Abstract
Dogs were the first animal to become domesticated by humans, and they represent a classic model system for unraveling the processes of domestication. We compare Australian dingo eye contact and socialization with Basenji and German Shepherd dog (GSD) breeds. Australian dingoes arrived in Australia 5,000–8,000 BP, and there is debate whether they were domesticated before their arrival. The Basenji represents a primitive breed that diverged from the remaining breeds early in the domestication process, while GSDs are a breed dog selected from existing domestic dogs in the late 1800s. We conducted a 4-phase study with unfamiliar and familiar investigators either sitting passively or actively calling each canid. We found 75% of dingoes made eye contact in each phase. In contrast, 86% of Basenjis and 96% of GSDs made eye contact. Dingoes also exhibited shorter eye-gaze duration than breed dogs and did not respond to their name being called actively. Sociability, quantified as a canid coming within 1 m of the experimenter, was lowest for dingoes and highest for GSDs. For sociability duration, dingoes spent less time within 1 m of the experimenter than either breed dog. When compared with previous studies, these data show that the dingo is behaviorally intermediate between wild wolves and Basenji dogs and suggest that it was not domesticated before it arrived in Australia. However, it remains possible that the accumulation of mutations since colonization has obscured historical behaviors, and dingoes now exist in a feralized retamed cycle. Additional morphological and genetic data are required to resolve this conundrum.
Keywords: Basenji, dingo, domestication, German Shepherd dog, eye gaze, sociability
Domestic mammals account for around 60% of mammalian biomass, double the amount of humans, and 15 times wild animals (Bar-On et al. 2018). Nevertheless, much still needs to be understood about the process of domestication. Frantz et al. (2020) review the process from ancient DNA genomes’ perspective and propose that the simplistic view of reproductive control and isolation from wild populations is no longer a valid interpretation of animal domestication. Instead, they suggest that domestication is a highly dynamic, nonlinear, and taxon-specific process. One understudied taxon that has the potential to provide insight into the processes of canid domestication is the Australian dingo. This study aims to experimentally compare eye contact and socialization of sanctuary dingoes with two breed dogs that represent distinct stages within the domestication process. We then contrast these results with those previously collected for tamed wolves. Here, we consider that eye contact and socialization are experimental proxies for domestication.
Charles Darwin (1868) proposed that humans can shape wild animals through the unconscious selection to become tame and then by artificial selection to be domesticated. Wild animals typically refer to animals that live or grow independently of people in natural conditions and with natural characteristics. Darwin purported that wild animals can become tamed to make them human-friendly without any thought to any predetermined purpose. Such tamed animals may have a relationship with humans for diet supplementation but avoid humans while breeding. Tamed animals are not expected to fear humans or respond to active calling but will initiate eye contact and be curious of humans (Darwin 1868; Belyaev 1979; Belyaev et al. 1985; Price 1999; Trut et al. 2009; Sanchez-Villagra et al. 2016; Range et al. 2019).
Darwin (1868) suggested that tamed animals may become domesticated through methodical or what we now call artificial selection. A long-term selection experiment on foxes demonstrated that selection on tame behavior alone was sufficient to drive correlated physiological and morphological changes characteristic of the domestication syndrome (Belyaev 1979; Belyaev et al. 1985; Trut et al. 2009). In domestic dogs, humans have selected some breeds for hunting, whereas others have been selected for guarding and protecting livestock, for example. One genetic prediction is that the suite of mutations associated with domestication will leave a statistical signature on the genome, but there is debate regarding which genes may have been selected (Wang et al. 2013; Frantz et al. 2020).
Domestication is not an evolutionary dead end as animals can return to living in the wild through the process of feralization (Clutton-Brock 1981). Feral canids are predicted to fear humans, avoid eye contact, and respond negatively to any attempt at active engagement (Smuts 2010; Gaynor et al. 2019). Gaynor et al. (2019) defined fear as spatial variation in the perception of risk influenced by, but distinct from, both the physical landscape and the actual risk of mortality. In Australia, humans are the “super-predator” (Darimont et al. 2015), are a driver of wildlife changes, and induce fear in large- and medium-sized carnivores (Suraci et al. 2019).
Feralized animals can revert to being domesticated through the processes of retaming and redomestication (Gering et al. 2019). They can be redomesticated within a generation or retamed and then redomesticated gradually over time (de Lavigne 2015; Gosling et al. 2013; McTavish et al. 2013). As epigenetic and genetic changes are expected to accumulate over time, it is most likely that the effort required to retame and then redomesticate an animal is proportional to the number of generations it has been feral. Gosling et al. (2013) suggested that it is difficult to socialize feral cats to humans after 2 months of age. Longhorn cattle were redomesticated from semiferal ancestors over multiple generations (McTavish et al. 2013). Canaan dogs were derived from feral dogs in Israel through a long-term selective breeding program (de Lavigne 2015).
We assay canid eye contact and sociability with humans in a four-phase study (Jakovcevic et al. 2012; vonHoldt et al. 2017). Canid eye contact and duration have been studied in a variety of scenarios with animals, including the detour task (Pongracz et al. 2005), object choice paradigm (Viranyi et al. 2008), and unsolvable task studies (Miklosi et al. 2003). Domestic dogs are expected to initiate and extend eye contact, be sociable to both unfamiliar and familiar people, and respond positively to active engagement (Gacsi et al. 2004; Miklosi et al. 2007; Udell and Wynne 2008; Udell et al. 2011). In an unresolvable task assay, Maglieri et al. (2019) compared eye-gaze duration for Czechoslovakian Wolfdogs, German Shepherd Dogs (GSD), and Labrador Retrievers with that previously collected from dingoes (Smith and Litchfield 2013). They reported that the amount of time spent gazing at humans by dingoes was comparable to that spent by Czechoslovakian Wolfdogs and less than for GSDs and Labrador Retrievers. Sociability may also differ within a breed or variety depending on their life histories. D’Aniello and Scandurra (2016) studied the behavior of Labrador Retrievers living in a kennel and those living in a house as pets when faced with an unsolvable task. They report that kennel dogs exhibited less gazing toward humans than pet dogs.
