Abstract
Rabies causes approximately 60,000 casualties annually and has a case fatality rate approaching 100% once clinical signs occur. The glycoprotein on the surface of the virion is important for the host immune response and facilitates interaction of the virion with host cell receptors. Nicotinic acetylcholine receptors were the first receptors identified as a molecular target for the rabies virus. Additional targets, including neural cell adhesion molecule, p75 neurotrophin receptor, metabotropic glutamate receptor subtype 2, and integrin β1, have been added to the list, all of which can mediate viral entry into the cell. Multiple receptors and different subtypes of nicotinic acetylcholine receptors result in a complex picture of virus-receptor interactions. In addition, some data suggest that the rabies virus glycoprotein inhibits cell signaling events mediated by various nicotinic receptor subtypes that have been implicated in altering behavior in unaffected animals. This review focuses on interactions between the rabies virus glycoprotein and nicotinic receptors and proposes possible functional consequences, including behavioral modifications and therapeutic approaches for future research.
Keywords: Rabies virus, Rabies virus glycoprotein, Nicotinic acetylcholine receptor, Behavior, nAChR
Graphical abstract
Highlights
-
•
Rabies virus glycoprotein ectodomain binds to nAChRs.
-
•
Glycoprotein and nAChR-selective α-neurotoxins share regions of sequence homology.
-
•
Peptide of the rabies virus glycoprotein modifies behavior in mice.
-
•
Nicotine and genetic deletion of α7 nAChRs modulates pathological aggression.
-
•
We hypothesize rabies virus glycoprotein-nAChR interactions modulate behavior.
Rabies virus, Rabies virus glycoprotein, Nicotinic acetylcholine receptor, Behavior, nAChR.
1. Rabies background and pathogenesis
The rabies virus (RABV) infects and kills between 50,000 and 70,000 humans each year; mostly in developing countries [1]. As most victims are children, the global impact of >3.7 million Disability Adjusted Life Years better conveys the real burden of dog-mediated rabies due to premature death and adverse events following sub-optimal human rabies vaccinations and treatments [1, 2]. Although rabies has been studied for over 150 years, we still lack a thorough understanding of rabies pathogenesis at the molecular level and thus satisfactory treatment options [3, 4].
The RABV belongs to the Lyssavirus genus of the Rhabdoviridae family. The virus is enveloped and has a single-stranded, negative-sense RNA genome, containing five genes which code for nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and a viral RNA polymerase (L) (see reviews Fooks et al. 2017 and Jackson 2020 for more detailed information). The RABV glycoprotein (RGP), which interacts with cellular targets, is arranged on the surface of virus particles forming trimers, containing cytoplasmic, transmembrane, and ectodomains [5, 6]. Specifically, the ectodomain homotrimers of the RGP are responsible for interacting with host cell receptors, including the nicotinic acetylcholine receptor (nAChR) [7], neural cell adhesion molecule (NCAM) [8], p75 neurotrophin receptor (p75NTR) [9], metabotropic glutamate receptor subtype 2 (mGluR 2) [10], and integrin β1 [11]. These receptor-RGP interactions initiate viral endocytosis to enter the cell [4, 12].
The RABV shows strong neurotropism, however, the histopathological findings seen after lethal RABV infection are often subtle and do not match the severity of the clinical signs [13]. RABV infection of neurons causes degenerative changes in dendrites and axons as well as oxidative stress caused by mitochondrial dysfunction [14, 15]. Further, neuronal blebbing, apoptosis, and altered neurotransmitter function have been observed after RABV infection [16, 17]. Apoptosis can be detected during late stages of the infection [18]. Nevertheless, how viral infection and neurotropism translate to disease is still unclear [3]. RABV infection in humans can lead to characteristic behavior changes such as hyperactivity, confusion, drowsiness, and agitation [4]. The molecular mechanisms leading to these extensive behavior changes, including aggression, hyper sociability, hydrophobia, and hypermobility, are uncertain [19], although newer reports have started to unveil some possible mechanisms [3, 4, 20].
Rabies has a case fatality rate approaching 100% once clinical signs occur owing to a lack of satisfactory treatment options once the virus has entered the central nervous system (CNS). However, the development of disease after exposure to the RABV can reliably be prevented with very effective post exposure prophylaxis. Limited access to post exposure prophylaxis is a major barrier to eliminating human disease and resulting fatalities. An improved understanding of the cellular mechanisms altered by RABV infection will likely lead to identification of new therapeutic targets and result in better treatment strategies as well as an amended general comprehension of rabies pathogenesis. Since several characteristics of rabies pathogenesis and ecology fall outside the norms of many viruses, an enhanced understanding of rabies pathogenesis will likely further our understanding of virus-host interactions. In this review, we will discuss the interactions between the RABV and host receptors with an emphasis on nAChRs, compare the behavioral modulation asserted by nAChR and RABV both individually and their possible interaction, as well as propose possible mechanisms and examples of the therapeutic implication of these interactions. These proposed mechanisms are based on a limited number of studies from different fields such as cancer biology and rabies pathogenesis, and open possible avenues for further research to better understand the pathogenesis of this important neglected infectious disease.
2. Cell surface receptors targeted by the RABV
The RGP is the only external protein on the rabies virus particle, making the RGP the key factor mediating virus entry into cells and the major target for neutralizing antibodies [5]. The function of the RGP as an antigen and the mechanism it utilizes to interact with cell surface receptors have been described [12, 21]. So far, four different receptors, in addition to nAChRs, have been identified as targets for RGP: NCAM, p75NTR, mGluR 2, and integrin β1.
The NCAM is a glycoprotein concentrated in synaptic regions and neuromuscular junctions (NMJs) [12]. There are three major isoforms of NCAM, -120, -140, and -180, which are generated by alternative mRNA splicing from a single gene [22, 23]. NCAM-140 and -180 are attached to their plasma membranes via transmembrane domains [24]. NCAM-120 has a glycosyl-phosphatidylinositol anchor that serves as the attachment point to the cell membrane. All three NCAM isoforms are targets for RABV [8, 25].
In vitro studies found that incubation with RABV decreased surface expression of NCAM and that treatment of susceptible cells with heparan sulfate, a ligand for NCAM, or with NCAM antibodies, significantly reduced RABV infection [8]. Pre-incubation of RABV inoculum with soluble NCAM protein, functioning as a receptor decoy, drastically neutralized the capacity of RABV to infect susceptible cells. In vivo studies using NCAM deficient mice showed a delay in rabies mortality as well as drastically restricted brain invasion by RABV [8]. These studies support the role of NCAM for RABV infection. Although, neuronal RABV infection in the absence of NCAM receptors can still occur [25], suggesting involvement of other receptors and mechanisms in neuronal RABV infection.
The p75NTR is also targeted by RABV. In vitro experiments showed that cells expressing p75NTR bind RGP, and that β-NGF, a p75NTR ligand, inhibits RABV infection [9]. p75NTR is mainly expressed during development, but in adult individuals it has important functions in hippocampal synapse modification [26, 27], the regulation of neurogenesis [28], and inhibition of axon regeneration after peripheral nerve injury [29]. Even though the p75NTR is not essential for RABV infection [30], it may be important for facilitating RABV transport to the CNS [31]. Live-cell imaging of sensory dorsal root ganglion neurons directly demonstrated that p75NTR co-internalizes with RABV and, subsequently, undergoes retrograde axonal transport [31]. The p75NTR binding site has been proposed to be located outside of the known antigenic site but within residues 318–352 of the RGP, since an anti-RGP antibody did not neutralize the p75NTR site [32].
