Abstract
Geomyoid rodents provide a great study system for the analysis of sexual dimorphism. They are polygynic and many inhabit harsh arid environments thought to promote sexual dimorphism. In fact, there has been extensive work published on the sexual size dimorphism of individual populations and species within this rodent clade. However, little work has been undertaken to assess the evolutionary patterns and processes associated with this sexual dimorphism. We use multivariate analyses of cranial measurements in a phylogenetic framework to determine the distribution of size and shape dimorphism among geomyoids and test for Rensch’s rule. Our results suggest that sexual dimorphism is more common in geomyids than heteromyids, but it is not in fact universal. There is evidence for variation in sexual dimorphism across populations. Additionally, in many taxa, geographic variation appears to overwhelm existing sexual dimorphism. We find support for the repeated independent evolution of shape and size dimorphism across geomyoid taxa, but we do not find support for an association between size and shape dimorphism. There is no evidence for Rensch’s rule in geomyoids, whether at the superfamily or family level. Together, our findings suggest that there is no single explanation for the evolution of sexual dimorphism in geomyoids and that, instead, it is the product of numerous evolutionary events. Future studies incorporating phylogenetic relationships will be necessary to paint a more complete picture of the evolution of sexual dimorphism in geomyoids.
Keywords: Geomyidae, Heteromyidae, phylogenetic comparative methods, morphometrics, Rensch’s rule
Geomyoid rodents number 109 species distributed across 2 families: Geomyidae and Heteromyidae (Mammal Diversity Database 2020). They have often been considered an ideal study system to investigate sexual dimorphism (Table 1). The reasons for this are manifold. Geomyoids are present across widely differing habitats, from mesic forests to xeric shrublands and deserts (e.g., Fernández et al. 2014). They also span a range of terrestrial locomotion; pocket gophers (Geomyidae) are fossorial whereas heteromyids are ricochetal, terrestrial, or semi-fossorial (Bartholomew and Caswell 1951; Bartholomew and Cary 1954; Djawdan 1993; Roberts et al. 1997; Wilkins and Roberts 2007; Calede et al. 2019). Harsh environments and resource scarcity/clustering have been suggested to promote polygyny and sexual size dimorphism (SSD) in mammals (Pérez-Barbería et al. 2002; Isaac 2005; Lukas and Clutton-Brock 2013); resource competition has specifically been suggested to play a role in controlling SSD in heteromyids (García-Navas 2017). Geomyoids are also polygynous (Daly and Patton 1986; Bradley et al. 1991; Patton and Smith 1993; García-Navas 2017). Polygynous species are associated with high levels of SSD (Cassini 2020).
Table 1.
Sample of past studies of sexual dimorphism in geomyoids
| F | Taxon | Variables | App | Dim? | Reference |
|---|---|---|---|---|---|
| G | Cratogeomys gymnurus | D, S | U | Y | Hafner et al. (2004) |
| G | Cratogeomys merriami | D, S | U | Y | Hafner et al. (2005) |
| G | Geomys spp. | S | U, M | Y | Mauk et al. (1999) |
| G | Geomys bursarius | E, Ma, S | U | Y | Hendricksen (1972) |
| G | Heterogeomys hispidus | D, S | U | Y | Spradling et al. (2016) |
| G | Heterogeomys hispidus | D, S | U | Y | Hafner et al. (2014) |
| G | Thomomys bottae | Ma, SL | U | Y | Patton and Brylski (1987) |
| G | Thomomys bottae | S | M | Y | Smith and Patton (1988) |
| G | Thomomys bottae | P | M | N | Dunmire (1955) |
| G | Thomomys bottae | Ma | U | Y | Daly and Patton (1986) |
| G | Thomomys umbrinus | S | U | Y | Castro-Campilllo and Ramírez-Pulido (2000) |