Sociability has been defined as the tendency to approach and interact with unfamiliar people (Bentosela et al. 2016). Bentosela et al. coded sociability as a “1” if the canid entered a 1 m semicircle around an investigator at any point in each phase and “0” if it did not. Sociability duration was calculated as the proportion of time each canid spent within the perimeter. Bentosela et al. (2016) examined the behavioral responses of wolves and mixed breed pet dogs in a 4-phase study. The wolves used in the study of Bentosela et al. (2016) were described as genetically wild and “human socialized (tame)” interacting with humans daily. They report that wolves spent a significantly shorter time in proximity to experimenters than did pet dogs and less time in the proximity of an unfamiliar experimenter than a familiar individual.
We compare behaviors of human socialized sanctuary-dwelling dingoes with two domestic dog breeds that were kenneled. We then compare our data with that previously published for wolves (Nagasawa et al. 2015; Bentosela et al. 2016; Lazzaroni et al. 2020). Johnston et al. (2017) assayed eye contact of dingoes and compared their results with those published for humanized wolves and pet dogs (Nagasawa et al. 2015). They reported that dingoes established eye contact more than wolves across two different studies with two different human handlers but held it for shorter times than dogs. The simplest explanation for this result is that dingoes were never genuinely domesticated and now exist in a wild-tamed cycle (Crowther et al. 2014; Jackson et al. 2017; Ballard and Wilson 2019; Smith et al. 2019). However, dingo ancestors may have been tamed and domesticated in South East (SE) Asia before they arrived in Australia (Ballard and Wilson 2019; Zhang et al. 2020). It is unlikely that dingoes were domesticated in Australia. Dingoes have played an essential role in the mobility and protection of Aboriginal women, and pups were frequently taken from the wild when they were very young (Philip 2017). The pups are a highly valued ritual food source, whereas some others are adopted into human society. The selected pups grow up in the company of women and children, providing an effective hunting aid, a living blanket, and guarding against intruders (Philip 2017). Puppies showing potential hunting ability are not selected as preferred breeding animals (White 1972), indicating no artificial selection has taken place (Smith and Litchfield 2009).
We include two breed dogs that represent distinct stages in the domestication process. Basenji dogs are an example of an ancient breed and are proposed to represent an early domestication stage (Parker et al. 2017; vonHoldt et al. 2010; Wheat et al. 2019). Basenjis are indigenous to the woodland savannah and rain forest areas of the Democratic Republic of Congo, with no other breed dogs in this region. Two of the 17 Basenjis studied here were born in the Democratic Republic of Congo and thus represent true African dogs. GSDs are a modern breed developed from livestock dogs in the 19th century (Parker et al. 2017; Field et al. 2020). They are not morphologically specialized and have been selected for intelligence and independence (Parker et al. 2017; Field et al. 2020).
We observed that dingoes exhibited shorter eye-gaze duration than Basenjis and GSDs and did not respond to their name being called actively. Furthermore, sociability was lowest for dingoes and highest for GSDs. When compared with previous studies, the combined evidence indicates that dingoes are behaviorally intermediate between socialized wolves and Basenjis (Nagasawa et al., 2015; Johnston et al., 2017). We suggest that it is most likely that dingoes were never domesticated, and living animals exist in a wild-tamed cycle. However, genetic mutations that have accumulated over the last 5,000–8,000 years have the potential to cloud predictions derived from extant organisms. Thus, we cannot reject the hypothesis that dingoes were domesticated in SE Asia and now exist in a feral-retamed cycle. Additional behavioral, genomic, and morphological data are required.
Materials and Methods
Animals
Seventeen dingoes were tested in December 2018 (Table 1). Of these, 14 were from Bargo Dingo Sanctuary (7 males and 7 females) and 3 were from Pure Dingo Sanctuary (1 male and 2 females). Both sanctuaries are in SE New South Wales (NSW), Australia. All dingoes were pure as determined by microsatellite testing (Wilton et al. 1999; Wilton 2001). No consistent differences were observed between wild-born and sanctuary-born dingoes, and they were pooled for analyses. One male was excluded from all analyses as his partner suffered a broken leg before the study, potentially modifying his behavior. The dingoes were housed in mated pairs and not kept as pets. Volunteers fed and walked the dingoes daily, but they rarely traveled from the sanctuary. The mean (SE) age was 3.8 ± 0.44 years.
Table 1.
Canids included in the study
| Canid | Location | Sex | Age | Local ID | Weight (kg) | |
|---|---|---|---|---|---|---|
| Dingo | Bargo | M | 8 | X3170 | 22 | |
| M | 4 | W0235 | 22 | |||
| M | 4 | W0380a | 20 | |||
| M | 3 | W0303 | 22 | |||
| M | 3 | X1039 | 22 | |||
| M | 3 | X3172 | 22 | |||
| M | 1 | W0363 | 17 | |||
| F | 8 | X3171 | 18 | |||
| F | 4 | W0381 | 18 | |||
| F | 4 | W0234 | 18 | |||
| F | 3 | W0379 | 18 | |||
| F | 3 | W0378 | 18 | |||
| F | 3 | W0383 | 18 | |||
| F | 1.5 | W0382 | 18 | |||
| Pure dingo | M | 4 | W0329 | 20 | ||
| F | 4 | W0330 | 16 | |||
| F | 4 | W0296 | 18 | |||
| GSD | Kingsvale | M | 5 | GSD16 | 43 | |
| M | 5 | GSD14 | 40 | |||
| M | 2 | GSD11 | 34 | |||
| F | 5.4 | GSD10 | 33 | |||
| F | 5.1 | GSD09 | 32 | |||
| F | 3.8 | GSD15 | 35 | |||
| F | 3.1 | GSD08 | 32 | |||
| F | 1.8 | GSD122 | 30 | |||
| F | 1.3 | GSD07 | 33 | |||
| F | 1.3 | GSD06 | 32 | |||
| F | 1.1 | GSD13 | 28 | |||
| Allendale | M | 5 | GSD03 | 38 | ||
| F | 6 | GSD02 | 34 | |||
| F | 5 | GSD04 | 34 | |||
| F | 4 | GSD05b | 34 | |||
| Basenji | Wuliango | M | 9 | BAS31c | 10 | |
| M | 6.8 | BAS07 | 10 | |||
| M | 1.6 | BAS32 | 10 | |||
| F | 2 | BAS03 | 9 | |||
| F | 2 | BAS02 | 9 | |||
| F | 8.9 | BAS05 | 9 | |||
| F | 3.4 | BAS04 | 9 | |||
| F | 2.5 | BAS06 | 9 | |||
| Zanzipow | M | 5.7 | BAS26 | 9 | ||
| M | 6.7 | BAS22 | 10 | |||
| M | 4 | BAS24 | 10 | |||
| M | 1 | BAS28 | 8 | |||
| F | 10 | BAS27 | 10 | |||
| F | 10 | BAS25 | 10 | |||
| F | 4 | BAS01 | 9 | |||
| F | 4 | BAS30 | 9 | |||
| F | 2.6 | BAS29 | 9 | |||
Partner injured so excluded.
