Abstract
Communication relies on signals that can be produced via different sensory modalities to modify receivers’ behavior. During social interactions, the possibility to perceive subtle visual cues enhances the use of facial expressions to exchange information. One of the most appropriate fields to explore the specific design features of visual signals is play fighting. Here, we explored the production and potential role of Relaxed Open Mouth (ROM) and Head Bobbing (HB) in regulating play fighting of wild spotted hyenas Crocuta crocuta, a highly hierarchical carnivore species. In accordance with the assumptions of the signal optimization theory, wild hyenas produced ROM and HB almost exclusively when the sender was in direct visual contact with the receiver thus suggesting that senders were attentive to the playmates’ face. Contrary to HB, the sequential analysis revealed that ROM often anticipated offensive patterns such as play biting thus supporting the hypothesis that ROM, but not HB, is a metacomunicative signal. Moreover, when the offensive patterns were biased toward one of the 2 players, the session was punctuated by a higher number of ROMs. Our findings support the general hypothesis that these 2 visual signals can play different roles in the management of play fighting in this carnivore species. The complementary use of ROM and HB would suggest that spotted hyenas are highly competent and fast in processing facial displays of different nature to correctly “read others’ intentions” and respond with appropriate motor actions to avoid misunderstanding during one of the most multifaceted and risky social interaction.
Keywords: Crocuta crocuta, head and facial signals, head bobbing, metacommunication, play fighting, relaxed open mouth display
Communication relies on signals (displays or actions) that are produced by a sender (hereafter, the sender) in the attempt to gain a behavioral response from a conspecific (hereafter, the receiver; Bradbury and Vehrencamp 1998). In communication, the transmission of the signal and its decoding must be adaptive to both parties (Hebets and Papaj 2005). Signals can convey information about the intrinsic characteristics of the sender (i.e., age, body size, and sex) or about the contexts under which the sender is behaving (i.e., motivation to compete, cooperate, and reproduce) (Hebets and Papaj 2005).
The signal optimization theory predicts that specific design features have evolved to maximize the probability of signal success in modifying a receivers’ behavior. Such features generally increase the optimality of the signal form and production (Bradbury and Vehrencamp 1998). The signal costs depend on the modality of transmission that can make the signal more likely to be detected by the receivers (Magnahagen 1991; Bradbury and Vehrencamp 1998). For example, to maximize detectability of the signal and reduce the costs of its production, a sender can emit the signal during particular periods of time or in presence of a specific audience (Hebets et al. 2016). A signal can be expressed through from different sensory modalities (visual, olfactory, and acoustic); therefore, gestures, postures, facial expressions, odors, or vocalizations can be used to convey different messages under different contexts (Lancaster 1971; Vankova and Bartos 2002; Burghardt 2005). On one side, a long distance between the sender and the receiver or the presence of visual barriers in the environment can preclude the possibility to use visual signals to communicate. On the other hand, when the sender and the receiver are interacting at a close distance, the possibility to perceive subtle visual cues enhances the use of facial expressions to exchange information (Bradbury and Vehrencamp 1998; Wiley 2006; Rosenthal 2007).
One of the most valuable behavioral contexts to explore the communicative potential of facial expressions in social mammals is play fighting. Because this activity implies close spatial proximity between the interacting subjects (Palagi et al. 2016) that engage in behavioral patterns borrowed from other functional competitive interactions (Burghardt 2005; Pellis and Pellis, 2017), play fighting is generally characterized by a redundancy of body postures and facial expressions (Pellis and Pellis 1998; van Hooff and Preuschoft 2003; Palagi 2008; Waller and Cherry 2012; Palagi et al. 2014; Weigel and Berman 2018). For this reason, play is a good model domain to test hypotheses on visual signals.
During social play, the motivational and intentional state of an individual can be expressed to a groupmate through the relaxed open mouth (ROM) display, which is considered a ritualized signal (sensuTinbergen 1952) that simulates the intention to bite during playful interactions in mammals (Fox 1970; van Hooff and Preuschoft 2003; Palagi 2006). ROM is widely reported in many social primate (e.g., Tonkean macaques Macaca tonkeana, Thierry et al. 1989; chimpanzees Pan troglodytes, Waller and Dunbar 2005; bonobos Pan paniscus, Palagi 2008) and non-primate species (foxes Vulpes vulpes, Fox 1970; black bears Ursus americanus, Henry and Herrero 1974; European polecats Mustela putorius, Poole 1978; coyotes Canis latrans, Way 2007; domestic dogs Canis familiaris, Cordoni et al. 2016; South American sea lions Otaria flavescens, Llamazares-Martin et al. 2017; sun bears Helarctos malayanus, Taylor et al. 2019). During play fighting ROM, which is generally considered a meta-communicative signal (Bekoff 1995), conveys a message of benign intent and appears to have a role in limiting the escalation of the playful session into real fighting (Bekoff 1995; Wright et al. 2018; Taylor et al. 2019). By performing a ROM, a playmate can inform about its own motivation to continue to play thus leading to prolonged play sessions (Waller and Dunbar 2005; Palagi 2008; Mancini et al. 2013; Davila-Ross and Dezecache, 2021).
Besides ROM, other types of signals can be displayed during playful interactions such as head and body gestures (Bekoff 1995; Yanagi and Berman 2014; Palagi et al. 2016; Call and Tomasello 2007). Among gestures, head movements seem to have a role in conveying motivation to play (hyacinth macao Anodorhyncus hyacinthinhusHick 1962; spider monkeys Ateles geoffroyi, Pellis and Pellis 1997, 2011; rhesus macaques Macaca mulattaSade 1973; spotted hyenas Crocuta crocuta, Drea et al. 1996; chimpanzees P.troglodytes, bonobos P.paniscusPollick and de Waal 2007; domestic dogs C.familiaris, horses Equus caballus, Maglieri et al. 2020). In particular, Head Bobbing (HB) is generally used to initiate a positive interaction between the interacting subjects and it has also been described also in phylogenetically very distant taxa from mammals such as in the genus Liolaemus (South American lizard, Labra et al. 2007).
The aim of our study is to explore the use and the potential role of ROM (Figure 1A; Supplementary Video S1) and HB (Figure 1B; Supplementary Video S2) in wild spotted hyenas C.crocuta. Like some primate species, spotted hyenas are organized in a fission–fusion society (Drea and Frank 2003; Smith et al. 2007) based on a strict and nepotistic dominance hierarchical system (Kruuk 1972; Tilson and Hamilton 1984; Frank 1986; Mills 1990; Wahaj et al. 2004). Despite such a crystallized hierarchy, hyenas rely on a complex network of cooperative behaviors and alliances that confer to the species a high level of social flexibility (Stratford and Périquet 2019; Vullioud et al. 2019). Hyena cooperation is evident in several behavioral domains such as rearing offspring (König 1997), hunting, and territorial defense (Holekamp et al. 2007). Moreover, in spotted hyenas play fighting seems to be an important behavior in the regulation and negotiation of social relationships at all stages of life (Drea et al. 1996; Nolfo et al. 2021). In a wild population, Nolfo et al. (2021) found that in spotted hyenas, play fighting never escalated into real aggression. Therefore, despite the high level of asymmetry and competitive elements characterizing hyena play fighting, it seems that players are able to manage their interactions. Here, we test 3 hypotheses including 5 predictions to clarify the nature of the 2 signals performed during play fighting in wild spotted hyenas (ROM and HB; Drea et al. 1996) that could have a potential role in conveying messages of playful motivation.