| H | Chaetodipus formosus | – | – | N | Hall (1946) † |
| H | Chaetodipus goldmani | S | U | N* | Straney and Patton (1980) |
| H | Chaetodipus hispidus | E, S | U | N* | Glass (1947) |
| H | Chaetodipus hispidus | S | U | N | Andersen and Light (2012) |
| H | Chaetodipus intermedius | E, S | U | Y | Wilkins and Schmidly (1979) |
| H | Chaetodipus intermedius | E, S | U, M | Y | Weckerly and Best (1985) |
| H | Chaetodipus nelsoni | E, S | U | Y | Wilkins and Schmidly (1979) |
| H | Chaetodipus penicillatus | E, S | U | Y | Wilkins and Schmidly (1979) |
| H | Chaetodipus penicillatus | – | – | N* | Hall (1946) † |
| H | Chaetodipus penicillatus | E, S | U | Y* | Hoffmeister and Lee (1967) |
| H | Dipodomys (Heermanni group) | E, S | U, M | Y | Best (1978) |
| H | Dipodomys agilis | E, S | U, M | Y | Best et al. (1986) |
| H | Dipodomys agilis | E, S | U, M | Y | Best (1983a) |
| H | Dipodomys californicus | E, S | U | Y | Dale (1939) |
| H | Dipodomys californicus | P | U | Y* | Dunmire (1955) |
| H | Dipodomys compactus | E, S | U | N | Baumgardner and Schmidly (1981) |
| H | Dipodomys deserti | E, S | U | Y | Nader (1978) |
| H | Dipodomys elator | E, S | U | Y | Best (1987) |
| H | Dipodomys elator | D, E, S | U | Y | Webster and Jones (1985) |
| H | Dipodomys gravipes | E, S | U | Y | Best (1983b) |
| H | Dipodomys heermanni | P | M | Y | Dunmire (1955) |
| H | Dipodomys merriami | – | – | Y | Hall (1946) † |
| H | Dipodomys merriami | D, E, S | U | Y | Lidicker (1960) |
| H | Dipodomys microps | KMa | U | N | Csuti (1979) |
| H | Dipodomys microps | E, S | U | N | Hall and Dale (1939) |
| H | Dipodomys ordii | Ma, E, S | U | Y | Desha (1967) |
| H | Dipodomys ordii | D, E, S | Y | Schmidly (1971) | |
| H | Dipodomys ordii | E, S | U | N* | Baumgardner and Schmidly (1981) |
| H | Dipodomys ordii | E, S | U | N* | Schmidly and Hendricks (1976) |
| H | Dipodomys ordii | D, S | U, M | Y | Kennedy and Schnell (1978) |
| H | Dipodomys ordii | E, S | U, M | Y | Robertson et al. (1992) |
| H | Dipodomys ordii | S | U | N | Setzer (1949) |
| H | Dipodomys phillipsii | D, E, S | U | N | Genoways and Jones (1971) |
| H | Dipodomys simulans | E, S | U, M | Y | Sullivan and Best 1997 |
| H | Dipodomys spectabilis | E, S | U | N | Nader (1978) |
| H | Heteromys adspersus | D, E, S | U | Y* | Genoways (1973) |
| H | Heteromys anomalus | S | U | N | Anderson and Gutiérrez (2009) |
| H | Heteromys australis | S | U | N | Anderson and Jarrin-V (2002) |
| H | Heteromys catopterius | S | U | N | Anderson and Gutiérrez (2009) |
| H | Heteromys gaumeri | E, S | U | N* | Engstrom et al. (1987) |
| H | Heteromys irroratus | D, E, S | U | Y* | Genoways (1973) |
| H | Heteromys pictus | D, E, S | U | Y* | Genoways (1973) |
| H | Heteromys salvini | D, E, S | U | Y* | Genoways (1973) |
| H | Heteromys teleus | S | U | N | Anderson and Jarrin-V (2002) |
| H | Microdipodops megacephalus | Ma, E, S | U | N | Hall (1941) |
| H | Microdipodops megacephalus | S | U | N | Schitoskey (1968) |
| H | Microdipodops pallidus | Ma, E, S | U | N | Hall (1941) |
| H | Perognathus flavescens | D, E, S | U | N* | Williams (1978) |
| H | Perognathus flavescens | D, E, S | U | N* | Reed and Choate (1986) |
| H | Perognathus flavescens | D, S | U | N* | Williams and Genoways (1979) |
| H | Perognathus flavus | E, S | U | N | Baker (1954) |
| H | Perognathus flavus | E, S | U | N* | Best and Skupski (1994) |
| H | Perognathus longimembris | – | – | N | Hall (1946) † |
| H | Perognathus parvus | – | – | Y | Hall (1946) † |
Indicates complex results or lack of statistical tests with the letter reflecting the author’s conclusion.
Conclusion taken from Best (1993). Best (1993, Tables 1 and 2) provides additional results of univariate analyses of SSD in 57 species of heteromyids.
