Abstract
Surface engineering of particles based on a polymeric coating is of great interest in materials design and applications. Due to the disadvantages of non-biodegradability and undesirable biocompatibility, the application of petroleum-based synthetic polymers coating in the biomedical field has been greatly limited. In addition, there is lack of a universal surface modification method to functionalize particles of different compositions, sizes, shapes, and structures. Thus, it is imperative to develop a versatile biopolymeric coating with good biocompatibility and tunable biodegradability for the preparation of functional particle materials regardless of their surface chemical and physical structures. Recently, the natural polysaccharide polymers (e.g. chitosan and cellulose), polyphenol-based biopolymers (e.g. polydopamine and tannic acid), and proteins (e.g. amyloid-like aggregates) have been utilized in surface modification of particles, and applications of these modified particles in the field of biomedicine have been also intensively exploited. In this review, the preparation of the above three coatings on particles surface are summarized, and the applications of these materials in drug loading/release, biomineralization, cell immobilization/protection, enzyme immobilization/protection, and antibacterial/antiviral are exemplified. Finally, the challenges and the future research directions on biopolymer coating for particles surface engineering are prospected.
Keywords: Biopolymer, Particle, Surface engineering, Bio-applications, Amyloid-like protein aggregates
Graphical abstract
Highlights
-
•
This review highlights the importance of particle surface engineering in the materials field. .
-
•
This review summarizes biopolymer coating for particle surface engineering and their biomedical applications. .
-
•
This review discusses the key challenges and directions for future research and development of particle surface engineering .
1. Introduction
Advances in material design and applications are highly dependent on the development of particle surface engineering strategies [[1], [2], [3], [4]], since micro/nanoparticles have found their significant values in broad and important applications, such as catalysis, sensors, coatings, composites, optoelectronics, energy, environment, and biomedicine [[5], [6], [7], [8], [9]]. The micro/nano particle surface chemistry and physical structure determine the key parameters of colloidal ion stability, bioactivity, and compatibility. Accordingly, by modifying the surface of micro/nano particles, the physical and chemical properties of the material surface can be controlled and new functions can be imparted, which thus derives an important field of particle surface engineering [[10], [11], [12], [13], [14], [15]].
The petroleum-based synthetic polymers are cost-efficient, readily available, and can be processed in various ways. For these reasons, they have been widely used to modify micro/nanoparticles aiming to improve their corresponding performance [16,17]. However, the non-biodegradability and poor biocompatibility have greatly limited the biomedical application of these synthetic polymers-modified micro/nanoparticles [[18], [19], [20]]. Moreover, the formation and stability of the synthetic polymer coatings are closely related to the physical and chemical properties of respective particle surface. In this regard, there are few general methods to functionalize particles of different compositions, sizes, shapes, and structures. Therefore, considering the crucial role of the advanced particle materials in biomedicine such as drug delivery, cancer therapy, bioimaging and so on, it is urgent to develop a universal surface engineering strategy based on natural polymers for the preparation of functional particle materials.
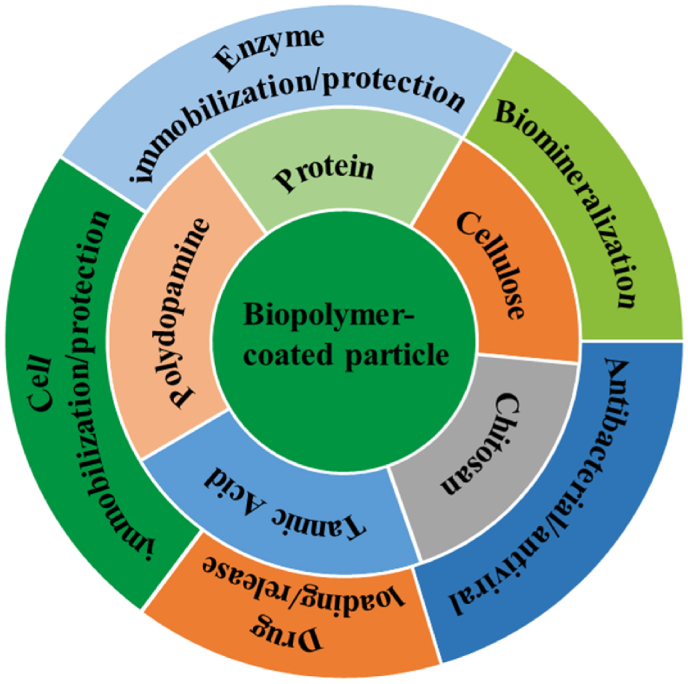
The above challenge has been addressed by developing biopolymer-based coating techniques due to excellent biocompatibility and biodegradability as well as mild preparation process of biopolymer materials [[21], [22], [23], [24], [25]]. Polysaccharides, polyphenol derivatives and proteins are typical commonly used natural biopolymers. Particularly, being inspired from natural adhesion, the polydopamine (PDA) and tannic acid (TA) systems as well as protein amyloid-like aggregates, could stably adhere onto virtually arbitrary particle surface to form a robust and conformal coating [[26], [27], [28], [29], [30]]. The various active functional groups such as hydroxyl, amino, and carboxyl groups introduced by these types of coatings not only endow the coated particles with multiple functions but also provide reactive sites to further chemical modification [[31], [32], [33], [34], [35], [36]]. This paper sets out to briefly introduce the structure and formation of the polysaccharide, polyphenol and protein coatings, and summarize the research progress on the particle surface engineering by these biopolymer coatings and their applications in the field of biomedicine (Fig. 1, Table 1). Especially, the newly discovered superfast amyloid-like protein aggregation process was specially underlined, in which the amyloid-like aggregation being rich in β-sheet structures can self-assemble into nanofilms on a range of particle surfaces with versatile compositions, sizes, shapes, and structures through a simple and rapid one-step aqueous coating process. At the end of this paper, the key challenges and directions for future research and developments of particle surface engineering are discussed.
Fig. 1.
Biopolymer coating for particle surface engineering and its biomedical application.
Table 1.
Progress summary of biomedical applications progress of different biopolymer coatings.
| Coatings | Methods | Applications | Reference |
|---|---|---|---|
| Chitosan | Dip coating, physical adsorption, electrostatic interaction |
Drug and gene delivery, tissue engineering, wound healing, antibacterial | [[37], [38], [39], [40], [41]] |
| Cellulose | Roller coating, dip coating, spray coating, spin coating and bar coating | Drug delivery, tissue engineering, antibiotic delivery, bio-sensing, antibacterial | [[42], [43], [44], [45], [46], [47], [48], [49], [50]] |
| Polydopamine | Polymerization | biomedicine, surface engineering | [[51], [52], [53], [54], [55]] |
| Tannic acid | Assembly | Drug delivery, antibacterial, clinical diagnosis, nanoprobe treatment, tooth desensitizers, living cell protection. | [[56], [57], [58], [59], [60], [61], [62], [63], [64]] |
| Protein | Dip coating, welding, self assembly | protein fixation, biomedical enzyme catalysis, enzyme fixation, coenzyme regeneration, cell protection, medical diagnostic | [[65], [66], [67], [68], [69], [70]] |
2. Polysaccharide-based coating for particle surface engineering and related biomedical application
Polysaccharides are natural macromolecular compounds formed by the polymerization of more than 10 monosaccharides through glycosidic bonds, and are widely found in animals, plants and microorganisms [[71], [72], [73]]. Different polysaccharides have a variety of chemical structures and versatile functions that determine their applications [[74], [75], [76]]. Chitosan and cellulose, as the two most common types of polysaccharide polymers, have excellent biocompatibility, biodegradability, film-forming, non-toxic, renewable and other excellent characteristics, which make them good candidates to modify particle surface and apply in biomedicine [37,38,[42], [43], [44], [45]].
2.1. Chitosan
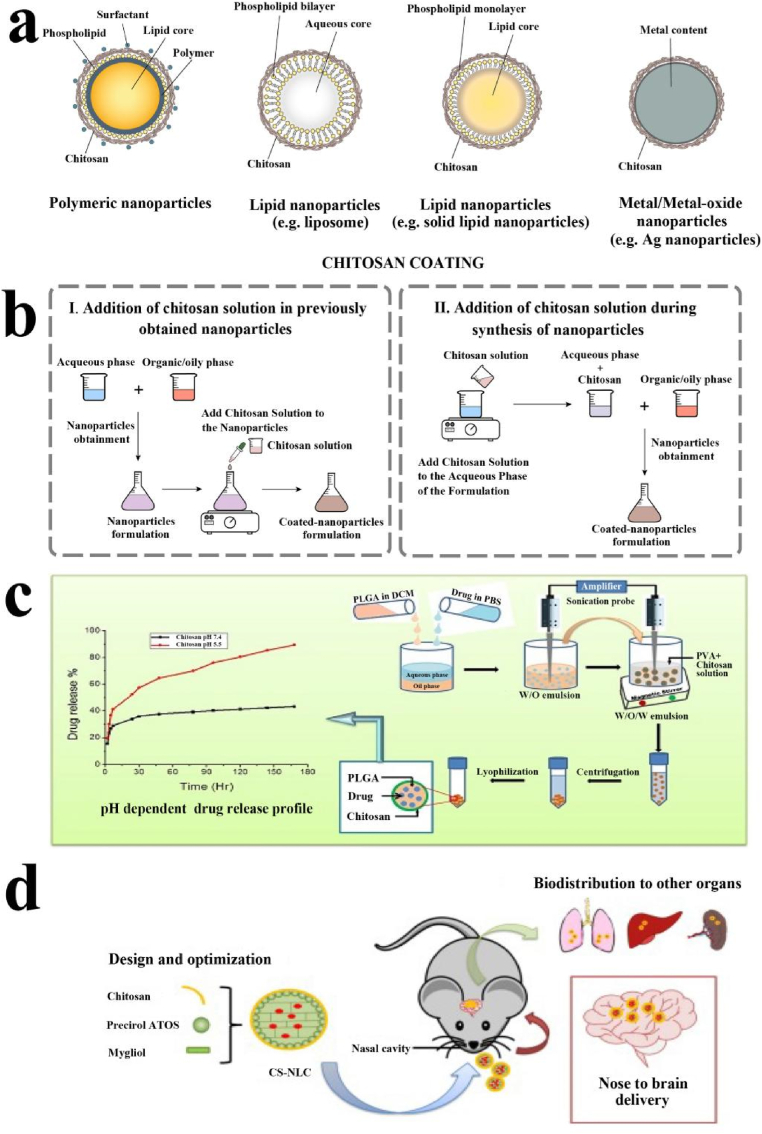
Chitosan is a natural polysaccharide derived from chitin, the second most abundant biopolymer in nature after cellulose. It can be obtained from the shells of marine crustaceans, insects, fungi, etc. [[77], [78], [79]] Its main features are high biodegradability, biocompatibility, antibacterial and neglectable toxicity. In recent years, chitosan is increasingly explored in a variety of biomedical applications, including drug and gene delivery, tissue engineering, wound healing, and antibacterial [[39], [40], [41]]. In the field of pharmaceutical nanotechnology, drug carriers made of polymer nanoparticles, lipid nanoparticles, and metal (or metal oxide)-based nanoparticles have been modified with chitosan (Fig. 2a) [[80], [81], [82], [83]]. The chitosan coating was usually formed on the particle surface through the following two methods: addition of chitosan solution in the previously obtained nanoparticles, addition of chitosan solution during the preparation of nanoparticles (Fig. 2b) [80]. When the cationic polymer chitosan is added to the nanoparticles formed or in formation, the chitosan turns into the shell that coats the nanoparticles. Under acid conditions, the positively charged ammonium groups of chitosan interact with the negative charges on the nanoparticle surface (such as the carboxylate end-group of the polymer or the phosphate group of the phospholipids), occurring an interfacial reaction, and such method commonly referred to as electrostatic or polyelectrolyte deposition or self-organized interaction between chitosan with negatively charged nanoparticles [80].
Fig. 2.
(a) Schematic illustration of chitosan coating in polymeric nanoparticles, lipid nanoparticles and metal/metal-oxide nanoparticles. (b) The two typical preparation methods of chitosan-coated nanoparticles. (c) Schematic representation of chitosan-coated drug nanoparticles and their pH-dependent drug release profile. (d) Schematic representation of the chitosan-coated nanostructured lipid drug carrier. Reproduced from refs 80 and 89 with permission from Elsevier.