The mGluR 2 is another cellular target for the RGP that facilitates virus entry into neuronal cells [10]. The mGluR2 belongs to a class of seven transmembrane domain receptors. It is abundant in the CNS and rarely expressed in other tissues [33]. Using flow cytometry, RABV infection decreases cell surface expression of mGluR2 [10,21]. Providing further support that mGluR2 are a RABV target, mGluR2 antibodies reduce RABV infection in vitro [10]. Additionally, mGluR2 ectodomain soluble protein neutralizes the infectivity of RABV cell-adapted strains in vitro and in vivo in a dose-dependent manner.
Most recently, integrin β1 was identified as a possible host-receptor for RABV and shown to be involved in peripheral infection [11]. Integrin β1 is abundantly expressed in skeletal muscle but is rare in the cerebral cortex [11]. RABV and integrin β1 directly interact via a coimmunoprecipitation, and co-internalize into cells via a clathrin-dependent endocytosis after RABV infection. Murine in vivo studies revealed by immunohistofluorescence that integrin β1 and RABV co-colocalized in muscle, but not in cerebral cortical neurons.
3. Neuromuscular nAChR
The first receptor identified as a molecular target for the RGP was the muscle subtype of nAChRs found at post-synaptic sites in the NMJ [7, 12]. Both the neuromuscular and the neuronal nAChRs are pentameric, cation-conducting channels which respond to the endogenous neurotransmitter acetylcholine and are involved in signal transduction. In mammals, there are 16 subunits, which are designated α1 – α7, α9, α10, β1 – β4, δ, ε, and γ based on sequence homology. The α2 – α7, α9 – α10, and β2 – β4 subunits are expressed by neurons and are referred to as the neuronal nAChR subunits [34, 35]. The mammalian muscle nAChR localized to the NMJ is composed of (α1)2β1δγ subunits in developing muscle, with the ε subunit substituted for the γ subunit in mature muscle [36].
Seminal work by Lentz and colleagues in the 1980s identified the NMJ nAChR to be important for RABV infection initially in muscle cells prior to infection of the CNS. Pretreatment of α-bungarotoxin and d-tubocurarine, ligands with high nAChR specificity, reduced attachment and infection of two strains of RABV in chick embryo myotubes with robust nAChR expression [37]. Further, gold-labeled RABV antigen and particles were found at the junctional folds of the NMJ, which highly express nAChRs [38]. In nerve-muscle coculture, the rabies virus preferentially localized to the NMJ which expressed α-bungarotoxin labeled nAChRs, and within endosomes at nerve terminals [39].
At the NMJ, the α1 subunit of the muscle subtype of nAChR is abundant and has been shown to facilitate RABV peripheral infection in vivo [37]. Interestingly, an interaction between integrin β1 and the α1 nAChR subunit was detected in a coimmunoprecipitation assay with transfected HEK293 cells, indicating that the integrin β1 role in RABV peripheral infection involves the muscle nAChR subtype [11].
4. Neuronal nAChR
Dorsal root ganglion (DRG) neurons are believed to be a crucial bridge allowing RABV to pass from the periphery into the CNS. Adult mouse DRG cells treated with non-nAChR subtype-selective antagonists mecamylamine and d-tubocurarine reduced the percentage of RABV infected neurons [40]. Interestingly, the DRG expresses a variety of neuronal nAChR subtypes, including α7, α3β4∗, and α6β4∗ (where ∗ indicates other subunits may be present) [41, 42]. These results suggest that neuronal nAChRs are possible targets for RABV.
Neuronal nAChRs located in the CNS have important physiological roles including mediating cholinergic excitatory neurotransmission, neurotransmitter release, and downstream intracellular pathways [43]. The nAChR subunit composition determines the pharmacological and biophysical properties of these receptors, and individual receptor subtypes are associated with a wide variety of biological processes, including regulation of animal behavior [34, 35], that are altered during RABV infection.
During rabies, the host has altered locomotor behavior, including increased ranging distance, hyperactivity, and ataxia [44, 45], processes which are also modulated by nAChRs. For example, the α4β2 and the α7 subtypes, the most common nAChRs in the CNS, are linked to hyperactivity, aggression, and anxiety [46, 47, 48, 49]. Locomotor behavior involves the nigrostriatal and mesolimbic dopaminergic systems which express the α6β2β3, α4α5β2, and α4α6β2β3 nAChR subtypes [50, 51, 52, 53, 54, 55, 56]. The α3β2 and α3β4 subtypes are expressed in the brainstem [49, 57, 58], which has integrative functions, including controlling the cardiovascular system, respiration, awareness, consciousness, and muscle contraction [59], processes also modified with RABV infection. These correlations, while not experimentally confirmed suggest a possible role for these neuronal receptors in rabies pathogenesis.
The function of neuronal nAChR subtypes in RABV infection is currently not well understood, although progress is being made [12, 21, 60]. Rat hippocampal cell culture neurons have been shown to be highly susceptible to infection by RABV [61]. Entry of the virus appears to predominantly occur in regions of the cell that highly express nAChRs, including the cell body, dendrites, and in synapses, with very little viral antigen present in axons.
A 2017 study by Hueffer and colleagues, using a combination of in vivo and in vitro methods, found that administration of the neurotoxin-like region of the RGP (see section below) led to behavior modifications, possibly through inhibition of the α4β2 nAChR subtype in C. elegans and mice [20]. The neurotoxin-like region of RGP inhibited the frequency of nAChR-meditated pharyngeal pumping in C. elegans and induced hyperactivity (a rabies-associated behavior) after intraventricular administration into the CNS of mice. Additionally, the α4β2 nAChR subtype was inhibited by both the neurotoxin-like region of RGP and the ectodomain of RGP in functional assays using a two-electrode voltage clamp electrophysiology [20]. RABV and host nAChR interactions are traditionally viewed to mediate mechanisms of viral cell entry and immune recognition [4]. However, the study by Hueffer et al. [20] suggests that this could be expanded to include functional modification of receptor signaling and even specific behavior modifications of the infected hosts.
5. RGP binds to nAChRs via a neurotoxin-like domain
The RGP contains a neurotoxin-like region which shows a significant sequence homology with snake α-neurotoxins that function as potent nAChR subtype selective antagonists [62, 63]. Several lines of evidence have identified the α1 nAChR subunit to contain the RGP binding site, which overlaps with the α-bungarotoxin site. nAChR α1 subunit monoclonal antibody prevented the attachment of radio-labeled RABV to cultured muscles cells with a high density of nAChRs [38]. RABV binding to the muscle-type nAChR was inhibited by nAChR antagonists, up to 50% by α-bungarotoxin and up to 30% by (+)-tubocurarine, but binding was not affected by the muscarinic acetylcholine receptor antagonist atropine [64]. Later, the RABV was confirmed to bind to the Torpedo californica electric organ α subunit, which is similar to the human α1 subunit, by a competitive mechanism, as α-bungarotoxin was able to compete for binding [65].