App =approach; D = dental measurements; Dim = dimorphic; E = external measurements; F = family; G = Geomyidae; H = Heteromyidae; K = kidney; M = multivariate; Ma = mass; N = No; P = postcranial measurements; S = skull measurements; SL = skull length; U = univariate; Y = Yes.
Multiple studies have analyzed the sexual dimorphism of populations, individual species, or small sets of species in both geomyoid families. Restricted for the most part to sexual dimorphism in size, they often incorporated multiple osteological measurements (Table 1) and led to the conclusion that geomyids have high sexual dimorphism (Hafner et al. 2004; Hafner et al. 2014; Spradling et al. 2016) whereas heteromyids are little to not sexually dimorphic (Schmidly and Hendricks 1976; Engstrom et al. 1987; Jones 1993; Anderson and Jarrin-V 2002; Anderson and Gutiérrez 2009; Andersen and Light 2012; García-Navas 2017). In both families, however, sexual dimorphism can be quite variable across taxa or even populations (Miller 1964; Hoffmeister and Lee 1967; Schmidly 1971; Baumgardner and Schmidly 1981; Daly and Patton 1986; Best 1987; Robertson et al. 1992; Best 1993). The existence of a sometimes-dramatic sexual dimorphism in Geomyidae remains largely puzzling. Although there have been 2 studies of SSD across multiple species of heteromyids (Best 1993; García-Navas 2017), none has ever been undertaken for geomyids.
There are however some data on the ontogeny of SSD in geomyids. Thus, in Thomomys bottae, the 2 sexes are significantly different by approximately 6.5 months of age, around the age at which males start reproducing; females start reproducing when they are 3 months old (Daly and Patton 1986; Patton and Brylski 1987). Males display a steeper growth curve than females through much of development and continue growing for a longer amount of time (Daly and Patton 1986; Patton and Brylski 1987). However, information on the evolution of sexual dimorphism in geomyoids is limited to the Heteromyidae. An analysis of SSD in heteromyids based on sex-specific species-level averages of snout to vent lengths demonstrates that resource competition as well as the balancing of premating and postmating sexual selection play a role in the low SSD of heteromyids (García-Navas 2017). Conspicuously missing from the literature is a comparison of sexual dimorphism across geomyoid species that incorporates shape in addition to size in a phylogenetic framework. Such work is critical to assessing the broad pattern of sexual dimorphism in families in which only a few species have been studied (Table 1), understanding the pattern of evolution of sexual dimorphism in geomyoids, and shedding light on the processes at play in its establishment. Such analyses have only been rarely undertaken in a diverse family of rodents (Matějů and Kratochvíl 2013; Martínez and Bidau 2016).
We use cranial measurements from several hundred specimens representing more than 35% of the species diversity of Geomyoidea to investigate the mode of evolution of SSD and shape dimorphism in a phylogenetic framework. We also explore whether geomyoids fit Rensch’s rule, an allometric pattern in which SSD increases with increasing mean body size in species with larger males and decreases with increased mean species size when females are the larger sex (Abouheif and Fairbairn 1997). Prior work has shown that heteromyids may not conform to Rensch’s rule (García-Navas 2017), but the high polygyny of geomyids warrants a rigorous analysis of Rensch’s rule. We specifically test the following hypotheses: (1) the apparent strong sexual dimorphism within Geomyidae is a feature of the family and includes both size and shape dimorphism; (2) in contrast, there is little evidence for sexual dimorphism in shape or size within Heteromyidae outside of Dipodomys; (3) the pattern of sexual dimorphism observed across the 2 families reflects the evolution of sexual dimorphism in the common ancestor of all geomyids whereas heteromyids display the ancestral condition for Geomyoidea of little to dimorphism between sexes; and (4) there is no evidence for Rensch’s rule in Geomyoidea or the families Heteromyidae and Geomyidae.
Material and Methods
Sampling and data collection
We sampled 39 species of geomyoids including 17 species of Geomyidae (out of 41) representing all 7 genera and 22 species (out of 68) of Heteromyidae spanning all 5 genera of the family (Mammal Diversity Database 2020). In total, we collected data from 813 specimens (mean 20.8 per species, median 18), 350 geomyids and 463 heteromyids (Table 2) from museum collections (Supplementary Data 1). We only measured adult specimens (based on the fusion of cranial sutures and the presence of fully erupted worn teeth) to avoid introducing ontogenetic variation in the dataset.
Table 2.