In the biomedical application, the in vitro and in vivo investigations have demonstrated that chitosan coating introduced many functions to particles, including: i) improving physicochemical stability, ii) regulating drug release profile, iii) improving tissue penetration, iv) changing the way to interact with cells, v) enhancing antibacterial capability, vi) improving bioavailability and drug efficacy. Chitosan-coated liposomes are stable during storage 15 weeks, such that over 85% of Vitamin C are protected against oxidation in both conditions room temperature or 4 °C [84]. Some authors have further shown that chitosan coated polymer nanoparticles can be used to release drugs in a specific environment (e.g., acidic pH). For instance, chitosan-coated poly (lactide-co-glycolide) (PLGA) nanoparticles released about 45% more sodium diclofenac at pH 5.5 compared to the release at pH 7.4 (Fig. 2c) [85]. An increase in permeation was further achieved by chitosan coating for niosomal nanoparticles in goat intestinal membrane and cornea and sheep nasal mucosae after coating by chitosan [[86], [87], [88]]. Chitosan could enhance cellular uptake of the coated nanoparticles, including polymer, lipid and metal-based nanoparticles. In these studies, the existence of chitosan on the surface of nanoparticles, whether natural or coupled with other molecules, is conducive to their interaction and internalization with the cell membrane, which is particularly relevant for the case that capsule drugs have intracellular targets. Nanostructured lipid carriers coated by chitosan (CS-NLCs) were further used for brain delivery of proteins through intranasal administration. In vitro experiments proved the biocompatibility of nanocarriers and their uptake by human bronchial epithelial cell (16HBE14o-). In addition, when the granules were incubated with erythrocytes, no hemagglutination or hemolysis was observed, and there was no toxic signal in the nasal mucosa of mice after administration of CS-NLCs (Fig. 2d) [89]. Chitosan are also commonly used for antimicrobial/antibacterial purposes. For instance, chitosan coatings could enhance the antibacterial activity of the metal nanoparticles such as gold (Au), silver (Ag), copper (Cu), platinum (Pt) and titanium (Ti). In this way, iron oxide nanoparticles modified with chitosan exhibited enhanced ROS production and antimicrobial activity [90]. Besides antibacterial ability, a study evaluating the effect of chitosan coating on the pharmacokinetic parameters of docetaxel-loaded nanoparticles further showed that the drug half-life, maximum drug concentration in blood of chitosan-coated nanoformulations were 21.9 times higher compared to uncoated nanoparticles and free drug [91]. In a mouse model of mucositis, rebamipide-loaded PLGA nanoparticles coated with chitosan decreased treatment time by 3.6 days compared to uncoated nanoparticles due to greater interaction with oral mucosa [92].
2.2. Cellulose
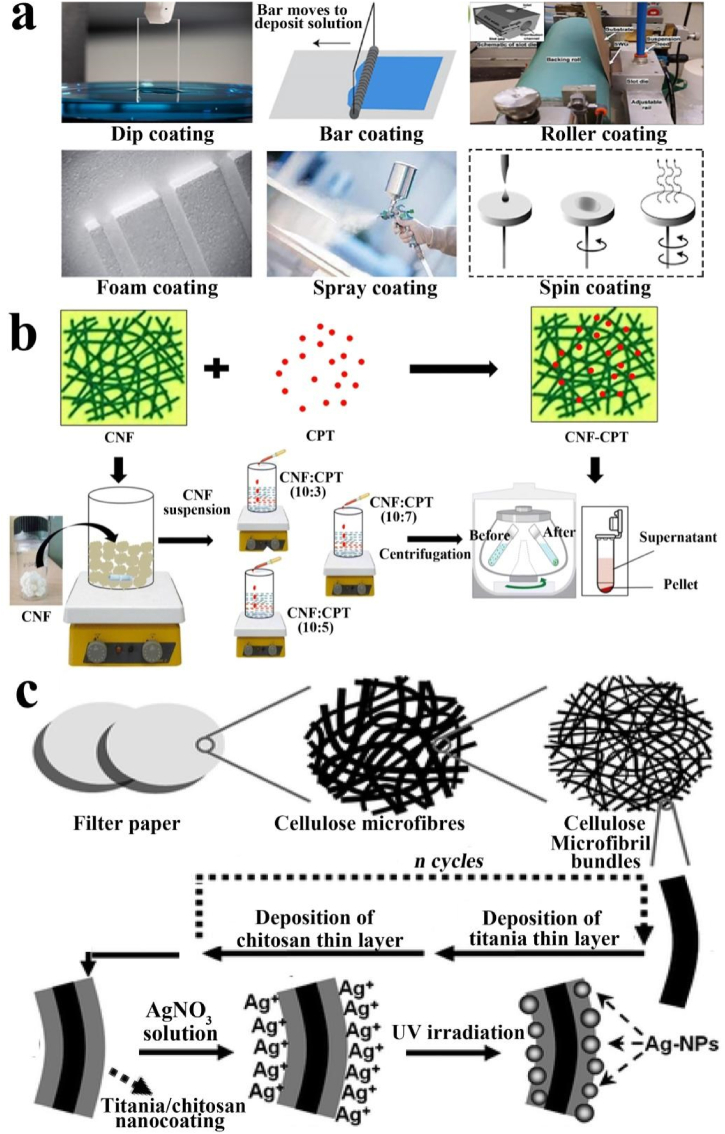
Cellulose is the most abundant natural polymer on earth and also an important biomass resources [[93], [94], [95]]. It is a homopolymer of chair conformed anhydrous-d-glucose units joined by β-1,4glycosidic linkages [96]. Due to the widespread presence of OH groups throughout polymer chain, a large number of inter and intramolecular hydrogen bonds are formed, so the structure of cellulose is relatively complex [97]. The presence of strong hydrogen bonds gives it a crystalline nature, rigidity and unreactiveness toward water and other chemical reagents. On the other hand, the weak hydrogen bonds presenting in the amorphous domains show hydrophilicity, flexibility and accessibility [98]. Due to the excellent properties, cellulose and its derivatives are now widely used as nano building blocks for many coatings, both in pristine form and as composites. Low cost, availability and renewability broaden the possibilities of cellulose in coatings applications. Different types of deposition technologies reported for cellulose based coatings include roller coating, dip coating, spray coating, spin coating and bar coating (Fig. 3a) [46].
Fig. 3.
(a) Schematic illustration of preparation method of cellulose coating. (b) Diagrammatic representation of the preparation of formulations using cellulose nanofibers (CNF). Camptothecin (CPT), and cellulose nanofibers and camptothecin composite (CNF-CPT). (c) Schematic illustration of cellulose composite coating. Reproduced from ref 46 with permission from Elsevier. Reproduced from ref 106 with permission from Springer Nature. Reproduced from ref 107 with permission from Royal Society of Chemistry.
To date, many reviews have been reported explaining the biomedical applications of cellulose in drug delivery systems, scaffolds for cell culture, tissue engineering, DNA extraction, antibiotic delivery, bio-sensing and anti-microbial activity [[47], [48], [49], [50],[99], [100], [101], [102]]. For the wound dressing, the composite material of cellulose-coated silver nanoparticles can not only show strong antibacterial activity against Staphylococcus aureus and Escherichia coli, but also provide a moist environment for the wound, resulting in better wound healing [103]. For the drug delivery system, cellulose coatings are used to encapsulate drugs in order to maintain stability of drugs towards variations in pH, ionic strength, and temperature around the target. It has been proven that these factors serve the key to control drug release [104]. In a recent study, drugs particles coated by cellulose/honey composite membranes showed constant release of drugs following a first-order release kinetics mechanism. In such a process, the drug concentration continually raised and then stabilized for roughly 48 h [105]. In another case, nanocellulose was used to encapsulate anticancer drugs (i.e, camptothecin), and the resultant camptothecin/cellulose nanofiber composites showed a high drug encapsulation efficiency of 65.28%, and tunable drug release under different physiological conditions (Fig. 3b) [106]. Cellulose was further used to immobilize silver nanoparticles, and the prepared composite showed excellent antibacterial activity against almost all inoculated bacteria (E. coli and S. aureus). Taken together, the developed particle materials based on the cellulose coating or hybridization have demonstrated their great potential and promise in antibacterial applications, such as antibacterial wound dressing, antibacterial packaging, antibacterial adhesion and so on (Fig. 3c) [107]. Although polysaccharide membrane materials have been widely used in various biomedical fields, the preparation of polysaccharide coatings still needs to be improved in the following aspects, such as complex extraction of polysaccharide monomers, long preparation time, special solvent needed to dissolve polysaccharides, poor universality and so on.
3. Polyphenol based coating for particle surface engineering and related biomedical application
Polyphenols belong to a broad group of chemical substances having one or more aromatic rings with two or more hydroxyl groups They are natural compounds found in plants and can be extracted from sources such as vegetables, grains, chocolates, fruits, tea, legumes, and seeds, among other sources [[108], [109], [110]]. Polyphenolic membrane materials are widely used because of their good universality and strong interaction to various substrates, and easy film formation in the aqueous phase [[111], [112], [113], [114], [115], [116]]. At present, the most investigated polyphenolic coatings are polydopamine and tannic acid systems.
3.1. Polydopamine
Polydopamine (PDA) is formed through oxidative polymerization of dopamine monomers at room temperature [[51], [52], [53]]. Its molecular structure mainly contains amine and catechol. Under alkaline conditions (pH > 7.5), dopamine was oxidized and spontaneously self-polymerized on the substrate surface to form a PDA coating. This self-polymerization reaction is mild and does not require any complicated instruments or harsh reaction conditions [54,55,[117], [118], [119]]. Compared with other polymer coatings, PDA coating can be formed on the surface of various materials (such as inorganic materials, organic materials, metals and metal oxides and even polymers). In addition, PDA coated materials can further react with functional molecules containing amino, sulfhydryl or carboxyl groups through Michael addition and/or Schiff base reaction to realize the advanced applications [[120], [121], [122], [123]]. Although the adhesion mechanism between PDA coating and surface have been not uncovered thoroughly, it is demonstrated that the catechol-metal coordination, electrostatic interactions, π-π interactions, hydrogen bonds play an important role [[124], [125], [126], [127]]. The manufacture and widespread application of PDA-based materials is developing rapidly, and a number of cost-effective PDA materials have been developed that are expected to be used in energy, biomedicine, water treatment, sensing, surface engineering, and other fields.
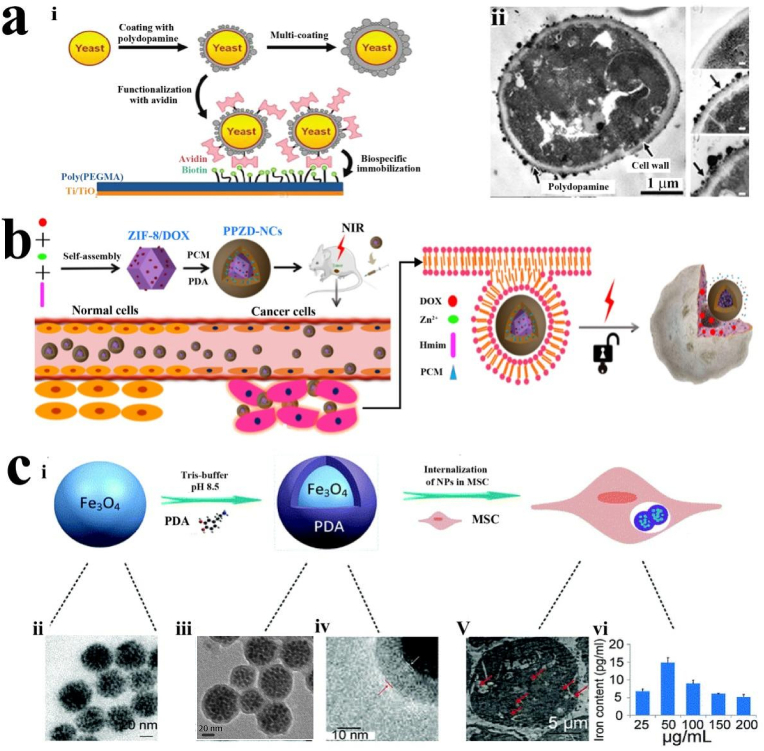
Recently, the PDA coating shows great potential in the protection or fixation of living cells. This area appears to be essential and a challenge for the development of cell-based devices and sensors, as well as investigation on cell-cell interactions and cell behavior modulation. For instance, Yang et al. proposed a biomimetic approach for individually encapsulating living yeast cells by coating them with a polydopamine layer and immobilizing the cells on a selected substrate. Artificial polydopamine shells can control cell division while preserving those cells from harsh environments, such as attack by foreign substances (Fig. 4a) [128]. The dopamine-modified nanoparticles, for instance, PDA-phase-change material@Zeolitic imidazolate framework-8/Doxorubicin hydrochloride nanocomposites (PPZD-NCs), exhibited biocompatibility, low toxicity, and high stability both in vitro and in vivo. They possessed great photothermal, thermal and pH-responsiveness, and effective synergistic thermo-chemotherapy properties (Fig. 4b) [129]. For bone regeneration, the PDA-coated HAp nanoparticles have been prepared, and then fixed the bone morphogenetic peptide on their surface through catechol linking with PDA. Such peptide-conjugated HAp@PDA nanoparticles promoted the adhesion and proliferation of MG-63 cells while maintaining the osteogenic activity of the grafted peptides. In another case, accelerating wound healing might be achieved by utilizing PDA-modified particles to enhance the migration of mesenchymal stem cells (MSCs) [130]. As shown in Fig. 4c, Li et al. found that Fe3O4@PDA nanoparticles could be internalized by MSCs without any negative effects on their stem cell properties, which could alleviate local inflammatory responses after implantation in burn skin [131].
Fig. 4.
(a-i) Schematic illustration of polydopamine encapsulation and surface functionalization of individual yeast cells. (a-ii) TEM images of polydopamine-coated yeast cells. (b) Schematic illustration of the synthesis of PDA-PCM@ZIF-8/DOX and controllable combination with thermo-chemotherapy. (c) Fe3O4@PDA nanoparticles prepared and internalized by MSCs: (c–i) Schematic illustration of the preparation of Fe3O4@PDA nanoparticles by thermal decomposition method; The TEM images of Fe3O4 nanoparticles (c-ii), Fe3O4@PDA nanoparticles (c-iii, iv), and Fe3O4@PDA internalization in MSCs (c-v); (c, vi) Iron concentration in cells determined by ICP-OES. Reproduced from ref 128 with permission from American Chemical Society. Reproduced from ref 129 with permission from Elsevier. Reproduced from ref 131 with permission from Royal Society of Chemistry.