In estrus.
Neutered male.
Seventeen Basenjis were assayed in January 2019 (Table 1). Of these, 8 were from Wuliango Kennels (3 males and 5 females), and 9 were from Zanzipow Kennels (4 males and 5 females). Wuliango Kennels had 2 Basenjis located in Townsville and 6 in Brisbane, Queensland, Australia, whereas Zanzipow Kennels is in SE NSW, Australia. The 2 Basenjis from Townsville were imported from Congo, Africa. Furthermore, 2 additional Basenjis from this kennel were F1’s of individuals from the Congo. These 4 did not show any unusual or distinctive behaviors from the remainder, which were registered with the Australian Kennel Club. One male from Zanzipow kennel was excluded from the study as he was neutered. The Basenjis are kept in kennel packs with frequent interactions between all dogs and their owners. They frequently traveled with their owners and were 4.95 ± 0.74 (SE) years of age.
Fifteen GSDs were tested in December 2018 (Table 1). Eleven were from Kingvale Kennels (3 males and 8 females) and 4 were from Allendell Kennels (1 male and 3 females). Both of these kennels are in SE NSW. Two female GSDs were subsequently excluded from the study as they came into estrus within 10 days of the study. All GSDs were registered with the Australian Kennel Club. GSDs were kept in large runs with fewer males than females. All of the GSDs were socialized and used to traveling distances in cars and trailers. The mean age was 3.66 ± 0.44 years.
All canids had been around humans from a young age and were used to being on a lead. Furthermore, every effort was made to ensure the sanctuaries and kennels were as similar as possible, but the canids were not kenneled in the same manner. As such, at least some of the variation observed between canids is likely to be due to their kenneling.
All experiments reported in this article were performed under the Association for the Study of Animal Behaviour and the Animal Behavior Society guidelines and were approved by the Institutional Animal Care and Use Committee of the University of New South Wales (18/148B for dingoes and German Shepherds and 19/171B for Basenjis).
Experimental design
A black iron mesh transportable run measuring 4 m long × 2 m wide × 2 m high was transported and built within each sanctuary and kennel (Figure 1). The same folding chair was placed at the back end of the run, and a 1 m semicircle was drawn around it. Cameras were positioned on the chest and head of the person entering the run. Tripods were also placed at the front (behind the gate), back, and on either side of the run (Figure 1). Video cameras then recorded each canid during the 4 phases of the study, each lasting for 2 min (Jakovcevic et al. 2012; Bentosela et al. 2016; vonHoldt et al. 2017).
Figure 1.
Experimental design showing camera positions at Pure Dingo Sanctuary. (A) Back camera without canid. (B) Front camera without canid (door open). (C) Back camera with dingo outside semicircle. (D) Front camera with dingo outside semicircle. (E) Head camera showing dingo eye gaze. (F) Chest camera with dingo eye gaze. Pictures have not been altered. Slight variances in color reflect lighting/camera-specific differences.
The 2-phase experimental design followed vonHoldt et al. (2017). Recordings from cameras’ recordings were analyzed for canid–human eye contact and time within a 1 m semicircle around the experimenter. Inclusion of multiple cameras enabled precise determination of eye contact, eye-contact duration, and time within the semicircle. Briefly, all canids were walked on a lead by their handler/owner to a transportable run and then released. The order of canids at each dingo sanctuary and each kennel was random. For each canid, the procedure was as follows:
Phase 1 (duration 2 min): An unfamiliar person entered the enclosure, sat on the chair, and looked at the floor.
Phase 2 (duration 2 min): The unfamiliar person repeatedly called the canid by name in an attempt to encourage it to approach, trying to keep it the circle without restraint. The unfamiliar person then left the run.
Phase 3 (duration 2 min): The animal’s usual (familiar) handler/owner entered and sat on the chair, looking at the floor.
Phase 4 (duration 2 min): The usual handler/repeatedly called the canid by name in an attempt to encourage it to approach, trying to keep it the circle without restraint. The familiar person then left the run.
J.W.O.B. was the unfamiliar person in all Phases 1 and 2 studies. He wore a blue dust-overall (Figure 1) and changed clothes between locations so canids would not be distracted by smells from another location, which may affect behavior. The most familiar person, their handler or owner, entered the run for Phases 3 and 4, so the level of familiarity was as constant as possible. The same instructions were given to all human participants; however, differences in behavior between humans remained. These included the frequency of calling the canids’ names and the amplitude of vocalizations. Collaborators and timers were out of sight of the canid being tested and instructed to keep quiet, not to cause a distraction.
The experimental run was isolated from all other canids, but differences occurred within and between sites. If a canid urinated or defecated in the cage during the study, it was cleaned and allowed to air dry before the next one entered the cage. The ambient temperature for studies was in the range of 20–30°C at all sites except at Zanzipow Kennels, where it was above 35°C during 4 assays. There was, however, a cool breeze, and the behaviors of these Basenjis did not appear compromised.
Coding
Videos from all 6 cameras were downloaded to a computer for analysis. The starting and finishing times of each phase were recorded onto an Excel spreadsheet. The videos from around the run were edited into a single channel to code eye-contact and eye-gaze duration. The editing was conducted to show the best angle of view, thereby enabling accurate assessment of eye gaze and body position. Eye contact was characterized to have occurred if an animal’s nose angled up toward the handler’s face (Johnston et al. 2017). It was coded as “0” if it did not make eye contact in each phase or “1” if it was observed. Eye-contact duration in each phase was then independently scored in seconds.