Sample of geomyoid rodents included in this study
| Family | Subfamily | Genus | Species | F | M | Abb. |
|---|---|---|---|---|---|---|
| Geomyidae | Geomyinae | Cratogeomys | castanops | 10 | 14 | Ccs |
| Geomyidae | Geomyinae | Cratogeomys | fumosus | 9 | 9 | Cfu |
| Geomyidae | Geomyinae | Cratogeomys | merriami | 11 | 10 | Cme |
| Geomyidae | Geomyinae | Geomys | arenarius | 9 | 8 | Gar |
| Geomyidae | Geomyinae | Geomys | bursarius | 7 | 9 | Gbu |
| Geomyidae | Geomyinae | Geomys | personatus | 7 | 8 | Gpe |
| Geomyidae | Geomyinae | Geomys | pinetis | 15 | 11 | Gpi |
| Geomyidae | Geomyinae | Heterogeomys | heterodus | 8 | 8 | Hhe |
| Geomyidae | Geomyinae | Heterogeomys | hispidus | 8 | 7 | Hhi |
| Geomyidae | Geomyinae | Orthogeomys | grandis | 12 | 11 | Ogr |
| Geomyidae | Geomyinae | Pappogeomys | bulleri | 12 | 12 | Pbu |
| Geomyidae | Geomyinae | Thomomys | bottae | 26 | 22 | Tbo |
| Geomyidae | Geomyinae | Thomomys | monticola | 6 | 8 | Tmo |
| Geomyidae | Geomyinae | Thomomys | talpoides | 10 | 10 | Tta |
| Geomyidae | Geomyinae | Thomomys | townsendii | 9 | 9 | Tto |
| Geomyidae | Geomyinae | Thomomys | umbrinus | 7 | 9 | Tum |
| Geomyidae | Geomyinae | Zygogeomys | trichopus | 10 | 9 | Ztr |
| Heteromyidae | Dipodomyinae | Dipodomys | deserti | 9 | 10 | Dde |
| Heteromyidae | Dipodomyinae | Dipodomys | heermanni | 8 | 13 | Dhe |
| Heteromyidae | Dipodomyinae | Dipodomys | ingens | 8 | 8 | Din |
| Heteromyidae | Dipodomyinae | Dipodomys | merriami | 25 | 25 | Dme |
| Heteromyidae | Dipodomyinae | Dipodomys | ordii | 20 | 20 | Dor |
| Heteromyidae | Dipodomyinae | Dipodomys | spectabilis | 9 | 8 | Dsp |
| Heteromyidae | Dipodomyinae | Microdipodops | megacephalus | 7 | 11 | Mme |
| Heteromyidae | Dipodomyinae | Microdipodops | pallidus | 9 | 10 | Mpa |
| Heteromyidae | Heteromyinae | Heteromys | anomalus | 8 | 8 | Han |
| Heteromyidae | Heteromyinae | Heteromys | desmarestianus | 9 | 8 | Hde |
| Heteromyidae | Heteromyinae | Heteromys | gaumeri | 8 | 8 | Hga |
| Heteromyidae | Heteromyinae | Heteromys | irroratus | 11 | 10 | Hir |
| Heteromyidae | Heteromyinae | Heteromys | pictus | 9 | 9 | Hpi |
| Heteromyidae | Perognathinae | Chaetodipus | baileyi | 8 | 7 | Cba |
| Heteromyidae | Perognathinae | Chaetodipus | californicus | 8 | 11 | Ccl |
| Heteromyidae | Perognathinae | Chaetodipus | hispidus | 8 | 8 | Chi |
| Heteromyidae | Perognathinae | Chaetodipus | intermedius | 13 | 17 | Cin |
| Heteromyidae | Perognathinae | Chaetodipus | penicillatus | 11 | 11 | Cpe |
| Heteromyidae | Perognathinae | Perognathus | flavescens | 8 | 8 | Pfl |
| Heteromyidae | Perognathinae | Perognathus | flavus | 8 | 9 | Pfu |
| Heteromyidae | Perognathinae | Perognathus | longimembris | 10 | 10 | Plo |
| Heteromyidae | Perognathinae | Perognathus | parvus | 9 | 11 | Ppa |
F = number of female specimens; M = number of male specimens; Abb. = Abbreviation used in the multivariate analysis.