Figure 1.
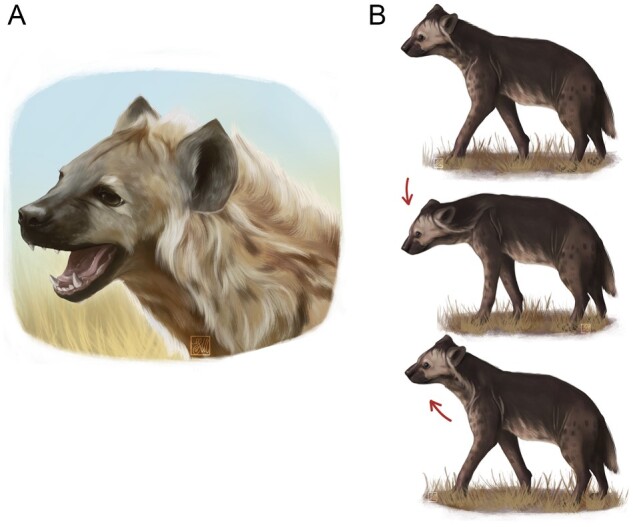
Illustration showing the 2 visual patterns analyzed in this study. A ROM performed by an adult (A) and an HB gesture performed by an immature subject (B). See the text for the definitions. Credits Fosca Mastrandrea.
Hypothesis—the optimization of the visual signals
Several researchers underlined the importance of the sender’s attention to the receiver’s attention in the production of both playful facial expressions and body gestures (Horowitz 2009; Demuru et al. 2015; Cordoni et al. 2016). The efficacy of visual signals such as ROM and HB strictly depends on the possibility for the receiver to intercept and decode the display emitted by the sender (signal optimization theory, Bradbury and Vehrencamp 1998; Hebets and Papaj 2005). If, in agreement with the optimization theory, the sender is attentive to the attention of the receiver whereas emitting the signal (i.e., intentional communication, Horowitz, 2009), we predict that both ROM and HB are mainly performed when the receiver is in direct visual contact with the sender (Figure 2A,C) (Prediction 1).
Figure 2.
Illustration showing the emission of the signals in the 2 different conditions: direct and indirect. ROM (A,B) and HB (C, D) were considered as detected when the sender was in front of the receiver (direct condition: A, C). ROM and HB were considered as not detected when the receiver was facing away from the sender or when the sender was in a lateral position with respect to the receiver (indirect condition: B,D). Credits Fosca Mastrandrea.
Hypothesis—the context of the signals
In a wide variety of mammal species, ROM is strictly performed during interactions of playful nature. For this reason, such facial expression has been characterized as a highly context-specific signal (Petrů et al. 2009; Scopa and Palagi 2016). On the contrary, HB seems to express motivation to engage in different positive social interactions (e.g., affiliation and play). If such signal contextualization is valid also for wild spotted hyenas, we predict ROM to be almost exclusively present during play fighting and HB also present during other positive contacts such as social affiliation (Prediction 2).
Hypothesis—metacommunicative nature of the signals
The term “metacommunication” was coined by Bateson (1951) to indicate that signals have underlying messages. Through the metacommunication process animals properly interpret playmates’ activities and realize that a behavioral pattern can have more than one meaning (Bateson 1972). If ROM and HB are metacommunicative signals anticipating and modifying the meaning of a competitive pattern, we expect such signals generally precede playful offensive patterns involving physical contact that would increase the probability of aggressive escalation if not preceded by an anticipatory signal (Prediction 3).
Due to the competitive nature of play fighting in spotted hyenas (Nolfo et al. 2021) and their ability to maintain a playful mood, we expected that those sessions showing the highest risk to escalate into real aggression (i.e., the most asymmetric and unbalanced), are also punctuated by the highest number of ROM and HB if both signals have a metacommunicative function (Prediction 4). Moreover, if ROM and HB share a metacommunicative function and reinforce each other in modulating the play fighting sessions, we expect to find them to co-variate (Prediction 5).
Materials and Methods
The reserve
Data collection was conducted at the Siyafunda Wildlife & Conservation research base (S -24.15029; E 30.65742), at the Greater Makalali Private Game Reserve (GMPGR, Limpopo, South Africa). The savannah biome characterizing the study area included herbaceous plants, tall trees, and bushes (Low and Rebelo 1996). The reserve is crossed by the Makhutswi River, a tributary of the Olifants River. Animals can find water also during the driest winter months (April–September) thanks to the presence of artificial waterholes. Spotted hyenas C.crocuta were introduced in 1995. The number of subjects forming the population of the GMPGR is still unknown.
Data collection
The data collection, 36 h of video recorded by A.P.N and G.C., covered the period from June to October 2019 during which animals were counted and individually recognized by the observers with the aid of the rangers of the reserve. By patrolling the various areas and known dens, we were able to identify 64 individuals (14 cubs, 5 juveniles, and 45 sub-adult/adults) on the basis of their peculiar morphological traits (e.g., scars, lack of fur patches, and spots on the fur; Holekamp et al. 1996; Holekamp and Smale 1998). Data on the number of males and females are not available, due to the difficulty to recognize the sex of the subjects.
During the observation period, we were able to follow 4 active dens and use them as observation spots. Observers recorded video from the vehicles to which animals were habituated. Videos were collected on the lactating females (LFs), their cubs, and all the subjects visiting the dens. The observation slots ranged from 2 to 3 per day (06.00–10.00 pm; 05.00–11.00 am; 03.00–06.00 pm). During the nocturnal slots (06.00–10.00 pm), to limit disturbance as much as possible, data were collected by using red illumination that was never directed toward the animal but on the ground around them (Finley 1959; Spoelstra et al. 2017). A 50× optical zoom and a tripod allowed recording of high-quality frames at long distances (up to 50 m). Video recordings were made using a Canon® EOS 110D camera. A second camera (Full HD Panasonic Lumix DC-FZ82) came into play when subjects were scattered around the observation spot. The concurrent use of 2 cameras allowed the video recording of all the activities of the group of subjects even when it split into subgroups. In this way, we were able to maximize the amount of time each subject was present in the observation spot. Observers directly recorded about 26 h of videos.
Videos were also collected by using camera traps (Ranger digital trail, BN056) provided by the Siyafunda research center. The camera traps were tied to trees approximately 10 m in front of the dens and 1.50 m above the ground. Each camera trap covered a range of 5 m around the den entrance. The camera traps were active 24 h/day, with no delay between consecutive videos (lasting from 40 to 60 s), and the sensitivity of the motion sensor was set to high. Through the camera traps, we collected a total of 12 h of videos. The 2 different methods did not have any effect on the distribution of play in the study group (Nolfo et al. 2021).
Only the individuals (N= 24) with at least 30 min of video recordings were included in the analyses (individual mean 109 ± 19 standard error (SE) minutes of videos). Our dataset includes 8 cubs, 2 juveniles, and 14 subadults/adults. In the analysis, the cubs and the juveniles were clustered as “immature subjects” and the subadults and adults as “adult subjects”.
