Abstract
The Brown–Vialetto–Van Laere syndrome or the riboflavin transporter deficiency syndrome is a neurodegenerative disorder initially reported by Brown in 1894, by Vialetto in 1936, and by Van Laere in 1966. The syndrome has been described in more than 100 patients since then. Hearing loss is the most common symptom of the syndrome, as most individuals have it through the development of the disease. Although there is a variation between the onset of hearing loss and the other possible symptoms, hearing loss usually begins in early childhood. Nevertheless, there are some cases describing hearing loss starting in adults. Hereby, we present a case report of a patient who started having the symptoms at the age of 14 and who had a mutation in the SLC52A3 gene, presenting with sensorineural hearing loss associated with cerebellar ataxia, who also underwent successful cochlear implant surgery.
Keywords: Cochlear implant, deafness, hearing loss, neuropathy
Introduction
Cochlear implant is a sensorial device that is capable of re-establishing hearing in individuals with severe to profound sensorineural hearing loss (SNHL), through electric stimulation of the spiral ganglion neurons.
The Brown–Vialetto–Van Laere syndrome (BVVLS) or the riboflavin transporter deficiency syndrome is a neurodegenerative disorder initially reported by Brown in 1894, by Vialetto in 1936, and by Van Laere in 1966. The syndrome has been described in more than 100 patients since then.1-3
In 2010, it was demonstrated that mutations in the riboflavin transporter genes, SLC52A2 (code of RFVT2) and SLC52A3 (code of RFVT3), are responsible for the syndrome.4 The riboflavin transporters are essential for normal cellular metabolism, indicating that the reduction of intracellular riboflavin levels is a critical pathologic indicator in BVVLS.3
A patient with BVVLS generally presents symptoms related to progressive bulbar dysfunction, SNHL, and respiratory dysfunction.2,5
Hearing loss is the most common symptom of the syndrome, as most individuals have it through the development of the disease. Although there is a variation between the onset of hearing loss and the other possible symptoms, SNHL usually begins in early childhood. Nevertheless, there are some cases describing SNHL starting in adults.6
Besides hearing loss, other cranial nerves including optic atrophy, upper and lower motor neurons involvement, and ataxia can be affected. The development of new symptoms can create a timeline like amyotrophic lateral sclerosis, Madras motor neuron disease, and Nathalie syndrome.7,8
The diagnosis is made through molecular analysis of the genes. Treatment with riboflavin oral reposition is efficient and retards the progression of the disease. The authors indicate treatment if there is suspicion of the transporter deficiency, even if the molecular results are not yet available.8
Hereby, we present a case report of a patient who started having the symptoms at the age of 14 and who had a mutation in the SLC52A3 gene, presenting with SNHL associated with cerebellar ataxia.
Case Presentation
A 31-year-old female presented with progressive bilateral SNHL that began when she was 14 years old.
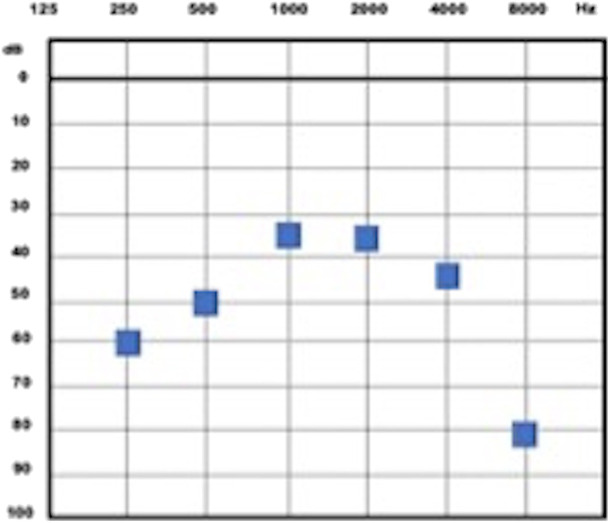
The pure tone audiometry presented a moderate to severe bilateral hearing loss (Figure 1).9 The speech perception test had no discrimination of the words presented (0% correct responses).
Figure 1.
Tone threshold audiometry. OD, right ear; OE, left ear.
The brainstem evoked response audiometry showed no waves on both sides and the otoacoustic emission in the distortion product was present on both sides.
She was diagnosed with auditive neuropathy at 21 years and started using a hearing amplification device in both ears, with the supervision of auditive training. After 6 months of therapy, there were still no benefits.
Aiming to screen her hearing abilities and to make fine adjustments in the hearing device, there was an auditory threshold test in an open field conducted, and in spite of a satisfactory threshold in the range of speech (1-4 kHz), the patient was referred no benefit from using the hearing devices, and also presented a poor performance in detection and comprehension of speech in closed set (Figure 2).
Figure 2.
Hearing threshold in an open field with amplification hearing device bilaterally.
Her head computed tomography scan was normal, and magnetic resonance imaging (MRI) showed a small reduction of the cochlear nerve signal bilaterally (Figure 3). After being evaluated by the multidisciplinary staff of the hearing group of our hospital, it was indicated to perform a cochlear implant (CI) surgery, which was made bilaterally, with an interval of 2 years between the surgeries (with 21 and 23 years old). Medel™ implants were used in the surgery.