Although PDA materials have been deeply studied, it seems unrealistic to transfer the application of PDA materials from laboratory research to commercial applications. Some practical application problems have attracted widespread attention, such as in the biomedical field, its biosafety has not been revealed especially whether its by-products will accumulate in cells or organs, and the potential negative impact on health. In the field of water treatment, the disposal of PDA modified materials after use has been widely concerned. In addition, the polymerization process, precise structure and composition of PDA are still lacking conclusive conclusions. Once the above problems can be successfully solved in the future, the research field of PDA has a very bright future, which will finally promote the rapid and interdisciplinary development of PDA materials.
3.2. Tannic acid
Tannic acid (TA is another classical polyphenolic polymer composed of a central glucose molecule with two esterified gallic acids (3,4,5-trihydroxybenzoic acid) on its five hydroxyl moieties [132]. The pyrogallol and catechol groups having high chemical reactivity provide TA with good coordination positions for biomimetic surface engineering and functional applications. Furthermore, due to its special structural properties, TA coatings are commonly used in materials engineering to facilitate interactions with various materials through multiple binding pathways, including coordination bonding, electrostatic, π-π stacking, hydrogen bonding and hydrophobic interaction [[56], [57], [58]].
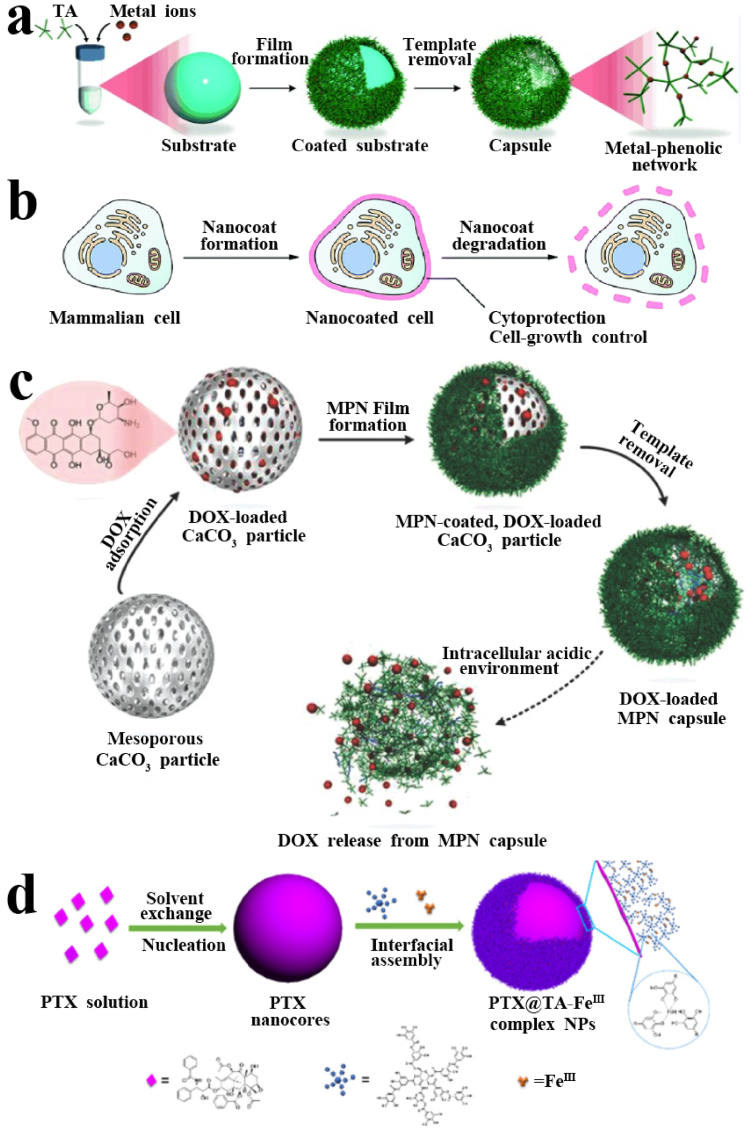
TA usually undergoes coordination assembly through the metal–phenolic network to form metal-organic supramolecular materials. Ejima et al. first proposed the synthesis of coordination complexes using natural polyphenol TA as an organic ligand and Fe(III) as the metallic crosslinking agent, which can be used to prepare film coating on a series of flat substrates and particles through one-step assembly [59]. Currently, many researches associated with the assembly of TA with iron or ferrous ions and their applications in the biomedical field have been explored, such as antibacterial coatings, clinical diagnosis, nanoprobe treatment, tooth desensitizers, separation and protection of living cells [[60], [61], [62], [63], [64]]. Guo et al. further developed a series of functional metal–phenolic network capsules prepared from TA and a range of metals (Fig. 5a) [133]. The properties of metal–phenolic network capsules were determined by coordinated metals, which can control film thickness, disassembly properties, and fluorescence behavior. Moreover, the functional properties of metal–phenolic network capsules were tailored for drug delivery, positron emission tomography, magnetic resonance imaging, and catalysis (Table 2). The ability to incorporate multiple metals into metal–phenolic network (MPN) capsules suggested that multiple functional materials can be generated.
Fig. 5.
(a) Schematic representation of controlled formation and degradation of TA–FeIII nanocoating on individual mammalian cells, mimicking sporulation and germination processes. (b) Schematic representation of the fabrication process of DOX-loaded MPN capsules and release mechanism of DOX from MPN capsules. (c) Assembly of TA and metal ions to form a MPN film on a particulate template, followed by the subsequent formation of a MPN capsule. (d) General procedure for the fabrication of PTX@TA−FeIII complex nanoparticles. Reproduced from refs 133 and 142 with permission from Wiley-VCH. Reproduced from ref 134 with permission from American Chemical Society. Reproduced from ref 141 with permission from Royal Society of Chemistry.
Table 2.
Progress summary of biomedical applications progress of TA coatings.
| TA coatings | Key applications | Reference |
|---|---|---|
| TA-FeIII | Surface engineering, Anticancer therapy, Living Cell protection, Drug delivery Anti-biofouling, Water treatment, Mechanical enhancement, Cell Imaging, Photoluminescence, Cellular surface engineering | [59,[133], [134], [135], [136], [137], [138], [139], [140], [141]] |
| TA-AlIII | Drug delivery | [142] |
| TA-CuII | Imaging, Antibacterial | [133] |
| TA-RhIII | Catalysis | [133] |
| TA-TiIV | Enzyme-immobilized capsules | [143] |
| TA-TbIII/EuIII | Biomedical imaging, Fluorescent capsules, Drug delivery | [133,144] |
| TA-GdIII/MnII | Magnetic resonance imaging | [133] |
| TA-VIII/CrIII/CoII/NiII/ZnII/ZrIV/MoII/RuIII/CdII/CeIII | Hollow capsules | [133] |
Based on the above fundamental studies, Lee et al. reported a cytocompatible method to form a degradable TA-FeIII coating on individual mammalian cells (HeLa, NIH 3T3, and Jurkat cells) by chemically mimicking the sporulation and germination processes in nature (Fig. 5b) [141]. The resultant TA–FeIII-coated mammalian cells were reasonably viable and had greatly enhanced resistance to other lethal agents, UV-C irradiation and toxic compounds. More importantly, the cell growth and proliferation, suppressed temporarily by the coating were restored by the controlled degradation of the coating. Ping et al. studied that drug-loaded metal–phenolic network capsules based on the formation of coordination complexes between TA and AlIII ions could be assembled and used as pH-responsive carriers for drug loading and intracellular drug release (Fig. 5c) [142]. Such metal–phenolic network capsules have a high drug loading (1.3 pg/capsule of DOX) and pH sensitivity, which give rise to pH-enhanced drug release kinetics and enhanced effectiveness in killing HeLa cells with the half-maximal inhibitory concentration (IC50) value of 0.20 μg/mL. In addition, such capsules are expected to be further applied in various biomedical applications, such as therapeutic nanomedicine, and multidrug combination delivery. Shen et al. used the coating of TA-FeIII supramolecular assembly to surface engineering the hydrophobic drug paclitaxel particles and applied it to the field of biomedical anti-tumor (Fig. 5d) [134]. Studies have demonstrated that the method is capable of effectively suppressing and retarding Ostwald ripening, providing drug nanoparticles with a small and uniform size and long-term colloidal stability. The final composite drug nanoparticles exhibited higher tumor accumulation, negligible toxicity, and enhanced antitumor activity, being superior to commercial formulations. The author's results further showed that the local on-demand coating of hydrophobic nanoparticles by TA-FeIII supramolecular complex can be realized through the cooperation and compromise of interfacial adhesion and assembly.
Although polyphenol coatings are the current research focus for the particle surface modification, there are still some problems that need to be solved. Typically, surface/interface after the modification by polyphenol coating is brown or black, the dopamine polymerization mechanism is complex, and the polymerization time is long (generally 10 h); unclear biodegradability of cross-linked products, poor coating stability (especially in alkaline environments), and rough coating surface/interface, etc. Therefore, it is particularly important to develop a new class of surface/interface modification method that has universal adhesion to all kinds of substrates and can overcome the shortcomings of polydopamine and TA coatings.
4. Protein-based coating for particle surface engineering and related biomedical application
As the essential constituent of organisms, proteins generally exhibit excellent biocompatibility and are considered to be an ideal candidate for developing biomaterials [[145], [146], [147]]. For instance, the application of collagen in scaffold for tissue engineering and wound dressing has been extensively reported [[148], [149], [150]]. However, it is always a challenge to fabricate a functional proteinaceous coating on micro/nano-particles surface. Amyloid is a kind of insoluble self-assembled protein aggregate displaying polymorphic mesoscopic structures. Even though the amyloids are originally discovered as the pathological hallmarks of human neurodegenerative diseases, it is found that there are also many nonpathogenic functional amyloid aggregates implicated in biomedical processes occurring in a variety of organisms ranging from simple bacteria to humans, such as the adhesive property of E. coil biofilms, biosynthesis of melanin in mammals, and human peptide hormone storage [151,152]. Since then, a particle surface coating technique based on protein amyloid aggregation have been created [65,66,153]. Amyloid has the structural characteristics of a multi-level assembly features: at the molecular and atomic level, it has a neatly arranged β-sheet structure; at the nano-level, the β-sheet structure self-assembles to form nanofibers with a high aspect ratio; at the micro-level, the nanofibers are stacked with each other into disordered bulk aggregates. This hierarchically ordered structural feature enables amyloid proteins to exhibit good structural stability and unique mechanical properties [67,68,154]. Amyloid aggregates usually result from the intermolecular interaction between unfolded or misfolded proteins. For the conventional amyloid aggregation of stable globular proteins, the native proteins need to unfold or hydrolyze to be polypeptide fragments (monomers) so as to overcome an energy barrier for amyloid aggregation, then the hydrophobic forces drive the nucleation of monomers and further transition to β-sheet-rich oligomers and protofibrils. Subsequently, the monomers join the nucleus end to grow slowly into a mature fibril [[155], [156], [157], [158]]. In the following section, two kinds of amyloid protein coatings fabricated through traditional and superfast amyloid protein aggregation and the biomedical application of the coated particles were summarized.
4.1. Traditional amyloid protein aggregation
Amyloid fibrils have been postulated to be the most thermodynamically stable protein unfolding state. They can be obtained by incubating the native protein aqueous solution at low pH and high temperature condition for several days [[159], [160], [161], [162], [163], [164]].
In antiviral area, Palika et al. introduced and developed a sustainable and biodegradable antiviral filtration membrane composed of amyloid nanofibrils made from food-grade milk proteins and iron oxyhydroxide nanoparticles. The membrane has excellent efficacy against a variety of viruses, including enveloped, non-enveloped, airborne and waterborne viruses such as SARS-CoV-2, H1N1 and enterovirus71, which highlights a possible role in fighting the current and future viral outbreaks and pandemics. In the field of iron-deficiency anaemia, the researchers studied the combination of organic and inorganic materials at the nanoscale to produce highly bioavailable and cost-effective iron–amyloid fibril hybrids [165]. Shen et al. used biodegradable amyloid fibrils from β-lactoglobulin, a low-cost milk protein with natural reducing effects, as anti-oxidizing nanocarriers and colloidal stabilizers for iron nanoparticles. The resulting hybrid materials form stable protein-iron colloidal dispersions that rapidly dissolve and release iron ions during acidic and enzymatic in vitro digestion. Importantly, this hybrid shows high in vivo iron bioavailability comparable to ferrous sulfate in haemoglobin-repletion and stable-isotope studies in rats, but with reduced organoleptic changes in foods. Feeding rats with these hybrid materials did not result in abnormal iron accumulation in any organs, or changes in whole blood glutathione concentrations, inferring their primary safety [166]. In terms of drug loading and release, Bajpai et al. used dialdehyde alginate to crosslink casein to prepared casein membrane material for loading and controlled release of cationic drugs, such as gentamicin sulfate [167].