Video analyses
A.P.N. and G.C. analyzed the videos by using VLC 2.1.5 Rincewind software and Jump-to-Time extension (0.02 s accuracy). Before starting the video analysis, the 2 observers underwent a training period lasting 30 h (the trainer was E.P.). The inter-observer reliability was checked by E.P. who randomly selected 10-min blocks for every 2 h of videos analyzed, to verify the correct classification of the behavioral patterns. Cohen’s kappa values did not score ˂ 0.93 for both the playful and affiliative pattern analyzed. The list of the playful and affiliative items observed and used for this study is shown in Supplementary Table S1.
By applying the all occurrences sampling method (Altmann 1974), we recorded and analyzed all the playful interactions occurring in the study subjects. The exact duration (0.02 s accuracy) of each session, the identity of the initiator and the receiver, the exact sequence of the playful patterns performed, and the time of the day were extracted for each play session analyzed. A dyadic playful session started with the first playful pattern (Supplementary Table S1) performed by one of the subjects toward the playmate. If the playmate did not respond with any playful action listed in Supplementary Table S1, such interaction was not included in the analysis. The session ended when one of the 2 hyenas moved away from the playmate or if a third subject interfered, starting a new session or interrupting the previous one (Llamazares-Martín et al. 2017).
From the 38-h of videos, we extracted all the ROMs and HBs and if they occurred during playful or affiliative interactions by the observed animals. During a ROM “the mouth is relaxed and kept open at different gradients; the mouth can be opened (a) just a little revealing only the upper parts of the most forward teeth of the lower jaw and (b) in a wider way completely revealing the lower and upper jaws” (e.g., Aloff 2005 pp. 284, 343). During a ROM (Figure 1A), a subject never closes its mouth even though a body target of the playmate is reached. The HB involves up-down movements of the head that are repeated in a stereotyped way whereas animals are walking or standing (Figure 1B).
To define the context in which the signals were emitted we verified if ROM and HB were followed by a behavioral pattern included in the affiliative or playful domain (see Supplementary Table S1 for a detailed description of the behavioral items).
To understand signal display across different contexts of play asymmetry we calculated the play asymmetry index (PAI). We divided the patterns into offensive (O), defensive (D), and neutral (Supplementary Table S1). We calculated PAI by applying the following formula:
The PAI absolute values (|PAI|) range from 0 (completely balanced session) to 1 (completely unbalanced session). It is worth noting that the item Rough&Tumble has been categorized as neutral, due to the impossibility to identify the exact pattern and direction engaged by the players (Supplementary Table S1). The attribution of offensive and/or defensive score to the players without actually seeing the pattern would have introduced a bias in the calculation of PAI.
To verify if the sender emitted the signal (ROM and HB) to maximize the probability of its detection by the receiver, we accurately registered the position of the receiver with respect to the sender. When the sender was in front of the receiver (i.e., within the range of its stereoscopic view), we considered ROM and HB as detected (Figure 2A,C). When the receiver was facing away from the sender (without direct visual contact) or when the sender was in a lateral position with respect to the receiver, we considered ROM and HB as not detected (Figure 2B,D).
Statistics
To evaluate whether ROM and HB occurred in different contexts (affiliative versus play fighting), via the paired sample randomization test (Manly 1991) we compared their rates between the 2 conditions (the level of significance set at 0.05).
With the software Behatrix version 0.9.11 (Friard and Gamba 2020), we conducted a sequential analysis to evaluate which playful patterns (Supplementary Table S1) were more likely to be enacted by the actor immediately after the emission of a ROM or an HB. For each ROM or HB event, we generated a string representing the ordered concatenation of patterns as they occurred after the occurrence of a ROM (e.g., ROM|play run; ROM|play bite) or an HB (e.g., HB|play run; HB|play bite). Then, via the same software, we created a flow diagram with the transitions from ROM and HB to the following pattern, with the percentage values of the relative occurrences of such transitions. Finally, we ran a permutation test based on the observed counts of the behavioral transitions (“Run random permutation test” Behatrix-function). We permuted the strings 10,000 times, obtaining P-values (0.001 accuracy) for each behavioral transition.
To evaluate which factor affected the total of ROM displays (number of ROM as response variable) and the total of HB displays (number of HB as response variable), we ran 2 Generalized Linear Mixed Models (LMMs) with a Poisson distribution by using the R-package glmmTMB 1.2.5042 (Brooks et al. 2017).
For the first model (response variable = number of ROM), we included the following fixed factors: the age combination of the players to account for age (immature→immature; immature→mature; mature→immature; mature→mature), Day/Night observations, LF (presence/absence), the logarithm of the absolute values of PAI (log|PAI|), and total number of HB.
For the second model (response variable = number of HB), the fixed factors considered were: the age combination of the players to account for age (immature→immature; immature→mature; mature→immature; mature→mature), Day/Night observations, LF (presence/absence), the logarithm of the values of PAI (log|PAI|), and total number of ROM.
The null model included the identity of the dyad involved in each play session as random factor and the logDURATION of the session as control predictor.
Via the likelihood ratio test (LRT; Dobson 2002) we compared the overall significance of the full model with the null model including only the random effects (Forstmeier and Schielzeth 2011). The LR test was applied also to check the significance of the fixed factors via the function Anova in the R-package car 3.0-10 (Fox and Weisberg 2019). To exclude the presence of collinearity between predictors, we scrutinized the variance inflation factors (VIF; Fox 2016) via the R-package performance 0.4.4 (Lüdecke et al. 2020). Model fit and overdispersion were tested by the use of the R-package DHARMa 0.3.3.0 (Hartig 2020). The marginal R2, representing the variance explained by fixed factors only, and the conditional R2, representing the variance explained by the entire model including both fixed and random effects (Nakagawa et al. 2017), were measured via the R-package MuMIn 1.43.17 (Bartoń 2020). Then, we employed the “confint(x)” function to evaluate the estimated effects as relative odds ratios. Relative odds ratios (i.e., the expected odds change for 1-unit increase in the explanatory variable when the remaining variables are set to their reference category) were used to measure the magnitude of the estimated effects. Via the Tukey’s test (Bretz et al. 2010), we ran all pairwise comparisons for the levels of the multilevel factor (R package emmeans, Lenth 2020).
Results
Hypothesis—the optimization of the signals
The mean number of ROM performed by adults was 1.923 ± 0.366 SE and by immature subjects was 1.600 ± 0.289 SE. The mean number of HB performed by adults was 1.667 ± 0.333 SE and by immature subjects was 1.933 ± 0.229 SE. All the ROM (N= 46) and HB events (N= 63) were performed when the sender was in direct visual contact with the receiver.
Hypothesis—the context of the signals
To verify if ROM and HB were expressed during a play context, we analyzed which pattern (affiliative versus playful) emitted by either the sender or the receiver immediately followed the ROM and HB emission. To be contextualized in play or affiliative domains, the signal had to be followed by at least 2 consecutive patterns of the same domain (Supplementary Table S1). After the emission of a ROM the subjects engaged in playful patterns more frequently than in affiliative patterns (ROMplay_domain > ROMaffiliative_domain: paired sample randomization test, t= 5.147; N= 46; P= 0.0001). HB was followed by affiliative and playful patterns at comparable frequencies (HBplay_domain ∼ HBaffiliative_domain: paired sample randomization test, t= −1.137; N= 63; P= 0.209).
