Abstract
Many methods have been used to synthesize xanthene derivatives using different catalysts. However, some of these methodologies have not been entirely satisfactory. Most of the mentioned methods have disadvantages such as low yields, prolonged reaction times, harsh reaction conditions and the requirement of expensive catalysis and use of toxic organic solvent. In this research, a green and highly efficient procedure for the one-pot synthesis of 1,8-dioxo-octahydro-xanthenes has been developed. Zr(HSO4)4 catalyst was used as an efficient and recoverable catalyst for synthesis of 1,8-dioxo-octahydro-xanthene derivatives via cyclocondensation of dimedone and aromatic aldehydes in solvent-free conditions. There are no examples of the use of Zr(HSO4)4 for the synthesis of 1,8-dioxo-octahydro-xanthene derivatives. The present method offers several advantages such as green, highly efficient, recoverable, reusable, simple work-up and simple purification of products. The structure of the synthesized products was confirmed by Fourier Transform Infrared (Ft-IR) and Proton nuclear magnetic resonance (1HNMR) analyzes. The antibacterial activity of the synthesized compounds was determined by agar disk diffusion method against gram-positive (S. aureus bacteria) and gram-negative (E. coli bacteria) microorganisms. Among the synthesized compounds (3a-3j), 3h compound showed the highest antibacterial effect by forming an inhibitory diameter zone of 15 mm around the disc containing 2000 mg of 3h-compound against gram-positive (S. aureus bacteria).
1. Use of Zr(HSO4)4 as a green and highly efficient and reusable heterogeneous catalyst.
2. Under solvent-free condition.
3. Simple work-up and Simple purification of products.
Keywords: Green; Highly efficient; One-pot synthesis; 1,8-dioxooctahydroxanthenes; Reusable heterogeneous catalyst; Solvent-free conditions
Graphical abstract

Specifications table
| Subject Area: | Chemistry |
| More specific subject area: | Medicinal Chemistry |
| Method name: | Aldol condensation reaction |
| Name and reference of original method: | Not applicable |
| Resource availability: | Not applicable |
Method details
Introduction
In recent years, synthetic chemists have shown tremendous interested in developing highly efficient transformation for the synthesis of xanthene derivatives due to their potential applications in the biological activities, pharmaceutical and industrial applications. These compounds have antibacterial properties due to the presence of reactive oxygen in their structure. For example, they have many biological and therapeutic properties such as anti-inflammatory [1], antiviral [2], antibacterial [3], as well as in photodynamic therapy [4] and as antagonists of the paralyzing action of zoxazolamine [5]. Xanthenes are also available from natural sources [6]. Many procedures are disclosed to synthesize xanthenes and benzoxanthenes such as montmorillonite K10 [7], nano-ZnAl2O4 [8], sodium hydrogen sulfate [9], cyclodehydrations [10], [C4dabco][BF4] ionic liquid [11], Smcl3 [12], acid activated clay [13], sulfonic acid functionalized silica [14], template-containing Zn/MCM-41 [15], Sulfamic acid [16], Nickel-Cobalt Ferrite [17], Many different methods have been reported for the synthesis of xanthenes; one of them is the condensation of aldehydes with cyclohexane-1,3-dione or 5,5-dimethylcyclohexane-1,3-dione to give 1,8-dioxooctahydroxanthene derivatives. This reaction has been conducted in the presence of strong protonic acids [18], Lewis acids such as InCl3.4H2O [19], FeCl3.6H2O [20] and heterogeneous catalysts like Dowex-50W [21], NaHSO4.SiO2 [22], Ambertyst-15 [23], HBF4/SiO2 [24] and Sulfonic acid-functionalized LUS-1 [25]. Other catalysts such as alum-promoted [26], potash alum [27], boric acid [28], ZrOCl2.8H2O [29], nano-Fe3O4 [30], B(HSO4)3 [31], TiO2 [32], nano-TiO2 [33], BiVO4-NPs [34] and InCl3 [35] and etc. [[36], [37], [38]].
