Abstract
Atopic dermatitis (AD) is a chronic and pruritic skin inflammatory disease causing a significant burden to health care management and patient’s quality of life. Seemingly healthy skin or non-lesional sites on AD patients still presents skin barrier defects and immune response, which can develop to AD at a later stage. To investigate further the balance between the epidermal barrier impairment and intrinsic immune dysregulation in AD, we exploited multispectral Raster-Scanning Optoacoustic Mesoscopy (ms-RSOM) to image lesional and non-lesional skin areas on AD patients of different severities non-invasively to elucidate their structural features and functional information. Herein, we demonstrate the objective assessment of AD severity using relative changes in oxygen saturation (δsO2) levels in microvasculature along with other structural parameters such as relative changes in epidermis thickness (δET) and total blood volume (δTBV) between the lesional and non-lesional areas of the skin. We could observe an increasing trend for δsO2 and δTBV, which correlated well with the subjective clinical Scoring Atopic Dermatitis (SCORAD) for evaluating the severity. Notably, δET showed a decreasing trend with AD severity, indicating that the difference in epidermal thickness between lesional and non-lesional area of the skin decreases with AD severity. Our results also correlated well with conventional metrics such as trans-epidermal water loss (TEWL) and erythrosine sedimentation rate (ESR). We quantified the δsO2 and δET changes to objectively evaluate the treatment response before and four months after treatment using topical steroids and cyclosporine in one severe AD patient. We observed reduced δsO2 and δET post treatment. We envision that in future, functional and structural imaging metrics derived from ms-RSOM can be translated as objective markers to assess and stratify the severity of AD and understand the function of skin barrier dysfunctions and immune dysregulation. It could also be employed to monitor the treatment response of AD in regular clinical settings.
Keywords: Multispectral optoacoustic imaging, Atopic dermatitis, Oxygen saturation, Epidermis thickness, Total blood volume, Non-lesional atopic dermatitis
1. Introduction
Atopic dermatitis (AD) is a chronic, pruritic skin inflammatory disease, which can create a huge burden for patients in terms of disease management as well as quality of life. Accurate and non-invasive evaluation of AD is vital in determining its severity, longitudinal response to therapeutic interventions and treatment management [1]. Currently, AD severity in the clinics is assessed using Eczema Area Severity Index (EASI) or Scoring AD (SCORAD), which have their own limitations [1], as they rely on direct inspection of the skin and are subjected to intra- and inter-observer variations depending on the assessors’ experience [2]. Partial EASI (p-EASI) uses serum biomarkers to assess disease severity objectively, but reflects clinical status in an indirect manner [3]. Biopsies allow direct objective assessment of the skin ultra-structures and even detection of subclinical skin inflammation in AD, but it is invasive and impractical in clinical settings [4]. There currently lacks an ideal objective scoring method that is easy to measure and yet sensitive to change with minimal bias, which would be of great value to the classification of severity of disease, prediction of disease trajectory and monitoring of treatment response [5]. Furthermore, there were studies reported that clinically normal-appearing skin or non-lesional areas in AD patients is characterized by the presence of inflammation albeit to a lesser extent than lesional area and that it is different from healthy normal skin [6], [7]. Non-lesional AD skin is also reported to exhibit a degree of skin barrier dysfunction coupled with increased water loss and decreased water content in the uppermost stratum corneum layer of the skin [8].
Optoacoustic (photoacoustic) imaging (OAI) is a non-invasive imaging technique using a pulsed laser for illumination and an ultrasound transducer for the detection of acoustic signals using the principle of thermoelastic expansion of tissue [9]. OAI can provide scalable imaging depth and resolution with structural, molecular and functional information. Optoacoustic tomography, a macroscopic version of OAI technique uses detector with lower central frequency collect the signals emitted by bigger vascular structures in longer wavelengths, which scatters less and resulting in deeper penetration depths, but poor lateral resolution. It was used for monitoring hemodynamic changes in foot and muscle tissues [10], [11], [12] and assessing breast tumor margins [13]. However, this configuration is not suitable to image and resolve the microvasculature in the dermis region owing to poor lateral resolution. On the contrary, optoacoustic microscopy, a higher resolution configuration of OAI system, whose resolution depending on optical focusing could be able to image microvasculature and its vascular response using two wavelengths [14]. However, the reported optoacoustic microscopy is limited by imaging depth of up to tens of microns below the skin surface [15]. The required imaging depth for the assessment of AD should provide detailed image of the blood vessels in the dermis region, usually 1 to 3 mm from the skin surface.
Raster-scanning optoacoustic mesoscopy (RSOM) is a novel high-resolution version of OAI system, ideally suited for imaging skin microvasculature using wideband ultrasound detector, allowing relatively deeper imaging (∼2 mm) with high lateral resolution of ∼40 µm [16]. A single wavelength RSOM was previously used to image and deduce specific structural metrics such as epidermis thickness, vessel diameter in the dermis, and capillary loop density in the dermal layers to study eczema and psoriasis [17], [18], [19]. The ability to monitor vascular structures and skin layers in a non-invasive manner allows clinicians to directly detect any subclinical inflammation.
In our previous study [17], using single wavelength RSOM, we have shown that there were apparent differences in specific metrics, such as epidermis thickness, total blood volume, vessel diameter in the dermis, and the ratio of low- and high-frequency signals in the dermis among AD skin of varying severities. These metrics was also used to monitor the response in an eczema subject after biologics treatment [20]. We have also proposed a quantitative classification of severity using structural features and explored multiple machine learning models for objective severity classification [17], [21]. Based on the findings that non-lesional AD skin is characterized by skin barrier dysfunction, the authors question the hypothesis that vascular structural and functional re-modelling is an essential pre-requisite in the pathogenesis of non-lesional areas in AD patients, regardless of AD severity. Herein, a multispectral Raster-Scanning Optoacoustic Mesoscopy (ms-RSOM) having high repetition rate laser with four distinct wavelengths was employed to spectrally unmix skin chromophores such as melanin, oxy-hemoglobin (HbO2), deoxy-hemoglobin (Hb) and to deduce functional information such as oxygen saturation (sO2) in skin microvasculature, which was unavailable in a single-wavelength RSOM system before. The acoustic signals generated at each individual wavelength were detected using a wideband transducer. The ms-RSOM system allows us to image oxygenation in tissue and characterize the morphological and physiological changes between non-lesional and lesional AD skins of varying severities.
