, Christoff K, 2017. Emotion and the prefrontal cortex: an integrative review. Psychol. Bull. doi: 10.1037/bul0000096. [DOI] [PubMed] [Google Scholar]
- Dodhia S, Hosanagar A, Fitzgerald DA, Labuschagne I, Wood AG, Nathan PJ, Phan KL, 2014. Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology doi: 10.1038/npp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC, 2007. Oxytocin Attenuates Amygdala Responses to Emotional Faces Regardless of Valence. Biol. Psychiatry doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC, 2010. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Ebner NC, Chen H, Porges E, Lin T, Fischer H, Feifel D, Cohen RA, 2016. Oxytocin’s effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology doi: 10.1016/j.psyneuen.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Fischer H, 2014. Studying the various facets of emotional aging. Front. Psychol. doi: 10.3389/fpsyg.2014.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Horta M, Lin T, Feifel D, Fischer H, Cohen RA, 2015. Oxytocin modulates meta-mood as a function of age and sex. Front. Aging Neurosci. 7 (SEP), 1–7. doi: 10.3389/fnagi.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Lin T, Muradoglu M, Weir DH, Plasencia GM, Lillard TS, …, Connelly JJ, 2019. Associations between oxytocin receptor gene (OXTR) methylation, plasma oxytocin, and attachment across adulthood. Int. J. Psychophysiol. doi: 10.1016/j.ijpsycho.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Maura GM, MacDonald K, Westberg L, Fischer H, 2013. Oxytocin and socioemotional aging: current knowledge and future trends. Front. Hum. Neurosci. doi: 10.3389/fnhum.2013.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M, Scheele D, Patin A, Preckel K, Becker B, Walter A, …, Hurlemann R, 2016. Oxytocin facilitates Pavlovian fear learning in fmales. Neuropsychopharmacology 41 (4), 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson EN, Emeis CL, 2017. Breastfeeding outcomes after oxytocin use during childbirth: an integrative review. J. Midwifery Women’s Health doi: 10.1111/jmwh.12601. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cognit. Sci. (Regul. Ed.) doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Herrera-Melendez AL, Pestke K, Feeser M, Aust S, Otte C, …, Grimm S, 2014. Early life stress modulates amygdala-prefrontal functional connectivity: implications for oxytocin effects. Hum. Brain Mapp. doi: 10.1002/hbm.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier I, Lin T, Liu P, Skarsten S, Feifel D, Ebner NC, 2021. Age and intranasal oxytocin effects on trust-related decisions after breach of trust: behavioral and brain evidence. Psychol. Aging doi: 10.1037/pag0000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Becker B, Luo L, Geng Y, Zhao W, Yin Y, …, Hurlemann R, 2016. Oxytocin, the peptide that bonds the sexes also divides them. Proc. Natl. Acad. Sci. 113 (27), 7650–7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Zhao W, Zhou F, Ma X, Yao S, Becker B, Kendrick KM, 2018. Oxytocin facilitates empathic-and self-embarrassment ratings by attenuating amygdala and anterior insula responses. Front. Endocrinol. (Lausanne) 9, 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ, 2001. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gooren L, 2003. Androgen deficiency in the aging male: benefits and risks of androgen supplementation. J. Steroid Biochem. Mol. Biol. 85 (2–5), 349–355. [DOI] [PubMed] [Google Scholar]
- Gossen A, Hahn A, Westphal L, Prinz S, Schultz RT, Gründer G, Spreckelmeyer KN, 2012. Oxytocin plasma concentrations after single intranasal oxytocin administration–a study in healthy men. Neuropeptides 46 (5), 211–215. [DOI] [PubMed] [Google Scholar]
- Gougelet R, Terzibas C, Voytek B, Callan D, 2018. Functional network activity mediating the shift of attentional resources during inattentional deafness in an aviation pursuit task. Front. Hum. Neurosci. doi: 10.3389/conf.fnhum.2018.227.00117. [DOI] [Google Scholar]
- Grace SA, Rossell SL, Heinrichs M, Kordsachia C, Labuschagne I, 2018. Oxytocin and brain activity in humans: a systematic review and coordinatebased meta-analysis of functional MRI studies. Psychoneuroendocrinology doi: 10.1016/j.psyneuen.2018.05.031. [DOI] [PubMed] [Google Scholar]
- Grainger SA, Henry JD, Steinvik HR, Vanman EJ, 2019. Intranasal oxytocin does not alter initial perceptions of facial trustworthiness in younger or older adults. J. Psychopharmacol. doi: 10.1177/0269881118806303. [DOI] [PubMed] [Google Scholar]