However, some of these methodologies have not been entirely satisfactory. Most of the mentioned methods have disadvantages such as low yields, prolonged reaction times, harsh reaction conditions and the requirement of expensive catalysis and use of toxic organic solvent. Therefore, the use of Zr (HSO4) 4 as a heterogeneous catalyst has been considered in this research to avoid these limitations and to create an alternative pathway for the synthesis of xanthene derivatives. Zr(HSO4)4 is a low-cost solid Brønsted acid. There are no examples of the use of Zr(HSO4)4 for the synthesis of 1,8-dioxooctahydroxanthene derivatives. In this research, Zr(HSO4)4 catalyst was used as an efficient, recoverable and reusable catalyst for synthesis of 1,8-dioxooctahydroxanthene derivatives via traditional route from cyclocondensation of dimedone and aromatic aldehydes in solvent-free conditions.
Experimental
All chemicals used were of synthetic grade and were used as received without any further purification. Melting points were determined in open glass capillaries on an Electrothermal 9100s apparatus and are uncorrected.
The IR spectra were recorded with FT-IR Shimadzu IR-470 instrument using potassium bromide pellets. The 1H-NMR spectra were determined on a Bruker Avance DRX-400 MHz instrument using TMS as the internal standard and CDCl3 as solvent. Chemical shifts are expressed as δ(ppm) and the coupling constant as J(H2). The progress of reaction was monitored by Thin-layer chromatography (TLC) using 0.2 mm Merck silicagel GF254 pre-coated plates and visualized by UV-light (254 nm).
Scheme 1.
synthesis of 1,8-dioxooctahydroxanthenes.
General procedure for the preparation of 1,8-dioxooctahydroxanthene (3a-j): To a mixture of dimedone 1 (2 mmol) and aldehyde 2 (1 mmol) was added Zr(HSO4)4 which prepared as reported [1] (20 mol%) and the mixture was allowed to stir at 110 °C for the total recorded time which indicated in Table 1. The progress of reactions was monitored by TLC (eluent: EtOAc/ Diethylether, 1:4). After completion of the reaction, the reaction mixture was cooled to room temperature. Then to mixture was added cold water and the product was extracted with ethyl acetate (3 × 5 mL). The organic layer was dried (MgSO4) and evaporated in vacuum. The crude product recrystallized by EtOH 96% and purified. All the desired products were characterized by comparison of their physical data with those reported compounds. For the total confirmed of synthetic compounds, the spectral data given below.
Table 1.
Screening of the catalyst and solvent for the reaction of benzaldehydes and dimedone catalyzed by Zr(HSO4)4.a
 | ||||
|---|---|---|---|---|
| Entry | Catalyst (mol%) | Solvent/condition | Time (min) | Yieldb (%) |
| 1 | 20 | water/reflux | 120 | trace |
| 2 | 20 | THF/reflux | 120 | trace |
| 3 | 20 | CHCl3/reflux | 120 | 20 |
| 4 | 20 | CH3CN/reflux | 120 | 15 |
| 5 | 20 | CH3CH2OH/reflux | 100 | 55 |
| 6 | 10 | solvent-free | 100 | 68 |
| 7 | 20 | solvent-free | 40 | 86 |
| 8 | 30 | solvent-free | 40 | 75 |
| 9 | 0 | solvent-free | 40 | trace |
Reaction condition: dimedone (2 mmol), aldehyde (1 mmol) and Zr(HSO4)4 as catalyst.
Isolated yield.
3,3,6,6-tetramethyl-9-phenyl-1,8-dioxooctahydroxanthene (3a): White powder, Yield (86%), mp 201-202 °C. FT-IR (ῡ, Cm−1) (KBr disc): 3040 (CHarom, Str.); 2990 (CHaliph, Str.); 1605 (C=O Str.); 1540 (C=C Str.); 1360 (C-O Str.). 1H NMR (400 MHz, CDCl3) δ(ppm): 1.12 (6H, s, 2CH3); 1.26 (6H, s, 2CH3); 2.30-2.50 (8H, m, 4CH2); 5.58 (1H, s, CH); 7.11-7.13 (2H, d, CHO, 3J = 6.8 Hz); 7.17-7.21 (1H, t, Hp, 3J = 7.8 Hz); 7.27-7.31 (2H, dd, Hm, 3J = 7.8 Hz, 3J = 6.8 Hz).