In this study, we present the functional information such as changes in oxygen saturation in the skin microvasculature as a biomarker for the first time to assess the severity of the AD inflammatory skin condition. For the first time to our knowledge, we also investigated non-invasively the differences in the subclinical structural features of the skin between lesional and non-lesional areas in different severities of AD. The objective quantification of treatment response using topical steroids in an AD patient was also demonstrated by the ms-RSOM derived metrics such as relative changes in oxygen saturation and epidermal thickness. The functional information of skin microvasculature (sO2) could be a novel, objective biomarker for non-invasive clinical investigation of inflammatory skin diseases and their response to treatment.
2. Methods
2.1. AD patients
Sixteen patients from the adult eczema clinic at National Skin Centre, Singapore, were recruited. Hanifin and Rajka diagnostic criteria was used for the diagnosis of AD. Out of the 16 AD patients, 5 had mild and moderate each while 6 had severe AD. The disease severity of each patient was assessed using the Scoring Atopic Dermatitis (SCORAD) and stratified as mild, moderate, or severe according to the SCORAD values of < 25, 25–50 and above 50 respectively [14]. Optoacoustic imaging using ms-RSOM was performed on all patients over two areas on the ventral forearm, one over a representative AD skin lesion and the other over a non-lesional area at least 2 cm away from the identified lesional area. Each image was acquired from a 5 mm × 3 mm area. The study was approved by National Healthcare Group (NHG) Domain Specific Review Board (DSRB), Singapore (Ref No. 2020/00079). Patient’s informed consent was obtained in compliance with the institutional approvals. Demographic details of the patients are provided in the supplementary information (SI).
2.2. Multispectral RSOM system
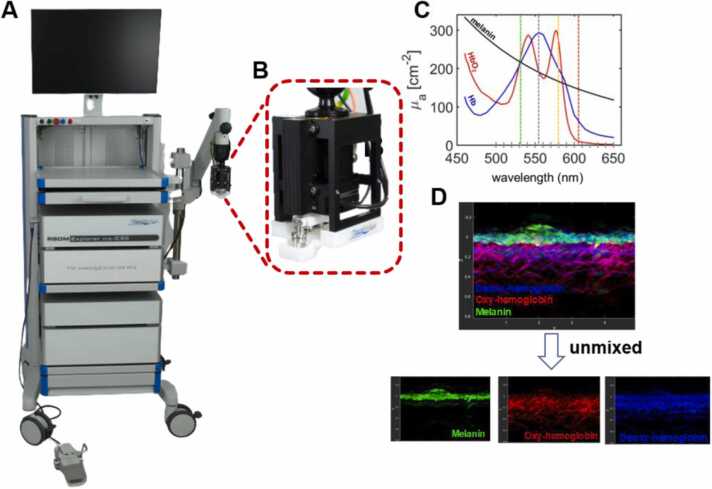
RSOM Explorer ms-C50 (iThera Medical GmbH, Munich, Germany) system used for imaging is shown in Fig. 1. We have described the system in detail in our previous publication [22]. Briefly, a nanosecond Raman laser having four distinct wavelengths (532, 555, 579, 606 nm) with a pulse energy ∼20 µJ, pulse width of ≤ 3.2 ns, repetition rate 1.3 kHz was used to illuminate the tissue and raster scanning was conducted over an area of 5 × 3 mm. An ultra-broadband transducer (center frequency 50 MHz, bandwidth 11–99 MHz) was used for detecting the acoustic signals. The scanning head (Fig. 1B) comprises of the two-axis motorized illumination and detection part, which will be in contact with the skin surface using a water-filled unit having an interchangeable polyethylene membrane in the bottom. The ms-RSOM system can provide high quality 3D images of skin morphology and vascular structures up to a depth of 2 mm from skin surface to dermis region, and with an axial and lateral resolution of up to 10 µm and 40 µm respectively. Depending on the intrinsic absorption coefficients in the visible wavelength range (Fig. 1C), visualization of different chromophores such as melanin, HbO2, Hb and the overlaid images was achieved (Fig. 1D). Functional information, oxygen saturation (sO2), of microvasculature can also be quantified using these parameters.
Fig. 1.
Experimental imaging (ms-C50) clinical prototype system. (A) Photograph and (B) imaging head of the system; (C) absorption spectra of endogenous chromophores in the skin from 450 to 650 nm and four laser wavelengths are shown in vertical dash line; (D) Representative vertical cross-sectional ms-RSOM image, with merged and unmixed images of melanin, oxy-hemoglobin and deoxy-hemoglobin.
2.3. Image processing
Multi-spectral RSOM system includes multi-wavelength pulsed laser source and ultra-wideband ultrasound detector. Absorption of light by different chromophores, such as melanin, deoxyhemoglobin and oxyhemoglobin, resulting in the generation of pressure waves that can be detected using an ultrasound transducer and reconstruct into images. The beam-forming algorithm was used to reconstruct each single wavelength image separately [23], [24]. Four distinct wavelengths (532 nm, 555 nm, 579 nm and 606 nm) were chosen in the volumetric imaging mode. Motion correction algorithm was applied onto the reconstructed multispectral data to inherently co-register with laser in wavelength sweep mode [25], followed by spatial fluence correction. A linear regression algorithm with non-negative constraint [26] was used to unmix the different skin chromophores based on their respective absorption spectra in the induced light wavelengths [27], shown in Fig. 1C. Fig. 1D shows the vertical maximum intensity projection of the volumetric multispectral 3D image along the depth. The top epidermal layer with strong absorption by melanin is primarily without any noticeable haemoglobin signal. The dermis region features vascular network characterized by absorption of both Hb and HbO2. Using ms-RSOM, the oxygen saturation could be measured in single vessel level. In this study, we calculated the oxygen saturation changes in the dermis region.
2.4. Analysis of specific imaging metrics using ms-RSOM
We investigated specific metrics derived from the ms-RSOM images from the subjects. We computed (1) differential oxygen saturation (δsO2), (2) differential epidermis thickness (δET), and (3) differential total blood volume (δTBV). Quantitative analysis of each metric for different AD severities were plotted.
2.4.1. Differential oxygen saturation
Depending on the absorption coefficient of hemoglobin from the representative individual wavelength images, HbO2 and Hb in the dermis were un-mixed. The oxygen saturation (sO2) was then calculated by the equation . Differential oxygen saturation (δsO2), the ratio of sO2 from the lesional area over that from non-lesional AD area were computed and plotted for each AD severity.