We collected 14 measurements from each set of skull and dentaries (Figure 1 and Table 3) based on measurements used in prior studies of geomyoid cranial morphology (e.g. Best 1978; Anderson 2003; Calede and Rasmussen 2020). These measurements were chosen to represent skull shape in three dimensions (length, width, and depth) across regions of the skull (rostrum, palate, braincase, and lower jaw) for all taxa. The linear measurement approach we adopt enables the future study of fossil specimens, which are often fragmentary and would be difficult to incorporate in a geometric morphometric analysis.
Figure 1.
Cranial measurements used in the analyses. (A) Cranium in dorsal view. (B) Cranium in ventral view. (C) Cranium in lateral view. (D) Dentary in medial view. Abbreviations defined in Table 3.
Table 3.
Description of the measurements used in the analyses
| Abb. | Description |
|---|---|
| GCL | Greatest cranium length from anterior edge of nasal to posterior edge of skull |
| NL | Nasal length |
| IMW | Intermaxillary width at M3 |
| MAW | Maxillary arch width at widest point |
| GCD | Greatest cranium depth from dorsal edge of parietal to ventral edge of auditory bulla |
| GCW | Greatest cranium width across tympanic bullae |
| RW | Rostral width |
| RD | Rostral depth |
| DM2 | Depth of skull at M2 alveolus |
| PW | Palatine width between toothrows at P4 |
| LD | Length of upper diastema |
| LDL | Length of lower diastema |
| DMND | Depth of dentary at M1 |
| MANL | Mandibular length from anterior face of incisor to posterior edge of condyloid process |
Abb. = abbreviation used in Figure 1.
Data were obtained from specimen photos using ImageJ 1.51 (Schneider et al. 2012) or directly measured on specimens using Mitutoyo CD-6” CSX digital calipers. Photos were taken using a Canon EOS Rebel SL2 camera or gathered from public online museum repositories. For each specimen, we calculated the geometric mean (GM) using the square root of the product of all 14 measurements (Jungers et al. 1995; Madar et al. 2002). Each original measurement was then divided by the GM to provide a size-corrected value for each morphological feature. All 14 resulting variables and the GM were log-transformed before analyses. The complete dataset is being analyzed to test other hypotheses, but it can be obtained from the corresponding author on reasonable request.
The phylogenetic framework used in our analyses is from Fabre et al. (2012). We randomly selected 100 trees from a selection of 1,000 time-calibrated trees developed by Price and Hopkins (2015) and pruned the trees to keep only the taxa with morphological data using the package ape 5.3 (Paradis et al. 2004) in R 4.0.3 (R Core Team 2019).
Measurement errors
Measurements were recorded to the nearest 0.01 mm. Data checks were performed across the entire dataset by the senior author to assess the reliability of measurements. These checks included remeasuring specimens measured once using ImageJ or calipers using the same technique and remeasuring specimens measured with calipers using photos (in ImageJ). A subset of specimens was selected at random from the entire dataset to assess possible errors in the measurements made from photographs. This sample covered all species included in this study measured using photographs. For the 2 comparisons involving calipers, a smaller number of specimens selected randomly across species covering both heteromyids and geomyids were selected. The absolute difference in millimeter between first and second measurements was computed. Errors reported as percentages were calculated relative to the initial measurement. The effects on the analyses were assessed by rerunning the analyses (including GM calculations, the SSD analysis, and the sexual shape dimorphism analysis).
SSD, sampling, and Rensch’s rule
Skull size is an accurate proxy for body mass in Rodentia (Millien and Bovy 2010; Bertrand et al. 2016). Additionally, unlike body mass, skull size does not vary seasonally or with gravidity. As such, it is a useful variable to investigate size differences within a species using museum specimens. We used the GM of all specimens in the dataset to assess sexual dimorphism in size in each species of geomyoid studied. For each taxon, we used a t-test to test for a significant difference in size between sexes. Additionally, for each species, we calculated the SSD using the approach adopted by García-Navas (2017). We predict that SSD will be significant in most species of Geomyidae studied as well as the heteromyid genus Dipodomys. In contrast, we predict that SSD will be nonsignificant in Heteromys, Microdipodops, Chaetodipus, and Perognathus.