3,3,6,6-tetramethyl-9-(4-methylphenyl)-1,8-dioxooctahydroxanthene (3b): White powder, Yield (81%), mp 173-175 °C. FT-IR (ῡ, Cm−1) (KBr disc): 3080 (CHarom, Str.); 2950 (CHaliph, Str.); 1670 (C=O Str.); 1540 (C=C Str.); 1380 (C-O Str.). 1H NMR (400 MHz, CDCl3) δ(ppm): 1.01 (6H, s, CH3); 1.11 (6H, s, CH3); 2.15 (4H, dd, CH2); 2.26 (3H, s, CH3); 2.47 (4H, s, CH2); 4.8 (1H, s); 7.02 (2H, d, CHarom, 3J = 8.2 Hz); 7.19 (2H, d, CHarom, 3J = 8.2 Hz).
3,3,6,6-tetramethyl-9-(4-methoxyphenyl)-1,8-dioxooctahydroxanthene (3c): Gray powder, Yield (82%), mp 238-239 °C. FT-IR (ῡ, Cm−1) (KBr disc): 3080 (CHarom, Str.); 3000 (CHaliph, Str.); 1685 (C=O Str.); 1530 (C=C Str.); 1380 (C-O Str.). 1H NMR (400 MHz, CDCl3) δ(ppm): 1.01 (6H, s, CH3); 1.11 (6H, s, CH3); 2.22 (4H, dd, CH2); 2.47 (4H, s, CH2); 2.47 (1H, s, CH); 3.74 (3H, s, OCH3); 6.7 (2H, d, CHarom, 3J = 8.4 Hz); 7.21 (2H, d, CHarom, 3J = 8.4 Hz).
3,3,6,6-tetramethyl-9-(3-methoxyphenyl)-1,8-dioxooctahydroxanthene (3d): White powder, Yield (92%), mp 159-160 °C. FT-IR (ῡ, Cm−1) (KBr disc): 3100 (CHarom, Str.); 3000 (CHaliph, Str.); 1580 (C=O Str.); 1520 (C=C Str.); 1370 (C-O Str.). 1H NMR (400 MHz, CDCl3) δ(ppm): 1.12 (6H, s, CH3); 1.25 (6H, s, CH3); 2.30-2.48 (8H, m, CH2); 3.75 (3H, s, OCH3); 5.53 (1H, s, CH); 6.68-7.22 (4H, m, CHarom).
Antibacterial activity
The antibacterial activity of the synthesized compounds was determined by agar disk diffusion method against gram-positive (S. aureus bacteria) and gram-negative (E. coli bacteria) microorganisms. For this purpose, Briefly, Mueller Hinton agar culture medium with a thickness of 5 mm was prepared first. Then a suspension of the desired bacteria was prepared with 0.5 McFarland turbidity standards. Next, 50 µl of fresh bacterial culture was pipetted in the center of sterile petri dish containing the prepared culture medium and dispersed well by sterile swap. Next, filter paper disks containing a concentration of 2000 µg/ml of the synthesized products (3a-3j) are placed on the surface of the agar previously inoculated with the desired bacterium. The plates were then incubated for 24 hours at 37°C. The inhibition diameters zones formed around each disk were measured by using a ruler. Each experiment was repeated three times and the average diameter of the growth inhibition zone was calculated.
Results and discussion
In order to optimize the reaction parameters, the catalytic activity of Zr(HSO4)4 in the synthesis of 1,8-dioxoctahydroxanthene derivatives under different reaction conditions was investigated. For this purpose, Reaction efficiency and reaction rate in different catalytic values and different solvents were investigated. Condensation reaction of benzaldehyde and dimedone was selected as a model reaction. As shown in Table 1, among the tested solvents such as water, THF, CHCl3, CH3CN, CH3CH2OH and a solvent-free system, the best result was obtained after 40 min under solvent-free conditions in good yield (86%) (Table 1, Entry 7). When the same reaction was performed in the absence of the catalyst, the corresponding product was obtained in trace (<10%) (Table 1, Entry 9). However, the presence of Zr(HSO4)4 in the amount of more than 30 mol%, the efficiency and reaction speed did not improve (Table 1, Entry 8)
After determining the optimal reaction conditions, aldol condensation reaction between dimedone and benzaldehyde derivatives was performed in the presence of Zr(HSO4)4 catalyst and in optimal reaction conditions (Table 2). All the products were characterized by 1HNMR and IR spectra and compared with previous articles.
Table 2.