The RGP neurotoxin-like domain is located between residues 175–203 of the mature RGP and has homologies to loop II (the central loop) of the α-neurotoxins and α-conotoxins (Table 1) [66-68]. A 2021 review contains detailed structural information of venom-derived neurotoxins and the ability of these proteins to target nAChRs [69]. Therefore, we have focused our discussion to possible parallels that can be surmised from α-neurotoxin loop II and interactions with nAChRs, and the similarities to the RGP.
Table 1.
Sequence comparison between RVG and loop II of α-bungarotoxin and α-cobratoxin. Bolded residues are conserved among RGP and at least one α-neurotoxin. The underlined residues are those important for mediating nAChR interactions. Snake toxin residues absent in RGP are represented with a dash space holder to facilitate sequence alignments.
| Virus/Toxin | Sequence | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RGP | Y | T | - | I | W | M | P | E | N | P | R | L | G | T | S | C | D | I | F | T | N | S | R | G | K | R | A | S | K | G | 203 |
| α-Bungarotoxin | Y | R | K | M | W | - | - | - | - | - | - | - | - | - | - | C | D | A | F | C | S | S | R | G | K | V | V | E | L | G | 43 |
| α-Cobratoxin | Y | T | K | T | W | - | - | - | - | - | - | - | - | - | - | C | D | A | F | C | S | I | R | G | K | R | V | D | L | G | 40 |
Loop II of α-bungarotoxin and α-cobratoxin have approximately 50% sequence homology to the RGP neurotoxin-like domain (Table 1). Circular dichroism spectroscopy identified that a 29 amino acid residue peptide of the neurotoxin-like domain of the RGP and an analogous king cobra loop II peptide were conformationally similar and were composed mostly of beta sheet structure [70]. This study showed that a peptide of the RGP neurotoxin domain is structurally similar to loop II of α-neurotoxins. Using a Asn194-Ser195-Arg196-Gly197 tetrapeptide, early molecular modeling studies showed that these RGP residues form an essential part of the binding site and that side chains Asn and Arg demonstrate molecular mimicry to the structure of ACh [71].
X-ray crystallography and cryo-EM structures have revealed great detail into the interactions of α-neurotoxin loop II with nAChRs [72, 73, 74, 75]. In the Torpedo receptor, α-bungarotoxin loop II reaches under loop C, penetrating deeply into the ACh binding pocket [74, 75]. The α-bungarotoxin Arg 36 guanidinium group is located far within the ACh binding pocket and contacts the principal face α subunit residues Tyr93, Tyr190, and Tyr198 and forms a cation-π sandwich with αTyr198. α-bungarotoxin loop II Phe 32 interacts with residues α1Trp149 and γTrp55 or δTrp57, which are important for ACh binding, while Val 40 makes important contacts with Tyr189 [74]. A cryo-EM structure of the α7 nAChR showed that the α-bungarotoxin Arg36 and Phe32 form a cation-π stack and aligns with Tyr187 in an edge-to-face orientation [75].
Several loop II residues of α-neurotoxins are important for ligand affinity and subtype specificity [68, 72, 76, 77, 78]. Mutating α-bungarotoxin residues Phe 32, Arg 36, and Gly 43 reduced binding affinity for Torpedo nAChR, while mutating similar RGP peptide residues showed analogous affects [68]. Similarly, α-cobratoxin residues Trp 25, Asp 27, Phe 29, Arg 33, and Arg36 are implicated in the binding of muscle and α7 nAChR subtypes [72]. Loop II residue Lys 23 is important for binding to the muscle subtype, while Ala 28, Cys26-Cys30, and Lys 35 are important for binding to the α7 nAChR subtype [72]. These findings show that functional similarities possibly exist between some α-neurotoxins and the RGP, but it remains to be determined if RGP selectively targets subtypes of neuronal nAChRs. The structural and sequence resemblances of α-neurotoxins and RGP opens the door to better understand the role of RGP in the pathogenesis of rabies beyond cell entry, as well as improving therapeutic approaches for rabies and other diseases associated with nAChR dysfunction, such as Alzheimer's disease, Parkinson's disease and schizophrenia [79, 80].
6. RGP structure
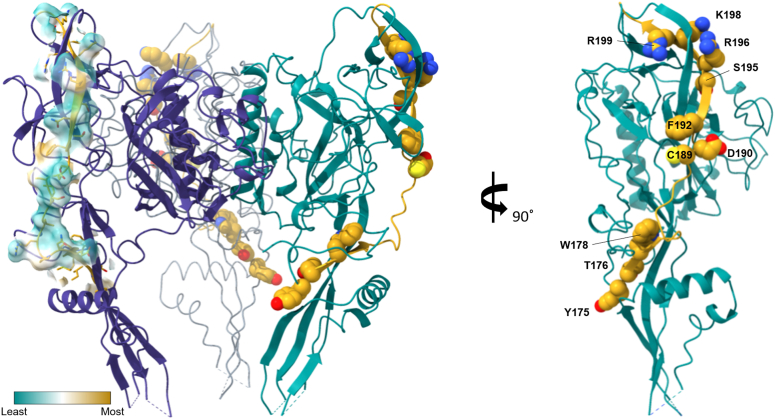
Homology modeling with the vesicular stomatitis virus (VSV) glycoprotein predicted that the RGP neurotoxin-like domain is exposed on the protein surface [81]. In 2020 x-ray crystallography studies, the structure of the RGP was solved [5, 82] and largely confirmed the predictions that were based on the VSV glycoprotein structure [83, 84]. In 2022, Callaway et al. published the structure of the RGP trimer bound to a prefusion-specific neutralizing antibody [6]. The glycoprotein ectodomain is divided into four [6, 82] linked domains: a central domain (CD, or domains I and II), a pleckstrin homology domain (PHD, or domain III) and a fusion domain containing two fusion loops (FD, or domain IV). The CD is connected to the transmembrane and cytoplasmic domains of the RGP at the C-terminus, with a central helix that elongates in the postfusion transition (domain II) and a solvent exposed upper half of the RGP (domain I). The presumed binding site of the RGP to nAChRs (see section above) is found in the PHD (domain III) which is also largely solvent exposed in the upper half of the RGP [5, 6] (Figure 1). However, it seems that in the Yang et al. [5] pre-fusion RGP structure, the neurotoxin-like domain does not fold into a loop structure that is typical for the homologous regions in neurotoxins, likely due to the lack of disulfide bonds. In this structure, the RGP neurotoxin-like region forms a β-strand, followed by a random coil, and ending with another β-strand (Figure 1), which is the same motif found in α-neurotoxins. Interestingly, the residues (175–203) that have been found to be critical for α-neurotoxin binding to nAChRs (see Table 1), are highly exposed to solvent, suggesting these residues to be accessible for nAChR interactions (Figure 1). Mapping the lipophilicity potential of this region shows that the region is highly hydrophilic, which makes sense due to its solvent accessibility, thus the residues likely participate in hydrogen and electrostatic interactions. Several residues including Cys 189. Phe 192, Arg 196, and Lys 198 likely interact with target residues via hydrophobic interactions. Residues Tyr 175 and Thr193 may also interact with nAChRs but are less obvious due to the residues positioning. Detailed structure and function studies of the RGP and nAChR interactions are needed to confirm these observations.
Figure 1.