Best (1993) reported that the detection of sexual dimorphism in heteromyids may be sample-size dependent. To address this concern, we ran 2 sets of analyses. First, we ran 2 reduced-major axis regressions with the package lmodel2 1.7-3 (Legendre 2018) in R. We used the absolute value of SSD we calculated for each species and our sample size data to assess the relationship between the degree of SSD detected and the sample size for each species across the entire dataset. We used the distance between mean male shape and mean female shape calculated for our shape analysis (see below) to determine the relationship between the degree of sexual shape dimorphism we calculated and the sample size for each species across our entire dataset. Second, we selected the 2 best sampled species representing 2 different genera from each family; all of which have more than 20 specimens and 2 of which have more than 40 specimens (Table 2). For each of those 4 species, we assessed the ratio of average female body size to average male body size for successively smaller sample sizes of an equal number of males and females. To do so, we resampled without replacement our full dataset (capped at X individuals per sex with X the smallest of the number of males or number of females) at 90 through 20% of the original dataset every 10%, 100 times. The final distribution of ratios was subjected to an analysis of variance (ANOVA), and, when appropriate, post hoc Tukey tests to assess differences in the accuracy of the sexual dimorphism among samples of different sizes of the same species. We undertook the same resampling (and statistical testing) strategy to assess the potential of sample size to affect our analysis of shape. To do so, we calculated the Euclidean distance (ED) between mean male and mean female in an ordination space (principal component analysis [PCA]) projected for successively smaller subsamples of the species. We then used an ANOVA and post hoc tests to determine if there were significant differences in shape difference between the 2 sexes as the sample of skulls studied gets smaller.
We used phylogenetic generalized least squares regressions (PGLS; Grafen 1989) of the SSD values on the GMs to test for Rensch’s rule while accounting for evolutionary history. We performed the PGLS for each of the 100 trees in our dataset. Lambda (λ) was optimized to find the maximum likelihood transformation for each tree and the mean of each statistic was used in our interpretations. We ran 3 different regressions, 1 for the entire geomyoid dataset and 1 each for the families Geomyidae and Heteromyidae. This decision was made because of the sensitivity of Rensch’s rule to the taxonomic level studied (Webb and Freckleton 2007) and because the locomotory and dietary differences between geomyids and heteromyids might influence the pattern of allometry of SSD (Bidau and Medina 2013; Johnson et al. 2017). The regressions were run using caper 1.0.1 (Orme 2018) using code modified from Famoso et al. (2016). We predict that there is no significant relationship between the log(GM) and SSD in Geomyoidea, Geomyidae, and Heteromyidae.
Rensch’s rule for shape and shape dimorphism
We determined sexual shape difference (SShD) using an ordination of the size-corrected morphological measurements. We first projected all specimens of the dataset into a PCA, then calculated mean PCA scores for each sex within each species. We used the mean PCA scores of the males and females to calculate the EDs between the means of each sex within each species as a measure of the sexual dimorphism in shape. After Astúa (2010), we assessed the relationship between body size and SShD to determine if sexual shape dimorphism is correlated with size (the “Rensch’s rule for shape” of Astúa [2010]). As for the analysis of Rensch’s rule described above, we used 3 PGLS regressions of the logged SShD values on the GMs for this analysis. We predict that there is no significant relationship between the log(GM) and SShD in Geomyoidea, Geomyidae, and Heteromyidae.
To determine whether or not the 2 sexes of a species are significantly different from one another in shape, we ran individual PCAs for each species using all 14 size-corrected variables. We tested for differences in PC scores between the 2 sexes using a MANOVA or an ANOVA when only 1 PC axis was significant. The significance of PC axes was determined using a Monte Carlo randomization test run using biostats McGarigal (2015). We predict that sexual shape dimorphism will be significant in most species of Geomyidae studied as well as the heteromyid genus Dipodomys. In contrast, we predict that sexual shape dimorphism will be nonsignificant in Heteromys, Microdipodops, Chaetodipus, and Perognathus.
Evolution of sexual dimorphism
As a step in understanding the establishment of sexual dimorphism in geomyoids, we investigated 2 elements of the pattern of evolution of sexual dimorphism in Geomyoidea. We used ancestral character state reconstruction to determine how many times SSD and SShD evolved in Geomyoidea, whether geomyids share significant sexual dimorphism because they inherited it from their common ancestor, and whether significant sexual dimorphism is a derived trait within Geomyoidea. We also used model fitting to explore the pattern of evolution of SSD and SShD within the superfamily. For both SSD and SShD, we tested the fit of different models of evolution (Brownian motion, directional evolution, early burst, and Ornstein–Uhlenbeck [OU]) using geiger 2.0.7 (Harmon et al. 2008). We ran these analyses for all 100 trees selected (see above). We evaluated the relative support for each model and each tree using Akaike weights calculated using qpcR 1.4-1 (Ritz and Spiess 2008) from the AICc. We then calculated the median weights for each model across all trees to assess overall support. We used maximum likelihood to reconstruct ancestral character states using phytools 0.7-70 (Revell 2012, 2013). We predict that significant sexual dimorphism in size and shape evolved once, in the common ancestor of all geomyids.