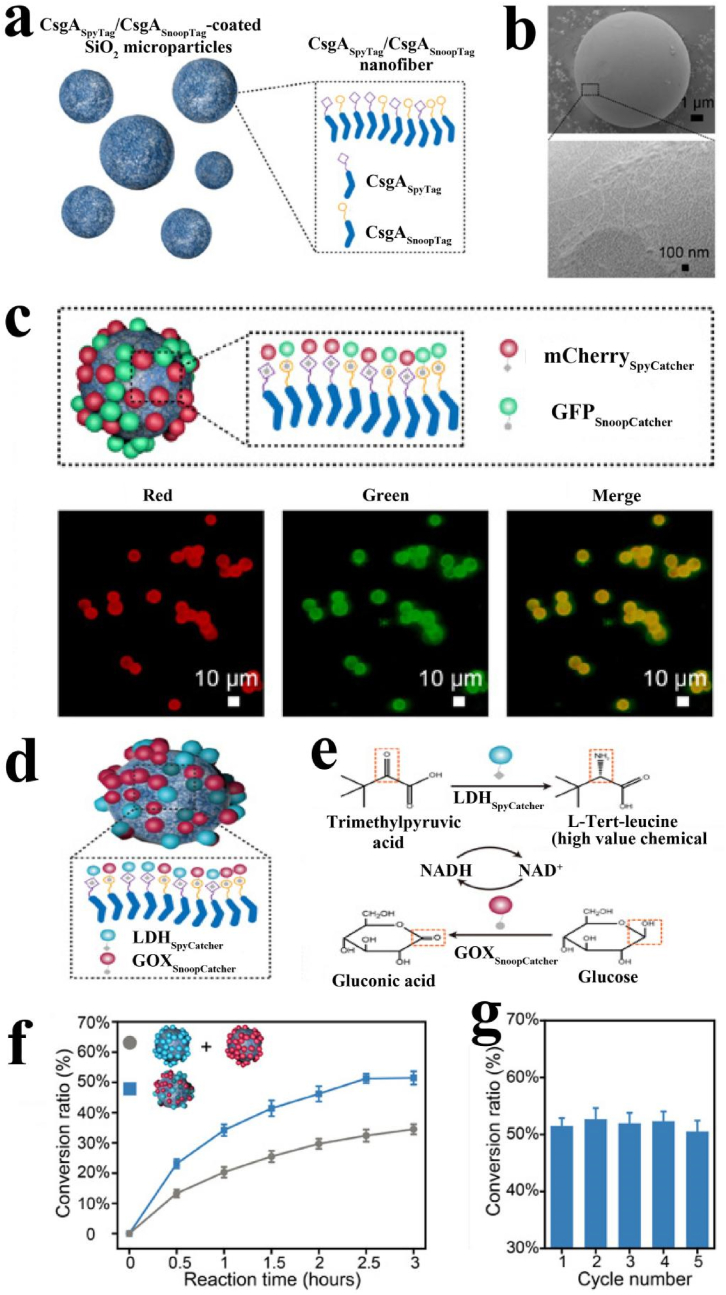
Additionally, a series of multifunctional amyloid materials were designed through genetic engineering [[168], [169], [170], [171], [172]]. Li et al. used protein-CsgASpyTag/CsgASnoopTag to modify the particles through the self-assembly of protein nanofibrils realizing the applications in the fields of protein fixation, biomedical enzyme catalysis, enzyme fixation and coenzyme regeneration (Fig. 6) [69]. CsgASpyTag/CsgASnoopTag modified particles were incubated with mCherrySpyCatcher and GFPSnoopCatcher (Fig. 6a–b). The fluorescence microscopy images showed that these particles displayed both the red fluorescence of mCherry and the green fluorescence of GFP (Fig. 6c). Therefore, it is confirmed that the CsgASpyTag/CsgASnoopTag nanofiber modified particles have the function of binding spycatcher and snoopcatcher fusion protein, which provides a basis for the functional application of particles.
Fig. 6.
Programmable amyloid coatings for tunable fluorescent materials and enzymatic biotransformation systems. (a) Illustration of microparticles coated with CsgASpyTag/CsgASnoopTag. (b) SEM images of SiO2 microparticles coated with CsgASpyTag/CsgASnoopTag. (c) Schematic showing fluorescent proteins conjugated on CsgASpyTag/CsgASnoopTag nanofiber (top) and fluorescence microscopy images of the corresponding fluorescent protein-conjugated CsgASpyTag/CsgASnoopTag-coated microparticles. (d) Schematic showing LDHSpyCatcher and GOXSnoopCatcher immobilized on CsgASpyTag/CsgASnoopTag-coated microparticles (e) Illustration of a dual-enzyme reaction system enabled by LDHSpyCatcher and GOXSnoopCatcher co-conjugated microparticles. (f) L-tert-leucine in two different microparticle systems (LDHSpyCatcher and GOXSnoopCatcher co-conjugated together on CsgASpyTag/CsgASnoopTag coatings versus LDHSpyCatcher-conjugated CsgASpyTag coatings along with GOXSnoopCatcher-conjugated CsgASnoopTag coatings) during the 3 h reaction conversion. (g) Conversion ratio of L-tert-leucine in the CsgASpyTag/CsgASnoopTag coating system over five cycles of 3 h reactions. Reproduced from ref 69 with permission from AAAS.
The series-parallel reaction catalyzed by immobilized multiple proteases is of tremendous importance in the field of biomedical enzyme catalysis. The immobilization of double enzyme and the regeneration of the coenzyme were realized by using the CsgASpyTag/CsgASnoopTag-coated SiO2 microspheres. Such study separately constructed the SpyCatcher domain-fused leucine dehydrogenase and SnoopCatcher domains-fused to glucose oxidase and co-immobilized them on CsgASpyTag/CsgASnoopTag-coated SiO2 microparticles surface (Fig. 6d). In the reaction system, trimethylpyruvate (TMP) acid is converted into the high value chemical L-tert-leucine by the leucine dehydrogenase (LDH) of the soil bacterium Lysobacter sphaericus, in which NADH [reduced form of nicotinamide adenine dinucleotide (NAD+)] as a coenzyme was required. Furthermore, GOX from Bacillus subtilis could regenerate NADH by oxidizing low-value glucose to gluconic acid. Therefore, these two enzymes can be assembled into an NADH recycling system (Fig. 6e). As shown in Fig. 6f, in the first 3 h reaction, the ratio to produce l-tert-leucine in the CsgASpyTag/CsgASnoopTag coating system was about 50%, while it was only 30% in the control system. In the CsgASpyTag/CsgASnoopTag coating system, the generated NADH could be immediately consumed by adjacent LDHSpyCatcher on the same particle surface. However, in the control group, the produced NADH was not consumed until it reached the surface of the LDHSpyCatcher-conjugated particles, resulting in a slower reaction rate. The relationship between the conversion rate of L-tert-leucine and the number of cycles indicated that the conversion did not significantly change over a series of five reaction cycles of 3 h each (Fig. 6g). Using the genetically engineered protein nanofibrils coating, the purpose of immobilization of double enzymes can be achieved, and the production cost can be reduced.
Notably, this conventional amyloid aggregation in vitro generally requires a time scale of hours and harsh incubation conditions such as an organic solvent, extreme low pH, high temperature, and ionic strength, which is not beneficial to the scale-up fabrication of amyloid-based surface coating.
4.2. Superfast amyloid protein aggregation
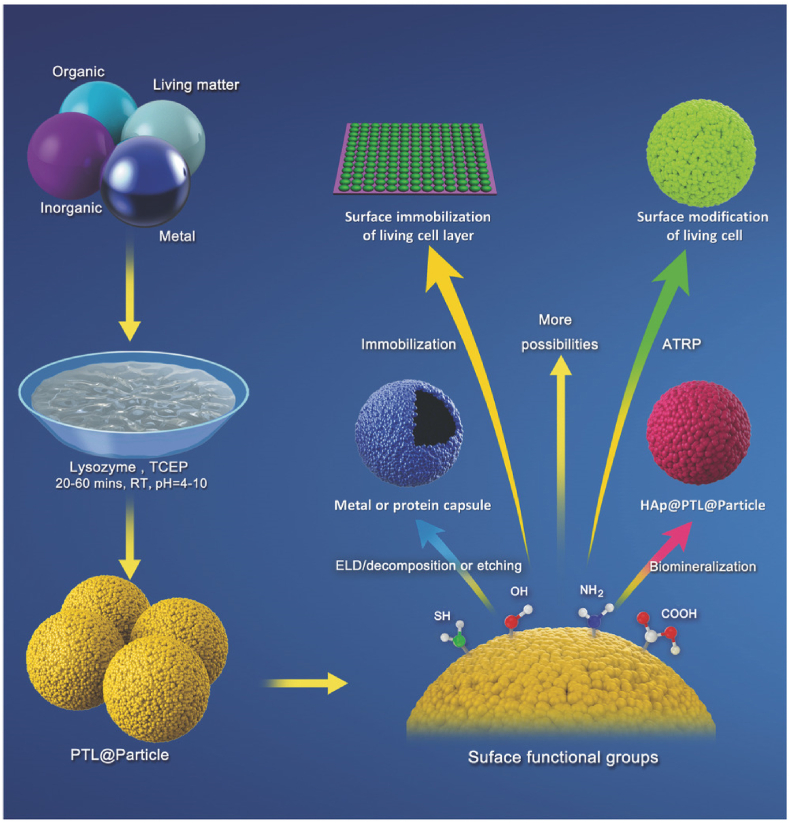
In contrast to the traditional protein amyloid aggregation, recently Yang group has developed superfast protein amyloid-like aggregation strategy. The disulfide bond breaking agent tris(2-carboxyethyl) phosphine (TCEP) or cysteine can induce a mild and fast protein unfolding in quasi-physiological aqueous solution. As a result, the high energy α-helix structure of protein was unlocked, which subsequently induced the spontaneous transition of the high energy α-helix conformation to the low energy β-sheet aggregate. Such process thus would produce insoluble protein aggregates, which shared the similar β-sheet stacking to that in classical amyloids. For this reason, we called this process as amyloid-like protein aggregation. This pathway is firstly discovered by our group in 2012 [65,66,70,[173], [174], [175], [176], [177]] Superfast amyloid-like protein aggregation can be finished in a very short time (about a few minutes or hours), and the conditions are mild (at room temperature and without any organic reagent); while the traditional amyloid protein aggregation process is usually complex, time consuming (tens of hours to days), low efficiency and requires harsh conditions (such as high temperature, organic solvents, extreme pH) [67,68,154]. Importantly, further work demonstrated that various globular proteins, such as lysozyme, insulin, bovine serum albumin, and α-lactalbumin, with a high fibrillation propensity (HFP) and abundant α-helix structures locked by intramolecular S–S bonds can undergo this rapid amyloid-like aggregation [[178], [179], [180], [181], [182], [183], [184]]. This strategy thereby exhibits its capability for the spontaneous and efficient formation of scalable amyloid-based biomaterials (e.g., nanofilms or nanocoatings), and versatile surface engineering method towards the functionalization of micro/nano-particles has been systematically founded (Fig. 7) [65].
Fig. 7.
Schematic illustration of the one-step aqueous formation of a phase-transitioned lysozyme (PTL) coating on micro/nanoparticles and subsequent functionalization for a range of technical applications. Reproduced from ref 65 with permission from Wiley-VCH.
The exposed multiple functional groups such as carboxyl, amine, hydroxyl, thiol, and aromatic rings on the surface of the phase-transitioned lysozyme (PTL) coating can produce different binding modes on the material surface, including metal-sulfur coordination bonding, hydrogen bonding, electrostatic and hydrophobic interaction [185]. Accordingly, the PTL nanofilm can coat a range of particles, including inorganic, organic, and metal particles and microscopic living particles such as microbes, demonstrating an extremely facile and rapid technique for the surface engineering of various living and nonliving colloids. The simultaneous exposure of polar and non-polar groups on the surface of the PTL nanofilm also endows the material with excellent chemical resistance against attack by a series of extreme conditions [183]. The PTL-coated polystyrene and silica particles (Fig. 8a–b) also showed better stability at extreme pH (e.g., pH12) than classical polydopamine that has weak adhesion with a surface under basic pH due to the ionization of phenolic groups in polydopamine. The abundant reactive sites on the surface of PTL coating provided micro/nanoparticles with great opportunities for further functionalization, such as radical living graft polymerization, the electroless deposition of metals, biomineralization and the facile synthesis of Janus particles. At the same time, protein capsules have been successfully prepared by a template method. The prepared PTL capsules were used as a micro reactor to successfully encapsulate various biomedical macromolecules, and isolate molecules with different functions in the microscopic field avoiding interference with each other, which provides a wide range of application prospects, e.g., the subsequent preparation of biochips.
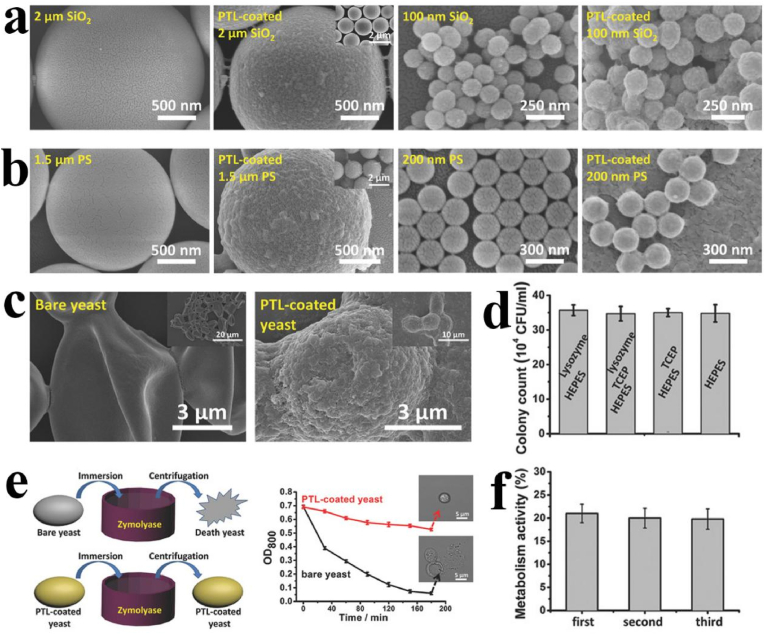
Fig. 8.