- Grainger SA, Henry JD, Steinvik HR, Vanman EJ, Rendell PG, Labuschagne I, 2018. Intranasal oxytocin does not reduce age-related diffculties in social cognition. Horm. Behav. doi: 10.1016/j.yhbeh.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, …, Schatzberg AF, 2007. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, …, Banati RB, 2013. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Guo Y, Friston K, Faisal A, Hill S, & Peng H (2015). Brain informatics and health: 8th international conference, BIH 2015 London, UK, august 30 – september 2, 2015 proceedings. In Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). 10.1007/978-3-31923344-4 [DOI] [Google Scholar]
- He X, Qin W, Liu Y, Zhang X, Duan Y, Song J, …, Yu C, 2014. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp. doi: 10.1002/hbm.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Domes G, 2008. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog. Brain Res. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Held L, Ott M, 2016. How the maximal evidence of p-values against point null hypotheses depends on sample size. Am. Stat. doi: 10.1080/00031305.2016.1209128. [DOI] [Google Scholar]
- Himberg J, Hyvärinen A, Esposito F, 2004. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos-Quiroga JA, Richarte Fernández V, Bosch R, Soliva JC, …, Vilarroya O, 2014. An independent components and functional connectivity analysis of resting state FMRI data points to neural network dysregulation in adult ADHD. Hum. Brain Mapp. doi: 10.1002/hbm.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta M, Pehlivanoglu D, Ebner NC, 2020. The role of intranasal oxytocin on social cognition: an integrative human lifespan approach. Curr. Behav. Neurosci. Rep doi: 10.1007/s40473-020-00214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta M, Ziaei M, Lin T, Porges EC, Fischer H, Feifel D, …, Ebner NC, 2019. Oxytocin alters patterns of brain activity and amygdalar connectivity by age during dynamic facial emotion identification. Neurobiol. Aging doi: 10.1016/j.neurobiolaging.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, …, Maier W, 2010. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J. Neurosci. 30 (14), 4999–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordan AD, Dolcos S, Dolcos F, 2013. Neural signatures of the response to emotional distraction: a review of evidence from brain imaging investigations. Front. Hum. Neurosci. doi: 10.3389/fnhum.2013.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys H, 1998. The Theory of Probability. OUP, Oxford. [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR, 2001. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging doi: 10.1016/S0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jurek B, Neumann ID, 2018. The oxytocin receptor: from intracellular signaling to behavior. Physiol. Rev. 98 (3), 1805–1908. [DOI] [PubMed] [Google Scholar]
- Kirsch P, 2005. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 25 (49), 11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch Peter., 2015. Oxytocin in the socioemotional brain: implications for psychiatric disorders. Dialogues Clin. Neurosci. doi: 10.31887/dcns.2015.17.4/pkirsch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács B, Kéri S, 2015. Off-label intranasal oxytocin use in adults is associated with increased amygdala-cingulate resting-state connectivity. Eur. Psychiatry 30 (4), 542–547. [DOI] [PubMed] [Google Scholar]
- Kumar J, Völlm B, Palaniyappan L, 2015. Oxytocin affects the connectivity of the precuneus and the Amygdala: a randomized, double-blinded, placebo-controlled neuroimaging trial. Int. J. Neuropsychopharmacol. doi: 10.1093/ijnp/pyu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurczek J, Wechsler E, Ahuja S, Jensen U, Cohen NJ, Tranel D, Duff M, 2015. Differential contributions of hippocampus and medial prefrontal cortex to self-projection and self-referential processing. Neuropsychologia 73, 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Pedder DJ, Henry JD, Terrett G, Rendell PG, 2020. Age differences in emotion regulation and facial muscle reactivity to emotional films. Gerontology 66 (1), 74–84. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, …, Nathan PJ, 2010. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, …, Nathan PJ, 2012. Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. Int. J. Neuropsychopharmacol. doi: 10.1017/S1461145711001489. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, 1995. Emotion: clues from the brain. Annu. Rev. Psychol. 46 (1), 209–235. [DOI] [PubMed] [Google Scholar]