Results
Sampling
There is no correlation between the size of the sample studied for a given species and the size difference between the 2 sexes detected for that species (Figure 2A). There is also no significant difference in size dimorphism among sample sizes (Figure 2B). In all species, no sample, however small, leads to a mean size ratio that differs significantly from the mean ratio found in the highest sample sizes. There is also no evidence for an important relationship between the sample size for each species and the shape dimorphism calculated for that species (Figure 3A). Although the regression calculated is significant (P = 0.03), sample size explains a very small amount of the variation in shape dimorphism (R2 = 0.072). However, post hoc tests show significant differences in shape dimorphism with varying sample sizes in all 4 species studied (Figure 3B). In Dipodomys merriami, a sample of 16 specimens is necessary to obtain shape dimorphism measurements that are indistinguishable from those calculated with 45 specimens. In T.bottae, reliable estimates of shape dimorphism are obtained with samples of 12 specimens or more. Samples of only 6 specimens yield estimates of shape dimorphism that are not significantly different from those obtained a sample of 20 specimens in Geomys pinetis. In Chaetodipus intermedius, at least half of the original number of specimens need to be included in the calculations; this corresponds to a sample of 12 specimens or more.
Figure 2.
Relationship between SSD and sampling. (A) Reduced major axis regression of the absolute value of SSD (SSD.abs) on the number of specimens for all species included in the analysis. (B) Resampling of 4 well-sampled species to investigate changes in the ratio of female size to male size across sample sizes (as a proportion of the full sample). Dark color represents geomyids. Light color represents heteromyids. N indicates size of full sample for each taxon.
Figure 3.
Relationship between sexual shape dimorphism and sampling. (A) Reduced major axis regression of the ED between the mean male shape and the mean female shape and number of specimens for all species studied. (B) Resampling of 4 well-sampled species to investigate changes in ED between mean male and mean female shapes across sample sizes. Dark color represents geomyids. Light color represents heteromyids. Asterisks represent sample sizes at which ED is significantly different than ED in the larger samples.
Measurement errors
Because the same measurements were taken following the same guidelines and in the same orientations (Figure 1 and Table 3) whether they were measured with calipers or through ImageJ, errors in the dataset studied are minimal; they range from < 0.2 to < 9%. There is no evidence that 1 of the 2 methods (measurements using calipers or from photographs) leads to higher errors. Errors are largest (as a percent) for the smallest variables measured (PW and DMND), which were sometimes <1.5 mm. There was no systematic pattern of bias between males and females. The largest error only affected the GM value analyzed by 1%; it does not affect the results of our analyses of SSD. A theoretical calculation of the effect of the worst measurement error detected applied uniformly to all 14 variables of the smallest specimen in the dataset (a specimen of Perognathus longimembris) yields a small change in the log of the GM for that specimen of 4.6%. We could not detect any effect on our multivariate analyses of shape of measurement errors.
Sexual size dimorphism
Within geomyids, 9 of the 17 species display a significant difference in logged GMs between males and females (Figure 4A and Table 4). These include 1 of the 3 species of Cratogeomys studied, C. merriami, 1 of the 4 species of Geomys studied, G. pinetis, both species of Heterogeomys as well as Orthogeomys grandis, 3 of the 4 species of Thomomys, and Zygogeomys trichopus. In all cases, the male is larger than the female. We do not detect any SSD in Pappogeomys. Within heteromyids, only 6 of the 22 species studied show significant sexual dimorphism (Figure 4B). These include 4 species within the genus Dipodomys and 2 of the 9 species of perognathines studied, Chaetodipus californicus and P.longimembris. In all species, the male is larger than the female. Neither Microdipodops nor Heteromys display size dimorphism.
Figure 4.
Size differences between males and females for each species studied. Size is represented by the logged GM. (A) Geomyidae. (B) Heteromyidae. Dark boxes represent males. Light boxes represent females. Asterisks indicates significant differences between sexes.
Table 4.