2.4.2. Differential epidermis thickness
An in-house developed automatic segmentation algorithm was first used on the unmixed melanin 3D ms-RSOM image stack, followed by calculating the melanin vertical optoacoustic profile along the direction of skin depth to define the boundary between the epidermis and dermis, obtained by averaging all pixels in x-y plane along the depth (z-axis). The first dominant peak represents the center of melanin layer. We obtained the bottom edge of melanin layer by using the full width at half maximum (FWHM). The distance from the skin surface to the end of melanin layer was taken as the epidermis thickness in our study since melanin is located in the basal layer of the epidermis. The ratio of epidermis thickness from the lesional area over that from non-lesional AD area were computed as differential epidermis thickness.
2.4.3. Differential total blood volume (TBV)
We calculated the TBV based on the in house-built image segmentation, followed by applying a threshold (20% of the maximum voxel value) to the dermal region of merged ms-RSOM 3D images. Then a binary mask was obtained by setting the voxels to be 0 if its value is below the threshold, and the rest of voxels to be 1. TBV is calculated as the total number of voxels with value of 1 in the segmented 3D dermal region, with the expression TBV = ∑ N × dV, where N is the number of voxels with value of one in the certain voxel volume dV. The ratio of TBV from the lesional area over that from non-lesional AD area was computed as differential total blood volume.
2.5. Transepidermal water loss measurement
Trans epidermal water loss (TEWL) is the amount of water that evaporates from the dermis through the epidermis into the external environment. TEWL was measured using a wireless portable device called VapoMeter (Delfin Technologies, Finland). An average of three separate measurements were taken for accurate results. Measuring TEWL is useful for identifying skin conditions like eczema, as the increasing rates of TEWL will indicate the damaged skin barrier level.
2.6. Erythrosine sedimentation rate measurement
Erythrosine sedimentation rate (ESR) indirectly measures the presence of inflammation in the body by detecting how fast erythrocytes settle to the bottom of a test tube containing blood. ESR was measured by ALCOR iSED erythrocyte sedimentation rate analyzer in unit of mm/hr.
2.7. Treatment response
One severe AD patient with SCORAD value 61.5 was imaged at baseline and after four months of treatment with topical steroids and cyclosporine. Images were acquired from the non-lesional and lesional areas of the patient and metrics such as δET and δsO2 were calculated at baseline and post-treatment.
3. Results and discussion
3.1. Biophysical and laboratory assessment of AD patients
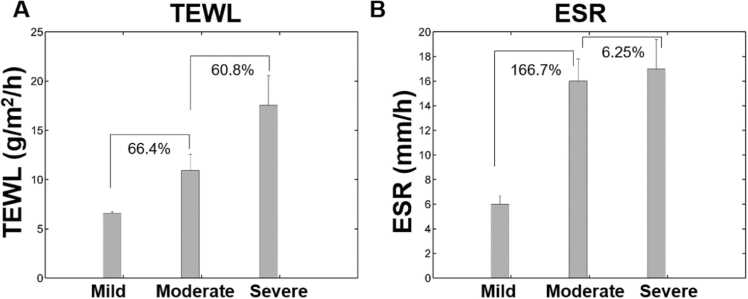
As an indicator of skin barrier function, TEWL measures the passive diffusion of water through the stratum corneum. Skin barrier dysfunction may be an important antecedent of dermatitis inflammation [28]. Fig. 2A suggests that the mean TEWL of the AD patients increased with AD severity as reported in literature [29]. TEWL increased by 66.4% from mild to moderate, and 60.8% from moderate to severe AD conditions.
Fig. 2.
Relation of (A) transepidermal water loss (TEWL) and (B) erythrosine sedimentation rate (ESR) with respect to AD severity based on SCORAD.
ESR on the other hand is an indicator of the systemic inflammatory activity in one’s body. We could observe that ESR values were significantly different among patients with varying AD severities. It increased by ∼167% from mild to moderate AD severity (Fig. 2B). Interestingly, the ESR increase was to a lesser degree (∼6%) from moderate to severe subgroups. This shows that ESR value correlated very well as reported by Jiang et al. [30]. Evidently, as TEWL and ESR were elevated with increasing disease severity, both skin barrier impairment and immune dysregulation form the hallmark etiologies of AD pathology.
3.2. ms-RSOM derived imaging metrics of AD skin
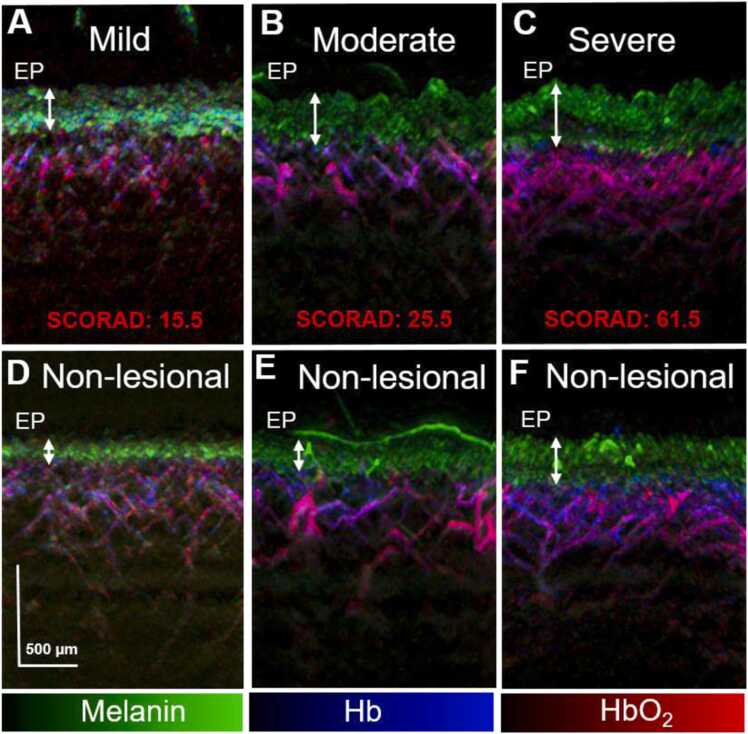
Fig. 3 shows representative ms-RSOM vertical cross-sectional images exhibiting the spatial maps for melanin, HbO2, and Hb in lesional and non-lesional areas in AD patients of different severities, revealing significant morphological and vasculature differences. The superficial epidermal layer is differentiated by the presence of melanin (green signals). The dermal layer, underneath the epidermis, features a vascular network distinguished by the absorption of Hb and HbO2 (blue and red signals respectively). In general, the epidermal layer thickness increased with AD severity in both lesional and non-lesional areas with the epidermal layer in the lesional area thicker than its corresponding non-lesional area for different severities. Interestingly, the epidermis thickness in the non-lesional area of the skin in the severe case exhibited almost the same value as its corresponding lesional area. This may indicate that the uninvolved AD areas appear to be characterized by subclinical (and thus undetectable by the naked eye) inflammation, which can potentially develop to symptomatic AD lesions. This is corroborated by the elevated ESR values exhibited by the severe AD subgroup (Fig. 2B). The vascular plexus in the dermis underwent remodelling as the AD severity increased with more intense haemoglobin signals present in the severe lesional case compared to that of mild and moderate cases (Fig. 3). In the pathogenesis of AD, there is disruption of the skin barrier, followed by sensitization and inflammation. In severe AD, as an inflammatory mediator, vascular endothelial growth factor is activated to a larger extent as indicated by its high ESR measurements, resulting in endothelial cell proliferation, increased blood flow due to vessel dilation and edema formation from increased vessel permeability [31].