Raw videos were used to determine the time within the 1 m semicircle. Most generally, we used the single video behind the experimenter; however, we used a second angle to corroborate time in the semicircle if there was any obstruction. Sociability contact was coded as “0” if it did not occur or a “1” if the canid entered the semicircle. Sociability duration, determined as the proportion of the duration of the phase each canid spent within the perimeter, was then independently calculated.
Interobserver reliability was performed between three observers, each coding at least 20% of the data. For the categorical data of eye contact and sociability Cohen’s Kappa was equal to or greater than 0.94. For the continuous variables of eye-gaze duration and time in the 1 m semicircle, the intraclass correlation coefficients were equal to or greater than 0.98. As a consequence, for each phase, results are an average of all coders.
Statistical analyses
Statistical analyses were completed in JMP 15 (SAS Institute, Cary, NC, USA). Logistic regression assessed eye contact and sociability contact as the dependent variable was binary. We followed the logistic regression with an odds ratio test on the significant parameters. The test provided pairwise odds ratios and probabilities >χ2. These probabilities were used to construct a compact letter display to indicate significance. A mixed-model Analysis of variance (ANOVA) assessed eye-gaze duration and sociability duration analyses. We considered the mixed-model ANOVA appropriate. It is relatively robust to the normality assumption (Kirk 1995) and enabled post hoc testing without repeated analyses of the same data. In both analyses, factors were canid variety (dingo, Basenji, and GSD), the experimenter (unfamiliar and familiar), and activity (passive and active). All interactions were included, and an alpha level of 0.05 was adopted. Preliminary analyses showed that age and sex did not affect any parameter tested. For visual clarity, nonsignificant effects were collapsed, and graphs were plotted in Prism 8 (GraphPad, San Diego, CA, USA).
Results
Eye-contact and eye-gaze duration
Eye contact was characterized to have occurred if an animal’s nose was observed to have angled up toward the handler’s face at any point in each phase. Dingoes made less eye contact than the domestic dog breeds (Figure 2). For dingoes, 15/16 made eye contact in phase 1, 13/16 in phase 2, 11/16 in phase 3, and 13/16 in phase 4. For Basenjis, 16/16 made eye contact in phase 1, 16/16 in phase 2, 14/16 in phase 3, and 14/16 in phase 4. For GSDs, 11/13 made eye contact in phase 1, 13/13 in phase 2, 12/13 in phase 3, and 13/13 in phase 4.
Figure 2.
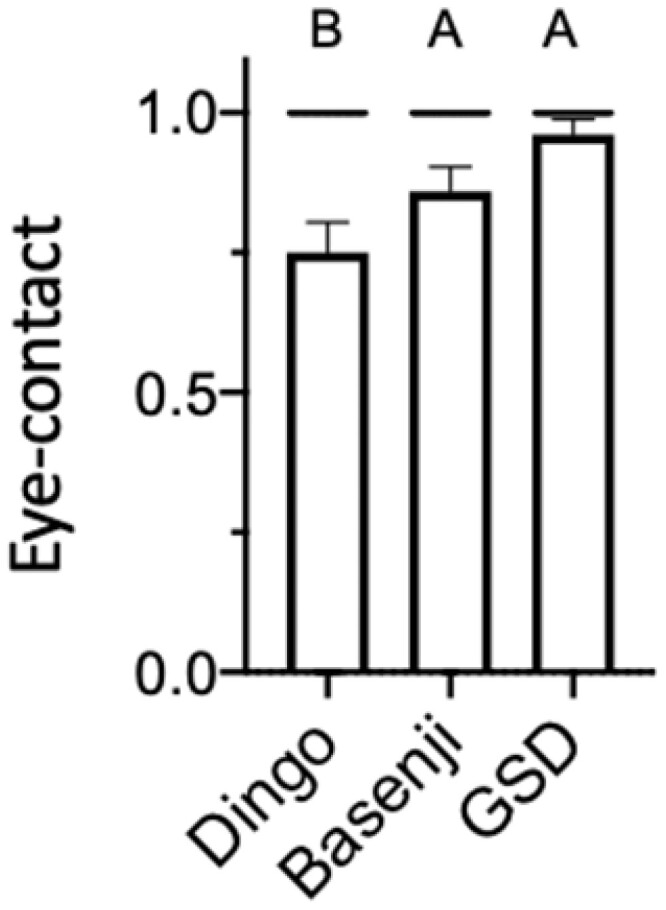
Proportion of dingoes, Basenji dogs, and GSDs making eye contact. Logistic regression shows canid had a significant effect on sociability (see text for details). Nonsignificant effects were collapsed (see text for details). Mean ± SE with all data points shown (either 0 or 1). Bars not connected by the same letter differ significantly according to odds ratio testing.
Logistic regression shows that canid variety had a significant effect on whether an animal made eye contact with the experimenter (χ22 = 8.86, P = 0.01). There were no significant effects of the experimenter (χ21 = 3.1e−6, P = 1.0), activity (χ21 = 9.67, P = 1.0), canid × experimenter (χ22 = 2.26, P = 0.32), canid × activity (χ22 = 3.90 P = 0.14), experimenter × activity (χ21 = 5.83e−8, P = 0.99), or the three-way interaction (χ22 = 1.07e−6, P = 1.00).
The eye-gaze duration was determined as the proportion of time that each canid spent in direct visual contact with the experimenter. Dingoes exhibited the shortest eye-gaze duration and did not respond strongly to their name being called actively. Basenjis held eye gaze for a shorter duration than GSDs, but both increased in duration when their names were called. Duration increased by about 8% for Basenjis and 11% for GSDs (Figure 3A). Eye-gaze duration toward the unfamiliar and familiar investigator was similar for dingoes and Basenjis (2% and 7%, respectively). However, for GSDs, it increased from 8% for the unfamiliar person to 12.5% for the familiar person (Figure 3B). In phase 1, mean eye-gaze duration of dingoes was 2.25 ± 0.58 s; in phase 2, it was 3.11 ± 0.78 s; in phase 3, it was 0.89 ± 0.35 s; and in phase 4, it was 1.96 ± 0.58 s. For Basenjis, mean eye-gaze duration was 4.2 ± 1.25 s in phase 1, 11.32 ± 2.17 s in phase 2, 2.67 ± 0.63 s in phase 3, and 11.17 ± 2.63 s in phase 4, For GSDs, mean eye-gaze duration was 2.5 ± 1.08 s in phase 1, 13.33 ± 3.18 s in phase 2, 7.62 ± 2.03 s in phase 3, and 17.31 ± 2.54 s in phase 4.