(a) SEM images of PS micro/nanoparticles before (left) and after (right) coating with the PTL membrane. (b) SEM images of SiO2 micro/nanoparticles before (left) and after (right) coating with the PTL membrane. (c) SEM images of bare yeast cells showing their deflated morphology due to destabilization during sample preparation and SEM characterization (left); SEM image of the PTL-coated yeast cells with improved mechanical stability during sample preparation and SEM characterization showing an intact morphology close to that of the native state (right). (d) Proliferative activity assay of yeast in different buffers. (e) Schematic illustration of PTL-coated yeast cells and their resistance to enzymatic digestion (left) and the corresponding contrasting survival ability of yeasts without and with the PTL coating in the presence of Zymolyase (right); SEM images inset in the curve show intact PTL-coated yeast and the lysis of bare yeast by Zymolyase. (f) Repeated measurements of the metabolic activity of an immobilized yeast layer by the PTL coating. Reproduced from ref 65 with permission from Wiley-VCH.
The PTL coating was further investigated to encapsulate and protect the life-active colloidal particles. In this regard, the PTL-coated yeast was obtained through a simple one-step aqueous coating method After repeated centrifugal separation and resuspension in deionized water for several times, it was found that the PTL-coated yeast maintained its natural shape, while the bare control yeast became deflated (Fig. 8c). In addition, because the only component of PTL is protein and the preparation conditions are mild, which is very different from other synthetic inorganic and polymer systems, the PTL system is biocompatible to living cells with ignorable influence on the activity of yeast (Fig. 8d). Moreover, it was found that the PTL film coated on yeast cells could function as an enhanced safeguard to protect living cells against external toxic substances, such as yeast enzymes and harmful or dangerous chemicals. It was found that bare yeast cells cannot survive in hypotonic buffer of Zymolyase (1 g/L), and the corresponding lysis of bare yeast cells was clearly recorded in the spectrophotometer as light scattering (optical density, OD) of the colloid rapid decreased with digestion time (Fig. 8e). In contrast, PTL-coated yeast cells exhibited a greatly attenuated decrease in OD value, and yeast cells with intact cell morphology were observed, suggesting that the PTL membrane effectively prevented digestion by external enzymatic enzymes. Further studies showed that after 12 h in the medium, the glucose concentration in the yeast medium decreased by 20% (formation of CO2 and water through aerobic metabolism), and this conversion was almost constant after multiple repetitions (Fig. 8f). This result further suggests that the PTL coating on yeast cells provides a biocompatible shell without significantly affecting their respiration and metabolism.
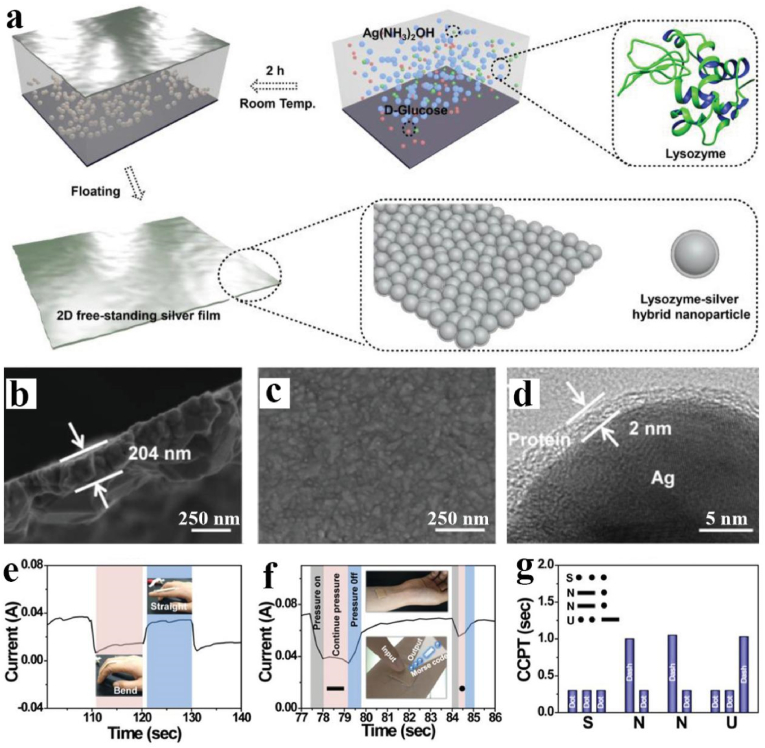
In order to further expand the application of amyloid-like protein materials, Yang's group further developed a class of amyloid-like protein-metal composite film materials for flexible electronics. Such study actually developed a facile, environmentally friendly, and bio-based redox system for fusing metal nanoparticles (e.g., Ag, Au and Cu) in an aqueous solution via protein (e.g., albumin, α-amylase, collagen, keratin and pepsin) bonding under ambient conditions, which differs from traditional nanomaterial welding at high pressures and temperatures [66]. Taking lysozyme film coated silver nanoparticles (AgNPs) as an example, according to the findings, AgNPs produced from the in-situ reduction of silver ammonium ions by glucose were bound by ultrathin amyloid-like β-sheet stacking of lysozyme to create a freestanding large-area 2D silver film (Fig. 9a). The thickness of the lysozyme/silver hybrid sheet may be precisely regulated at the nanoscale, and its reflectivity and conductivity can also be adjusted. Within 2 h, a large-area 2D silver film with a thickness of 204 nm could be manufactured, and the lysozyme bonding layer around AgNPs in the conductive silver film could be seen clearly with a thickness of 2 nm (Fig. 9b–d). The sensor, which is made by lysozyme welding a freestanding large-area 2D silver film, has a sensitive pressure-responsive resistance change and may then transmit Morse code by detecting the pressure change (e.g., resistance or current change) produced by a small tap of the finger (Fig. 9e–g). The approaches described in this study provide new ideas for the transfer of encrypted information for use in human movement detection, health-monitoring systems, medical diagnostic equipment, and the design of proteins to weld certain materials to tailor material functions.
Fig. 9.
(a) Schematic illustration of preparation of 2D free-standing silver film. (b) Cross-sectional image of the conductive 2D free-standing silver film showing the thickness of the film. (c) SEM image of the conductive 2D free-standing silver film. (d) HR-TEM image of the conductive 2D free-standing silver film showing the protein-binding layer. (e) The response current of out-of-plane sensors monitoring finger movements. (f) The basic “dash” and “dot” are transmitted by recording the continuous pressing time of the finger. (g) Transmission of Morse code (SNNU) by recording CCPT. Reproduced from ref 66 with permission from Wiley-VCH.
At present, the superfast amyloid protein aggregates materials have been realized in the fields of surface engineering, molecular separation and controlled release, antibacterial, antifouling, adsorption, biomineralization and so on. Polydopamine and tannic acid coatings generally appear brown or black color. The polymerization mechanism of polydopamine is complicate and the polymerization time is long (generally more than 10 h). Moreover, the biodegradability of the polydopamine and tannic acid coatings is not clear, and the coating surface is rough and stability is poor (especially in alkaline environment). On the contrary, the amyloid-like protein coating is colorless, and can be formed on the surface in a short time (several minutes) under mild aqueous solution; moreover, it shows excellent structural stability under severe harsh conditions (such as pH 12, pH 2, organic solvents, ultrasonic, etc.) and good biocompatibility in vivo and vitro. However, there are still some problems to be solved, such as finding cheaper proteins, in-deep investigation on the surface adhesion mechanism, exploiting protein reducing agents without cytotoxicity, long-term stability and toxicity of coatings in vivo are unclear, developing more functional amyloid materials and so on.
5. Conclusion and outlook
Micro/nanoparticles are very useful for a broad spectrum of biomedical applications, ranging from drug delivery to cancer therapy, bioimaging and anti-bacteria. The surface chemical and physical structure of particles is identified as one of the key parameter that determines, for example, colloidal stability, bioactivity, and compatibility. Thus, particle surface engineering has become a very important field. Due to the non-biodegradability and poor biocompatibility, petroleum-based synthetic polymers severely limit their further advance as biomaterials. Alternatively, natural polymers show unique biodegradable and biocompatible advantages and the development and use of biopolymer-based coatings have attracted extensive attention. In this paper, the research status of polysaccharides, polyphenolic polymers and proteins in particle surface modification and their applications in biomedicine was summarized. Chitosan and cellulose are two of the most common polysaccharide polymers, which can form films on the surface of particles through various processing methods, but their poor processability, solubility and universality seriously limit their application scenarios. Polyphenolic polymers can be coated on the surface of various particles by simple solution immersion method regardless of their physical and chemical properties, however, the polymerization process is time-consuming and the coating colour is dark. Protein amyloid aggregates, especially the new type of superfast amyloid aggregation, show great advantages and potential in particle surface engineering. The amyloid protein coatings not only exhibit the universality and adhesion stability similar to polyphenol system, but also overcome the shortcomings of polyphenol system, providing a new avenue for particle surface functionalization (Table 3).
Table 3.
Summary of the main advantages and disadvantages of common coatings.
| Coatings | Disadvantages | Advantages |
|---|---|---|
| Synthetic polymer | Non-biodegradability, poor biocompatibility | Cost-efficient, readily available, easy processing |
| Polysaccharide | Complex extraction of polysaccharide monomers, long preparation time, special solvent needed to dissolve polysaccharides, poor universality |
Biocompatibility, biodegradability, film-forming, non-toxic, renewable |
| Polyphenolic | polyphenol coating is brown or black, dopamine polymerization mechanism is complex, long preparation time, unclear biodegradability of cross-linked products, rough coating surface/interface |
Good universality, easy film formation, multifunctional application |
| Protein | Adhesion mechanism is not clear, long-term stability and toxicity of coatings in vivo are unclear | Good universality, easy film formation, biodegradable, biocompatibility, short preparation time, low cost, mild preparation conditions |
Despite the above advances, there are still several challenges that restrict the further development of surface coating materials. (1) The mechanisms for protein amyloid aggregation and the strong substrate-independent adhesion have not yet been thoroughly understood, more works should be focused on using advanced characterization methods and computer simulations technologies to uncover these mysteries. (2) It is urgent to explore more inexpensive and easily available plant protein precursors and other fascinating biofunctions to meet the needs of large-scale production and complicated biomedical environments. (3) Although the biocompatibility of polyphenol materials and protein amyloid aggregates has been extensively studied, their stability, degradation behaviours and toxicity of degradation products in the body have received little attention, which is exactly the most critical challenge in their practical clinical applications. In conclusion, biopolymer coating especial protein amyloid aggregates hold great promise in particle surface engineering and biomedical application, and at the same time, more efforts should be focused on overcoming the related challenges. In addition to the good development of biopolymer coatings in biomedical engineering, biopolymer coatings also have good application prospects in the following fields, such as water treatment, sensing, wettability modulation, micro/nanosurface construction, energy, catalysis, adsorption, and other fields.
Credit author statement
Qingmin Yang: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. Jian Zhao: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. Arif Muhammad: Investigation, Writing – review & editing. Lihua Tian: Investigation, Writing – review & editing. Yongchun Liu: Funding acquisition, Investigation, Writing – review & editing. Lixin Chen: Conceptualization, Supervision, Writing – review & editing. Peng Yang: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
P.Y. is grateful for funding from the National Key R&D Program of China (Nos. 2020YFA0710400, 2020YFA0710402), the National Natural Science Foundation of China (No. 21875132), the 111 Project (No. B14041), the Fundamental Research Funds for the Central Universities (No. GK201801003), the Innovation Capability Support Program of Shaanxi (No. 2020TD-024), the Science and Technology Innovation Team of Shaanxi Province (No. 2022TD-35). J.Z. appreciates funding from the National Natural Science Foundation of China (No. 51903146), the Natural Science Foundation of Shaanxi Province (No. 2020JQ-420), the National Key R&D Program of China (Nos. 2020YFA0710400 and 2020YFA0710403), the Fundamental Research Funds for the Central Universities (No. GK202205017). Y.L. appreciates funding from the National Natural Science Foundation of China (No. 51903147).
Contributor Information
Jian Zhao, Email: zhaojian@snnu.edu.cn.
Lixin Chen, Email: lixin@nwpu.edu.cn.
Peng Yang, Email: yangpeng@snnu.edu.cn.