Fig. 3.
Maximum intensity projection (MIP) images from lesional area from a representative (A) mild, (B) moderate (C) severe AD subject, and from their corresponding non-lesional areas respectively (D-F), showing the merged melanin (green), Hb (blue) and HbO2 (red) spatial optoacoustic signals. The epidermal (EP) layer is denoted with white arrows. Scale bar; 500 µm.
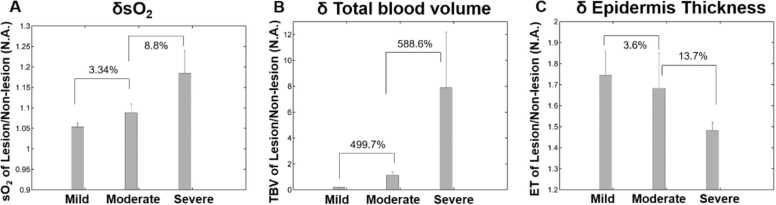
Fig. 4 shows the differential sO2, ET and TBV values calculated for varying AD severities. Notably, the sO2 of lesional areas are higher than that of non-lesional areas (as δsO2 >1) for all severities of AD, possibly due to the high blood flow towards the sites involved in inflammation (Fig. 4A), which is consistent with previous reports utilizing other optical imaging modalities [32]. Importantly, the ratio of sO2 between lesional and non-lesional areas increased with AD severity. The δsO2 in severe AD was 8.8% higher than that of moderate case, while the δsO2 in moderate severity was 3.34% higher than that of mild AD skin. Similar to δsO2, the δTBV values also increased with increasing AD severity. δTBV in severe AD was 5.9 times higher than that in moderate AD and approximately 30 times higher compared to that of mild AD (Fig. 4B). The increasing TBV is indicative for one of the inflammatory responses in which the vessels get dilated due to blood flow to the sites of inflammation to get more nutrients. The ratio of TBV between the lesion and non-lesion areas dramatically increased with AD severity showing that non-lesional areas in severe cases had less inflammatory response than the lesional areas.
Fig. 4.
Relation of (A) differential oxygen saturation (δsO2); (B) differential total blood volume (δTBV) and (C) differential epidermal thickness (δET) with respect to AD severity.
On the other hand, δET decreased with increasing severity of AD patients (Fig. 4C), while values are larger than 1 for all severities. It showed that the ET of lesional areas are thicker than that of non-lesional areas. The mean differential epidermal thickness for mild eczema skin was 3.6% higher than that of moderate AD, which in turn was 13.7% higher than that of severe AD. The thicker epidermal layer in lesional area compared to non-lesional area can be related to lichenification, which is due to chronic inflammation and scratching. However, this indicates that the difference in epidermal thickness between involved and uninvolved AD areas became less pronounced and similar to each other as the severity of AD increases. Compared to the corresponding lesional areas, uninvolved areas in severe AD cases may not display acute intrinsic inflammation (lower sO2 values of vasculature) but may still present thickened epidermis. The thickened epidermis may limit the imaging depth due to high melanin absorption in the visible wavelengths. In this study, we recruited subjects of Fitzpatrick skin types from III to IV to minimize the potential impact of difference in light fluence at all wavelengths. It is well established from literature that non-lesional skin areas of AD patients can remain abnormal compared to healthy skin. These subclinical abnormalities are perhaps largely due to the epidermal barrier dysfunction and less so from the systemic immune response [33]. Identification of the factors contributing to the subclinical structural irregularities in the unaffected skin can expedite the management of severe AD with effective treatment response.
The strengths of our study includes the assessment of oxygen saturation in microvessels at high resolution and its comparison with non-lesional and lesional skin. However, imaging was done at a single time point and therefore we could not assess the sensitivity of the imaging system to longitudinal changes. While skin biopsies to allow a direct histopathological and imaging correlation were not done in this study, the structural features observed with ms-RSOM have been previously validated against histology and video capillaroscopy [34]. The ability of ms-RSOM to image the skin at multiple wavelengths allow for the spatial visualization of the different chromophores, so as to quantify the oxygen saturation of the microvasculature. This enables the diagnosis and surveillance of associated inflammatory conditions. Compared to capillaroscopy, ms-RSOM provides high quality 3D images of the microvasculature of the encompassing deep dermis.