Hypothesis—metacommunicative nature of the signals
Within the playful context, the sequential analysis revealed that after the emission of a ROM, the sender significantly performed the following play patterns (transition ROM→nose-to-nose contact: percentage of occurrence = 17.391%, P= 0.0061; transition ROM→play bite: percentage of occurrence = 34.783%; P= 0.0001, transition ROM→play run: percentage of occurrence = 26.087%; P= 0.0008). The sequential analysis revealed that after the emission of an HB, the sender significantly performed the following play patterns (transition HB→nose-to-nose contact: percentage of occurrence = 17.857%, P= 0.0026; transition HB→play run: percentage of occurrence = 50.000%; P= 0.0001).
Variables affecting ROM
The full model, including all the fixed factors (see Materials and Methods for the definitions) was statistically different from the null model, comprising only the random factor (dyads) and the control factor (LOGduration) (LRT: χ2 = 15.801, df = 7, P= 0.027). No collinearity was found between the fixed factors (low correlation, range VIFmin= 1.10; VIFmax = 3.35). We did not find any effect of HB on the number of ROMs. The fixed factor “age combination” (Table 1; Figure 3) and log|PAI| had a significant effect on the number of ROMs performed (Table 1; Figure 4). There was a positive correlation between the number of ROMs and the log|PAI| values thus indicating that when the session became more unbalanced, the animals increased the emission of facial expressions (Figure 4). The play sessions initiated by adults tended to be punctuated by a higher number of ROMs compared with the play sessions involving only immature subjects (Tukey test timmature-immature versus adult-immature = −2.478, df= 167, P= 0.067; timmature-immature versus adult-adult = −2.452, df= 167, P= 0.071). We did not find any significant difference in the number of ROMs performed between all the other age–class combinations (t-ratioimmature-immature versus immature-adult = −0.334, df= 167, P= 0.987; t-ratio immature-adult versus adult-immature = −1.572, df= 167, P= 0.398; t-ratioimmature-adult versus adult-adult = −2.128, df= 167, P= 0.148; t-ratioadult-immature versus adult-adult= −0.1.041, df= 167, P= 0.725; Figure 3).
Table 1.
Results of the generalized LMM analysis (response variable: total ROM displays, Poisson distribution)
| Fixed effects | Coeff | SE | 2.5% CI | 97.5% CI | χ 2 | df | P-value |
|---|---|---|---|---|---|---|---|
| Intercept | −5.372 | 1.186 | −7.695 | −3.048 | — | — | — |
| Log|PAI| | 0.879 | 0.355 | 0.183 | 1.576 | 6.131 | 1 | 0.013 |
| TotHB | 0.017 | 0.370 | −0.742 | 0.707 | 0.002 | 1 | 0.962 |
| AC | — | — | — | — | 9.572 | 3 | 0.022 |
| AC [immature→mature]a, b | 0.442 | 1.325 | −2.154 | 3.038 | — | — | — |
| AC [mature→immature]a, b | 2.286 | 0.922 | 0.478 | 4.094 | — | — | — |
| AC [mature→mature]a, b | 3.490 | 1.423 | 0.701 | 6.280 | — | — | — |
| Day/night | −1.392 | 1.186 | −2.880 | 0.095 | 3.368 | 1 | 0.066 |
| LF [presence/absence] | −1.692 | 0.927 | −3.509 | 0.125 | 2.291 | 1 | 0.068 |
Estimated parameters (Coeff), SE, 95% confidence intervals (2.5–97.5% CI), and results of the LRTs of the best Generalized LMM (with a Poisson distribution) investigating the effect of the following variables on the: log|PAI|; total HB displays (totHB); AC (immature→immature; immature→mature; mature→immature; mature→mature); Day/Night; LF (presence/absence); marginal R2 = 0.284; conditional R2 = 0.704; Ncases = 177; Ndyads = 62. Variance for the random factors: dyads = 2.572 (±1.604 SD). Significant P-values are shown in bold
Estimate parameters ± SE refer to the difference of the response between the reported level of this categorical predictor and the reference category of the same predictor
These predictors were dummy coded, with the “AC [immature→immature]” being the reference category.
Figure 3.
Raincloud ridge plot showing the total number of ROM events in the 4 age–class combinations (green density curve = immature → immature play; light blue density curve = immature → adult play; blue density curve = adult → immature play; grey density curve = adult → adult play).
Figure 4.
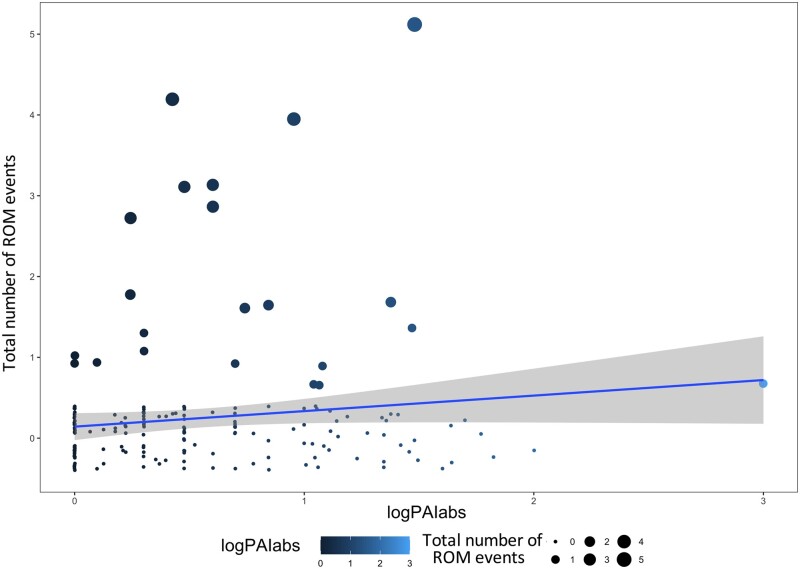
Scatter plot showing the relationship between the total number of ROM events and the logarithm transformation of |PAI| values (logPAIabs). Dot size follows the total number of ROMs, whereas dot color follows the logarithm of |PAI| values. The blue line represents the linear regression between the variables and the respective 95% CI.
Variables affecting HB
The full model, including all the fixed factors (see “Materials and methods” for the definitions) was statistically different from the null model, comprising only the random factor (dyads) and the control factor (LOGduration) (LRT: χ2 = 34.146, df = 7, P= 0.00001). No collinearity was found between the fixed factors (low correlation, range VIFmin= 1.10; VIFmax = 2.03). We did not find any effect of ROM and log|PAI| on the number of HBs. The only fixed factor with a significant effect on the number of HB performed was “age combination” (Table 2; Figure 5). The sessions including and initiated by immature subjects were punctuated by a higher number of HB (Tukey’s test timmature-immature versus immature-adult= −4.54; df= 167; P= 0.0001; t-ratioimmature-adult versus adult-immature = 3.429, df= 167, P= 0.004; t-ratioimmature-adult versus adult-adult = 2.915, df= 167, P= 0.021). All the other age–class combinations did not differ in the number of HB diplayed (timmature-immature versus adult-adult = −0.416, df= 167, P= 0.976; t-ratioimmature-immature versus adult-immature = −0.382, df= 167, P= 0.981; t-ratioadult-immature versus adult-adult= −0.100, df= 167, P= 0.999; Figure 5).