Figure 3.
Eye-gaze duration of dingoes, Basenji dogs, and GSDs in seconds. ANOVA shows canid, activity, canid × activity, and canid × experimenter had significant effects on eye-gaze duration. Nonsignificant effects were collapsed (see text for details). (A) When the experimenter was passive and when they actively called the animal. (B) When the experimenter was unfamiliar and when they were familiar. Mean ± SE with all data points shown. Bars not connected by the same letter differ significantly according to post hoc Student’s t-tests.
ANOVA shows canid variety (F2,168 = 23.97, P < 0.0001), activity (F1,168 = 42.71, P < 0.0001), canid × activity (F2,168 = 8.25, P = 0.0004), and canid × experimenter (F2,168 = 3.47, P = 0.03) had significant effects on eye-gaze duration. Experimenter (F1,168 = 0.74, P = 0.39), experimenter × activity (F1,168 = 0.008, P = 0.93), and the three-way interaction (F2,168 = 0.15, P = 0.86) did not significantly influence duration of eye gaze.
Sociability
Sociability was initially determined by calculating the proportion of canids coming within 1 m of the experimenter. Overall, dingoes spent less time close to the unfamiliar investigators than Basenjis or GSD’s and all canids spent a higher proportion of time close to the familiar person (Figure 4). For dingoes, 8/16 came within 1 m of the experimenter in phase 1, 9/16 in phase 2, 15/16 in phase 3, and 15/16 in phase 4. For Basenjis, 12/16 entered the semicircle in phase 1, 11/16 in phase 2, 14/16 in phase 3, and 14/16 in phase 4. For GSDs, 12/13 were sociable in phase 1, 12/13 in phase 2, 13/13 in phase 3, and 13/13 in phase 4.
Figure 4.

The sociability of dingoes, Basenji dogs, and GSDs determined by venturing within 1 m of the experimenter. Logistic regression shows canid and experimenter had significant effects on sociability. Nonsignificant effects were collapsed (see text for details). Mean ± SE with all data points shown (either 0 or 1). Bars not connected by the same letter differ significantly according to odds ratio testing.
Logistic regression shows canid variety and experimenter had significant effects on whether an animal came within 1 m of the experimenter (χ22 = 8.72, P = 0.01 and χ21 = 18.97, P < 0.0001, respectively). There were no significant effects of activity (χ21 = 1.5e−10, P = 1.0), canid × experimenter (χ22 = 1.62, P = 0.44), canid × activity (χ22 = 3.6e−8 P = 1.0), experimenter × activity (χ21 = 1.54e−10, P = 1.0), or the three-way interaction (χ22 = 3.67e−8, P = 1.00).
For sociability duration, the canid, experimenter, and calling activity influenced the time animals spent within 1 m of the investigator (Figure 5). Overall dingoes spent less time within 1 m of the experimenter than Basenjis or GSDs (27, 59, and 46%, respectively). Canids spent less time within 1 m of the unfamiliar than the familiar investigator (28 and 60%, respectively) and less time close to the experimenter during the passive phases than active phases (31 and 52%, respectively). Dingoes spent 0.11 ± 0.04 proportion of phase 1 within the semicircle, 0.1 ± 0.07 proportion of phase 2, 0.39 ± 0.07 proportion of phase 3, and 0.49 ± 0.07 proportion of phase 4. Basenjis spent 0.29 ± 0.09 proportion of phase 1 within 1 m of the investigator, 0.1 ± 0.11 proportion of phase 2, 0.70 ± 0.07 s proportion of phase 3, and 0.88 ± 0.047 s proportion of phase 4. GSDs spent 0.26 ± 0.05 proportion of phase 1 within the semicircle, 0.47 ± 0.09 proportion of phase 2, 0.45 ± 0.1 s proportion of phase 3, and 0.69 ± 0.07 proportion of phase 4.
Figure 5.

The sociability of dingoes, Basenji dogs, and GSDs determined as the proportion of time canids spent within 1 m of an investigator. ANOVA shows main effects of canid, experimenter, and activity with no significant interactions (see text for details). Mean ± SE with all data points shown. Bars not connected by the same letter differ significantly according to post hoc Student’s t-tests.
The main effects of canid (F2,168 = 22.24, P < 0.001), experimenter (F1,168 = 56.97, P < 0.0001), and activity (F1,168 = 14.29, P < 0.0002) influenced the amount of time spent within the 1 m semicircle. There were no significant effects of canid × activity (F2,168 = 1.88, P = 0.16), canid × experimenter (F2,168 = 1.74, P = 0.18), experimenter × activity (F2,168 = 0.18, P = 0.67), and the three-way interaction (F2,168 = 0.36, P = 0.70). Consistent with the eye-gaze duration assay, a post hoc t-test comparing dingoes in the passive and active phases shows that actively calling them did not increase the time spent in the circle (t62 = 0.63, P = 0.53).
Discussion
Domestication of the Grey wolf to yield the domestic dog is arguably one of the best characterized evolutionary processes in evolutionary biology (vonHoldt et al. 2010). Nevertheless, there are multiple gaps in our understanding. One understudied taxon that has the potential to provide insight into the process is the Australian dingo. Here, we experimentally compare eye contact and socialization of sanctuary dingoes with kennel Basenji dogs and kennel GSDs that represent different stages of the domestication process. Basenjis are a primitive breed that represents an early stage in the domestication process. In contrast, GSDs have been strongly selected by humans over the past 150 years. Eye contact and sociability data show that Australian dingoes do not exhibit behaviors typical of a feral dog nor are they intermediate in behavior between the breed dogs. In combination, these results suggest that dingoes were never domesticated. Nevertheless, it remains possible that mutations have accumulated since dingoes colonized Australia and obscure predicted feralization behaviors.