References
- 1.Decher G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science. 1997;277(5330):1232–1237. [Google Scholar]
- 2.Caruso F., Caruso R.A., Mohwald H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science. 1998;282(5391):1111–1114. doi: 10.1126/science.282.5391.1111. [DOI] [PubMed] [Google Scholar]
- 3.Ryu D.Y., Shin K., Drockenmuller E., et al. A generalized approach to the modification of solid surfaces. Science. 2005;308(5719):236–239. doi: 10.1126/science.1106604. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A.K., Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Fan J.B., Song Y., Liu H., et al. A general strategy to synthesize chemically and topologically anisotropic Janus particles. Sci. Adv. 2017;3(6) doi: 10.1126/sciadv.1603203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su H., Price C.A.H., Jing L., et al. Janus particles: design, preparation, and biomedical applications. Mater. Today Bio. 2019;4 doi: 10.1016/j.mtbio.2019.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karimi M., Ghasemi A., Zangabad P.S., et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016;45(5):1457–1501. doi: 10.1039/c5cs00798d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J., Han S., Zheng H., et al. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr. Polym. 2015;123:53–66. doi: 10.1016/j.carbpol.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Hao N., Nie Y., Zhang J.X.J. Microfluidic synthesis of functional inorganic micro-/nanoparticles and applications in biomedical engineering. Int. Mater. Rev. 2018;63(8):461–487. [Google Scholar]
- 10.Petros R.A., DeSimone J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P., Xia J., Luo S. Generation of well-defined micro/nanoparticles via advanced manufacturing techniques for therapeutic delivery. Materials. 2018;11(4):623. doi: 10.3390/ma11040623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapsford K.E., Algar W.R., Berti L., et al. Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem. Rev. 2013;113(3):1904–2074. doi: 10.1021/cr300143v. [DOI] [PubMed] [Google Scholar]
- 13.Z Nie Z., Fava D., Kumacheva E., et al. Self-assembly of metal–polymer analogues of amphiphilic triblock copolymers. Nat. Mater. 2007;6(8):609–614. doi: 10.1038/nmat1954. [DOI] [PubMed] [Google Scholar]
- 14.Cerroni B., Chiessi E., Margheritelli S., et al. Polymer shelled microparticles for a targeted doxorubicin delivery in cancer therapy. Biomacromolecules. 2011;12(3):593–601. doi: 10.1021/bm101207k. [DOI] [PubMed] [Google Scholar]
- 15.Schwemmer T., Baumgartner J., Faivre D., et al. Peptide-mediated nanoengineering of inorganic particle surfaces: a general route toward surface functionalization via peptide adhesion domains. J. Am. Chem. Soc. 2012;134(4):2385–2391. doi: 10.1021/ja2104944. [DOI] [PubMed] [Google Scholar]
- 16.Jainae K., Sukpirom N., Fuangswasdi S., et al. Adsorption of Hg (II) from aqueous solutions by thiol-functionalized polymer-coated magnetic particles. J. Ind. Eng. Chem. 2015;23:273–278. [Google Scholar]
- 17.Liao M.H., Chen D.H. Fast and efficient adsorption/desorption of protein by a novel magnetic nano-adsorbent. Biotechnol. Lett. 2002;24(22):1913–1917. [Google Scholar]
- 18.Galiano F., Briceño K., Marino T., et al. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018;564:562–586. [Google Scholar]
- 19.Gandini A., Lacerda T.M. From monomers to polymers from renewable resources: recent advances. Prog. Polym. Sci. 2015;48:1–39. [Google Scholar]
- 20.Diyana Z.N., Jumaidin R., Selamat M.Z., et al. Physical properties of thermoplastic starch derived from natural resources and its blends: a review. Polymers. 2021;13(9):1396. doi: 10.3390/polym13091396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopalakrishnan S., Xu J., Zhong F., et al. Strategies for fabricating protein films for biomaterial applications. Adv. Sustain. Syst. 2021;5(1) doi: 10.1002/adsu.202000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muir V.G., Burdick J.A. Chemically modified biopolymers for the formation of biomedical hydrogels. Chem. Rev. 2020;121(18):10908–10949. doi: 10.1021/acs.chemrev.0c00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coltelli M.B., Wild F., Bugnicourt E., et al. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: an extensive review. Coatings. 2015;6(1):1. [Google Scholar]
- 24.Zink J., Wyrobnik T., Prinz T., et al. Physical, chemical and biochemical modifications of protein-based films and coatings: an extensive review. Int. J. Mol. Sci. 2016;17(9):1376. doi: 10.3390/ijms17091376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamróz E., Kulawik P., Kopel P. The effect of nanofillers on the functional properties of biopolymer-based films: a review. Polymers. 2019;11(4):675. doi: 10.3390/polym11040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H.A., Ma Y., Zhou F., et al. Material-independent surface chemistry beyond polydopamine coating. Acc. Chem. Res. 2019;52(3):704–713. doi: 10.1021/acs.accounts.8b00583. [DOI] [PubMed] [Google Scholar]
- 27.Liang H., Zhou B., Wu D., et al. Supramolecular design and applications of polyphenol-based architecture: a review. Adv. Colloid Interface Sci. 2019;272 doi: 10.1016/j.cis.2019.102019. [DOI] [PubMed] [Google Scholar]
- 28.Sathishkumar G., Kasi G., Zhang K., et al. Recent progress in Tannic Acid-driven antimicrobial/antifouling surface coating strategies. J. Mater. Chem. B. 2022;10:2296–2315. doi: 10.1039/d1tb02073k. [DOI] [PubMed] [Google Scholar]
- 29.Frank L.A., Onzi G.R., Morawski A.S., et al. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020;147 [Google Scholar]
- 30.Shen Y., Levin A., Kamada A., et al. From protein building blocks to functional materials. ACS Nano. 2021;15(4):5819–5837. doi: 10.1021/acsnano.0c08510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Rijn P., Tutus M., Kathrein C., et al. Challenges and advances in the field of self-assembled membranes. Chem. Soc. Rev. 2013;42(16):6578–6592. doi: 10.1039/c3cs60125k. [DOI] [PubMed] [Google Scholar]
- 32.Salehi E., Daraei P., Shamsabadi A.A. A review on chitosan-based adsorptive membranes. Carbohydr. Polym. 2016;152:419–432. doi: 10.1016/j.carbpol.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Yan W., Shi M., Dong C., et al. Applications of tannic acid in membrane technologies: a review. Adv. Colloid Interface Sci. 2020;284 doi: 10.1016/j.cis.2020.102267. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Ai K., Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014;114(9):5057–5115. doi: 10.1021/cr400407a. [DOI] [PubMed] [Google Scholar]
- 35.Wei G., Su Z., Reynolds N.P. Self-assembling peptide and protein amyloids: from structure to tailored function in nanotechnology. Chem. Soc. Rev. 2017;46(15):4661–4708. doi: 10.1039/c6cs00542j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang F., Yang P. Biopolymer-based membrane adsorber for removing contaminants from aqueous solution: progress and prospects. Macromol. Rapid Commun. 2022;43(3) doi: 10.1002/marc.202100669. [DOI] [PubMed] [Google Scholar]
- 37.Rezaei F.S., Sharifianjazi F., Esmaeilkhanian A., et al. Chitosan films and scaffolds for regenerative medicine applications: a review. Carbohydr. Polym. 2021;273 doi: 10.1016/j.carbpol.2021.118631. [DOI] [PubMed] [Google Scholar]
- 38.Mohebbi S., Nezhad M.N., Zarrintaj P., et al. Chitosan in biomedical engineering: a critical review. Curr. Stem Cell Res. Ther. 2019;14(2):93–116. doi: 10.2174/1574888X13666180912142028. [DOI] [PubMed] [Google Scholar]
- 39.Ngah W.S.W., Teong L.C., Hanafiah M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr. Polym. 2011;83(4):1446–1456. [Google Scholar]
- 40.Agnihotri S.A., Mallikarjuna N.N., Aminabhavi T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Contr. Release. 2004;100(1):5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Suh J.K.F., Matthew H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 42.Pandey A. Pharmaceutical and biomedical applications of cellulose nanofibers: a review. Environ. Chem. Lett. 2021;19(3):2043–2055. [Google Scholar]
- 43.Boonmahitthisud A., Soykeabkaew N., Ongthip L., et al. Review of the recent developments in all-cellulose nanocomposites: properties and applications. Carbohydr. Polym. 2022;286 doi: 10.1016/j.carbpol.2022.119192. [DOI] [PubMed] [Google Scholar]
- 44.Moon R.J., Martini A., Nairn J., et al. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev. 2011;40(7):3941–3994. doi: 10.1039/c0cs00108b. [DOI] [PubMed] [Google Scholar]
- 45.Nechyporchuk O., Belgacem M.N., Bras J. Production of cellulose nanofibrils: a review of recent advances. Ind. Crop. Prod. 2016;93:2–25. [Google Scholar]
- 46.Cherian R.M., Tharayil A., Varghese R.T., et al. A review on the emerging applications of nano-cellulose as advanced coatings. Carbohydr. Polym. 2022;282 doi: 10.1016/j.carbpol.2022.119123. [DOI] [PubMed] [Google Scholar]
- 47.Subhedar A., Bhadauria S., Ahankari S., et al. Nanocellulose in biomedical and biosensing applications: a review. Int. J. Biol. Macromol. 2021;166:587–600. doi: 10.1016/j.ijbiomac.2020.10.217. [DOI] [PubMed] [Google Scholar]
- 48.Picheth G.F., Pirich C.L., Sierakowski M.R., et al. Bacterial cellulose in biomedical applications: a review. Int. J. Biol. Macromol. 2017;104:97–106. doi: 10.1016/j.ijbiomac.2017.05.171. [DOI] [PubMed] [Google Scholar]
- 49.Liu W., Du H., Zhang M., et al. Bacterial cellulose-based composite scaffolds for biomedical applications: a review. ACS Sustain. Chem. Eng. 2020;8(20):7536–7562. [Google Scholar]
- 50.Seddiqi H., Oliaei E., Honarkar H., et al. Cellulose and its derivatives: towards biomedical applications. Cellulose. 2021;28(4):1893–1931. [Google Scholar]
- 51.Ryu J.H., Messersmith P.B., Lee H. Polydopamine surface chemistry: a decade of discovery. ACS Appl. Mater. Interfaces. 2018;10(9):7523–7540. doi: 10.1021/acsami.7b19865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Q., Chen J., Liu M., et al. Polydopamine-based functional materials and their applications in energy, environmental, and catalytic fields: state-of-the-art review. Chem. Eng. J. 2020;387 [Google Scholar]
- 53.Yang P., Zhu F., Zhang Z., et al. Stimuli-responsive polydopamine-based smart materials. Chem. Soc. Rev. 2021;50(14):8319–8343. doi: 10.1039/d1cs00374g. [DOI] [PubMed] [Google Scholar]
- 54.Lee H., Dellatore S.M., Miller W.M., et al. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu Y., Yang L., Zhang J., et al. Polydopamine antibacterial materials. Mater. Horiz. 2021;8(6):1618–1633. doi: 10.1039/d0mh01985b. [DOI] [PubMed] [Google Scholar]
- 56.Liu L., Shi H., Yu H., et al. One-step hydrophobization of tannic acid for antibacterial coating on catheters to prevent catheter-associated infections. Biomater. Sci. 2019;7(12):5035–5043. doi: 10.1039/c9bm01223k. [DOI] [PubMed] [Google Scholar]
- 57.Guo Z., Xie W., Lu J., et al. Tannic acid-based metal phenolic networks for bio-applications: a review. J. Mater. Chem. B. 2021;9(20):4098–4110. doi: 10.1039/d1tb00383f. [DOI] [PubMed] [Google Scholar]
- 58.Sathishkumar G., Kasi G., Zhang K., et al. Recent progress in Tannic Acid-driven antimicrobial/antifouling surface coating strategies. J. Mater. Chem. B. 2022;10:2296–2315. doi: 10.1039/d1tb02073k. [DOI] [PubMed] [Google Scholar]
- 59.Ejima H., Richardson J.J., Liang K., et al. One-step assembly of coordination complexes for versatile film and particle engineering. Science. 2013;341(6142):154–157. doi: 10.1126/science.1237265. [DOI] [PubMed] [Google Scholar]
- 60.Xu L.Q., Pranantyo D., Neoh K.G., et al. Thiol reactive maleimido-containing tannic acid for the bioinspired surface anchoring and post-functionalization of antifouling coatings. ACS Sustain. Chem. Eng. 2016;4(8):4264–4272. [Google Scholar]
- 61.Chang T.W., Ko H., Huang W.S., et al. Tannic acid-induced interfacial ligand-to-metal charge transfer and the phase transformation of Fe3O4 nanoparticles for the photothermal bacteria destruction. Chem. Eng. J. 2022;428 [Google Scholar]
- 62.Wang Y., Zou Y., Wu Y., et al. Universal antifouling and photothermal antibacterial surfaces based on multifunctional metal–phenolic networks for prevention of biofilm formation. ACS Appl. Mater. Interfaces. 2021;13(41):48403–48413. doi: 10.1021/acsami.1c14979. [DOI] [PubMed] [Google Scholar]
- 63.Zou L., Shao P., Zhang K., et al. Tannic acid-based adsorbent with superior selectivity for lead (II) capture: adsorption site and selective mechanism. Chem. Eng. J. 2019;364:160–166. [Google Scholar]
- 64.Chen C., Yang H., Yang X., et al. Tannic acid: a crosslinker leading to versatile functional polymeric networks: a review. RSC Adv. 2022;12(13):7689–7711. doi: 10.1039/d1ra07657d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu R., Zhao J., Han Q., et al. One-step assembly of a biomimetic biopolymer coating for particle surface engineering. Adv. Mater. 2018;30(38) doi: 10.1002/adma.201802851. [DOI] [PubMed] [Google Scholar]