3.3. Treatment response monitoring
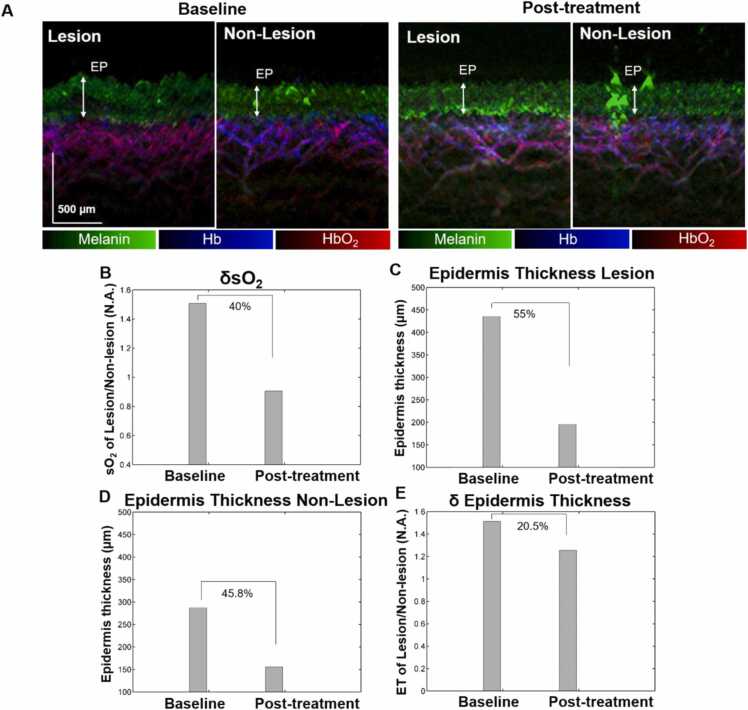
A single severe AD patient undergone treatment with topical steroids and cyclosporine was imaged at baseline and post-treatment (Fig. 5). The difference between the vascular structures of lesional and non-lesional areas at baseline became less pronounced after treatment (Fig. 5A). After treatment, the vascular structure of the lesional area became more regular with less HbO2 (red) signals , presumably due to the reduced inflammation. After treatment, the epidermal layer of the lesional area became thinner, making the difference in epidermal thickness between lesional and non-lesional area smaller. Notably, the non-lesional area has a thinner epidermal layer than the lesional area at baseline and post-treatment. It was observed that the δsO2 was reduced significantly (40%) after treatment indicating reduced inflammation in the lesional AD sites when compared to unaffected skin areas (Fig. 5B). ET in the lesional area reduced from 436 µm to 196 µm post treatment (Fig. 5C), while ET in the non-lesional area is reduced slightly from 288 µm to 156 µm post treatment (Fig. 5D) as application of topical steroids to uninvolved skin areas are known to result in skin thinning [35]. Overall, after the treatment, δET was reduced by 20.5% (Fig. 5E) indicating a lower difference in ET between lesional and non-lesional skin sites.
Fig. 5.
(A) Maximum intensity projection (MIP) images of lesional and non-lesional areas at baseline and post-treatment using topical steroids and cyclosporine; Quantifications of (B) differential oxygen saturation (δsO2); Epidermis thickness of lesional area (C) and non-lesional area (D) before and after treatment; (E) differential epidermal thickness (δET) of lesional AD and non-lesional AD areas before and after treatment. The epidermal (EP) layer is denoted with white arrows. Scale bar; 500 µm.
In general, for AD treatment, topical steroids result in localized vasoconstriction by reducing the size of keratinocytes and inhibiting their proliferation [36]. It has potentially irreversible atrophogenic effects. While oral cyclosporine as an immunosuppressant [37] is very useful to provide rapid relief in patients with severe AD, but it is not long-lasting. The quantification of cutaneous atrophy in the context of topical steroids application could be achieved by ultrasonography and confocal laser scanning microscopy limited by the low-resolution 2D images or high-resolution images at shallow imaging depths. Our ms-RSOM system can provide high-resolution 3D images of the skin morphology and vascular structures up to 2 mm. It could also provide the functional information of microvasculature. A higher percentage of decrease in δsO2 can be observed, which shows the immune response is most affected by the treatments and less to the epidermal thickness. This is probably because alleviation of topical steroids will induce highly proliferating cells in the epidermis, increasing the epidermal thickness due to the viable epidermis with larger cells of irregular sizes and shapes [38]. Therefore, the balance between atrophogenic effect of topical steroids and the rebounding effect of epidermal hyperplasia might result in less dramatic decrease in epidermal thickness from the treatment [39]. The main side effect of topical steroids is dermal thinning, which we did not observe for this AD patient.
Summary
In this study, the use of a novel high-resolution multispectral optoacoustic system for imaging inflammatory skin conditions was presented. Quantitative analysis of ms-RSOM derived imaging metrics including relative changes in oxygen saturation, epidermal thickness, and total blood volume, could quantitatively differentiate the lesional and non-lesional skin areas. This technique provides imaging derived metrics in an objective and non-invasive manner in a short turn-around time with low variability. This study presented the first report on the functional changes of oxygen saturation in skin microvasculature as a marker to gauge the severity of AD. Our study also revealed that in the case of severe AD, the difference in the subclinical structural features of the skin between lesional and non-lesional part is indeed minimal. The differences in the structural features between lesional and unaffected areas is thought to be mostly by virtue of epidermal barrier dysfunction and less so from the systemic immune response. This study also demonstrated the significance of ms-RSOM derived metrics to objectively quantify the treatment response using topical steroids in an AD patient. In the era of targeted and personalized therapy, non-invasive skin imaging technologies such as ms-RSOM offers great potential in better understanding of the disease and its response to treatment. In this context, this study can open up new avenues for understanding inflammatory skin conditions and also for the formulation of new therapies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Medical Research Council [grant number NMRC OF-IRG Grant OFIRG18nov-0101] and funding support from the Agency for Science, Technology and Research’s (A*STAR) BMRC Central Research Fund (CRF, UIBR) Award. Authors would like to acknowledge the support from iThera Medical as a part of the SBIC-iThera joint medical lab.
Biographies

Xiuting Li is currently a senior research fellow in Institute of Bioengineering and Bioimaging (IBB), A*STAR. She received her Ph.D. degree in Physics and Applied Physics from School of Physical and Mathematical Sciences (SPMS), Nanyang Technological University (NTU), 2013. Her research interests are in bioinformatics, image processing and analysis, machine learning and artificial intelligence.

Mohesh Moothanchery graduated with an Integrated Masters in Photonics from Cochin University of Science and Technology (CUSAT), India in 2009. He was awarded his doctoral degree (PhD) from Technological University Dublin (TUD), Ireland in October 2013. He worked as a Senior Researcher at the HiLASE Centre, Academy of Sciences of the Czech Republic (ASCR) and as a Research Fellow at Nanyang Technological University (NTU), Singapore before joining, Agency for Science, Technology and Research (A*STAR), Singapore. He is currently the Technical Manager at the Centre for Preclinical Imaging, University of Liverpool, United Kingdom. His research interests are in the field of biomedical instrumentation and imaging.