Figure 3.
Coronal plane of MRI T2 sequence, demonstrating the bilateral slight reduction in the cochlear nerve signal. MRI, magnetic resonance imaging.
After the first CI was activated, auditory comprehension and hearing recognition abilities were significantly improved. Nevertheless, there were no improvements in hearing of noise with bimodal adapting (using both, CI and hearing amplification device). For this reason, the second CI was implanted. After that, the subject had a remarkable improvement, especially in those daily activities that are acknowledged as challenging: hearing and understanding song’s lyrics and hearing in noisy environments such as the workplace.
After 3 years of CI bilateral adapting, the patient clinically evolved with fallings, reduced articulatory patterns, ataxic movements, loss of muscular strength in lower limbs, and need the support of crutches to walk. She was diagnosed with progressive cerebellar ataxy, beginning a genetic investigation that discovered the SLC52A3 gene mutation. Three years after the riboflavin reposition, there was a march significant improvement.
After 5 years of the first surgery, the patient presented a mean of 28 dB of tone threshold in an open field, using bilateral CI devices (Figure 4). In speech perception, there was a 64% of auditive detection of dissyllables and 100% of trisyllables, both in open set.
Figure 4.
Open field audiometry showing bilateral CI auditive threshold. CI, cochlear implant.
Discussion
The BVVLS or the riboflavin transporter deficiency syndrome is a motor neuropathy that manifests with weakness of both upper and lower limbs, respiratory distress due to diaphragm palsy, sensorial neuropathy that manifests as march ataxy and cranial nerves neuropathy, such as optic atrophy, SNHL, and bulbar palsy.5
The riboflavin (7,8-dimethyl-10-[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]benzo[g]pteridine-2,4-dione) is a hydrophilic vitamin, which is converted into important coenzymes that are enrolled in the metabolic pathways of carbohydrates, amino acids, and lipids.3 The mutated gene of one of both related to riboflavin transport causes reduced absorption and a systemic level deficiency, as this vitamin is not produced by humans.
Riboflavin byproducts are critical components of the transport chain of the mitochondrial electrons. The reduced transport results in impaired mitochondrial activity.3,4,7
This deficiency is considered a recessive autosome neurodegenerative disorder, treatable, and rare.1,2 Bosh et al1 presented a revision paper with data of 74 subjects with BVVLS in 34 publications up to 2012. In this revision, the authors conclude that the absence of the treatment can lead to a lethal ending and indicate that treatment with reposition of riboflavin must be initiated as soon as there is a probability of the syndrome, even though molecular results are not yet available.
In this case report, the patient started having auditive symptoms when she was 14 years old, and the molecular result of the SLC52A3 gene mutation was only diagnosed after the cerebellar ataxy.
Hearing loss is one of the first symptoms of BVVLS and can precede other symptoms by years,1,2 which is a contributing factor to delayed diagnosis, increasing molecular testing importance in SNHL, especially those related to auditory neuropathy as in this case. Bosch et al1 described the mean age of onset of symptoms to be 8.2 years.
Neural degeneration, neuron decreased number, and gliosis are the most common findings in the brainstem and cerebral medulla, which can be detected in an MRI as the brainstem and cerebellar atrophy, respectively.7
The MRI of the case reported central atrophy at the beginning of the cochlear nerve.
The first case report that underwent a cochlear implant surgery was presented by Sinnathuray et al10 with a poor CI response. Authors allege that central alterations did not contribute to a good CI performance.11
Other studies demonstrate speech auditory perception improvement with the surgical intervention after CI surgery with riboflavin supplement. Salmira et al12 demonstrated the low benefits of speech recognition in an open set with hearing aid and emphasized the importance of additional image evaluation to indicate CI in those cases.
There is a recent systematic review13 regarding this subject, collecting 14 articles of BVVLS and CI performance, demonstrating a variation of auditory results, but with a generally good response, with hearing threshold and speech recognition improvement.
Furthermore, CI use and auditory rehabilitation in children with BVVLS is found in specialized literature, demonstrating improvement in both auditory threshold and speech detection.14,15
Some studies demonstrate that oral riboflavin treatment allows an ataxy improvement, although with no delay of hearing loss progression. The oral reposition must undergo throughout the life of the patients.5,6,8
Conclusion
The BVVLS is a rare pathology that must be remembered when there is an association of SNHL and auditory neuropathy with neurological findings. The correct diagnostic is of extreme importance, as the treatment is simple and can interrupt the disease’s progression, improving the patient’s quality of life.
Footnotes
Ethics Committee Approval: The study was approved by the Ethics Committee of the University Hospital, Medical School of Ribeirão Preto (CAAE: 36784620.1.0000.5440 , CEP HCRP USP).