- 66.Qin R., Liu Y., Tao F., et al. Protein-bound freestanding 2D metal film for stealth information transmission. Adv. Mater. 2019;31(5) doi: 10.1002/adma.201803377. [DOI] [PubMed] [Google Scholar]
- 67.Wei G., Su Z., Reynolds N.P., et al. Self-assembling peptide and protein amyloids: from structure to tailored function in nanotechnology. Chem. Soc. Rev. 2017;46(15):4661–4708. doi: 10.1039/c6cs00542j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ke P.C., Zhou R., Serpell L.C., et al. Half a century of amyloids: past, present and future. Chem. Soc. Rev. 2020;49(15):5473–5509. doi: 10.1039/c9cs00199a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y., Li K., Wang X., et al. Conformable self-assembling amyloid protein coatings with genetically programmable functionality. Sci. Adv. 2020;6(21) doi: 10.1126/sciadv.aba1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y., Tao F., Miao S., et al. Controlling the structure and function of protein thin films through amyloid-like aggregation. Acc. Chem. Res. 2021;54(15):3016–3027. doi: 10.1021/acs.accounts.1c00231. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Y., Chen X., Chen T., et al. A review of the antibacterial activity and mechanisms of plant polysaccharides. Trends Food Sci. Technol. 2022;123:264–280. [Google Scholar]
- 72.Cazón P., Velazquez G., Ramírez J.A., et al. Polysaccharide-based films and coatings for food packaging: a review. Food Hydrocolloids. 2017;68:136–148. [Google Scholar]
- 73.Chen X., Yang J., Shen M., et al. Structure, function and advance application of microwave-treated polysaccharide: a review. Trends Food Sci. Technol. 2022;123:198–209. [Google Scholar]
- 74.Yu Y., Shen M., Song Q., et al. Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohydr. Polym. 2018;183:91–101. doi: 10.1016/j.carbpol.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 75.Vatanpour V., Gul B.Y., Zeytuncu B., et al. Polysaccharides in fabrication of membranes: a review. Carbohydr. Polym. 2021;281 doi: 10.1016/j.carbpol.2021.119041. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y., Wu L., Li P., et al. Polysaccharide based hemostatic strategy for ultrarapid hemostasis. Macromol. Biosci. 2020;20(4) doi: 10.1002/mabi.201900370. [DOI] [PubMed] [Google Scholar]
- 77.Muxika A., Etxabide A., Uranga J., et al. Chitosan as a bioactive polymer: processing, properties and applications. Int. J. Biol. Macromol. 2017;105:1358–1368. doi: 10.1016/j.ijbiomac.2017.07.087. [DOI] [PubMed] [Google Scholar]
- 78.Dash M., Chiellini F., Ottenbrite R.M., et al. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011;36(8):981–1014. [Google Scholar]
- 79.Rinaudo M. Chitin and chitosan: properties and applications. Prog. Polym. Sci. 2006;31(7):603–632. [Google Scholar]
- 80.Frank L.A., Onzi G.R., Morawski A.S., et al. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020;147 [Google Scholar]
- 81.Bruinsmann F.A., Pigana S., Aguirre T., et al. Chitosan-coated nanoparticles: effect of chitosan molecular weight on nasal transmucosal delivery. Pharmaceutics. 2019;11(2):86. doi: 10.3390/pharmaceutics11020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo Y., Teng Z., Li Y., et al. Solid lipid nanoparticles for oral drug delivery: chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr. Polym. 2015;122:221–229. doi: 10.1016/j.carbpol.2014.12.084. [DOI] [PubMed] [Google Scholar]
- 83.Hauksdóttir H.L., Webster T.J. Selenium and iron oxide nanocomposites for magnetically-targeted anti-cancer applications. J. Biomed. Nanotechnol. 2018;14(3):510–525. doi: 10.1166/jbn.2018.2521. [DOI] [PubMed] [Google Scholar]
- 84.Liu N., Park H.J. Factors effect on the loading efficiency of Vitamin C loaded chitosan-coated nanoliposomes. Colloids Surf. B Biointerfaces. 2010;76(1):16–19. doi: 10.1016/j.colsurfb.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 85.Khanal S., Adhikari U., Rijal N.P., et al. pH-responsive PLGA nanoparticle for controlled payload delivery of diclofenac sodium. J. Funct. Biomater. 2016;7(3):21. doi: 10.3390/jfb7030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khalifa A.L.Z.M., Abdul Rasool B.K. Optimized mucoadhesive coated niosomes as a sustained oral delivery system of famotidine. AAPS PharmSciTech. 2017;18(8):3064–3075. doi: 10.1208/s12249-017-0780-7. [DOI] [PubMed] [Google Scholar]
- 87.Verma A., Sharma G., Jain A., et al. Systematic optimization of cationic surface engineered mucoadhesive vesicles employing Design of Experiment (DoE): a preclinical investigation. Int. J. Biol. Macromol. 2019;133:1142–1155. doi: 10.1016/j.ijbiomac.2019.04.118. [DOI] [PubMed] [Google Scholar]
- 88.Khallaf R.A., Aboud H.M., Sayed O.M. Surface modified niosomes of olanzapine for brain targeting via nasal route; preparation, optimization, and in vivo evaluation. J. Liposome Res. 2020;30(2):163–173. doi: 10.1080/08982104.2019.1610435. [DOI] [PubMed] [Google Scholar]
- 89.Gartziandia O., Herran E., Pedraz J.L., et al. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf. B Biointerfaces. 2015;134:304–313. doi: 10.1016/j.colsurfb.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 90.Arakha M., Pal S., Samantarrai D., et al. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015;5(1):1–12. doi: 10.1038/srep14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alshraim M.O., Sangi S., Harisa G.I., et al. Chitosan-coated flexible liposomes magnify the anticancer activity and bioavailability of docetaxel: impact on composition. Molecules. 2019;24(2):250. doi: 10.3390/molecules24020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takeuchi I., Kamiki Y., Makino K. Therapeutic efficacy of rebamipide-loaded PLGA nanoparticles coated with chitosan in a mouse model for oral mucositis induced by cancer chemotherapy. Colloids Surf. B Biointerfaces. 2018;167:468–473. doi: 10.1016/j.colsurfb.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 93.Klemm D., Kramer F., Moritz S., et al. Nanocelluloses: a new family of nature-based materials. Angew. Chem. Int. Ed. 2011;50(24):5438–5466. doi: 10.1002/anie.201001273. [DOI] [PubMed] [Google Scholar]
- 94.Thomas P., Duolikun T., Rumjit N.P., et al. Comprehensive review on nanocellulose: recent developments, challenges and future prospects. J. Mech. Behav. Biomed. Mater. 2020;110 doi: 10.1016/j.jmbbm.2020.103884. [DOI] [PubMed] [Google Scholar]
- 95.Dhali K., Ghasemlou M., Daver F., et al. A review of nanocellulose as a new material towards environmental sustainability. Sci. Total Environ. 2021;775 doi: 10.1016/j.scitotenv.2021.145871. [DOI] [PubMed] [Google Scholar]
- 96.Lu P., Hsieh Y.L. Preparation and properties of cellulose nanocrystals: rods, spheres, and network. Carbohydr. Polym. 2010;82(2):329–336. [Google Scholar]
- 97.Gupta P.K., Raghunath S.S., Prasanna D.V., et al. An update on overview of cellulose, its structure and applications. Cellulose. 2019:846–1297. [Google Scholar]
- 98.Kargarzadeh H., Ioelovich M., Ahmad I., et al. Methods for extraction of nanocellulose from various sources. Handb. Nanocellulose Cellul. Nanocompo. 2017;1:1–51. [Google Scholar]
- 99.Jorfi M., Foster E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015;132 [Google Scholar]
- 100.Xue Y., Mou Z., Xiao H. Nanocellulose as a sustainable biomass material: structure, properties, present status and future prospects in biomedical applications. Nanoscale. 2017;9(39):14758–14781. doi: 10.1039/c7nr04994c. [DOI] [PubMed] [Google Scholar]
- 101.Carlström I.E., Rashad A., Campodoni E., et al. Cross-linked gelatin-nanocellulose scaffolds for bone tissue engineering. Mater. Lett. 2020;264 [Google Scholar]
- 102.Long W., Ouyang H., Hu X., et al. State-of-art review on preparation, surface functionalization and biomedical applications of cellulose nanocrystals-based materials. Int. J. Biol. Macromol. 2021;186:591–615. doi: 10.1016/j.ijbiomac.2021.07.066. [DOI] [PubMed] [Google Scholar]
- 103.Maneerung T., Tokura S., Rujiravanit R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008;72(1):43–51. [Google Scholar]
- 104.Hoare T.R., Kohane D.S. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- 105.Abu T.M., Zahan K.A., Rajaie M.A., et al. Nanocellulose as drug delivery system for honey as antimicrobial wound dressing. Mater. Today Proc. 2020;31:14–17. [Google Scholar]
- 106.Kumari P., Meena A. Application of enzyme-mediated cellulose nanofibers from lemongrass waste for the controlled release of anticancer drugs. Environ. Sci. Pollut. Control Ser. 2021;28:46343–46355. doi: 10.1007/s11356-020-08358-3. [DOI] [PubMed] [Google Scholar]
- 107.Xiao W., Xu J., Liu X., et al. Antibacterial hybrid materials fabricated by nanocoating of microfibril bundles of cellulose substance with titania/chitosan/silver-nanoparticle composite films. J. Mater. Chem. B. 2013;1(28):3477–3485. doi: 10.1039/c3tb20303d. [DOI] [PubMed] [Google Scholar]
- 108.Sileika T.S., Barrett D.G., Zhang R., et al. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew. Chem. Int. Ed. 2013;125(41):10966–10970. doi: 10.1002/anie.201304922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Durazzo A., Lucarini M., Souto E.B., et al. Polyphenols: a concise overview on the chemistry, occurrence, and human health. Phytother Res. 2019;33(9):2221–2243. doi: 10.1002/ptr.6419. [DOI] [PubMed] [Google Scholar]
- 110.Chen Y., Cheng S., Dai J., et al. Molecular mechanisms and applications of tea polyphenols: a narrative review. J. Food Biochem. 2021;45(10) doi: 10.1111/jfbc.13910. [DOI] [PubMed] [Google Scholar]
- 111.Zhou J., Lin Z., Ju Y., et al. Polyphenol-mediated assembly for particle engineering. Acc. Chem. Res. 2020;53(7):1269–1278. doi: 10.1021/acs.accounts.0c00150. [DOI] [PubMed] [Google Scholar]
- 112.De Araújo F.F., de Paulo Farias D., Neri-Numa I.A., et al. Polyphenols and their applications: an approach in food chemistry and innovation potential. Food Chem. 2021;338 doi: 10.1016/j.foodchem.2020.127535. [DOI] [PubMed] [Google Scholar]
- 113.Yan Z., Zhong Y., Duan Y., et al. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nut. 2020;6(2):115–123. doi: 10.1016/j.aninu.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Behboodi-Sadabad F., Li S., Lei W., et al. High-throughput screening of multifunctional nanocoatings based on combinations of polyphenols and catecholamines. Mater. Today Bio. 2021;10 doi: 10.1016/j.mtbio.2021.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Quan T.H., Benjakul S., Sae-leaw T., et al. Protein–polyphenol conjugates: antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019;91:507–517. [Google Scholar]
- 116.Xu L.Q., Neoh K.G., Kang E.T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Prog. Polym. Sci. 2018;87:165–196. [Google Scholar]
- 117.Deng Z., Wang W., Xu X., et al. Biofunction of polydopamine coating in stem cell culture. ACS Appl. Mater. Interfaces. 2021;13(9):10748–10759. doi: 10.1021/acsami.0c22565. [DOI] [PubMed] [Google Scholar]
- 118.Bernsmann F., Ball V., Addiego F., et al. Dopamine-melanin film deposition depends on the used oxidant and buffer solution. Langmuir. 2011;27(6):2819–2825. doi: 10.1021/la104981s. [DOI] [PubMed] [Google Scholar]
- 119.Quaroni L., Chumanov G. Preparation of polymer-coated functionalized silver nanoparticles. J. Am. Chem. Soc. 1999;121(45):10642–10643. [Google Scholar]
- 120.Wu D., Zhou J., Creyer M.N., et al. Phenolic-enabled nanotechnology: versatile particle engineering for biomedicine. Chem. Soc. Rev. 2021;50(7):4432–4483. doi: 10.1039/d0cs00908c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qu K., Wang Y., Vasileff A., et al. Polydopamine-inspired nanomaterials for energy conversion and storage. J. Mater. Chem. 2018;6(44):21827–21846. [Google Scholar]
- 122.Lee H.A., Park E., Lee H. Polydopamine and its derivative surface chemistry in material science: a focused review for studies at KAIST. Adv. Mater. 2020;32(35) doi: 10.1002/adma.201907505. [DOI] [PubMed] [Google Scholar]
- 123.Cheng W., Zeng X., Chen H., et al. Versatile polydopamine platforms: synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano. 2019;13(8):8537–8565. doi: 10.1021/acsnano.9b04436. [DOI] [PubMed] [Google Scholar]
- 124.Yang P., Zhang S., Chen X., et al. Recent developments in polydopamine fluorescent nanomaterials. Mater. Horiz. 2020;7(3):746–761. [Google Scholar]
- 125.Wang Z., Zou Y., Li Y., et al. Metal-containing polydopamine nanomaterials: catalysis, energy, and theranostics. Small. 2020;16(18) doi: 10.1002/smll.201907042. [DOI] [PubMed] [Google Scholar]
- 126.Kwon I.S., Bettinger C.J. Polydopamine nanostructures as biomaterials for medical applications. J. Mater. Chem. B. 2018;6(43):6895–6903. doi: 10.1039/C8TB02310G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Siciliano G., Monteduro A.G., Turco A., et al. Polydopamine-coated magnetic iron oxide nanoparticles: from design to applications. Nanomaterials. 2022;12(7):1145. doi: 10.3390/nano12071145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang S.H., Kang S.M., Lee K.B., et al. Mussel-inspired encapsulation and functionalization of individual yeast cells. J. Am. Chem. Soc. 2011;133(9):2795–2797. doi: 10.1021/ja1100189. [DOI] [PubMed] [Google Scholar]