Eye contact data support the hypothesis that sanctuary dingoes are intermediate between wolves and domestic dogs. In the 2 min phase, when the familiar investigator actively called the canid (phase 4), we observed ∼81% dingoes, 87.5% of Basenjis, and 100% of GSDs made eye contact with their usual handler. Phase 4 of our study is similar to the protocols previously used to study eye contact in various canids where they employed a 5-min testing window (Johnston et al. 2017; Nagasawa et al. 2015). Johnston et al. (2017) recorded 96% of sanctuary dingoes made eye contact when the dingo was on a leash and 91% when it was off-leash (their experiments 1A and B). Nagasawa (2015) observed 55% of wolves and 100% of pet dogs made eye contact. The observation that dingoes initiated eye contact at a level intermediate between wolves and domestic dogs prompted Johnston et al. (2017) to suggest that the motivation to initiate eye contact in canids evolved early in the domestication process.
Combining our data with previously published results, we observe that eye-gaze duration is lowest for wolves, intermediate for dingoes, and increases for kennel dogs and pet dogs. Here, phase 4 of our study, when a familiar person actively calls to the canid, is similar to previous studies, thereby provides an informed comparison (Johnston et al. 2017; Nagasawa et al. 2015). There is, however, a difference in the interaction period, so we standardize studies to duration/min to enable comparison. In human socialized wolves, eye-gaze duration was 0.09 s/min (Nagasawa et al. 2015). In dingoes, we observed that eye-median gaze duration is 0.9 s/min, whereas Johnston et al. (2017) reported that it is 0.54 s/min. In kennel dogs, we observed median eye contact duration of 3.8 s/min in Basenjis, and 8.23 s in GSDs. For pet dogs, median eye contact time was 8 s (Nagasawa et al. 2015; Johnston et al. 2017).
Eye-contact data provide initial evidence to suggest that dingoes have never been domesticated and exist in a wild-tamed cycle in Australia. They did not avoid human eye contact, and eye gaze did not change when their name was called. A significant decrease in eye contact was not observed and is predicted if dingoes existed in a recently feralized-retamed cycle (Smuts 2010; Gaynor et al. 2019). In contrast, Basenjis and GSDs had greater eye contact and eye gaze and responded when their name was called. Minimally, this indicates that dingoes are less responsive to social cues than domestic dogs and this suggests they are more independent than breed dogs who expect a reward for their attentive behavior (Gacsi et al. 2004).
Sociability and sociability duration assays also show that sanctuary dingoes are intermediate between human-socialized wolves and domestic dogs. We observe sanctuary dingoes spent significantly less time in the proximity of experimenters than breed dogs (all phases, 27% dingo, 60% Basenji, and 47% GSD). Further dingoes spent 10% of their time close to an unfamiliar experimenter and 44% within the proximity of a familiar individual. Bentosela et al. (2016) conducted a similar experiment with genetically wild and human-socialized wolves and a broad set of domestic dogs, but their conditions differed slightly. Wolves were tested in familiar outdoor enclosures, whereas dogs were tested in the part of their home that they felt most comfortable as such slight differences are expected. Bentosela et al. (2016) report wolves spent significantly less time in the proximity of experimenters than did pet dogs (25 and 70%, respectively). They also spent less time close to an unfamiliar experimenter than a familiar individual (31 and 64%, respectively). Showing the same trend, Lazzaroni et al. (2020) found that human-socialized wolves seemed to be less attracted to familiar humans than similarly raised dogs, highlighting the critical role of domestication in affecting dogs’ social behaviors toward humans.
There are notable strengths and several limitations of the ethology study. The same cage was built in each sanctuary, and animals entered the cage without distress. No canids were under 18 months and were all within an age range of 8 years, so results are unlikely to be affected by age-related differences. All canids had been around humans from a young age, and no individual family pets were included. However, there were differences in the canids’ kenneling, and the familiar person differed slightly in their behaviors increasing variation within sites. A limitation of the study is that we did not include wolves. Therefore, it remains possible that the differences we observed may be accounted by methodological differences.
These data indicate that the behavior of dingoes is functionally intermediate between wolves and breed dogs. Ockham’s razor suggests that dingoes were never domesticated and now exist in a wild-tamed cycle (Crowther et al. 2014; Jackson et al. 2017; Ballard and Wilson 2019; Smith et al. 2019). Our data support this hypothesis as dingoes did not avoid eye contact when they were actively called, which is predicted for feralized and retamed canids (Smuts 2010; Gaynor et al. 2019). However, it is not clear that predictions derived from recently feralized animals’ events will apply to those feralized centuries ago. Thus, it remains possible that dingo ancestors were tamed and domesticated in SE Asia before they arrived in Australia, and they now exist in a feral-retamed cycle (Ballard and Wilson 2019; Zhang et al. 2020). Additional studies with brain imaging, the examination of DNA methylation patterns, expression profiling, and genomic tests of allele frequencies data are required to test the hypothesis that dingoes were never domesticated and will facilitate additional impetrations of behavioral data (Scott and Fuller 1965; Klinghammer and Goodman 1987; Trut 1999; Persson et al. 2017; Smith et al. 2018; Ballard and Wilson 2019; Zhang et al. 2020).
Supplementary Material
Acknowledgments
Bargo Dingo Sanctuary volunteers and Barry and Lyn Eggleton from Pure Dingo Sanctuary made dingoes available. GSDs were made available by Ivan and Sonya Pacek and by Rhonda Rhalps. Jennifer Power and Ethel Blair gave us access to the Basenjis. Bridget vonHoldt advised on the Socialization studies design and Angie Johnston commented on the manuscript. Lynne Gardner, Teagan Levin, Stephanie Summersby, and Christel MacDonald helped with the execution of the studies. Peter Geelan-Small advised on the statistics. Two reviewers made comments that improved the manuscript.
Funding
The study was supported by Australian Research Council Discover Project 150102038.