Rabies glycoprotein neurotoxin-like loop residues are accessible to solvent in the solved prefusion RGP structure. The yellow region represents the neurotoxin-like domain (residues 175–203) found in the PHD (domain III). Space filled and labeled residues correspond to those important for α-neurotoxin interactions with nAChRs. In the trimer model, the hydrophobicity of the neurotoxin-like domain of the left protomer is displayed demonstrating that the region is largely hydrophilic (cyan), with several key exposed residues being hydrophobic (yellow). A single protomer rotated 90° is shown on the right. Model was built using UCSF ChimeraX (version: 1.40.93 (2020-06-03)) and the solved cryo-EM structure of RGP timer (PDB 7U9G) [6].
7. nAChR modulational of behavior
The traditional virus interaction with cell surface receptors as a mechanism of cell entry may not be the only consequence of RGP-nAChR interactions. Here we discuss the possibility that inhibition of nAChRs by the RGP may play a key role in the dramatic behavior changes observed with RABV infection [20].
The neuroanatomical bases and neurological mechanisms for the behavioral changes observed during RABV infection are not well understood. Infection of the limbic system and subsequent dysfunction is suspected to play an important role in host behavior [3]. Two-thirds of humans infected with dog RABV variants present with the classic furious rabies, characterized by fluctuating consciousness, changed mental status, aggression, hydrophobia, and autonomic stimulation signs [19]. The remaining third develop paralytic rabies progressing to coma [19]. The mechanisms determining the form of RABV infection is not known but does not seem to be associated with RGP sequence [85].
Increased movement, aggression, and biting in animal hosts are important for transmission of the RABV to new hosts at a time when the virus is secreted in saliva. This aggressive behavior may be associated with low serotonergic activity in the brain [86, 87]. Accumulation of RABV antigen was found in the midbrain raphe nuclei in experimentally infected skunks, indicating impaired serotonin neurotransmission from the brainstem may contribute to the aggressive behavior [88]. Combining molecular techniques and animal models revealed that the RGP derived neurotoxin-like peptides modify host behavior, such as inducing hyperactivity in mice, possibly by inhibition of neuronal nAChRs [20]. However, this study did not specifically antagonize nAChR subtypes, or other RABV receptors.
nAChRs are known to modulate behaviors including aggression, attention, mood, and impulsivity. Nicotine and other drugs targeting nAChRs can reduce offensive, defensive, and predatory aggression in animal models [89]. Correspondingly, in human laboratory and clinical settings, nicotine may reduce aggressive behavior [90, 91, 92, 93, 94, 95], and the α7 nAChR subtype may be a critical component to modulating this aggressive behavior. The α7 nAChR is necessary for the anti-aggressive or ‘serenic’ effects of systemic administration of nicotine, and an α7 nAChR partial agonist (GTS-21) can reestablish this serenic nicotinic effect [46].
The hippocampal α7 nAChR seems to directly regulate aggression in mice [96]. The loss of α7 nAChR subtype function, either by pharmacological means in mice or by genetic deletion in humans as occurs with the 15q13.3 microdeletion syndrome, increases aggression [96, 97, 98, 99]. What remains to be determined is whether RGP functionally interacts with the neuronal α7 nAChR subtype and can modify animal behaviors including aggression. These rodent and human studies provide evidence that the neuronal α7 nAChR plays a critical role in modulating aggression behaviors and that this behavior can be modified with exogenous ligands. The RGP with its neurotoxin-like region could be one such ligand. Further experimental support is needed to test this hypothesis. These experiments should include specific behavioral assays to test effects on aggression as well as knock out mice to evaluate the role of different subtypes of nicotinic receptors.
8. Therapy development based on functional RGP and nAChR interactions
In addition to providing a possible mechanistic explanation for rabies-associated behavioral changes, the functional RABV interaction with nAChR is being used for the development of therapeutic approaches to treat cancer and rabies.
Controlling cancer cell replication by limiting cell division through modification of intracellular signaling pathways provides a promising avenue for the development of cancer treatment. For some lung cancers, upregulation of α7 nAChRs has been observed compared to precancerous cells and is related to smoking [100]. The RGP's affinity to the nAChR has been utilized in the development of possible lung cancer treatments. The oncolytic Newcastle Disease Virus expressing the RGP enhances apoptosis and inhibits migration of lung adenoma cells by regulating α7 nAChR signaling pathways [100]. Similarly, by antagonizing α7 nAChR, the RGP promotes apoptosis in gastric carcinoma cells, demonstrating the potential of RGP for treatment of gastric cancer [101]. These interaction between RGP and α7 nAChR not only provide some evidence of the usefulness of RGP nAChR interaction in developing therapeutic approaches to non-rabies diseases but also further strengthen the proposed role in rabies pathogenesis suggested in this review article.
Interactions between α1 nAChR derived peptides with the RGP were investigated for the purpose of designing potential anti-rabies agents. α1 nAChR peptide sequences from different host species (bovine, human, electric fish/torpedo) were tested, and both the bovine and torpedo peptides bound and inhibited the RGP [102]. Encouragingly, α1 nAChR peptides and their analogs may serve as potential leads in developing antiviral agents against RABV infection.
In order to develop better treatment strategies, it is critical to understand the process by which the RABV is able to evade the host immune response and gain access to the CNS. A recombinant trimeric RGP binds to α7 nAChRs expressed on monocyte-derived macrophages [103]. This interaction induced the cholinergic anti-inflammatory pathway, including suppression of macrophages to function as T-cell activators, and may affect macrophage polarization. These findings suggest that RABV could evade the immune system by inducing an anti-inflammatory state in human macrophages through interactions with α7 nAChR.
9. Conclusion
The studies reviewed in this manuscript show that rabies virus interacts with multiple host target receptors, including nAChRs. The effects of RGP binding to neuronal nAChRs possibly results in inhibition of receptor function and alterations of associated signaling pathways, and we propose a potential link to RGP modifying animal behavior. These findings suggest the possibility of a complex relationship of RABV and its interaction with host cells through the RGP.
We propose that the RGP can function as a neuronal nAChR antagonist and could induce aggression, and possibly other behaviors associated with rabies. Our suggestion is based on five main conclusions: (1) The RABV binds to muscle and neuronal (α4β2) nAChRs via the RGP ectodomain [20, 65, 68, 70, 71, 104]. (2) α-neurotoxins are known to bind at the orthosteric binding site on muscle and neuronal (α7) nAChRs [72, 74, 75]. (3) RGP and snake α-neurotoxins have relatively high sequence homologies, and α-neurotoxins can compete for binding with RGP on muscle nAChRs [64, 65, 66, 67, 68, 77, 105]. (4) Nicotine and genetic deletion of the α7 nAChR can significantly modulate pathological aggression [46, 90, 91, 92, 94, 95, 96, 97, 98, 106]. (5) RABV binds to the α7 nAChR, inducing an anti-inflammatory state and altering intracellular signaling in several cell types [100, 103, 107]. Together, these outcomes suggest the possibility that the RGP binds to neuronal nAChRs, to possibly modulate aggressive behavior in host animals, which would aid in the transmission of the virus. These proposed mechanisms currently lack strong experimental support but could provide a fruitful avenue to better understand rabies pathogenesis, and encourages further experimental research on the topic.