- 129.Wu Q., Niu M., Chen X., et al. Biocompatible and biodegradable zeolitic imidazolate framework/polydopamine nanocarriers for dual stimulus triggered tumor thermo-chemotherapy. Biomaterials. 2018;162:132–143. doi: 10.1016/j.biomaterials.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 130.Sun Y., Deng Y., Ye Z., et al. Peptide decorated nano-hydroxyapatite with enhanced bioactivity and osteogenic differentiation via polydopamine coating. Colloids Surf. B Biointerfaces. 2013;111:107–116. doi: 10.1016/j.colsurfb.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 131.Li X., Wei Z., Li B., et al. In vivo migration of Fe3O4@polydopamine nanoparticle-labeled mesenchymal stem cells to burn injury sites and their therapeutic effects in a rat model. Biomater. Sci. 2019;7(7):2861–2872. doi: 10.1039/c9bm00242a. [DOI] [PubMed] [Google Scholar]
- 132.Ma W., Guo A., Zhang Y., et al. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014;40(1):6–19. [Google Scholar]
- 133.Guo J., Ping Y., Ejima H., et al. Engineering multifunctional capsules through the assembly of metal–phenolic networks. Angew. Chem. Int. Ed. 2014;53(22):5546–5551. doi: 10.1002/anie.201311136. [DOI] [PubMed] [Google Scholar]
- 134.Shen G., Xing R., Zhang N., et al. Interfacial cohesion and assembly of bioadhesive molecules for design of long-term stable hydrophobic nanodrugs toward effective anticancer therapy. ACS Nano. 2016;10(6):5720–5729. doi: 10.1021/acsnano.5b07276. [DOI] [PubMed] [Google Scholar]
- 135.Rahim M.A., Ejima H., Cho K.L., et al. Coordination-driven multistep assembly of metal–polyphenol films and capsules. Chem. Mater. 2014;26(4):1645–1653. [Google Scholar]
- 136.Li W., Bing W., Huang S., et al. Mussel byssus-like reversible metal-chelated supramolecular complex used for dynamic cellular surface engineering and imaging. Adv. Funct. Mater. 2015;25(24):3775–3784. [Google Scholar]
- 137.Hu M., Ju Y., Liang K., et al. Void engineering in metal–organic frameworks via synergistic etching and surface functionalization. Adv. Funct. Mater. 2016;26(32):5827–5834. [Google Scholar]
- 138.Dong C., Wang Z., Wu J., et al. A green strategy to immobilize silver nanoparticles onto reverse osmosis membrane for enhanced anti-biofouling property. Desalination. 2017;401:32–41. [Google Scholar]
- 139.Wei J., Liang Y., Hu Y., et al. A versatile iron–tannin-framework ink coating strategy to fabricate biomass-derived iron carbide/Fe-N-carbon catalysts for efficient oxygen reduction. Angew. Chem. Int. Ed. 2016;55(4):1355–1359. doi: 10.1002/anie.201509024. [DOI] [PubMed] [Google Scholar]
- 140.Wu J., Wang Z., Yan W., et al. Improving the hydrophilicity and fouling resistance of RO membranes by surface immobilization of PVP based on a metal-polyphenol precursor layer. J. Membr. Sci. 2015;496:58–69. [Google Scholar]
- 141.Lee J., Cho H., Choi J., et al. Chemical sporulation and germination: cytoprotective nanocoating of individual mammalian cells with a degradable tannic acid–FeIII complex. Nanoscale. 2015;7(45):18918–18922. doi: 10.1039/c5nr05573c. [DOI] [PubMed] [Google Scholar]
- 142.Ping Y., Guo J., Ejima H., et al. pH-responsive capsules engineered from metal–phenolic networks for anticancer drug delivery. Small. 2015;11(17):2032–2036. doi: 10.1002/smll.201403343. [DOI] [PubMed] [Google Scholar]
- 143.Yang C., Wu H., Yang X., et al. Coordination-enabled one-step assembly of ultrathin, hybrid microcapsules with weak pH-response. ACS Appl. Mater. Interfaces. 2015;7(17):9178–9184. doi: 10.1021/acsami.5b01463. [DOI] [PubMed] [Google Scholar]
- 144.Liang H., Li J., He Y., et al. Engineering multifunctional films based on metal-phenolic networks for rational pH-responsive delivery and cell imaging. ACS Biomater. Sci. Eng. 2016;2(3):317–325. doi: 10.1021/acsbiomaterials.5b00363. [DOI] [PubMed] [Google Scholar]
- 145.Pieters B.J.G.E., Van Eldijk M.B., Nolte R.J.M., et al. Natural supramolecular protein assemblies. Chem. Soc. Rev. 2016;45(1):24–39. doi: 10.1039/c5cs00157a. [DOI] [PubMed] [Google Scholar]
- 146.Bai Y., Luo Q., Liu J. Protein self-assembly via supramolecular strategies. Chem. Soc. Rev. 2016;45(10):2756–2767. doi: 10.1039/c6cs00004e. [DOI] [PubMed] [Google Scholar]
- 147.Luo Q., Hou C., Bai Y., et al. Protein assembly: versatile approaches to construct highly ordered nanostructures. Chem. Rev. 2016;116(22):13571–13632. doi: 10.1021/acs.chemrev.6b00228. [DOI] [PubMed] [Google Scholar]
- 148.Sharma S., Rai V.K., Narang R.K., et al. Collagen-based formulations for wound healing: a literature review. J. Life Sci. 2021;290 doi: 10.1016/j.lfs.2021.120096. [DOI] [PubMed] [Google Scholar]
- 149.Busra M.F.M., Lokanathan Y. Recent development in the fabrication of collagen scaffolds for tissue engineering applications: a review. Curr. Pharmaceut. Biotechnol. 2019;20(12):992–1003. doi: 10.2174/1389201020666190731121016. [DOI] [PubMed] [Google Scholar]
- 150.Sionkowska A., Skrzyński S., Śmiechowski K., et al. The review of versatile application of collagen. Polym. Adv. Technol. 2017;28(1):4–9. [Google Scholar]
- 151.Li C., Xu L., Zuo Y.Y., et al. Tuning protein assembly pathways through superfast amyloid-like aggregation. Biomater. Sci. 2018;6(4):836–841. doi: 10.1039/c8bm00066b. [DOI] [PubMed] [Google Scholar]
- 152.Cao Y., Mezzenga R. Design principles of food gels. Nat. Food. 2020;1(2):106–118. doi: 10.1038/s43016-019-0009-x. [DOI] [PubMed] [Google Scholar]
- 153.Wang D., Ha Y., Gu J., et al. 2D protein supramolecular nanofilm with exceptionally large area and emergent functions. Adv. Mater. 2016;28(34):7414–7423. doi: 10.1002/adma.201506476. [DOI] [PubMed] [Google Scholar]
- 154.Daggett V., Fersht A.R. Is there a unifying mechanism for protein folding? Trends Biochem. Sci. 2003;28(1):18–25. doi: 10.1016/s0968-0004(02)00012-9. [DOI] [PubMed] [Google Scholar]
- 155.Adamcik J., Mezzenga R. Amyloid polymorphism in the protein folding and aggregation energy landscape. Angew. Chem. Int. Ed. 2018;57(28):8370–8382. doi: 10.1002/anie.201713416. [DOI] [PubMed] [Google Scholar]
- 156.Toyama B.H., Weissman J.S. Amyloid structure: conformational diversity and consequences. Annu. Rev. Biochem. 2011;80:557–585. doi: 10.1146/annurev-biochem-090908-120656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chiti F., Dobson C.M. Amyloid formation by globular proteins under native conditions. Nat. Chem. Biol. 2009;5(1):15–22. doi: 10.1038/nchembio.131. [DOI] [PubMed] [Google Scholar]
- 158.Arosio P., Knowles T.P.J., Linse S. On the lag phase in amyloid fibril formation. Phys. Chem. Chem. Phys. 2015;17(12):7606–7618. doi: 10.1039/c4cp05563b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Knowles T.P.J., Mezzenga R. Amyloid fibrils as building blocks for natural and artificial functional materials. Adv. Mater. 2016;28(31):6546–6561. doi: 10.1002/adma.201505961. [DOI] [PubMed] [Google Scholar]
- 160.Li J., Zhang F. Amyloids as building blocks for macroscopic functional materials: designs, applications and challenges. Int. J. Mol. Sci. 2021;22(19) doi: 10.3390/ijms221910698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ridgley D.M., Claunch E.C., Barone J.R. The effect of processing on large, self-assembled amyloid fibers. Soft Matter. 2012;8(40):10298–10306. [Google Scholar]
- 162.Das S., Jacob R.S., Patel K., et al. Amyloid fibrils: versatile biomaterials for cell adhesion and tissue engineering applications. Biomacromolecules. 2018;19(6):1826–1839. doi: 10.1021/acs.biomac.8b00279. [DOI] [PubMed] [Google Scholar]
- 163.Jones O.G., Mezzenga R. Inhibiting, promoting, and preserving stability of functional protein fibrils. Soft Matter. 2012;8(4):876–895. [Google Scholar]
- 164.Gopalakrishnan S., Xu J., Zhong F., et al. Strategies for fabricating protein films for biomaterial applications. Adv. Sustain. Syst. 2021;5(1) doi: 10.1002/adsu.202000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Palika A., Armanious A., Rahimi A., et al. An antiviral trap made of protein nanofibrils and iron oxyhydroxide nanoparticles. Nat. Nanotechnol. 2021;16(8):918–925. doi: 10.1038/s41565-021-00920-5. [DOI] [PubMed] [Google Scholar]
- 166.Shen Y., Posavec L., Bolisetty S., et al. Amyloid fibril systems reduce, stabilize and deliver bioavailable nanosized iron. Nat. Nanotechnol. 2017;12(7):642–647. doi: 10.1038/nnano.2017.58. [DOI] [PubMed] [Google Scholar]
- 167.Bajpai S.K., Shah F.F., Bajpai M. Dynamic release of gentamicin sulfate (GS) from alginate dialdehyde (AD)-crosslinked casein (CAS) films for antimicrobial applications. Des. Monomers Polym. 2017;20(1):18–32. doi: 10.1080/15685551.2016.1231037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhong C., Gurry T., Cheng A.A., et al. Self-assembling multi-component nanofibers for strong bioinspired underwater adhesives. Nat. Nanotechnol. 2014;9(10):858–866. doi: 10.1038/nnano.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.An B., Wang X., Cui M., et al. Diverse supramolecular nanofiber networks assembled by functional low-complexity domains. ACS Nano. 2017;11(7):6985–6995. doi: 10.1021/acsnano.7b02298. [DOI] [PubMed] [Google Scholar]
- 170.Li Y., Li K., Wang X., et al. Patterned amyloid materials integrating robustness and genetically programmable functionality. Nano Lett. 2019;19(12):8399–8408. doi: 10.1021/acs.nanolett.9b02324. [DOI] [PubMed] [Google Scholar]
- 171.Duraj-Thatte A.M., Courchesne N.M.D., Praveschotinunt P., et al. Genetically programmable self-regenerating bacterial hydrogels. Adv. Mater. 2019;31(40) doi: 10.1002/adma.201901826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang X., Li Z., Xiao H., et al. A universal and ultrastable mineralization coating bioinspired from biofilms. Adv. Funct. Mater. 2018;28(32) [Google Scholar]
- 173.Yang P. Direct Biomolecule binding on nonfouling surfaces via newly discovered supramolecular self-assembly of lysozyme under physiological conditions. Macromol. Biosci. 2012;12(8):1053–1059. doi: 10.1002/mabi.201200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xu Y., Liu Y., Hu X., et al. The Synthesis of a 2D Ultra-large protein supramolecular nanofilm by chemoselective thiol-disulfide exchange and its emergent functions. Angew. Chem. Int. Ed. 2020;59(7):2850–2859. doi: 10.1002/anie.201912848. [DOI] [PubMed] [Google Scholar]
- 175.Yang F., Tao F., Li C., et al. Self-assembled membrane composed of amyloid-like proteins for efficient size-selective molecular separation and dialysis. Nat. Commun. 2018;9(1):1–11. doi: 10.1038/s41467-018-07888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li C., Lu D., Deng J., et al. Amyloid-like rapid surface modification for antifouling and in-depth remineralization of dentine tubules to treat dental hypersensitivity. Adv. Mater. 2019;31(46) doi: 10.1002/adma.201903973. [DOI] [PubMed] [Google Scholar]
- 177.Chen M., Yang F., Chen X., et al. Crack suppression in conductive film by amyloid-like protein aggregation toward flexible device. Adv. Mater. 2021;33(44) doi: 10.1002/adma.202104187. [DOI] [PubMed] [Google Scholar]
- 178.Wu Z., Yang P. Simple multipurpose surface functionalization by phase transited protein adhesion. Adv. Mater. Interfac. 2015;2(2) [Google Scholar]
- 179.Gao A., Wu Q., Wang D., et al. A superhydrophobic surface templated by protein self-assembly and emerging application toward protein crystallization. Adv. Mater. 2016;28(3):579–587. doi: 10.1002/adma.201504769. [DOI] [PubMed] [Google Scholar]
- 180.Yang Q., Cao J., Yang F., et al. Amyloid-like aggregates of bovine serum albumin for extraction of gold from ores and electronic waste. Chem. Eng. J. 2021;416 [Google Scholar]
- 181.Hu X., Tian J., Li C., et al. Amyloid-like protein aggregates: a new class of bioinspired materials merging an interfacial anchor with antifouling. Adv. Mater. 2020;32(23) doi: 10.1002/adma.202000128. [DOI] [PubMed] [Google Scholar]
- 182.Yang Q., Liu Y., Chen L., et al. Study on the Amyloid-like fibrinogen-based nanofilm. Acta Polym. Sin. 2020;51(8):890–900. [Google Scholar]
- 183.Zhao J., Yang P. Amyloid-mediated Fabrication of organic-inorganic hybrid materials and their biomedical applications. Adv. Mater. Interfac. 2020;7(19) [Google Scholar]
- 184.Tao F., Han Q., Liu K., et al. Tuning crystallization pathways through the mesoscale assembly of biomacromolecular nanocrystals. Angew. Chem. Int. Ed. 2017;56(43):13440–13444. doi: 10.1002/anie.201706843. [DOI] [PubMed] [Google Scholar]
- 185.Gu J., Miao S., Yan Z., et al. Multiplex binding of amyloid-like protein nanofilm to different material surfaces. Colloid Interface Sci. Commun. 2018;22:42–48. [Google Scholar]