Informed Consent: Written informed consent has been obtained from the Ethics Committee.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.A.H., M.S.A.A; Design – M.S.A.A.; G.H.M.F.; Supervision - M.A.H., A.C.M.B.R.; Resources – M.A.H., A.C.M.B.R., E.T.M.; Materials – M.S.A.A.; Data Collection and/or Processing – M.S.A.A.; G.H.M.F.; Literature Search – M.A.H., A.C.M.B.R., E.T.M.; Writing Manuscript – M.S.A.A.; G.H.M.F.; Critical Review – M.A.H., M.S.A.A., A.C.M.B.R.
Acknowledgments: Authors manifest their regards to Maria Cecilia Onofre for text correction and reference adjustments.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. Bosch AM, Stroek K, Abeling NG, Waterham HR, Ijlst L, Wanders RJ. The Brown-Vialetto-Van Laere and Fazio-Londe syndrome revisited: natural history, genetics, treatment and future perspectives. Orphanet J Rare Dis. 2012;7:83. 10.1186/1750-1172-7-83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis A, Josifova D, Lloyd-Owen S, Radunovic A, Swash M. Brown-Vialetto-Van Laere syndrome: a 28-year follow-up. J Neurol Neurosurg Psychiatry. 2016;87(6):681–682.. 10.1136/jnnp-2014-310088) [DOI] [PubMed] [Google Scholar]
- 3. Manole A, Jaunmuktane Z, Hargreaves I, et al. Clinical, pathological and functional characterization of riboflavin-responsive neuropathy. Brain. 2017;140(11):2820–2837.. 10.1093/brain/awx231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cosgrove J, Datta S, Busby M. Adult onset Brown-Vialetto-Van Laere syndrome with opsoclonus and a novel heterozygous mutation: a case report. Clin Neurol Neurosurg. 2015;128:1–3.. 10.1016/j.clineuro.2014.10.016) [DOI] [PubMed] [Google Scholar]
- 5. Manole A, Houlden H. Riboflavin transporter deficiency neuronopathy. GeneReviewsÕ [Internet]. Seattle, WA: University of Washington; 2015:1993–2016.. [Google Scholar]
- 6. Anand G, Hasan N, Jayapal S, et al. Early use of high-dose riboflavin in a case of Brown-Vialetto-Van Laere syndrome. Dev Med Child Neurol. 2012;54(2):187–189.. 10.1111/j.1469-8749.2011.04142.x) [DOI] [PubMed] [Google Scholar]
- 7. Manole A, Fratta P, Houlden H. Recent advances in bulbar syndromes: genetic causes and disease mechanisms. Curr Opin Neurol. 2014;27(5):506–514.. 10.1097/WCO.0000000000000133) [DOI] [PubMed] [Google Scholar]
- 8. Jaeger B, Bosch AM. Clinical presentation and outcome of riboflavin transporter deficiency: mini review after five years of experience. J Inherit Metab Dis. 2016;39(4):559–564.. 10.1007/s10545-016-9924-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. International Bureau for Audiophonologie ( BIAP ). BIAP Recommendation 02/1: Audiometric Classification of Hearing Disabilities. 1996. Available at: http://www.biap.org/fr/recommandations/recommendations/tc-02-classification. [Google Scholar]
- 10. Sinnathuray AR, Watson DR, Fruhstorfer B, Olarte JR, Toner JG. Cochlear Implantation in Brown-Vialetto-Van-Laere syndrome. J Laryngol Otol. 2011;125(3):314–317.. 10.1017/S0022215110001982) [DOI] [PubMed] [Google Scholar]
- 11. Chandran R, Alexander M, Naina P, Balraj A. Auditory neuropathy spectrum disorder with Brown-Vialetto-Van Laere syndrome: challenges in hearing rehabilitation. J Laryngol Otol. 2015;129(5):504–508.. 10.1017/S0022215114003375) [DOI] [PubMed] [Google Scholar]
- 12. Salmina C, Wagner F, Wiest R, et al. Neurotologic and functional MRI findings in a patient with bilateral profound deafness having Brown-Vialetto-Van Leare syndrome. Otol Neurotol. 2014;35(9):1495–1500.. 10.1097/MAO.0000000000000572) [DOI] [PubMed] [Google Scholar]
- 13. Chaudhry D, Chaudhry A, Muzaffar J, Monksfield P, Bance M. Cochlear implantation outcomes in post synaptic auditory neuropathies: a systematic review and narrative synthesis. J Int Adv Otol. 2020;16(3):411–431.. 10.5152/iao.2020.9035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Menezes MP, O'Brien K, Hill M, et al. Auditory neuropathy in Brown-Vialetto-Van Laere syndrome due to riboflavin transporter RFVT2 deficiency. Dev Med Child Neurol. 2016;58(8):848–854.. 10.1111/dmcn.13084) [DOI] [PubMed] [Google Scholar]
- 15. Anderson P, Schaefer S, Henderson L, Bruce IA. Cochlear implantation in children with auditory neuropathy: lessons from Brown-Vialetto-Van Laere syndrome. Cochlear Implants Int. 2019;20(1):31–38.. 10.1080/14670100.2018.1534035) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a