The existence of multiple receptors for the rabies virus on host cell membranes complicates interpretations of some in vitro and in vivo results. Expanding our understanding of virus-receptor interactions beyond cell entry to include alterations in host cell biology and behavioral modifications could lead to fundamental advances in the field of virial pathogenesis and host-pathogen interactions. These findings suggest the need for further studies of the functional aspects of virus-receptor interactions and how changes in receptor function alter host cell biology beyond virus entry. Only with a better understanding of host factors and RABV pathogenetic mechanisms can a safe and effective antiviral therapy be developed.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Karsten Hueffer was supported by National Institute of General Medical Sciences of the National Institutes of Health; Institutional Development Award (IDeA) [P20GM103395].
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors would like to thank Lahra Weber for her careful review of the manuscript, and BioRender for use of their program to develop the graphical abstract.
References
- 1.Hampson K., Coudeville L., Lembo T., Sambo M., Kieffer A., Attlan M., et al. Estimating the global burden of endemic canine rabies. PLoS Neglected Trop. Dis. 2015;9(4) doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hampson K., Dobson A., Kaare M., Dushoff J., Magoto M., Sindoya E., et al. Rabies exposures, post-exposure prophylaxis and deaths in a region of endemic canine rabies. PLoS Neglected Trop. Dis. 2008;2(11):e339. doi: 10.1371/journal.pntd.0000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson A.C. In: Rabies Scientific Basis of the Disease and its Management. 4. Fooks A.R., Jackson A.C., editors. Academic Press; London: 2020. Pathogenesis; p. 698. [Google Scholar]
- 4.Fooks A.R., Cliquet F., Finke S., Freuling C., Hemachudha T., Mani R.S., et al. Rabies. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.91. [DOI] [PubMed] [Google Scholar]
- 5.Yang F., Lin S., Ye F., Yang J., Qi J., Chen Z., et al. Structural analysis of rabies virus glycoprotein reveals pH-dependent conformational changes and interactions with a neutralizing antibody. Cell Host Microbe. 2020;27(3):441–453 e7. doi: 10.1016/j.chom.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Callaway H.M., Zyla D., Larrous F., Melo GDd, Hastie K.M., Avalos R.D., et al. Structure of the rabies virus glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Sci. Adv. 2022;8(24) doi: 10.1126/sciadv.abp9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lentz T.L., Burrage T.G., Smith A.L., Tignor G.H. The acetylcholine receptor as a cellular receptor for rabies virus. Yale J. Biol. Med. 1983;56(4):315–322. [PMC free article] [PubMed] [Google Scholar]
- 8.Thoulouze M.I., Lafage M., Schachner M., Hartmann U., Cremer H., Lafon M. The neural cell adhesion molecule is a receptor for rabies virus. J. Virol. 1998;72(9):7181–7190. doi: 10.1128/jvi.72.9.7181-7190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuffereau C., Bénéjean J., Blondel D., Kieffer B., Flamand A. Low-affinity nerve-growth factor receptor (P75NTR) can serve as a receptor for rabies virus. EMBO J. 1998;17(24):7250–7259. doi: 10.1093/emboj/17.24.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Wang Z., Liu R., Shuai L., Wang X., Luo J., et al. Metabotropic glutamate receptor subtype 2 is a cellular receptor for rabies virus. PLoS Pathog. 2018;14(7) doi: 10.1371/journal.ppat.1007189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shuai L., Wang J., Zhao D., Wen Z., Ge J., He X., et al. Integrin beta 1 promotes peripheral entry by rabies virus. J. Virol. 2020;94(2) doi: 10.1128/JVI.01819-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lafon M. Rabies virus receptors. J. Neurovirol. 2005;11(1):82–87. doi: 10.1080/13550280590900427. [DOI] [PubMed] [Google Scholar]
- 13.Laothamatas J., Wacharapluesadee S., Lumlertdacha B., Ampawong S., Tepsumethanon V., Shuangshoti S., et al. Furious and paralytic rabies of canine origin: neuroimaging with virological and cytokine studies. J. Neurovirol. 2008;14(2):119–129. doi: 10.1080/13550280701883857. [DOI] [PubMed] [Google Scholar]
- 14.Alandijany T., Kammouni W., Roy Chowdhury S.K., Fernyhough P., Jackson A.C. Mitochondrial dysfunction in rabies virus infection of neurons. J. Neurovirol. 2013;19(6):537–549. doi: 10.1007/s13365-013-0214-6. [DOI] [PubMed] [Google Scholar]
- 15.Kammouni W., Wood H., Saleh A., Appolinario C.M., Fernyhough P., Jackson A.C. Rabies virus phosphoprotein interacts with mitochondrial Complex I and induces mitochondrial dysfunction and oxidative stress. J. Neurovirol. 2015;21(4):370–382. doi: 10.1007/s13365-015-0320-8. [DOI] [PubMed] [Google Scholar]
- 16.Jackson A., Wunner W. Academic Press; 2007. Rabies; p. 660. [Google Scholar]
- 17.Scott C.A., Rossiter J.P., Andrew R.D., Jackson A.C. Structural abnormalities in neurons are sufficient to explain the clinical disease and fatal outcome of experimental rabies in yellow fluorescent protein-expressing transgenic mice. J. Virol. 2008;82(1):513–521. doi: 10.1128/JVI.01677-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietzschold B., Li J., Faber M., Schnell M. Concepts in the pathogenesis of rabies. Future Virol. 2008;3(5):481–490. doi: 10.2217/17460794.3.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemachudha T., Ugolini G., Wacharapluesadee S., Sungkarat W., Shuangshoti S., Laothamatas J. Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol. 2013;12(5):498–513. doi: 10.1016/S1474-4422(13)70038-3. [DOI] [PubMed] [Google Scholar]
- 20.Hueffer K., Khatri S., Rideout S., Harris M.B., Papke R.L., Stokes C., et al. Rabies virus modifies host behaviour through a snake-toxin like region of its glycoprotein that inhibits neurotransmitter receptors in the CNS. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-12726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belot L., Albertini A., Gaudin Y. Structural and cellular biology of rhabdovirus entry. Adv. Virus Res. 2019;104:147–183. doi: 10.1016/bs.aivir.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Barbas J.A., Chaix J.C., Steinmetz M., Goridis C. Differential splicing and alternative polyadenylation generates distinct NCAM transcripts and proteins in the mouse. EMBO J. 1988;7(3):625–632. doi: 10.1002/j.1460-2075.1988.tb02856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santoni M.J., Barthels D., Vopper G., Boned A., Goridis C., Wille W. Differential exon usage involving an unusual splicing mechanism generates at least eight types of NCAM cDNA in mouse brain. EMBO J. 1989;8(2):385–392. doi: 10.1002/j.1460-2075.1989.tb03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olive S., Dubois C., Schachner M., Rougon G. The F3 neuronal glycosylphosphatidylinositol-linked molecule is localized to glycolipid-enriched membrane subdomains and interacts with L1 and fyn kinase in cerebellum. J. Neurochem. 1995;65(5):2307–2317. doi: 10.1046/j.1471-4159.1995.65052307.x. [DOI] [PubMed] [Google Scholar]