Synthesis of 1,8-dioxooctahydroxanthene derivatives in the presence of Zr(HSO4)4 and in optimal reaction conditions.
| Entry | R | Product | Time (min) | Yield % | Mp (˚C) | Mp (Lit) |
|---|---|---|---|---|---|---|
| 1 | C6H5 | 3a | 40 | 86 | 201-203 | 205-206 (37) |
| 2 | p-CH3C6H4 | 3b | 50 | 81 | 229-230 | 222-225 (38) |
| 3 | p- CH3OC6H4 | 3c | 55 | 82 | 251-252 | 248-250 (37) |
| 4 | m- CH3OC6H4 | 3d | 35 | 92 | 304-305 | 308-310 (38) |
| 5 | p-Cl C6H4 | 3e | 40 | 88 | 240-241 | 237-238 (37) |
| 6 | p-NO2 C6H4 | 3f | 35 | 94 | 230-032 | 226-228 (37) |
| 7 | p-OHC6H4 | 3g | 60 | 80 | 254-255 | 250-251 (37) |
| 8 | 2,4- di-Cl-C6H4 | 3h | 30 | 95 | 239-241 | 247-248 (37) |
| 9 | p-(Me)2N C6H4 | 3i | 60 | 72 | 295-297 | - |
| 10 | m-Cl C6H4 | 3j | 40 | 88 | 179-181 | 185-186 (39) |
Antimicrobial activities of 1,8-dioxo-octahydroxanthene derivatives
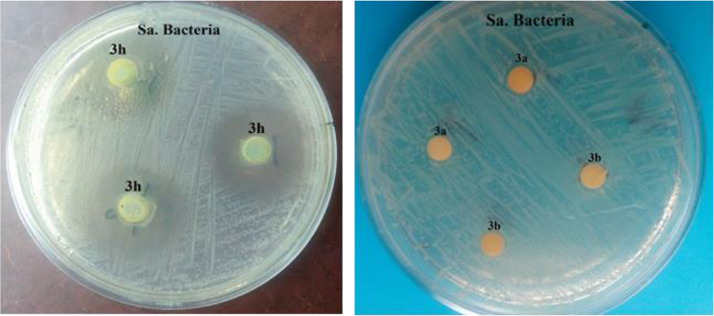
The antibacterial properties of all synthesized compounds (3a-3j) were investigated by agar disk diffusion method against gram-positive (S. aureus bacteria) and gram-negative (E. coli bacteria) microorganisms. The results are reported in Table 3 and Fig. 1.
Table 3.
Inhibition zones (mm) of synthesized 1,8-dioxooctahydroxanthenes derivatives against against gram-positive (S. aureus bacteria) and gram-negative (E. coli bacteria) microorganisms by the disc diffusion method.
| Entry | Product | S. aureus | E. coli |
|---|---|---|---|
| 1 | 3a | 4* | 0⁎⁎ |
| 2 | 3b | 3.5 | 0 |
| 3 | 3c | 0 | 0 |
| 4 | 3d | 0 | 0 |
| 5 | 3e | 0 | 0 |
| 6 | 3f | 0 | 0 |
| 7 | 3g | 0 | 0 |
| 8 | 3h | 15 | 7.5 |
| 9 | 3i | 4 | 0 |
| 10 | 3j | 3 | 0 |
Numbers are reported in millimeters.
The numbers reported are the inhibitions halos formation around the disk.
Fig. 1.
Perform antibacterial test by disk diffusion method against gram-positive (S. aureus bacteria) and gram-negative (E. coli bacteria) microorganisms.
Recovery of catalyst
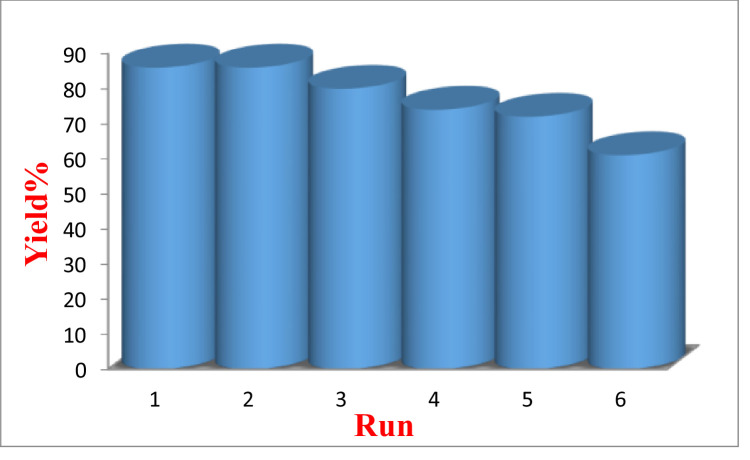
Recycling of heterogeneous catalysts after the end of the reaction and its reusability with the least change in the reaction efficiency can be considered one of the most important advantages of this type of catalyst. In order to investigate the recovery of Zr(HSO4)4 catalyst, after each use of this catalyst, we washed it with ethanol and water and dried it in the oven and then participated in the same reaction. The results show that the catalyst can be used up to three run without significantly changing the reaction efficiency (Fig. 2).