- Leppanen J, Ng KW, Tchanturia K, Treasure J, 2017. Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neurosci. Biobehav. Rev. doi: 10.1016/j.neubiorev.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Levy J, Goldstein A, Zagoory-Sharon O, Weisman O, Schneiderman I, Eidelman-Rothman M, Feldman R, 2016. Oxytocin selectively modulates brain response to stimuli probing social synchrony. Neuroimage 124, 923–930. [DOI] [PubMed] [Google Scholar]
- Li P, Fan TT, Zhao RJ, Han Y, Shi L, Sun HQ, …, Lu L, 2017. Altered brain network connectivity as a potential endophenotype of schizophrenia. Sci. Rep. doi: 10.1038/s41598-017-05774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Xiao H, Wang K, Zheng Y, Chen P, Wang X, …, Zhang X, 2018. Upregulation of oxytocin receptor in the hyperplastic prostate. Front. Endocrinol. doi: 10.3389/fendo.2018.00403, (Lausanne). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Pehlivanoglu D, Ziaei M, Liu P, Woods AJ, Feifel D, Fischer H, Ebner NC (under review). Age-Related Differences in Amygdala Activation Associated with Face Trustworthiness but No Evidence of Oxytocin Modulation. Frontiers in Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Liu GA, Perez E, Rainer RD, Febo M, Cruz-Almeida Y, Ebner NC, 2018. Systemic inflammation mediates age-related cognitive deficits. Front. Aging Neurosci. doi: 10.3389/fnagi.2018.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Feng T, 2017. The overlapping brain region accounting for the relationship between procrastination and impulsivity: a voxel-based morphometry study. Neuroscience 360, 9–17. doi: 10.1016/j.neuroscience.2017.07.042. [DOI] [PubMed] [Google Scholar]
- Luo L, Becker B, Geng Y, Zhao Z, Gao S, Zhao W, …, Gao Z, 2017. Sex-dependent neural effect of oxytocin during subliminal processing of negative emotion faces. Neuroimage 162, 127–137. [DOI] [PubMed] [Google Scholar]
- Luo Y, Qin S, Fernandez G, Zhang Y, Klumpers F, Li H, 2014. Emotion perception and executive control interact in the salience network during emotionally charged working memory processing. Hum. Brain Mapp. 35 (11), 5606–5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH, 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marrelec G, Perlbarg V, 2008. Contribution of exploratory methods to the investigation of extended large-scale brain networks in functional MRI: methodologies, results, and challenges. Int. J. Biomed. Imaging doi: 10.1155/2008/218519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Carstensen LL, 2005. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cognit. Sci. (Regul. Ed.) 9 (10), 496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Hansen LK, Sejnowsk TJ, 2003. Independent component analysis of functional MRI: what is signal and what is noise? Curr. Opin. Neurobiol. 13 (5), 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Wildenberg JC, Liu J, Chen J, Calhoun VD, Biswal BB, …, Prabhakaran V, 2012. Parallel ICA identifies sub-components of resting state networks that covary with behavioral indices. Front. Hum. Neurosci. doi: 10.3389/fnhum.2012.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2015). Salience network. In Brain Mapping: An Encyclopedic Reference. 10.1016/B978-0-12-397025-1.00052-X [DOI] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M, 2011. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mierop A, Mikolajczak M, Stahl C, Béna J, Luminet O, Lane A, Corneille O, 2020. How can intranasal oxytocin research be trusted? A systematic review of the interactive effects of intranasal oxytocin on psychosocial outcomes. Perspect. Psychol. Sci. doi: 10.1177/1745691620921525. [DOI] [PubMed] [Google Scholar]
- Naveau M, Doucet G, Delcroix N, Petit L, Zago L, Crivello F, …, Joliot M, 2012. A novel group ICA approach based on multi-scale individual component clustering. application to a large sample of fMRI data. Neuroinformatics doi: 10.1007/s12021-012-9145-2. [DOI] [PubMed] [Google Scholar]
- Noh SR, Isaacowitz DM, 2013. Emotional faces in context: age differences in recognition accuracy and scanning patterns. Emotion 13 (2), 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SK, Dunlop K, Downar J, 2016. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front. Syst. Neurosci. doi: 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasencia G, Luedicke JM, Nazarloo HP, Carter CS, Ebner NC, 2019. Plasma oxytocin and vasopressin levels in young and older men and women: functional relationships with attachment and cognition. Psychoneuroendocrinology doi: 10.1016/j.psyneuen.2019.104419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF, 2007. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivar. Behav. Res. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Procyshyn T, Lombardo Mi., Lai M-C, Auyeung B, Crockford S, Deakin J, … Bethlehem R (2020). Intranasal oxytocin differentially affects resting-state functional connectivity of social brain regions in autistic and non-autistic women. [Google Scholar]