Kwa Cheng Yi earned his BEng (Hons) in Bioengineering from School of Chemistry, Chemical Engineering and Biotechnology (CCEB), formerly known as School of Chemical and Biomedical Engineering (SCBE), at Nanyang Technological University (NTU) in 2021. He is currently a Research Assistant in National Skin Centre (NSC). He is trained to operate various imaging modalities and is actively involved in skin imaging research at NSC.

Wan Ling Tan is a research officer of A*STAR Skin Research Labs (A*SRL), A*STAR. She graduated with BSc (Hons) in Biomedical Science from Universiti Tunku Abdul Rahman (UTAR), Malaysia. Her research interests are imaging for skin cancer and skin diseases.

Yik Weng Yew (Asst Prof) is a Consultant Dermatologist at the National Skin Centre (NSC) with strong clinical and research interests in atopic dermatitis (AD), psoriasis dermato-epidemiology and disease outcomes research, with close to 60 peer reviewed scientific publications. He is currently the deputy head of the NSC Research Division and head of Eczema clinic, NSC. He obtained his MBBS from the Yong Loo Lin School of Medicine, NUS in 2006 and completed his training as a dermatologist in 2014 at NSC. After his dermatology training, he obtained a Master of Public Health to further his training in epidemiology research from the Harvard School of Public Health, USA in 2015 as part of his Health Manpower Development Plan award. He was awarded the National Healthcare Group Clinician Scientist Career Scheme award in 2016 and started his PhD studies at Lee Kong Chian School of Medicine, NTU, Singapore to study disease prevalence and risk factors associations of atopic dermatitis in a general adult population cohort. He has also obtained numerous grants, notably the National Medical Research Council (NMRC) New Investigator Grant in 2018 to study treatment of nail psoriasis with microneedles and recently the NMRC Transitional Award in July 2020 to investigate the effects of adiposity on skin barrier function in a general population cohort. He is active in various types of research in AD and collaborates with various local and overseas institutes on population-based cohort studies, clinical trials, basic and translational AD research on omics, skin microbiome and skin bioimaging.

Steven Tien Guan Thng (Assoc Prof) graduated from National University of Singapore in 1992 and went on to pursue his interest in Humanitarian and Disaster medicine with the Singapore Armed Forces from 1992 to 2002. After completing his dermatology training in Singapore, Dr Steven Thng went on to Amsterdam Medical Centre, Netherlands, to continue his training in pigmentary disorder, under Professor Westerhof. Upon returning from his training, Dr Steven Thng started the pigment clinic in National Skin Centre as well as set up the melanocyte culture lab to start his work on cultured melanocytes grafting for vitiligo patients. Currently, Dr Thng is the head of the pigment clinic, in charge of managing all complex pigmentary disorders in National Skin Centre, and he is also the principal surgeon for tissue grafting for vitiligo. In 2012, he has also started a research clinic for hyperpigmentary disorders exploring novel ways to manage difficult hyperpigmentary conditions. He took over as Executive Director of Skin Research Institute of Singapore from Jan 2017 to Feb 2020, and was responsible for the transformation of SRIS from a virtual institute to a dynamic institute, working on 4 main multi-institutional, interdisciplinary research programs of chronic wound, acne, skin microbiome and atopic dermatitis. His current research interest is on skin pigmentary disorder, novel drug delivery systems, as well as advanced imaging for skin cancer and skin diseases. He has translated much of his research to improve patient care in dermatology. He has been serving as the Chief Dermatologist of Skin Research Institute of Singapore from Feb 2020. For his dedication in research and patient care, he was awarded Excellence in Public Service Award in 2013, Healthcare Humanity Award in 2014, Singapore Clinician Investigator Award in 2015 and 2016.

U. S. Dinish is the Group Leader in Translational Biophotonics Laboratory at Institute of Bioengineering and Bioimaging (IBB), A*STAR, Singapore. He also holds a joint adjunct faculty position at School of Physical and Mathematical Sciences (SPMS), Nanyang Technological University (NTU), Singapore. He has extensive expertise in various preclinical and clinical studies using Photoacoustic imaging, diffuse reflectance spectroscopy (DRS), Raman spectroscopy, surface enhanced Raman scattering (SERS, fluorescence imaging and multimodal imaging approaches. He is the lead PI/Co-PI of many national and international research grants on preclinical and clinical bioimaging/sensing studies. He holds 19 patents/patent applications and has published 4 book chapters, over 130 international journal papers and conference proceedings. He co-edited a book titled ‘frontiers in biophotonics for translational medicine’ (Springer) in 2015. Currently, he is serving as the editorial board member of ‘Scientific Reports’ (Nature Publishing Group) and also as a consulting editor for ‘International Journal of Nanomedicine’ (Dove press). He is also acting as series editor for Springer’s book series titled, ‘Progress in Optical Science and Photonics’.

Amalina Ebrahim Attia earned her BEng (Hons) in Chemical Engineering from National University of Singapore (NUS). She received her PhD in Drug Delivery from NUS Graduate School for Integrative Sciences and Engineering. She is currently a Research Scientist in Institute of Bioengineering and Bioimaging (IBB), Singapore. Her current research focuses on design and development of pre-clinical and clinical applications for photoacoustic and other optical imaging modalities.

Malini Olivo (Prof) is the Deputy Executive Director of Institute of Bioengineering and Bioimaging (IBB), Agency for Science Technology and Research (A*STAR) Singapore and the Director of Biophotonics and Head of the Translational Biophotonics Laboratory at IBB, A*STAR where she leads efforts to establish a clinical biophotonics translational platform programme. Concurrently, she is also the Co-Executive Director of the A*STAR Health & MedTech Horizontal Technology Programme Office, where she spearheads and coordinates MedTech R&D between A*STAR and the national healthcare ecosystem. Prof. Malini Olivo is also an Adjunct Professor, Lee Kong Chian School of Medicine, NTU, Department of Obstetrics & Gynaecology, National University Health System, NUS, Singapore and Royal College of Surgeons Ireland, Dublin Ireland. She has published over 400 papers (including several contributions in high impact journals), 3 books and 10 book chapters, and filed 30 patents on technology platforms and devices. She has initiated 3 first-in-human studies using photoacoustic technology in humans for skin and breast cancer and eczema in the last 3 years. She is also founder of 2 MedTech start-ups in Singapore. For her pioneering work on developing biophotonics and decades of experience in translating biophotonics from bench to the clinic, Prof. Malini Olivo was elected to the American Institute for Medical and Biological Engineering (AIMBE) College of Fellows in Washing D.C., U.S.A., in 2019. She is also a fellow member of the Optical Society of America for pioneering and contributing to the field of photomedicine in the seminal area of cancer diagnostics and therapeutics. She is a Fellow of institute of Physics in UK and Ireland. She champions women in STEM research as a pioneer in the field of biomedical physics.