Fig. 2.
The reusability of Zr(HSO4)4 catalyst for the preparation of 3,3,6,6-tetramethyl-9-phenyl-1,8-dioxoocta hydroxanthene (3a).
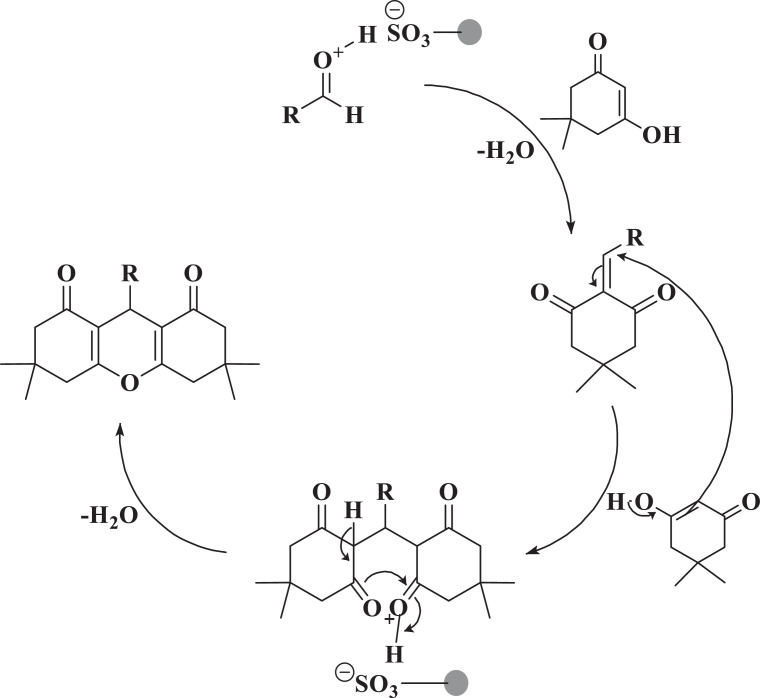
The proposed mechanism is shown in Scheme 2. At first, SO3H groups, as an acidic agent, activates the carbonyl aldehyde group. Then dimedone attacks this intermediate. In the next step, by increasing Michael and the removal of a water molecule, the products are synthesized.
Scheme 2.
Proposed mechanism for the synthesis of 1,8-dioxooctahydroxanthene in the presence of Zr(HSO4)4 as catalyst.
Table 4 shows a comparison between the methods reported in the articles and the method performed in this work.
Table 4.
A comparison of the methods used in the articles and the method used in this work.
| Entry | Catalyst | Conditions | Reaction time (min) | Yields% | Ref. |
|---|---|---|---|---|---|
| 1 | PEG | H2O/reflux | ∼ 180 | ∼ 90 | [39] |
| 2 | activated zinc metal and solid NH4C | Solvent free /MW | ∼ 50 | ∼ 90 | [40] |
| 3 | 1-butyl-3-methylimidazoliumtetrafluoroborate (BMIF) | solvent free/∼200 ˚C | ∼ 60 | ∼ 90 | [41] |
| 4 | Fe nanoparticles loaded in zeolite X (Fe-X) | Solvent-free, 90°C | ∼ 40 | ∼ 90 | [42] |
| 5 | SO3H@Fe3O4 magnetic nanocatalyst | Solvent-free, 80 ˚C | ∼ 20 | ∼ 90 | [43] |
| 6 | Zr(HSO4)4 | Solvent-free, 110°C | 35-60 | 72-95 | This work |
Conclusion
In summary, in this study we introduced compound Zr(HSO4)4 as a green, effective and recyclable catalyst for the one pot synthesis of various 1,8-dioxooctahydroxanthenes derivatives. Some advantages of this protocol include a simple reaction set-up, high products yields and elimination of toxic solvents.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported in part by the research and technology of Islamic Azad University. Tonekabon branch.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2022.101832.