Table 2.
Results of the generalized LMM analysis (response variable: total HB displays, Poisson distribution)
| Fixed effects | Coeff | SE | 2.5% CI | 97.5% CI | χ 2 | df | P-value |
|---|---|---|---|---|---|---|---|
| Intercept | −1.800 | 0.637 | −3.046 | −0.549 | |||
| Log|PAI| | 0.258 | 0.325 | −0.379 | 0.894 | 0.629 | 1 | 0.427 |
| totROM | 0.151 | 0.191 | −0.223 | 0.525 | 0.626 | 1 | 0.428 |
| AC | 27.620 | 3 | < 0.0001 | ||||
| AC [immature→mature]a, b | 2.698 | 0.606 | 1.510 | 3.885 | — | — | — |
| AC [mature→immature]a, b | 0.290 | 0.759 | −1.196 | 1.775 | — | — | — |
| AC [mature→mature]a, b | 0.383 | 0.919 | −1.419 | 2.184 | — | — | — |
| Day/Night | 0.851 | 0.453 | −0.037 | 1.739 | 3.527 | 1 | 0.061 |
| LF [presence/absence] | −0.695 | 0.525 | −1.725 | 0.335 | 1.751 | 1 | 0.186 |
Estimated parameters (Coeff), SE, 95% CIs (2.5–97.5% CI), and results of the LRTs of the best Generalized LMM (with a Poisson distribution) investigating the effect of the following variables on the: log|PAI|; total ROM displays (totROM); AC (immature→immature; immature→mature; mature→immature; mature→mature); Day/Night; LF (presence/absence); marginal R2 = 0.302; conditional R2 = 0.542; Ncases = 177; Ndyads = 62;. Variance for the random factors: dyads = 0.720 (±0.848 SD).
Estimate parameters ± SE refer to the difference of the response between the reported level of this categorical predictor and the reference category of the same predictor.
These predictors were dummy coded, with the “AC [immature→immature]” being the reference category.
Figure 5.
Raincloud ridge plot showing the total number of HB events in the 4 age–class combinations (green density curve = immature → immature play; light blue density curve = immature → adult play; blue density curve = adult → immature play; grey density curve = adult → adult play).
A summary of the hypotheses, predictions, and outcomes is shown in Table 3.
Table 3.
Summary of the hypotheses, predictions, and outcomes presented in the study
| Hypotheses | Predictions | Outcomes |
|---|---|---|
| The efficacy of visual signals such as ROM and HB strictly depends on the possibility for the receiver to intercept and decode the display emitted by the sender; the sender is attentive to the attention of the receiver whereas emitting the signal (optimization of the visual signal) | (P1) - ROM and HB are mainly performed when the receiver is in direct visual contact with the sender | Supported |
| ROM is a highly specific signal, strictly performed during interactions of playful nature; HB expresses motivation to engage in positive social interactions (e.g., affiliation, play) | (P2) - ROM is exclusively present during play fighting and HB is also present during other positive contacts such as social affiliation | Supported |
| ROM and HB are metacommunicative signals | (P3) - ROM and HB precede playful offensive patterns involving physical contact that would increase the probability of aggressive escalation | Partially supported |
| (P4) - The session with the highest risk to escalate into real aggression are also punctuated by the highest number of both ROM and HB | Partially supported | |
| (P5) - If ROM and HB share a metacommunicative function and reinforce each other in modulating the play fighting sessions, we expect to find them to co-variate | Not supported |
Discussion
Here, we found that visual signals have an important role in the management of play fighting of wild spotted hyenas thus stressing the role of visual communication in this large carnivore species. At the same time, the study permitted to test general hypotheses on the designed features on the basis of the evolution of visual signals in mammals. Whereas emitting ROM (Figure 1A) and HB (Figure 1B), the sender was attentive to the position of the receiver’s face (Figure 2A,C) thus increasing the likelihood for both visual signals to be intercepted and properly decoded by the receiver (Prediction 1 supported, Table 3). Therefore, the emission of these playful signals supports the optimization theory predicting that maximizing the detectability of the signal significantly optimizes the cost and benefit ratio of its production (Bradbury and Vehrencamp 1998; Hebets and Papaj 2005). Similar findings have been also reported for several mammal species, where facial expressions and body gestures are generally produced in an “attentive to attention condition” especially during play fighting (domestic dogs, C.familiaris, Horowitz 2009; bonobos, P.paniscus; Demuru et al. 2015; South American sea lions, O.flavescens, Llamazares-Martìn et al. 2017; lowland gorillas, Gorilla gorilla gorilla; Palagi et al. 2019a, 2019b, meerkats, Suricata suricatta; Palagi et al. 2019a, 2019b, sun bears, H.malayanus; Taylor et al. 2019). The “attentive to attention condition” (Horowitz 2009) is the one of the building blocks for the development of intentional communication (Ben Mocha and Burkart 2021). Intentional communication plays not only a crucial role in increasing the detection probability, but also informs about animal competence in signal transmission. Our findings strongly suggest that maximizing the probability to be seen whereas producing the signal (Hebets and Papaj, 2005) can be particularly relevant when the action requires a rapid and proper reply by the playmate thus increasing motor synchronization and reducing the risk of misunderstanding.
Although both visual signals were present during playful context in wild spotted hyenas, ROM was more context-dependent than HB, which was also performed under the affiliative context (e.g., mother–infant interactions, greeting ceremonies) (Prediction 2 supported, Table 3). The use of head gestures has been reported in great apes not only to initiate a play session (bonobos; Demuru et al. 2015) but also to get attention from the receiver and start a positive interaction (e.g., chimpanzees; Hobaiter and Byrne 2011). Also in monkeys, head gestures can be used in >1 context. For example, they can have a role in inviting a groupmate to initiate a play session (rhesus macaques, M.mulatta; Sade 1973; spider monkeys, A.geoffroy, Pellis and Pellis 1997, 2011) and a partner to engage in a sexual interaction (M.mulatta, Michael and Zumpe 1970).