- 25.Hotta K., Motoi Y., Okutani A., Kaku Y., Noguchi A., Inoue S., et al. Role of GPI-anchored NCAM-120 in rabies virus infection. Microb. Infect. 2007;9(2):167–174. doi: 10.1016/j.micinf.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Woo N.H., Teng H.K., Siao C.J., Chiaruttini C., Pang P.T., Milner T.A., et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 2005;8(8):1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 27.Zagrebelsky M., Holz A., Dechant G., Barde Y.A., Bonhoeffer T., Korte M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J. Neurosci. 2005;25(43):9989–9999. doi: 10.1523/JNEUROSCI.2492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernabeu R.O., Longo F.M. The p75 neurotrophin receptor is expressed by adult mouse dentate progenitor cells and regulates neuronal and non-neuronal cell genesis. BMC Neurosci. 2010;11:136. doi: 10.1186/1471-2202-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGregor C., Sabatier M., English A. Early regeneration of axons following peripheral nerve injury is enhanced if p75(NTR) is eliminated from the surrounding pathway. Eur. J. Neurosci. 2021;53(2):663–672. doi: 10.1111/ejn.14943. [DOI] [PubMed] [Google Scholar]
- 30.Tuffereau C., Schmidt K., Langevin C., Lafay F., Dechant G., Koltzenburg M. The rabies virus glycoprotein receptor p75NTR is not essential for rabies virus infection. J. Virol. 2007;81(24):13622–13630. doi: 10.1128/JVI.02368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gluska S., Zahavi E.E., Chein M., Gradus T., Bauer A., Finke S., et al. Rabies Virus Hijacks and accelerates the p75NTR retrograde axonal transport machinery. PLoS Pathog. 2014;10(8) doi: 10.1371/journal.ppat.1004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langevin C., Tuffereau C. Mutations conferring resistance to neutralization by a soluble form of the neurotrophin receptor (p75NTR) map outside of the known antigenic sites of the rabies virus glycoprotein. J. Virol. 2002;76(21):10756–10765. doi: 10.1128/JVI.76.21.10756-10765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohishi H., Shigemoto R., Nakanishi S., Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR 2, in the central nervous system of the rat. Neuroscience. 1993;53(4):1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- 34.Wu J., Lukas R.J. Naturally-expressed nicotinic acetylcholine receptor subtypes. Biochem. Pharmacol. 2011;82(8):800–807. doi: 10.1016/j.bcp.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurst R., Rollema H., Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol. Ther. 2013;137(1):22–54. doi: 10.1016/j.pharmthera.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Albuquerque E.X., Pereira E.F., Alkondon M., Rogers S.W. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 2009;89(1):73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lentz T.L., Burrage T.G., Smith A.L., Crick J., Tignor G.H. Is the acetylcholine receptor a rabies virus receptor? Science. 1982;215(4529):182–184. doi: 10.1126/science.7053569. [DOI] [PubMed] [Google Scholar]
- 38.Burrage T.G., Tignor G.H., Smith A.L. Rabies virus binding at neuromuscular junctions. Virus Res. 1985;2(3):273–289. doi: 10.1016/0168-1702(85)90014-0. [DOI] [PubMed] [Google Scholar]
- 39.Lewis P., Fu Y., Lentz T.L. Rabies virus entry at the neuromuscular junction in nerve-muscle cocultures. Muscle Nerve. 2000;23(5):720–730. doi: 10.1002/(sici)1097-4598(200005)23:5<720::aid-mus9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Castellanos J.E., Castaneda D.R., Velandia A.E., Hurtado H. Partial inhibition of the in vitro infection of adult mouse dorsal root ganglion neurons by rabies virus using nicotinic antagonists. Neurosci. Lett. 1997;229(3):198–200. doi: 10.1016/s0304-3940(97)00440-0. [DOI] [PubMed] [Google Scholar]
- 41.Genzen J.R., Van Cleve W., McGehee D.S. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J. Neurophysiol. 2001;86(4):1773–1782. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- 42.Hone A.J., Meyer E.L., McIntyre M., McIntosh J.M. Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the alpha6beta4∗ subtype. Faseb. J. 2012;26(2):917–926. doi: 10.1096/fj.11-195883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gotti C., Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog. Neurobiol. 2004;74(6):363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Rupprecht C.E., Hanlon C.A., Hemachudha T. Rabies re-examined. Lancet Infect. Dis. 2002;2(6):327–343. doi: 10.1016/s1473-3099(02)00287-6. [DOI] [PubMed] [Google Scholar]
- 45.Jackson A.C. Diabolical effects of rabies encephalitis. J. Neurovirol. 2016;22(1):8–13. doi: 10.1007/s13365-015-0351-1. [DOI] [PubMed] [Google Scholar]
- 46.Lewis A.S., Mineur Y.S., Smith P.H., Cahuzac E.L.M., Picciotto M.R. Modulation of aggressive behavior in mice by nicotinic receptor subtypes. Biochem. Pharmacol. 2015;97(4):488–497. doi: 10.1016/j.bcp.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picciotto M.R., Lewis A.S., van Schalkwyk G.I., Mineur Y.S. Mood and anxiety regulation by nicotinic acetylcholine receptors: a potential pathway to modulate aggression and related behavioral states. Neuropharmacology. 2015;96(Pt B):235–243. doi: 10.1016/j.neuropharm.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King S.L., Caldarone B.J., Picciotto M.R. Beta2-subunit-containing nicotinic acetylcholine receptors are critical for dopamine-dependent locomotor activation following repeated nicotine administration. Neuropharmacology. 2004;47(Suppl 1):132–139. doi: 10.1016/j.neuropharm.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 49.Champtiaux N., Gotti C., Cordero-Erausquin M., David D.J., Przybylski C., Lena C., et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J. Neurosci. 2003;23(21):7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quik M., Perez X.A., Grady S.R. Role of alpha 6 nicotinic receptors in CNS dopaminergic function: relevance to addiction and neurological disorders. Biochem. Pharmacol. 2011;82(8):873–882. doi: 10.1016/j.bcp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grady S.R., Salminen O., Laverty D.C., Whiteaker P., McIntosh J.M., Collins A.C., et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem. Pharmacol. 2007;74(8):1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gotti C., Guiducci S., Tedesco V., Corbioli S., Zanetti L., Moretti M., et al. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2∗ receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J. Neurosci. : the official journal of the Society for Neuroscience. 2010;30(15):5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luetje C.W. Getting past the asterisk: the subunit composition of presynaptic nicotinic receptors that modulate striatal dopamine release. Mol. Pharmacol. 2004;65(6):1333–1335. doi: 10.1124/mol.65.6.1333. [DOI] [PubMed] [Google Scholar]