Contributor Information
U.S. Dinish, Email: dinish@ibb.a-star.edu.sg.
Amalina Binte Ebrahim Attia, Email: Amalina_Attia@ibb.a-star.edu.sg.
Malini Olivo, Email: Malini_Olivo@ibb.a-star.edu.sg.
Data availability
The authors do not have permission to share data.
References
- 1.Paller A.S., Kabashima K., Bieber T. Therapeutic pipeline for atopic dermatitis: End of the drought. J. Allergy Clin. Immunol. 2017;140:633–643. doi: 10.1016/J.JACI.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt J., Langan S., Williams H.C. What are the best outcome measurements for atopic eczema? A systematic review. J. Allergy Clin. Immunol. 2007;120:1389–1398. doi: 10.1016/J.JACI.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Thijs J.L., Fiechter R., Bruijnzeel-Koomen C.A.F.M., Knol E.F., Giovannone B., de Bruin-Weller M.S., Hijnen D.J., Thijs J.L., Drylewicz J., Fiechter R., Nierkens S., Hijnen D.J., Strickland I., Sleeman M.A., Herath A., May R.D., Nierkens S. EASI p-EASI: utilizing a combination of serum biomarkers offers an objective measurement tool for disease severity in atopic dermatitis patients. J. Allergy Clin. Immuno. 2017;140:1703. doi: 10.1016/J.JACI.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 4.Kim B.E., Goleva E., Kim P.S., Norquest K., Bronchick C., Taylor P., Leung D.Y.M. Side-by-side comparison of skin biopsies and skin tape stripping highlights abnormal stratum corneum in atopic dermatitis. J. Invest. Dermatol. 2019;139:2387–2389. doi: 10.1016/J.JID.2019.03.1160. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chopra R., Silverberg J.I. Assessing the severity of atopic dermatitis in clinical trials and practice. Clin. Dermatol. 2018;36:606–615. doi: 10.1016/J.CLINDERMATOL.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Hamid Q., Boguniewicz M., Leung D.Y.M. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J. Clin. Investig. 1994;94:870–876. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung D.Y.M., Boguniewicz M., Howell M.D., Nomura I., Hamid Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cork M.J., Danby S.G., Vasilopoulos Y., Hadgraft J., Lane M.E., Moustafa M., Guy R.H., MacGowan A.L., Tazi-Ahnini R., Ward S.J. Epidermal barrier dysfunction in atopic dermatitis. J. Invest. Dermatol. 2009;129:1892–1908. doi: 10.1038/JID.2009.133. [DOI] [PubMed] [Google Scholar]
- 9.Moothanchery M., Bi R., Kim J.Y., Balasundaram G., Kim C., Olivo M.C. High-speed simultaneous multiscale photoacoustic microscopy. J. Biomed. Opt. 2019;24:1–7. doi: 10.1117/1.JBO.24.8.086001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlas A., Kallmayer M., Fasoula N.A., Liapis E., Bariotakis M., Krönke M., Anastasopoulou M., Reber J., Eckstein H.H., Ntziachristos V. Multispectral optoacoustic tomography of muscle perfusion and oxygenation under arterial and venous occlusion: a human pilot study. J. Biophotonics. 2020;13 doi: 10.1002/JBIO.201960169. [DOI] [PubMed] [Google Scholar]
- 11.J. Yang, G. Zhang, G. Zhang, W. Chang, W. Chang, Z. Chi, Q. Shang, Q. Shang, M. Wu, M. Wu, T. Pan, T. Pan, L. Huang, L. Huang, H. Jiang, Photoacoustic imaging of hemodynamic changes in forearm skeletal muscle during cuff occlusion, Biomedical Optics Express, Vol. 11, Issue 8, Pp. 4560–4570. 11 (2020) 4560–4570. https://doi.org/10.1364/BOE.392221. [DOI] [PMC free article] [PubMed]
- 12.Yang J., Zhang G., Shang Q., Wu M., Huang L., Jiang H. Detecting hemodynamic changes in the foot vessels of diabetic patients by photoacoustic tomography. J. Biophotonics. 2020;13 doi: 10.1002/JBIO.202000011. [DOI] [PubMed] [Google Scholar]
- 13.Goh Y., Ghayathri B., et al. Multispectral optoacoustic tomography in assessment of breast tumor margins during breast-conserving surgery: a first-in-human case study. Clin. Breast Cancer. 2018;18(6):e1247–e1250. doi: 10.1016/j.clbc.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Favazza C.P., Cornelius L.A., Wang L. v. In vivo functional photoacoustic microscopy of cutaneous microvasculature in human skin. J. Biomed. Opt. 2011;16 doi: 10.1117/1.3536522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao J., Wang L.V. Sensitivity of photoacoustic microscopy. Photoacoustics. 2014;2:87–101. doi: 10.1016/j.pacs.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omar M., Soliman D., Gateau J., Ntziachristos V. Ultrawideband reflection-mode optoacoustic mesoscopy. Opt. Lett. 2014;39:3911. doi: 10.1364/OL.39.003911. [DOI] [PubMed] [Google Scholar]
- 17.Yew Y.W., Dinish U.S., Kuan A.H.Y., Li X., Dev K., Attia A.B.E., Bi R., Moothanchery M., Balasundaram G., Aguirre J., Ntziachristos V., Olivo M., Thng S.T.G. Raster-scanning optoacoustic mesoscopy (RSOM) imaging as an objective disease severity tool in atopic dermatitis patients. J. Am. Acad. Dermatol. 2020 doi: 10.1016/j.jaad.2020.06.045. [DOI] [PubMed] [Google Scholar]
- 18.Aguirre J., Schwarz M., Garzorz N., Omar M., Buehler A., Eyerich K., Ntziachristos V. Precision assessment of label-free psoriasis biomarkers with ultra-broadband optoacoustic mesoscopy. Nat. Biomed. Eng. 2017;1:1–8. doi: 10.1038/s41551-017-0068. [DOI] [Google Scholar]
- 19.Schwarz M., Buehler A., Aguirre J., Ntziachristos V. Three-dimensional multispectral optoacoustic mesoscopy reveals melanin and blood oxygenation in human skin in vivo. J. Biophotonics. 2016;9:55–60. doi: 10.1002/JBIO.201500247. [DOI] [PubMed] [Google Scholar]