Appendix. Supplementary materials
Data Availability
Data will be made available on request.
References
- 1.Poupelin J.P., Saint-Rut G., Foussard-Blanpin O., Narcisse G., Uchida-Ernouf G., Lacroix R. Eur. J. Med. Chem. 1978;13:67. doi: 10.1002/CHIN.197825154. [DOI] [Google Scholar]
- 2.Naidu K.R.M., Krishna B.S., Kumar M.A., Arulselvan P., Khalivulla S.I., Lasekan O. Molecules. 2012;17:7543. doi: 10.3390/molecules17067543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaya M., Demir E., Bekci H. J. Enzyme Inhib. Med. Chem. 2012;28:885. doi: 10.3109/14756366.2012.692087. [DOI] [PubMed] [Google Scholar]
- 4.Buck S.T.G., Bettanin F., Orestes E., Homem-de-Mello P., Imasato H., Viana R.B., Perussi J.R., da Silva A.B.F. J. Chem. 2017;2017:1. doi: 10.1155/2017/7365263. [DOI] [Google Scholar]
- 5.Maleki B., Barzegar S., Sepehr Z., Kermanian M., Tayebee R. J.Iran. Chem. Soc. 2012;9:757. doi: 10.1007/s13738-012-0092-5. [DOI] [Google Scholar]
- 6.Ravindranath B., Seshadri T.R. Phytochemistry. 1973;12:2781. doi: 10.1016/0031-9422(73)85099-X. [DOI] [Google Scholar]
- 7.Dabiri M., Azimi S.C., Bazgir A. Chem. Zvesti. 2008;62:522. doi: 10.2478/s11696-008-0050-y. [DOI] [Google Scholar]
- 8.Mandlimath T.R., Umamahesh B., Sathiyanarayanan K.I. J. Mol. Catal. A. Chem. 2014;391:198. doi: 10.1016/j.molcata.2014.04.030. [DOI] [Google Scholar]
- 9.Karimi-Jaberi Z., Hashemi M.M. Monatsh. Chem. 2008;139:605. doi: 10.1007/s00706-007-0786-z. [DOI] [Google Scholar]
- 10.Barnerjee A., Mukherjee A.K. Stain. Technol. 2009;56:83. doi: 10.3109/10520298109067286. [DOI] [PubMed] [Google Scholar]
- 11.Da-Zhen X., Liu Y., Shi S., Wang Y. Green. Chem. 2010;12:514. doi: 10.1039/B918595J. [DOI] [Google Scholar]
- 12.Ilangovan A., Malayappasamy S., Muralidharan S., Maruthamuthu S. Chem. Cent. J. 2011;5:81. doi: 10.1186/1752-153X-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouhani H., Sarrafi A., Tahmooresi M. Chem Eng Commun. 2016;203:289. doi: 10.1080/00986445.2014.975890. [DOI] [Google Scholar]
- 14.Ziarani G.M., Badiei A.R., Azizi M. Sci. Iran. 2011;18:453. doi: 10.1016/j.scient.2011.05.008. [DOI] [Google Scholar]
- 15.Pirouzmand M., Gharehbaba A.M., Ghasemi Z. J. Mex. Chem. Soc. 2016;60:4. [Google Scholar]
- 16.Heravi M.M., Alinejhad H., Bakhtiari K., Oskooie H.A. Mol. Divers. 2010;14:621. doi: 10.1007/s11030-009-9196-y. [DOI] [PubMed] [Google Scholar]
- 17.Maripi S., Korupolu R.B., Madasu S.B. Curr. Opin. Green Sustain. Chem. 2017;7:70. doi: 10.4236/gsc.2017.71006. [DOI] [Google Scholar]
- 18.Rouhani H., Sarrafi A., Tahmooresi M. Chem Eng Commun. 2016;203:289. doi: 10.1080/00986445.2014.975890. [DOI] [Google Scholar]
- 19.Fan X., Hu X., Zhang X., Wang J. Can. J. Chem. 2005;83:16. doi: 10.1139/v04-155. [DOI] [Google Scholar]
- 20.Fan X.S., Li Y.Z., Zhang X.Y., Hu X.Y., Wang J.J. Chin. J. Org. Chem. 2005;16:897. [Google Scholar]