Author contributions
J.W.O.B. designed the study. J.W.O.B., C.G., and L.E. collected the data. J.W.O.B. and R.I.K. conducted the analysis. S.Y. conducted additional analyses. J.W.O.B. wrote the manuscript. All authors read and approved the final version of the manuscript.
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
Ethics
Ethics has been approved by the Research Ethics and Compliance Support at UNSW (18/148B, dingoes and GSDs and 19/171B for Basenjis).
Conflict of Interest
The authors declare no competing or financial interests.
Contributor Information
J William O Ballard, Department of Ecology, Environment, and Evolution, Latrobe University, Melbourne, VIC 3086, Australia; Department of BioSciences, The University of Melbourne, Melbourne, VIC 3010, Australia.
Chloe Gardner, School of Life and Environmental Sciences, University of Sydney, Camperdown, NSW 2006, Australia.
Lucille Ellem, Bargo Dingo Sanctuary, Bargo, NSW 2574, Australia.
Sonu Yadav, School of Biotechnology and Biomolecular Sciences, UNSW Sydney, Sydney, NSW 2052, Australia.
Richard I Kemp, School of Psychology, UNSW Sydney, Sydney, NSW 2052, Australia.
References
- Ballard JWO, Wilson LAB, 2019. The australin dingo: untamed or feral? Front Zool 16:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On YM, Phillips R, Milo R, 2018. The biomass distribution on earth. Proc Natl Acad Sci U S A 115:6506–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaev DK, 1979. Destabilizing selection as a factor in domestication. J Hered 70:301–308. [DOI] [PubMed] [Google Scholar]
- Belyaev DK, Plyusnina IZ, Trut LN, 1985. Domestication in the silver fox Vulpes fulvus desm: changes in physiological boundaries of the sensitive period of primary socialization. Appl Anim Behav Sci 13:359–370. [Google Scholar]
- Bentosela M, Wynne CD, D'Orazio M, Elgier A, Udell MA, 2016. Sociability and gazing toward humans in dogs and wolves: simple behaviors with broad implications. J Exp Anal Behav 105:68–75. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock J, 1981. Domesticated Animals from Early Times. Austin: University of Texas Press. [Google Scholar]
- Crowther MS, Fillios M, Colman N, Letnic M, 2014. An updated description of the Australian dingo (Canis dingo Meyer, 1793). J Zool 293:192–203. [Google Scholar]
- D’Aniello A, Scandurra A, 2016. Ontogenetic effects on gazing behaviour: a case study of kennel dogs (Labrador Retrievers) in the impossible task paradigm. Anim Cogn 19:565–570. [DOI] [PubMed] [Google Scholar]
- Darimont CT, Fox CH, Bryan HM, Reimchen TE, 2015. Human impacts: the unique ecology of human predators. Science 349:858–860. [DOI] [PubMed] [Google Scholar]
- Darwin C, 1868. The Variation of Animals and Plants under Domestication. London: John Murray. [Google Scholar]
- de Lavigne G, 2015. Free Ranging Dogs: Stray, Feral or Wild? San Francisco, CA: Lulu Press, Inc. [Google Scholar]
- Field MA, Rosen BD, Dudchenko O, Chan EKF, Minoche AE. et al. , 2020. Canfam_GSD: de novo chromosome - length genome assembly of the German Shepherd dog Canis lupus familiaris using a combination of long reads, optical mapping and Hi–C. GiGaScience 9: giaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz LAF, Bradley DG, Larson G, Orlando L. 2020. Animal domestication in the era of ancient genomics. Nat Rev Genet 21:449–460. [DOI] [PubMed] [Google Scholar]
- Gacsi M, Miklosi A, Varga O, Topal J, Csanyi V, 2004. Are readers of our face readers of our minds? Dogs Canis familiaris show situation-dependent recognition of human's attention. Anim Cogn 7:144–153. [DOI] [PubMed] [Google Scholar]
- Gaynor KM, Brown JS, Middleton AD, Power ME, Brashares JS, 2019. Landscapes of fear: spatial patterns of risk perception and response. Trends Ecol Evol 34:355–368. [DOI] [PubMed] [Google Scholar]
- Gering E, Incorvaia D, Henriksen R, Conner J, Getty T. et al. , 2019. Getting back to nature: feralization in animals and plants. Trends Ecol Evol 34:1137–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling L, Stavisky J, Dean R, 2013. What is a feral cat? Variation in definitions may be associated with different management strategies. J Feline Med Surg 19:759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SM, Groves CP, Fleming PJS, Aplin KP, Eldridge MDB. et al. , 2017. The wayward dog: is the Australian native dog or dingo a distinct species? Zootaxa 4317:201–224. [Google Scholar]
- Jakovcevic A, Mustaca A, Bentosela M, 2012. Do more sociable dogs gaze longer to the human face than less sociable ones? Behav Process 90:217–222. [DOI] [PubMed] [Google Scholar]
- Johnston AM, Turrin C, Watson L, Arre AM, Santos LR, 2017. Uncovering the origins of dog - human eye contact: dingoes establish eye contact more than wolves, but less than dogs. An Behav 133:123–129. [Google Scholar]
- Kirk R, 1995. Experimental Design: Procedures for the Behavioral Sciences. Pacific Grove: Brooks/Cole. [Google Scholar]
- Klinghammer E, Goodman P, 1987. Socialization and management of wolves in captivity. In: Frank E editor. Man and Wolf: Advances, Issues and Problems in Captive Wolf Research. Amsterdam: Springer. [Google Scholar]
- Lazzaroni M, Range F, Backes J, Portele K, Scheck K. et al. , 2020. The effect of domestication and experience on the social interaction of dogs and wolves with a human companion. Front Psychol 11:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglieri V, Prato-Previde E, Tommasi E, Palagi E, 2019. Wolf-like or dog-like? A comparison of gazing behaviour across three dog breeds tested in their familiar environments. R Soc Open Sci 6:190946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTavish EJ, Decker JE, Schnabel RD, Taylor JF, Hillis DM, 2013. New World cattle show ancestry from multiple independent domestication events. Proc Natl Acad Sci USA 110:E1398–E1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklosi A, Kubinyi E, Topal J, Gacsi M, Viranyi Z. et al. , 2003. A simple reason for a big difference: wolves do not look back at humans, but dogs do. Curr Biol 13:763–766. [DOI] [PubMed] [Google Scholar]
- Miklosi A, Topal J, Csanyi V, 2007. Big thoughts in small brains? Dogs as a model for understanding human social cognition. Neuroreport 18:467–471. [DOI] [PubMed] [Google Scholar]
- Nagasawa M, Mitsui S, En S, Ohtani N, Ohta M. et al. , 2015. Oxytocin-gaze positive loop and the coevolution of human - dog bonds. Science 348:333–336. [DOI] [PubMed] [Google Scholar]
- Parker HG, Dreger DL, Rimbault M, Davis BW, Mullen AB. et al. , 2017. Genomic analyses reveal the influence of geographic origin, migration, and hybridization on modern dog breed development. Cell Rep 19:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson ME, Trottier AJ, Belteky J, Roth LSV, Jensen P, 2017. Intranasal oxytocin and a polymorphism in the oxytocin receptor gene are associated with human-directed social behavior in golden retriever dogs. Horm Behav 95:85–93. [DOI] [PubMed] [Google Scholar]
- Philip J, 2017. Living blanket, water diviner, wild pet: a cultural history of the dingo. The conversation [August 7]. Available from: https://theconversation.com/living-blanket-water-diviner-wild-pet-a-cultural-history-of-the-dingo-80189. 1 November 2020.