- 20.Yew Y.W., Dinish U.S., Choi E.C.E., Bi R., Ho C.J.H., Dev K., Li X., Attia A.B.E., Wong M.K.W., Balasundaram G., Ntziachristos V., Olivo M., Thng S.T.G. Investigation of morphological, vascular and biochemical changes in the skin of an atopic dermatitis (AD) patient in respose to dupilumab using raster scanning optoacoustic mesoscopy (RSOM) and handheld confocal Raman spectroscopy (CRS) J. Dermatol. Sci. 2019;95:123–125. doi: 10.1016/j.jdermsci.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Park S., Saw S.N., Li X., Paknezhad M., Coppola D., Dinish U.S., Attia A.B.E., Yew Y.W., Thng S.T.G., Lee H.K., Olivo M. Model learning analysis of 3D optoacoustic mesoscopy images for the classification of atopic dermatitis. Biomed. Opt. Express. 2021;12:3671. doi: 10.1364/BOE.415105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attia A.B.E., Moothanchery M., Li X., Yew Y.W., Thng S.T.G., Dinish U.S., Olivo M. Microvascular imaging and monitoring of hemodynamic changes in the skin during arterial-venous occlusion using multispectral raster-scanning optoacoustic mesoscopy. Photoacoustics. 2021;22 doi: 10.1016/j.pacs.2021.100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kermany D.S., Goldbaum M., Cai W., Valentim C.C.S., Liang H., Baxter S.L., McKeown A., Yang G., Wu X., Yan F., Dong J., Prasadha M.K., Pei J., Ting M., Zhu J., Li C., Hewett S., Dong J., Ziyar I., Shi A., Zhang R., Zheng L., Hou R., Shi W., Fu X., Duan Y., Huu V.A.N., Wen C., Zhang E.D., Zhang C.L., Li O., Wang X., Singer M.A., Sun X., Xu J., Tafreshi A., Lewis M.A., Xia H., Zhang K. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell. 2018;172:1122–1124. doi: 10.1016/j.cell.2018.02.010. e9. [DOI] [PubMed] [Google Scholar]
- 24.Xu M., Wang L.V. Universal back-projection algorithm for photoacoustic computed tomography. Phys. Rev. E - Stat. Nonlinear, Soft Matter Phys. 2005;71:1–7. doi: 10.1103/PhysRevE.71.016706. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz M., Garzorz-Stark N., Eyerich K., Aguirre J., Ntziachristos V. Motion correction in optoacoustic mesoscopy. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-11277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ntziachristos V., Razansky D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT. Chem. Rev. 2010;110:2783–2794. doi: 10.1021/cr9002566. [DOI] [PubMed] [Google Scholar]
- 27.Jacques S.L. Optical properties of biological tissues: a review. Phys. Med. Biol. 2013;58 doi: 10.1088/0031-9155/58/11/R37. [DOI] [PubMed] [Google Scholar]
- 28.Flohr C., England K., Radulovic S. Filaggrin loss‐of‐function mutations are associated with early‐onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br. J. Dermatol. 2010;163:1333–1336. doi: 10.1111/j.1365-2133.2010.10068.x. [DOI] [PubMed] [Google Scholar]
- 29.Chamlin S.L., Kao J., Frieden I.J., Sheu M.Y., Fowler A.J., Fluhr J.W., Williams M.L., Elias P.M. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J. Am. Acad. Dermatol. 2002;47:198–208. doi: 10.1067/mjd.2002.124617. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Y., Ma W. Assessment of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in atopic dermatitis patients. Med. Sci. Monit. 2017;23:1340–1346. doi: 10.12659/MSM.900212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chopra R., Vakharia P.P., Sacotte R., Patel N., Immaneni S., White T., Kantor R., Hsu D.Y., Silverberg J.I. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br. J. Dermatol. 2017;177:1316–1321. doi: 10.1111/bjd.15641. [DOI] [PubMed] [Google Scholar]
- 32.Oi M., Maruhashi T., Kumazawa K. Diagnosis of skin and soft tissue infections using near‐infrared spectroscopy. Acute Med. Surg. 2021;8 doi: 10.1002/ams2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Czarnowicki T., Krueger J.G., Yassky E.G. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J. Allerg. Clin. Immuno. Pract. 2014;2(4):371–379. doi: 10.1016/j.jaip.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Hughes M., Moore T., O’Leary N., Tracey A., Ennis H., Dinsdale G., Murray A., Roberts C., Herrick A.L. A study comparing videocapillaroscopy and dermoscopy in the assessment of nailfold capillaries in patients with systemic sclerosis-spectrum disorders. Rheumatology. 2015;54:1435–1442. doi: 10.1093/rheumatology/keu533. [DOI] [PubMed] [Google Scholar]
- 35.Schmitt J., Von Kobyletzki L., Svensson Å., Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: Systematic review and meta-analysis of randomized controlled trials. Br. J. Dermatol. 2011;164:415–428. doi: 10.1111/j.1365-2133.2010.10030.x. [DOI] [PubMed] [Google Scholar]
- 36.Schoepe S., Schacke H., May E., Asadullah K. Glucocorticoid therapy-induced skin atrophy. Exp. Derm. 2006;15:406. doi: 10.1111/j.0906-6705.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee S.S., Tan A.W.H., Giam Y.C. Cyclosporin in the treatment of severe atopic dermatitis: a retrospective study. Ann. Acad. Med Singa. 2004;33(3):311–313. [PubMed] [Google Scholar]
- 38.Zheng P., Lavker R.M., Lehmann P., Kligman A.M. Morphologic investigations on the rebound phenomenon after corticosteroid-induced atrophy in human skin. J. Invest. Dermatol. 1984;82:345–352. doi: 10.1111/1523-1747.ep12260665. [DOI] [PubMed] [Google Scholar]
- 39.Aschoff R., Lang A., Koch E. Effects of intermittent treatment with topical corticosteroids and calcineurin inhibitors on epidermal and dermal thickness using optical coherence tomography and ultrasound. Ski. Pharm. Physiol. 2022;35:41–50. doi: 10.1159/000518214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.