Within the playful context, the sequential analysis revealed that both ROM and HB were significantly followed by nose-to-nose contact (neutral pattern) and play run (no-contact offensive pattern) (Supplementary Table S1) compared with the other playful patterns recruited in the session. However, contrary to HB, ROM also significantly preceded a play bite action which is classified as a contact offensive pattern (Supplementary Table S1) (Prediction 3 supported for ROM and not for HB, Table 3). Although in spotted hyenas play bites and aggressive bites differ in their performance (Drea et al. 1996), ROM can clarify the meaning of the biting pattern immediately before its execution and such anticipation can prevent possible misunderstanding between the 2 players. This is the first empirical evidence that a facial expression (ROM) can be used as a metacommunicative signal in wild spotted hyenas. The use of signals in clarifying the intentions of animals during play (Bekoff 1972, 1995; Pellis and Pellis 1996) seems to acquire even more importance in species whose play modality is rough and competitive (Nolfo et al. 2021). During play fighting, spotted hyenas engage in a variety of offensive patterns that are often performed in an unbalanced way between the players. One of most unpredictable offensive patterns is play biting which is characterized by a high variability in the targeted body parts of the receiver (see the illustrated ethogram in Nolfo et al. 2021). Although standardized sequential analyses illustrating the temporal relation between facial expressions and the subsequent playful patterns are missing in the literature, to our knowledge, the linkage between the frequency of facial displays punctuating the session and the competitive nature of the interaction has been reported. Bonobos, for example, increase their play faces when they need to cope with rough play under risky situations, such as when the escape opportunities are reduced (Tacconi and Palagi 2009). Young lowland gorilla males engage more in playful facial expressions during contact than during locomotor play (Palagi et al. 2007). Outside the primate order, ROM has been observed during social play in the American black bear U.americanus. In this species, play fighting is particularly rough and ROM has been found to anticipate biting actions. Moreover, bears can engage in long-lasting ROMs (for up to 30 s) probably to make the signal more conspicuous and easily detectable by the partner (Henry and Herrero 1974).
Whereas the level of play fighting asymmetry had no effect on the rates of HB, it significantly affected the frequency of ROM (Prediction 4 supported for ROM and not for HB). When the playful offensive patterns of a session were strongly biased toward one of the 2 players (high levels of |PAI|), such session was characterized by a high number of ROMs (Figure 4). Moreover, ROM tended to be more frequent when the session involved age-mismatched subjects and when it was initiated by an adult (Figure 3). Our results on the use of ROM in spotted hyenas suggest that the maintenance of the positive mood during strongly unbalanced interactions (high PAI scores, age-mismatched players) requires not only a significant investment in terms of attention to others’ faces, a basic element of intentional communication, but also the ability to “place” the signal in a proper temporal context (e.g., before play biting). Future studies on the direction of the signal emission as a function of the advantageous or disadvantageous positions of each player could help to clarify if ROM is really used to de-escalate the roughness of the session.
Contrary to ROM, HB did not show any variations as a function of play asymmetry and was more frequent when the sessions included and were initiated by the immature subjects (Figure 5). It is possible that this head gesture, being also present during affiliative interactions, can be used more to invite to initiate an interaction rather than to manage the roughness of the play session. However, further analyses on larger datasets will be necessary to verify this hypothesis.
The 2 signals do not seem to reinforce each other during play fighting, because none of the statistical models revealed a positive relation between HB and ROM emission frequency (Prediction 5 not supported). Although the absence of evidence is not the evidence of absence, these findings taken together with all the others support the general hypothesis that ROM and HB can play different roles in the management of play fighting in this competitive and highly hierarchical species. However, considering that play fighting in our groups of spotted hyenas involved all age classes and never escalated into overt aggression (Nolfo et al. 2021), we can reasonably assume that these animals are highly competent and fast in emitting and decoding facial displays of different nature to correctly “read” others’ intentions and respond with appropriate motor actions. The next step will be verifying which level of intentionality (Ben Mocha and Burkart 2021) is involved in the emission of these signals and whether, as it occurs in other carnivores (dogs; Palagi et al. 2015; sun bears, Taylor et al. 2019; meerkats, Palagi et al. 2019a, 2019b), some forms of mimicry can help spotted hyenas to synchronize their motor actions thus favoring playful activity at any stage of their life.
Supplementary Material
Acknowledgments
We thank the Siyafunda Wildlife and Conservation and, in particular, the director Michael Job for his kind support and hospitality during the data collection. Moreover, we wish to thank the rangers who provided invaluable help in tracking animals (in alphabetical order): Sam Adams, Chaz Domijan, Kai Harris, Emma Jenkins, Jelle Linssen, Kayla McClelland, Lukas Schefer, and Derek Smith. Veronica Maglieri for her help in statistics and Fosca Mastrandrea for the drawings illustrating the behavioral items defined in this study. Finally, we wish to thank Cheryl Sanchez for her accurate linguistic revision of the manuscript. This study was supported by the University of Pisa (Italy).
Authors’ Contribution
Conceptualization: E.P.; Data collection: G.C. and A.P.N.; Training for behavioral data collection and video-analysis, E.P.; video-analysis, G.C and A.P.N.; data analysis: A.P.N. and E.P.; Writing: A.P.N., G.C., and E.P.; Revision: A.P.N., G.C., and E.P.
Ethical Note
The data reported here derive from non-invasive research that is compliant with the current South African law and Pisa University regulations. Thus, no permit from the Bio-Ethical Committee was needed.
Supplementary Material
“Supplementary material can be found at https://academic.oup.com/cz”.
Conflict of interests
The authors declare that they have no conflict of interest.
Contributor Information
Andrea Paolo Nolfo, Department of Biology, Unit of Ethology, University of Pisa, Via A. Volta 6, Pisa, 56126, Italy.
Grazia Casetta, Department of Biology, Unit of Ethology, University of Pisa, Via A. Volta 6, Pisa, 56126, Italy.
Elisabetta Palagi, Department of Biology, Unit of Ethology, University of Pisa, Via A. Volta 6, Pisa, 56126, Italy; Natural History Museum, University of Pisa, Via Roma 79, Calci, Pisa, 56011, Italy.
References
- Aloff B, 2005. Canine Body Language. A Photographic Guide Interpreting the Native Language of the Domestic Dog. Wenatebee (WA): Dogwise Publishers. [Google Scholar]
- Altmann J, 1974. Observational study of behaviour sampling methods. Behaviour 49:227–265. [DOI] [PubMed] [Google Scholar]
- Bartoń K, 2020. MuMIn: multi-model inference. R package version 1.43.17. Available form: https://CRAN.R-project.org/package=MuMIn.