- Quintana DS, Alvares GA, Hickie IB, Guastella AJ, 2015. Do delivery routes of intranasally administered oxytocin account for observed effects on social cognition and behavior? A two-level model. Neurosci. Biobehav. Rev. doi: 10.1016/j.neubiorev.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ, 2020. An allostatic theory of oxytocin. Trends Cognit. Sci. (Regul. Ed.) 24 (7), 515–528. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Smerud KT, Andreassen OA, Djupesland PG, 2018. Evidence for intranasal oxytocin delivery to the brain: recent advances and future perspectives. Ther. Deliv. doi: 10.4155/tde-2018-0002. [DOI] [PubMed] [Google Scholar]
- Rachakonda S, Egolf E, Correa N, Calhoun V, & Neuropsychiatry O (2007). Group ICA of fMRI toolbox (GIFT) manual. Computer (Long Beach Calif). [Google Scholar]
- Reed AE, Chan L, Mikels JA, 2014. Meta-analysis of the age-related positivity effect: age differences in preferences for positive over negative information. Psychol. Aging doi: 10.1037/a0035194. [DOI] [PubMed] [Google Scholar]
- Reed SC, Haney M, Manubay J, Campagna BR, Reed B, Foltin RW, Evans SM, 2019. Sex differences in stress reactivity after intranasal oxytocin in recreational cannabis users. Pharmacol. Biochem. Behav. doi: 10.1016/j.pbb.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A (1964). Auditory-verbal learning test (AVLT). Press Universitaire de France. [Google Scholar]
- Riem MME, Bakermans-Kranenburg MJ, Huffmeijer R, van IJzendoorn MH, 2013. Does intranasal oxytocin promote prosocial behavior to an excluded fellow player? A randomized-controlled trial with Cyberball. Psychoneuroendocrinology doi: 10.1016/j.psyneuen.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Riem MME, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MAS, Vermeiren RRJM, …, Rombouts SARB, 2011. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol. Psychiatry doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Riem MME, Bakermans-Kranenburg MJ, Voorthuis A, van IJzendoorn MH, 2014. Oxytocin effects on mind-reading are moderated by experiences of maternal love withdrawal: an fMRI study. Prog. Neuropsychopharmacol. Biol. Psychiatry 51, 105–112. [DOI] [PubMed] [Google Scholar]
- Riem MME, Van Ijzendoorn MH, Tops M, Boksem MAS, Rombouts SARB, Bakermans-Kranenburg MJ, 2012. No laughing matter: intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology doi: 10.1038/npp.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, …, Pagnoni G, 2014. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissanen J, 1983. A universal prior for integers and estimation by minimum description length. Ann. Stat. doi: 10.1214/aos/1176346150. [DOI] [Google Scholar]
- Schmitz TW, Johnson SC, 2006. Self-appraisal decisions evoke dissociated dorsalventral aMPFC networks. Neuroimage doi: 10.1016/j.neuroimage.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, 2011. The neural bases for empathy. Neuroscientist doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, 2006. Estrogen and cognitive aging in women. Neuroscience 138 (3), 1021–1026. [DOI] [PubMed] [Google Scholar]
- Shi L, Sun J, Xia Y, Ren Z, Chen Q, Wei D, …, Qiu J, 2018. Large-scale brain network connectivity underlying creativity in resting-state and task fMRI: cooperation between default network and frontal-parietal network. Biol. Psychol. doi: 10.1016/j.biopsycho.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Singer T, Snozzi R, Bird G, Petrovic P, Silani G, Heinrichs M, Dolan RJ, 2008. Effects of oxytocin and prosocial behavior on brain responses to direct and vicariously experienced pain. Emotion doi: 10.1037/a0014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Phan KL, Labuschagne I, Welsh R, Nathan PJ, Wood AG, 2013. Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. Int. J. Neuropsychopharmacol. doi: 10.1017/S1461145712000533. [DOI] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, Hurlemann R, 2013. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep. 3 (1), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter M, Kocher MG, 2007. Trust and trustworthiness across different age groups. Games Econ. Behav. doi: 10.1016/j.geb.2006.07.006. [DOI] [Google Scholar]