- 54.Salminen O., Murphy K.L., McIntosh J.M., Drago J., Marks M.J., Collins A.C., et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol. Pharmacol. 2004;65(6):1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 55.Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur. J. Pharmacol. 2000;393(1-3):295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- 56.Drenan R.M., Grady S.R., Steele A.D., McKinney S., Patzlaff N.E., McIntosh J.M., et al. Cholinergic modulation of locomotion and striatal dopamine release is mediated by alpha6alpha4∗ nicotinic acetylcholine receptors. J. Neurosci. : the official journal of the Society for Neuroscience. 2010;30(29):9877–9889. doi: 10.1523/JNEUROSCI.2056-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gotti C., Moretti M., Clementi F., Riganti L., McIntosh J.M., Collins A.C., et al. Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Mol. Pharmacol. 2005;67(6):2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- 58.Hogg R.C., Raggenbass M., Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev. Physiol. Biochem. Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- 59.Purves D., Augustine G.J., Fitzpatrick D., Hall W.H., LaMantia A., McNamara J.O., Williams S.M., editors. Neuroscience. third ed. ed. Sinauer; Sunderland, Massachusetts: 2004. [Google Scholar]
- 60.Hanham C.A., Zhao F., Tignor G.H. Evidence from the anti-idiotypic network that the acetylcholine receptor is a rabies virus receptor. J. Virol. 1993;67(1):530–542. doi: 10.1128/jvi.67.1.530-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis P., Lentz T.L. Rabies virus entry into cultured rat hippocampal neurons. J. Neurocytol. 1998;27(8):559–573. doi: 10.1023/a:1006912610044. [DOI] [PubMed] [Google Scholar]
- 62.Barber C.M., Isbister G.K., Hodgson W.C. Alpha neurotoxins. Toxicon. 2013;66:47–58. doi: 10.1016/j.toxicon.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 63.Kasheverov VTaI. In: Neurochemistry. Heinbockel T., editor. InTech; 2014. Peptide and protein neurotoxin toolbox in research on nicotinic acetylcholine receptors. [Google Scholar]
- 64.Lentz T.L., Benson R.J., Klimowicz D., Wilson P.T., Hawrot E. Binding of rabies virus to purified Torpedo acetylcholine receptor. Brain Res. 1986;387(3):211–219. doi: 10.1016/0169-328x(86)90027-6. [DOI] [PubMed] [Google Scholar]
- 65.Gastka M., Horvath J., Lentz T.L. Rabies virus binding to the nicotinic acetylcholine receptor alpha subunit demonstrated by virus overlay protein binding assay. J. Gen. Virol. 1996;77(Pt 10):2437–2440. doi: 10.1099/0022-1317-77-10-2437. [DOI] [PubMed] [Google Scholar]
- 66.Galat A., Gross G., Drevet P., Sato A., Menez A. Conserved structural determinants in three-fingered protein domains. FEBS J. 2008;275(12):3207–3225. doi: 10.1111/j.1742-4658.2008.06473.x. [DOI] [PubMed] [Google Scholar]
- 67.Lentz T.L., Wilson P.T., Hawrot E., Speicher D.W. Amino acid sequence similarity between rabies virus glycoprotein and snake venom curaremimetic neurotoxins. Science. 1984;226(4676):847–848. doi: 10.1126/science.6494916. [DOI] [PubMed] [Google Scholar]
- 68.Lentz T.L. Structure-function relationships of curaremimetic neurotoxin loop 2 and of a structurally similar segment of rabies virus glycoprotein in their interaction with the nicotinic acetylcholine receptor. Biochemistry. 1991;30(45):10949–10957. doi: 10.1021/bi00109a020. [DOI] [PubMed] [Google Scholar]
- 69.Bekbossynova A., Zharylgap A., Filchakova O. Venom-derived neurotoxins targeting nicotinic acetylcholine receptors. Molecules. 2021;26(11) doi: 10.3390/molecules26113373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Donnelly-Roberts D.L., Lentz T.L. Structural and conformational similarity between synthetic peptides of curaremimetic neurotoxins and rabies virus glycoprotein. Brain Res Mol Brain Res. 1991;11(2):107–113. doi: 10.1016/0169-328x(91)90112-b. [DOI] [PubMed] [Google Scholar]
- 71.Rustici M., Bracci L., Lozzi L., Neri P., Santucci A., Soldani P., et al. A model of the rabies virus glycoprotein active site. Biopolymers. 1993;33(6):961–969. doi: 10.1002/bip.360330612. [DOI] [PubMed] [Google Scholar]
- 72.Antil-Delbeke S., Gaillard C., Tamiya T., Corringer P.J., Changeux J.P., Servent D., et al. Molecular determinants by which a long chain toxin from snake venom interacts with the neuronal alpha 7-nicotinic acetylcholine receptor. J. Biol. Chem. 2000;275(38):29594–29601. doi: 10.1074/jbc.M909746199. [DOI] [PubMed] [Google Scholar]
- 73.Servent D., Antil-Delbeke S., Gaillard C., Corringer P.J., Changeux J.P., Menez A. Molecular characterization of the specificity of interactions of various neurotoxins on two distinct nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2000;393(1-3):197–204. doi: 10.1016/s0014-2999(00)00095-9. [DOI] [PubMed] [Google Scholar]
- 74.Rahman M.M., Teng J., Worrell B.T., Noviello C.M., Lee M., Karlin A., et al. Structure of the native muscle-type nicotinic receptor and inhibition by snake venom toxins. Neuron. 2020;106(6):952–962 e5. doi: 10.1016/j.neuron.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noviello C.M., Gharpure A., Mukhtasimova N., Cabuco R., Baxter L., Borek D., et al. Structure and gating mechanism of the alpha 7 nicotinic acetylcholine receptor. Cell. 2021;184(8):2121–2134 e13. doi: 10.1016/j.cell.2021.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bourne Y., Talley T.T., Hansen S.B., Taylor P., Marchot P. Crystal structure of a Cbtx-AChBP complex reveals essential interactions between snake alpha-neurotoxins and nicotinic receptors. EMBO J. 2005;24(8):1512–1522. doi: 10.1038/sj.emboj.7600620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fruchart-Gaillard C., Gilquin B., Antil-Delbeke S., Le Novere N., Tamiya T., Corringer P.J., et al. Experimentally based model of a complex between a snake toxin and the alpha 7 nicotinic receptor. Proc. Natl. Acad. Sci. U. S. A. 2002;99(5):3216–3221. doi: 10.1073/pnas.042699899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiappinelli V.A., Weaver W.R., McLane K.E., Conti-Fine B.M., Fiordalisi J.J., Grant G.A. Binding of native kappa-neurotoxins and site-directed mutants to nicotinic acetylcholine receptors. Toxicon. 1996;34(11-12):1243–1256. doi: 10.1016/s0041-0101(96)00110-9. [DOI] [PubMed] [Google Scholar]
- 79.Dineley K.T., Pandya A.A., Yakel J.L. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 2015;36(2):96–108. doi: 10.1016/j.tips.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Picciotto M.R., Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front. Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- 81.Nagarajan Tm W.E., Rupprecht C.R. Monoclonal atnibodies for the prevention of rabies: theory and clinical practice. Antib. Technol. J. 2014;4:1–12. [Google Scholar]
- 82.Hellert J., Buchrieser J., Larrous F., Minola A., de Melo G.D., Soriaga L., et al. Structure of the prefusion-locking broadly neutralizing antibody RVC20 bound to the rabies virus glycoprotein. Nat. Commun. 2020;11(1):596. doi: 10.1038/s41467-020-14398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roche S., Bressanelli S., Rey F.A., Gaudin Y. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein. Guangxi Sci. 2006;313(5784):187–191. doi: 10.1126/science.1127683. [DOI] [PubMed] [Google Scholar]