- Bateson G, 1951. Information and codification; and conventions of communication. Communication: The Social Matrix of Psychiatry. New York (NY): Norton and Co. 168–227. [Google Scholar]
- Bateson G, 1972. Steps to an Ecology of Mind: Collected Essays in Anthropology, Psychiatry, Evolution, and Epistemology. Chicago (IL): University of Chicago Press. [Google Scholar]
- Bekoff M, 1995. Play signals as punctuation: the structure of social play in canids. Behaviour 132:419–429. [Google Scholar]
- Bekoff M, 1972. The development of social interaction, play, and metacommunication in mammals: an ethological perspective. Q Rev Biol 47:412–434. [Google Scholar]
- Ben Mocha Y, Burkart JM, 2021. Intentional communication: solving methodological issues to assigning first-order intentional signalling. Biol Rev 96:903–921. doi: 10.1111/brv.12685. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL, 1998. Principles of Animal Communication. Sunderland (MA): Sinauer Associates, Inc. [Google Scholar]
- Bretz F, Maurer W, Hommel G, 2010. Test and power considerations for multiple endpoint analyses using sequentially rejective graphical procedures. Stat Med 30:1489–1501. [DOI] [PubMed] [Google Scholar]
- Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW. et al. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated Generalized Linear Mixed Modeling. The R Journal 9:378–400. [Google Scholar]
- Burghardt GM, 2005. The Genesis of Animal Play: Testing the Limits. Cambridge: The MIT Press [Google Scholar]
- Fox MW, 1970. A comparative study of the development of facial expression in canids: wolf, coyote and foxes. Behaviour 36:49–73. [Google Scholar]
- Call J, Tomasello M, 2007. The Gestural Communication of Apes and Monkeys. Mahwah (NJ): Lawrence Erlbaum. [Google Scholar]
- Cordoni G, Nicotra V, Palagi E, 2016. Unveiling the secret of play in dogs Canis lupus familiaris: asymmetry and signals. J Comp Psychol 130:278–287. [DOI] [PubMed] [Google Scholar]
- Davila-Ross M, Dezecache G, 2021. The complexity and phylogenetic continuity of laughter and smiles in hominids. Front Psychol 12:648497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuru E, Ferrari PF, Palagi E, 2015. Emotionality and intentionality in bonobo playful communication. Anim Cogn 18:333–344. [DOI] [PubMed] [Google Scholar]
- Dobson AJ, 2002. An Introduction to Generalized Linear Models. 2nd edn. Boca Raton (FL): Chapman and Hall/CRC Press. [Google Scholar]
- Drea CM, Frank LG, 2003. The social complexity of spotted hyenas. In: FBM Waal, de Waal F, Tyack PL, editors. Tyack Animal Social Complexity. Cambridge: Harvard University Press. 121–148. [Google Scholar]
- Drea CM, Hawk JE, Glickman SE, 1996. Aggression decreases as play emerges in infant spotted hyaenas: preparation for joining the clan. Anim Behav 51:1323–1336. [Google Scholar]
- Finley RB, 1959. Observation of nocturnal animals by red light. J Mammal 40:591–594. [Google Scholar]
- Frank LG, 1986. Social organization of the spotted hyena Crocuta crocuta dominance and reproduction. Anim Behav 34:1510–1527. [Google Scholar]
- Friard O, Gamba M, 2020. Behatrix: behavioral sequences analysis with permutations test. [accessed 20 July 2021]. Available from: http://www.boris.unito.it/pages/behatrix.
- Forstmeier W, Schielzeth H, 2011. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol 65:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S, 2019. An R Companion to Applied Regression. 3rd edn. Los Angeles (CA): Sage Publications. [Google Scholar]
- Fox J, 2016. Applied Regression Analysis and Generalized Linear Models. 2nd edn. Los Angeles (CA): Sage Publications. [Google Scholar]
- Hartig F, 2020. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models version 0.3.3.0 R package. Available from: https://CRAN.R-project.org/package=DHARMa.
- Hebets EA, Papaj DR, 2005. Complex signal function: developing a framework of testable hypotheses. Behav Ecol Sociobiol 57:197–214. [Google Scholar]
- Hebets EA, Barron AB, Balakrishnan CN, Hauber ME, Mason PH. et al. 2016. A systems approach to animal communication. Proc R Soc Lond B Biol Sci 283:20152889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, Herrero SM, 1974. Social play in the American Black Bear: its similarity to canid social play and an examination of its identifying characteristics. Am Zool 14:371–389. [Google Scholar]
- Hick U, 1962. Beobachtungen uber das spielverhalten unseres hyazinth ara Anodorhynchus hyacinthus. Freunde d. Kolner Zoo 5:8–9. [Google Scholar]
- Hobaiter C, Byrne RW, 2011. Serial gesturing by wild chimpanzees: its nature and function for communication. Anim Cogn 14:827–838. [DOI] [PubMed] [Google Scholar]
- Holekamp KE, Smale L, Szykman M, 1996. Rank and reproduction in the female spotted hyaena. J Reprod Fertil 108:229–237. [DOI] [PubMed] [Google Scholar]
- Holekamp KE, Smale L, 1998. Behavioral development in the spotted hyena. Bioscience 48:997–1005. [Google Scholar]
- Holekamp KE, Sakai ST, Lundrigan BL, 2007. Social intelligence in the spotted hyena Crocuta crocuta. Phil Trans R Soc B 362:523–538. doi: 10.1098/rstb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A, 2009. Attention to attention in domestic dog Canis familiaris dyadic play. Anim Cogn 12:107–118. [DOI] [PubMed] [Google Scholar]
- König B, 1997. Cooperative care of young in mammals. Naturwissenschaften 84:95–104. [DOI] [PubMed] [Google Scholar]
- Kruuk H, 1972. The Spotted Hyena: A Study of Predation and Social Behavior. Chicago (IL): University of Chicago Press [Google Scholar]
- Labra A, Carazo P, Desfilis E, Font E, 2007. Agonistic interactions in a Liolaemus lizard: structure of head bob displays. Herpetologica 63:11–18. [Google Scholar]
- Lancaster J, 1971. Play-mothering: the relations between juvenile females and young infants among free-ranging vervet monkeys Cercopithecus aethiops. Folia Primatol 15:161–182. [PubMed] [Google Scholar]
- Lenth R, 2020. emmeans: estimated marginal means, aka least-squares means. Available from: https://cran.r-project.org/package=emmeans.
- Low AB, Rebelo AG, 1996. Vegetation of South Africa, Lesotho and Swaziland. Pretoria, South Africa: Department of Environmental Affairs and Tourism. [Google Scholar]
- Lüdecke D, Makowski D, Waggoner P, 2020. Performance: assessment of regression models performance version 0.4.4 R package. Available from: https://CRAN.R-project.org/package=performance.