- Sze JA, Gyurak A, Goodkind MS, Levenson RW, 2012. Greater emotional empathy and prosocial behavior in late life. Emotion doi: 10.1037/a0025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD, 2009. Two systems of resting state connectivity between the insula and cingulate cortex. Hum. Brain Mapp. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ, 2009. Oxytocin and social perception: oxytocin increases perceived facial trustworthiness and attractiveness. Horm. Behav. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, Van Boxtel MPJ, Evans AC, Jolles J, Uylings HBM, 2002. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage doi: 10.1016/S1053-8119(02)91173-0. [DOI] [PubMed] [Google Scholar]
- Tohka J, Foerde K, Aron AR, Tom SM, Toga AW, Poldrack RA, 2008. Automatic independent component labeling for artifact removal in fMRI. Neuroimage 39 (3), 1227–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L, 2012. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse WS, Siu AFY, Zhang Q, Chan HYE, 2018. Maternal oxytocin responsiveness improves specificity of positive social memory recall. Psychoneuroendocrinology doi: 10.1016/j.psyneuen.2018.08.026. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, 2015. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V, 2010. Typical and atypical development of functional human brain networks: insights from resting-state fMRI. Front. Syst. Neurosci. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE, 2010. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Davidkova G, Zhu YS, Koibuchi N, Chin WW, Pfaff D, 2001. Differential interaction of estrogen receptor and thyroid hormone receptor isoforms on the rat oxytocin receptor promoter leads to differences in transcriptional regulation. Neuroendocrinology doi: 10.1159/000054698. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, VanEssen DC, …, Raichle ME, 2007. Intrinsic functional architecture in the anaesthetized monkey brain. Nature doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Walum H, Waldman ID, Young LJ, 2016. Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol. Psychiatry doi: 10.1016/j.biopsych.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A, 1988. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981). The digit symbol substitution test. Wechsler Adult Intelligence. [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, Monk CS, 2010. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 1313, 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A, 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2 (3), 125–141. [DOI] [PubMed] [Google Scholar]
- Wittfoth-Schardt D, Gründing J, Wittfoth M, Lanfermann H, Heinrichs M, Domes G, …, Waller C, 2012. Oxytocin modulates neural reactivity to children’s faces as a function of social salience. Neuropsychopharmacology doi: 10.1038/npp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Feng C, Lu X, Liu X, & Liu Q (2018). Oxytocin effects on the resting-state mentalizing brain network. BioRxiv. 10.1101/465658 [DOI] [PubMed] [Google Scholar]
- Wu Q, Huang Q, Liu C, Wu H, 2022. Oxytocin modulates social brain network correlations in resting and task state. bioRxiv 2021-12. [DOI] [PubMed] [Google Scholar]
- Xin F, Zhou F, Zhou X, Ma X, Geng Y, Zhao W, …, Becker B, 2018. Oxytocin modulates the intrinsic dynamics between attention-related large-scale networks. Cereb. Cortex doi: 10.1093/cercor/bhy295. [DOI] [PubMed] [Google Scholar]
- Yao S, Becker B, Zhao W, Zhao Z, Kou J, Ma X, …, Kendrick KM, 2018a. Oxytocin modulates attention switching between interoceptive signals and external social cues. Neuropsychopharmacology 43 (2), 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Zhao W, Geng Y, Chen Y, Zhao Z, Ma X, …, Kendrick KM, 2018b. Oxytocin facilitates approach behavior to positive social stimuli via decreasing anterior insula activity. Int. J. Neuropsychopharmacol. doi: 10.1093/ijnp/pyy068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Doñamayor N, Münte TF, 2014. Brain network of semantic integration in sentence reading: insights from independent component analysis and graph theoretical analysis. Hum. Brain Mapp. doi: 10.1002/hbm.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Ma X, Geng Y, Zhao W, Zhou F, Wang J, …, Kendrick KM, 2019. Oxytocin differentially modulates specific dorsal and ventral striatal functional connections with frontal and cerebellar regions. Neuroimage 184, 781–789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and code can be found at GitHub and OpenNeuro (upon publication of the manuscript).
The data and code can be found at GitHub and OpenNeuro (upon publication of the manuscript).