- Pongracz M, Miklosi A, Vida V, Csanyi V, 2005. The pet dogs ability for learning from a human demonstrator in a detour task is independent from the breed and age. Appl Anim Behav Sci 90:309–323. [Google Scholar]
- Price EO, 1999. Behavioral development in animals undergoing domestication. Appl Anim Behav Sci 65:245–271. [Google Scholar]
- Range F, Marshall-Pescini S, Kratz C, Viranyi Z, 2019. Wolves lead and dogs follow, but they both cooperate with humans. Sci Rep 9:3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Villagra MR, Geiger M, Schneider RA, 2016. The taming of the neural crest: a developmental perspective on the origins of morphological covariation in domesticated mammals. R Soc Open Sci 3:160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JP, Fuller JL, 1965. Genetics and the Social Behavior of the Dog. 1st edn. Chicago, IL: University of Chicago Press. [Google Scholar]
- Smith BP, Cairns KM, Adams JW, Newsome TM, Fillios M. et al. , 2019. Taxonomic status of the Australian dingo: the case for Canis dingo Meyer, 1793. Zootaxa 4564:173–197. [DOI] [PubMed] [Google Scholar]
- Smith BP, Litchfield CA, 2009. A review of the relationship between indigenous Australians, dingoes Canis dingo and domestic dogs Canis familiaris. Anthrozoös 22:111–128. [Google Scholar]
- Smith BP, Litchfield CA, 2013. Looking back at ‘looking back’: operationalising referential gaze for dingoes in an unsolvable task. Anim Cogn 16:961–971. [DOI] [PubMed] [Google Scholar]
- Smith BP, Lucas TA, Norris RM, Henneberg M, 2018. Brain size/body weight in the dingo Canis dingo: comparisons with domestic and wild canids. Aust J Zool 65:292–301. [Google Scholar]
- Smuts B, 2010. Domestic dogs. In: Choe JC editor. Encyclopedia of Animal Behavior. Cambridge: Academic Press. 73–79. [Google Scholar]
- Suraci JP, Clinchy M, Zanette LY, Wilmers CC, 2019. Fear of humans as apex predators has landscape-scale impacts from mountain lions to mice. Ecol Lett 22:1578–1586. [DOI] [PubMed] [Google Scholar]
- Trut L, 1999. Early canid domestication: the farm - fox experiment. Am Sci 87:160–169. [Google Scholar]
- Trut L, Oskina I, Kharlamova A, 2009. Animal evolution during domestication: the domesticated fox as a model. Bioessays 31:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udell MA, Wynne CD, 2008. A review of domestic dogs' Canis familiaris human-like behaviors: or why behavior analysts should stop worrying and love their dogs. J Exp Anal Behav 89:247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udell MAR, Dorey NR, Wynne CDL, 2011. Can your dog read your mind? Understanding the causes of canine perspective taking. Learn Behav 39:289–302. [DOI] [PubMed] [Google Scholar]
- Viranyi Z, Gacsi M, Kubinyi E, Topal J, Belenyi B. et al. , 2008. Comprehension of human pointing gestures in young human-reared wolves Canis lupus and dogs Canis familiaris. Anim Cogn 11:373–387. [DOI] [PubMed] [Google Scholar]
- vonHoldt BM, Pollinger JP, Lohmueller KE, Han E, Parker HG. et al. , 2010. Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature 464:898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vonHoldt BM, Shuldiner E, Koch IJ, Kartzinel RY, Hogan A. et al. , 2017. Structural variants in genes associated with human Williams-Beuren syndrome underlie stereotypical hypersociability in domestic dogs. Sci Adv 3:e1700398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GD, Zhai W, Yang HC, Fan RX, Cao X. et al. , 2013. The genomics of selection in dogs and the parallel evolution between dogs and humans. Nat Commun 4:1860. [DOI] [PubMed] [Google Scholar]
- Wheat CH, Fitzpatrick JL, Rogell B, Temrin H, 2019. Behavioural correlations of the domestication syndrome are decoupled in modern dog breeds. Nat Commun 10:2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IM, 1972. Hunting dogs at yalata. Mankind 8:201–205. [Google Scholar]
- Wilton AN, 2001. DNA methods of assessing dingo purity. In: Dickman CR, Lunney D editors. A Symposium on the Dingo. Sydney: R Zool Soc NSW, 49–56. [Google Scholar]
- Wilton AN, Steward DJ, Zafiris K, 1999. Microsatellite variation in the Australian dingo. J Hered 90:108–111. [DOI] [PubMed] [Google Scholar]
- Zhang S-J, Wang GD, Ma P, Zhang LL, Yin TT. et al. , 2020. Genomic regions under selection in the feralization of the dingoes. Nat Comm 11:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