- 84.Roche S., Rey F.A., Gaudin Y., Bressanelli S. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein. Guangxi Sci. 2007;315(5813):843–848. doi: 10.1126/science.1135710. [DOI] [PubMed] [Google Scholar]
- 85.Hemachudha T., Wacharapluesadee S., Lumlertdaecha B., Orciari L.A., Rupprecht C.E., La-Ongpant M., et al. Sequence analysis of rabies virus in humans exhibiting encephalitic or paralytic rabies. J. Infect. Dis. 2003;188(7):960–966. doi: 10.1086/378415. [DOI] [PubMed] [Google Scholar]
- 86.Kalin N.H. Primate models to understand human aggression. J. Clin. Psychiatr. 1999;60(Suppl 15):29–32. [PubMed] [Google Scholar]
- 87.Klasen M., Wolf D., Eisner P.D., Eggermann T., Zerres K., Zepf F.D., et al. Serotonergic Contributions to human brain aggression networks. Front. Neurosci. 2019;13:42. doi: 10.3389/fnins.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Charlton K.M., Casey G.A., Campbell J.B. Experimental rabies in skunks: effects of immunosuppression induced by cyclophosphamide. Can. J. Comp. Med. 1984;48(1):72–77. [PMC free article] [PubMed] [Google Scholar]
- 89.de Boer S.F. Animal models of excessive aggression: implications for human aggression and violence. Curr Opin Psychol. 2018;19:81–87. doi: 10.1016/j.copsyc.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 90.Cherek D.R. Effects of smoking different doses of nicotine on human aggressive behavior. Psychopharmacology (Berl) 1981;75(4):339–345. doi: 10.1007/BF00435849. [DOI] [PubMed] [Google Scholar]
- 91.Cherek D.R., Bennett R.H., Grabowski J. Human aggressive responding during acute tobacco abstinence: effects of nicotine and placebo gum. Psychopharmacology (Berl) 1991;104(3):317–322. doi: 10.1007/BF02246030. [DOI] [PubMed] [Google Scholar]
- 92.Jamner L.D., Shapiro D., Jarvik M.E. Nicotine reduces the frequency of anger reports in smokers and nonsmokers with high but not low hostility: an ambulatory study. Exp. Clin. Psychopharmacol. 1999;7(4):454–463. doi: 10.1037//1064-1297.7.4.454. [DOI] [PubMed] [Google Scholar]
- 93.Van Schalkwyk G.I., Lewis A.S., Qayyum Z., Koslosky K., Picciotto M.R., Volkmar F.R. Reduction of aggressive episodes after repeated transdermal nicotine administration in a hospitalized adolescent with autism spectrum disorder. J. Autism Dev. Disord. 2015;45(9):3061–3066. doi: 10.1007/s10803-015-2471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lewis A.S., van Schalkwyk G.I., Lopez M.O., Volkmar F.R., Picciotto M.R., Sukhodolsky D.G. An exploratory trial of transdermal nicotine for aggression and irritability in adults with autism spectrum disorder. J. Autism Dev. Disord. 2018;48(8):2748–2757. doi: 10.1007/s10803-018-3536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Allen M.H., Debanne M., Lazignac C., Adam E., Dickinson L.M., Damsa C. Effect of nicotine replacement therapy on agitation in smokers with schizophrenia: a double-blind, randomized, placebo-controlled study. Am. J. Psychiatr. 2011;168(4):395–399. doi: 10.1176/appi.ajp.2010.10040569. [DOI] [PubMed] [Google Scholar]
- 96.Lewis A.S., Pittenger S.T., Mineur Y.S., Stout D., Smith P.H., Picciotto M.R. Bidirectional regulation of aggression in mice by hippocampal alpha-7 nicotinic acetylcholine receptors. Neuropsychopharmacology. 2018;43(6):1267–1275. doi: 10.1038/npp.2017.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shinawi M., Schaaf C.P., Bhatt S.S., Xia Z., Patel A., Cheung S.W., et al. A small recurrent deletion within 15q13.3 is associated with a range of neurodevelopmental phenotypes. Nat. Genet. 2009;41(12):1269–1271. doi: 10.1038/ng.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fejgin K., Nielsen J., Birknow M.R., Bastlund J.F., Nielsen V., Lauridsen J.B., et al. A mouse model that recapitulates cardinal features of the 15q13.3 microdeletion syndrome including schizophrenia- and epilepsy-related alterations. Biol. Psychiatr. 2014;76(2):128–137. doi: 10.1016/j.biopsych.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 99.Gillentine M.A., Berry L.N., Goin-Kochel R.P., Ali M.A., Ge J., Guffey D., et al. The cognitive and behavioral phenotypes of individuals with CHRNA7 duplications. J. Autism Dev. Disord. 2017;47(3):549–562. doi: 10.1007/s10803-016-2961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yan Y., Su C., Hang M., Huang H., Zhao Y., Shao X., et al. Recombinant Newcastle disease virus rL-RVG enhances the apoptosis and inhibits the migration of A549 lung adenocarcinoma cells via regulating alpha 7 nicotinic acetylcholine receptors in vitro. Virol. J. 2017;14(1):190. doi: 10.1186/s12985-017-0852-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bu X., Yin C., Zhang X., Zhang A., Shao X., Zhang Y., et al. LaSota strain expressing the rabies virus glycoprotein (rL-RVG) suppresses gastric cancer by inhibiting the alpha 7 nicotinic acetylcholine receptor (α7 nAChR)/phosphoinositide 3-kinase (PI3K)/AKT pathway. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2019;25:5482–5492. doi: 10.12659/MSM.915251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sajjanar B., Dhusia K., Saxena S., Joshi V., Bisht D., Thakuria D., et al. Nicotinic acetylcholine receptor alpha 1(nAChRα1) subunit peptides as potential antiviral agents against rabies virus. Int. J. Biol. Macromol. 2017;104(Pt A):180–188. doi: 10.1016/j.ijbiomac.2017.05.179. [DOI] [PubMed] [Google Scholar]
- 103.Embregts C.W.E., Begeman L., Voesenek C.J., Martina B.E.E., Koopmans M.P.G., Kuiken T., et al. Street RABV induces the cholinergic anti-inflammatory pathway in human monocyte-derived macrophages by binding to nAChr alpha 7. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.622516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huey R., Hawthorne S., McCarron P. The potential use of rabies virus glycoprotein-derived peptides to facilitate drug delivery into the central nervous system: a mini review. J. Drug Target. 2017;25(5):379–385. doi: 10.1080/1061186X.2016.1223676. [DOI] [PubMed] [Google Scholar]
- 105.Antil S., Servent D., Menez A. Variability among the sites by which curaremimetic toxins bind to torpedo acetylcholine receptor, as revealed by identification of the functional residues of alpha-cobratoxin. J. Biol. Chem. 1999;274(49):34851–34858. doi: 10.1074/jbc.274.49.34851. [DOI] [PubMed] [Google Scholar]
- 106.Gillentine M.A., White J.J., Grochowski C.M., Lupski J.R., Schaaf C.P., Calarge C.A. CHRNA7 deletions are enriched in risperidone-treated children and adolescents. J. Child Adolesc. Psychopharmacol. 2017;27(10):908–915. doi: 10.1089/cap.2017.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bu X., Yin C., Zhang X., Zhang A., Shao X., Zhang Y., et al. LaSota strain expressing the rabies virus glycoprotein (rL-RVG) suppresses gastric cancer by inhibiting the alpha 7 nicotinic acetylcholine receptor (alpha 7 nAChR)/phosphoinositide 3-kinase (PI3K)/AKT pathway. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2019;25:5482–5492. doi: 10.12659/MSM.915251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.