- 21.Shakibaei G.I., Mirzaei P., Bazgir A. Appl. Catal. A: Gen. 2007;325:188. doi: 10.1016/j.apcata.2007.03.008. [DOI] [Google Scholar]
- 22.Das B., Thirupathi P., Reddy K.R., Ravikath B., Nagarapu L. Catal. Commun. 2007;8:535. doi: 10.1016/j.catcom.2006.02.023. [DOI] [Google Scholar]
- 23.Das B., Thirupathi P., Mahender I., Reddy V.S., Rao Y.K. J. Mol. Catal. A: Chem. 2006;247:233. doi: 10.1016/j.molcata.2005.11.048. [DOI] [Google Scholar]
- 24.Zhang Z.H., Tao X.Y. Aust. J. Chem. 2008;61:77. doi: 10.1071/CH07274. [DOI] [Google Scholar]
- 25.Rahimifard M., Mohammadi Ziarani G., Badiei A., Asadi S., Abdolhasani A. Res. Chem. Inter. 2016;42:3847. https://link.springer.com/article/10.1007/s11164-015-2248-2 [Google Scholar]
- 26.Madje B.R., Ubale M.B., Bharad J.V., Shingare M.S. S. Afr. J. Chem. 2010;63:36. http://journals.sabinet.co.za/sajchem [Google Scholar]
- 27.Aakruti R.S., Meryl Maria G., Iniyavan P., Sreekath A., Vijayakumar V. Int. J. Chem. Tech. Res. 2014;6:3979. https://www.researchgate.net/publication/268148287 [Google Scholar]
- 28.Rezayati S., Hajinasiri R., Erfani Z., Rezayati S., Afshari-Sharifabad S. Iran. H. Catal. 2014;4:157. [Google Scholar]
- 29.Massouri B., Ahmadynezhad M., Poor Heravi M.R. Lett. Org. Chem. 2013;10:302. [Google Scholar]
- 30.Nemati F., Sabaqian S. J.Saudi. Chem. Soc. 2013;21:383. doi: 10.1016/j.jscs.2014.04.009. [DOI] [Google Scholar]
- 31.Moghanian H., Mobinikhaledi A., Deinavizadeh M. Res. Chem. Intermed. 2014;10:1007. doi: 10.1016/j.cclet.2014.12.007. [DOI] [Google Scholar]
- 32.Kumari S., Shekhar A., Pathak D. Chem. Sci. Trans. 2014;3:652. doi: 10.7598/cst2014.783. [DOI] [Google Scholar]
- 33.Rahmani S., Amoozadeh A. J Nanostruct. 2014;4:91. [Google Scholar]
- 34.Shoja A., Shirini F., Abedini M., Zanjanchi M.A. J. Nanostruct. Chem. 2014;4:110. doi: 10.1007/s40097-014-0110-5. [DOI] [Google Scholar]
- 35.Karami B., Nejati S., Eskandari K. Cur. Chem. Lett. 2015;4:169. doi: 10.5267/j.ccl.2015.5.001. [DOI] [Google Scholar]
- 36.Zhou Z., Deng X. J. Mol. Catal. A Chem. 2013;367:99. doi: 10.1016/j.molcata.2012.11.002. [DOI] [Google Scholar]
- 37.Kantevari S., Bantu R., Nagarapu L. J. Mol. Catal. A Chem. 2007;269:53. doi: 10.1016/j.molcata.2006.12.039. [DOI] [Google Scholar]
- 38.Das P.J., Das J. RSC Adv. 2015;5:11745. doi: 10.1039/C4RA12298D. [DOI] [Google Scholar]
- 39.Chavan A.P. Bull Korean Chem Soc. 2003;53:4. doi: 10.3184/030823403103174209. [DOI] [Google Scholar]
- 40.Gholap A.R., Chavan AP. J. Chem. Research. 2003;2003(6) doi: 10.3184/030823403103174209. [DOI] [Google Scholar]
- 41.Sangwan R., Saini M., Verma R., Kumar S., Banerjee M., Jain S. J. Mol. Struct. 2020;1208 doi: 10.1016/j.molstruc.2020.127786. [DOI] [Google Scholar]
- 42.Hojati S.F., Moosavifar M., Ghbali N.M. J. Chem. Sci. 2020;132:38. doi: 10.1007/s12039-020-1736-0. [DOI] [Google Scholar]
- 43.Wu X., Peng W.X. J Chin Chem Soc. 2020;67:11. doi: 10.1002/jccs.202000087. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.