- Llamazares-Martin C, Scopa C, Guillen-Salazar F, Palagi E, 2017. Relaxed open mouth reciprocity favours playful contacts in South American sea lions Otaria flavescens. Behav Process 140:87e95. [DOI] [PubMed] [Google Scholar]
- Maglieri V, Bigozzi F, Riccobono MG, Palagi E, 2020. Levelling playing field: synchronization and rapid facial mimicry in dog-horse play. Behav Process doi: 10.1016/j.beproc.2020.104104. [DOI] [PubMed] [Google Scholar]
- Magnahagen C, 1991. Predation risk as a cost of reproduction. Trends Ecol Evol 6:183–186. [DOI] [PubMed] [Google Scholar]
- Mancini G, Ferrari PF, Palagi E, 2013. In play we trust. Rapid facial mimicry predicts the duration of playful interactions in geladas. PLoS ONE 8:66481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly BFJ, 1991. Randomization and Monte Carlo Methods in Biology. New York (NY): Chapman & Hall. [Google Scholar]
- Michael RP, Zumpe D, 1970. Sexual initiating behaviour by female rhesus monkeys Macaca mulatta under laboratory conditions. Behaviour 36:168–186. [Google Scholar]
- Mills MGL, 1990. Kalahari Hyaenas: The Behavioural Ecology of Two Species. London: Unwin Hyman. [Google Scholar]
- Nakagawa S, Johnson PCD, Schielzeth H, 2017. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface 14:20170213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolfo AP, Casetta G, Palagi E, 2021. Play fighting in wild spotted hyenas: like a bridge over the trouble water of a hierarchical society. Anim Behav (in press) doi.org/10.1016/j.anbehav.2021.07.012 [Google Scholar]
- Palagi E, 2006. Social play in bonobos Pan paniscus and chimpanzees Pan troglodytes: implications for natural social system and inter-individual relationships. Am J Phys Anthropol 129:418–426. [DOI] [PubMed] [Google Scholar]
- Palagi E, 2008. Sharing the motivation to play: the use of signals in adult bonobos. Anim Behav 75:887–896. [Google Scholar]
- Palagi E, Antonacci D, Cordoni G, 2007. Fine-tuning of social play in juvenile lowland gorillas Gorilla gorilla gorilla. Dev Psychobiol 49:433–445. [DOI] [PubMed] [Google Scholar]
- Palagi E, Norscia I, Spada G, 2014. Relaxed open mouth as a playful signal in wild ring-tailed lemurs. Am J Primatol 76:1074– 1083. [DOI] [PubMed] [Google Scholar]
- Palagi E, Nicotra V, Cordoni G, 2015. Rapid mimicry and emotional contagion in domestic dogs. R Soc Open Sci 2:150505. doi: 10.1098/rsos.150505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palagi E, Burghardt GM, Smuts B, Cordoni G, Dall’Olio S, 2016. Rough-and-tumble play as a window on animal communication. Biol Rev 91:311–327. [DOI] [PubMed] [Google Scholar]
- Palagi E, Marchi E, Cavicchio P, Bandoli F, 2019a. Sharing playful mood: rapid facial mimicry in Suricata suricatta. Anim Cogn 22:719–732. [DOI] [PubMed] [Google Scholar]
- Palagi E, Norscia I, Pressi S, Cordoni G, 2019b. Facial mimicry and play: a comparative study in chimpanzees and gorillas. Emotion 19:665–681. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC, 1996. On knowing it’s only play: the role of play signals in play fighting. Aggress Violent Behav 1:249–268. [Google Scholar]
- Pellis SM, Pellis VC, 1997. Targets, tactics and the open mouth face during play fighting in three species of primates. Aggr Behav 23:41–57. [Google Scholar]
- Pellis SM, Pellis VC, 1998. Play fighting of rats in comparative perspective: a schema for neurobehavioral analyses. Neurosci Biobehav Rev 23:87–101. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC, 2011. To whom the play signal is directed: a study of headshaking in black-handed spider monkeys Ateles geoffroyi. J Comp Psychol 125:1–10. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC, 2017. What is play fighting and what is it good for? Learn Behav 45:355–366. [DOI] [PubMed] [Google Scholar]
- Petrů M, Spinka M, Charvátová V, Lhota S, 2009. Revisiting play elements and self-handicapping in play: a comparative ethogram of five Old World monkey species. J Comp Psychol 123:250–263. doi: 10.1037/a0016217 [DOI] [PubMed] [Google Scholar]
- Pollick AS, de Waal FBM, 2007. Ape gestures and language evolution. Proc Natl Acad Sci USA 104:8184–8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole TB, 1978. An analysis of social play in polecats (Mustelidae) with comments on the form and evolutionary history of the open mouth play face. Anim Behav 26:36–49. [Google Scholar]
- Rosenthal GG, 2007. Spatiotemporal dimensions of visual signals in animal communication. Annu Rev Ecol Evol Syst 38:155–178. [Google Scholar]
- Sade DS, 1973. An ethogram for rhesus monkeys. Antithetical contrast in posture and movement. Am J Phys Anthropol 38:537–542. [DOI] [PubMed] [Google Scholar]
- Scopa C, Palagi E, 2016. Mimic me while playing! Social tolerance and rapid facial mimicry in macaques (Macaca tonkeana and Macaca fuscata). J Comp Psychol 130:153–161. [DOI] [PubMed] [Google Scholar]
- Smith JE, Memenis SK, Holekamp KE, 2007. Rank-related partner choice in the fission - fusion society of spotted hyenas Crocuta crocuta. Behav Ecol Sociobiol 61:753–765. [Google Scholar]
- Spoelstra K, van Grunsven RHA, Ramakers JJC, Ferguson KB, Raap T. et al. 2017. Response of bats to light with different spectra: light-shy and agile bat presence is affected by white and green, but not red light. Proc Royal Soc B: Biol Sci 284:20170075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford K, Périquet S, 2019. Dyadic associations reveal clan size and social network structure in the fission - fusion society of spotted hyaenas. Afr J Ecol 58:182–192. [Google Scholar]
- Tacconi G, Palagi E, 2009. Play behavioural tactics under space reduction: social challenges in bonobos Pan paniscus. Anim Behav 78:469–476. [Google Scholar]
- Taylor D, Haltmann D, Dezecache G, Wong ST, Davila-Ross M, 2019. Facial complexity in sun bears: exact facial mimicry and social sensitivity. Sci Rep 9:4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry B, Demaria C, Preuschoft S, Desportes C, 1989. Structural convergence between silent bared-teeth display and relaxed open-mouth display in the Tonkean macaque Macaca tonkeana. Folia Primatol 52:178–184. [DOI] [PubMed] [Google Scholar]
- Tinbergen N, 1952. ‘Derived’ activities: their causation, biological significance, origin, and emancipation during evolution. Q Rev Biol 27:1–32. [DOI] [PubMed] [Google Scholar]
- Tilson RT, Hamilton WJ, 1984. Social dominance and feeding patterns of spotted hyaenas. Anim Behav 32:715–724. [Google Scholar]
- van Hooff J, Preuschoft S, 2003. Laughter and Smiling: The Intertwining of Nature and Culture. Animal Social Complexity: Intelligence, Culture, and Individualized Societies. Cambridge (MA): Harvard University Press. [Google Scholar]
- Vankova D, Bartos L, 2002. The function of mounting behaviour in farmed red deer calves. Ethology 108:473–482. [Google Scholar]
- Vullioud C, Davidian E, Wachter B, Rousset F, Courtiol A. et al. 2019. Social support drives female dominance in the spotted hyaena. Nat Ecol Evol 3:71–76. [DOI] [PubMed] [Google Scholar]
- Wahaj SA, Van Horn RC, Van Horn TL, Dreyer R, Hilgris R. et al. 2004. Kin discrimination in the spotted hyena Crocuta crocuta: nepotism among siblings. Behav Ecol Sociobiol 56:237–247. [Google Scholar]
- Waller BM, Dunbar RIM, 2005. Differential behavioural effects of silent bared-teeth display and relaxed open-mouth display in chimpanzees Pan troglodytes. Ethology 111:129–142. [Google Scholar]
- Waller BM, Cherry L, 2012. Facilitating play through communication: significance of teeth exposure in the gorilla play face. Am J Primatol 74:157–164. [DOI] [PubMed] [Google Scholar]
- Way JG, 2007. Social and play behavior in a wild Eastern coyote Canis latrans pack. Can Field Nat 121:397–401. [Google Scholar]
- Weigel EA, Berman CM, 2018. Body signals used during social play in captive immature western lowland gorillas. Primates 59:253–265. [DOI] [PubMed] [Google Scholar]
- Wiley RH, 2006. Signal detection and animal communication. Adv Study Behav 36:217–247. [Google Scholar]
- Wright KR, Mayhew JA, Sheeran LK, Funkhouser JA, Wagner RS. et al. 2018. Playing it cool: characterizing social play, bout termination, and candidate play signals of juvenile and infant Tibetan macaques Macaca thibetana. Zool Res 39:272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi A, Berman CM, 2014. Body signals during social play in free-living rhesus macaques Macaca mulatta: a systematic analysis. Am J Primatol 76:168–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.