Summary of results of analyses of size and shape sexual dimorphism across geomyoid species studied
| Family | Subfamily | Genus | Species | Abb. | Size | Shape |
|---|---|---|---|---|---|---|
| Geomyidae | Geomyinae | Cratogeomys | castanops | Ccs | N | N |
| Geomyidae | Geomyinae | Cratogeomys | fumosus | Cfu | N | N |
| Geomyidae | Geomyinae | Cratogeomys | merriami | Cme | Y | Y |
| Geomyidae | Geomyinae | Geomys | arenarius | Gar | N | (Y) |
| Geomyidae | Geomyinae | Geomys | bursarius | Gbu | N | N |
| Geomyidae | Geomyinae | Geomys | personatus | Gpe | N | Y |
| Geomyidae | Geomyinae | Geomys | pinetis | Gpi | Y | N |
| Geomyidae | Geomyinae | Heterogeomys | heterodus | Hhe | Y | Y |
| Geomyidae | Geomyinae | Heterogeomys | hispidus | Hhi | Y | Y |
| Geomyidae | Geomyinae | Orthogeomys | grandis | Ogr | Y | Y |
| Geomyidae | Geomyinae | Pappogeomys | bulleri | Pbu | N | N |
| Geomyidae | Geomyinae | Thomomys | bottae | Tbo | Y | Y |
| Geomyidae | Geomyinae | Thomomys | monticola | Tmo | N | N |
| Geomyidae | Geomyinae | Thomomys | talpoides | Tta | Y | Y |
| Geomyidae | Geomyinae | Thomomys | townsendii | Tto | Y | N |
| Geomyidae | Geomyinae | Thomomys | umbrinus | Tum | N | Y |
| Geomyidae | Geomyinae | Zygogeomys | trichopus | Ztr | Y | N |
| Heteromyidae | Dipodomyinae | Dipodomys | deserti | Dde | Y | N |
| Heteromyidae | Dipodomyinae | Dipodomys | heermanni | Dhe | N | N |
| Heteromyidae | Dipodomyinae | Dipodomys | ingens | Din | Y | N |
| Heteromyidae | Dipodomyinae | Dipodomys | merriami | Dme | Y | Y |
| Heteromyidae | Dipodomyinae | Dipodomys | ordii | Dor | N | N |
| Heteromyidae | Dipodomyinae | Dipodomys | spectabilis | Dsp | Y | N |
| Heteromyidae | Dipodomyinae | Microdipodops | megacephalus | Mme | N | N |
| Heteromyidae | Dipodomyinae | Microdipodops | pallidus | Mpa | N | N |
| Heteromyidae | Heteromyinae | Heteromys | anomalus | Han | N | N |
| Heteromyidae | Heteromyinae | Heteromys | desmarestianus | Hde | N | N |
| Heteromyidae | Heteromyinae | Heteromys | gaumeri | Hga | N | N |
| Heteromyidae | Heteromyinae | Heteromys | irroratus | Hir | N | N |
| Heteromyidae | Heteromyinae | Heteromys | pictus | Hpi | N | N |
| Heteromyidae | Perognathinae | Chaetodipus | baileyi | Cba | N | N |
| Heteromyidae | Perognathinae | Chaetodipus | californicus | Ccl | Y | Y |
| Heteromyidae | Perognathinae | Chaetodipus | hispidus | Chi | N | Y |
| Heteromyidae | Perognathinae | Chaetodipus | intermedius | Cin | N | (Y) |
| Heteromyidae | Perognathinae | Chaetodipus | penicillatus | Cpe | N | N |
| Heteromyidae | Perognathinae | Perognathus | flavescens | Pfl | N | N |
| Heteromyidae | Perognathinae | Perognathus | flavus | Pfu | N | N |
| Heteromyidae | Perognathinae | Perognathus | longimembris | Plo | Y | N |
| Heteromyidae | Perognathinae | Perognathus | parvus | Ppa | N | N |
Parentheses indicate taxa with sexes significantly different at α > 0.06.
Abb. = abbreviation used in the multivariate analysis.
Our calculations of SSD show an overwhelming pattern of males being larger than females across Geomyoidea (Figure 5A). Of the 39 species studied, only 7 have a positive SSD (i.e. the female is larger than the male); 6 of those are heteromyids. Females are, on average, larger than males in both species of Microdipodops, 3 species of perognathines, and Dipodomys ordii. Within Geomyidae, only Thomomys townsendii has an average female larger than the average male. An analysis of the SSD for all species pooled by family shows that geomyids have significantly larger males (relative to females) than heteromyids (ANOVA: F = 6.295, P = 0.017; Figure 5B).
Figure 5.
SSD (SSD) for the taxa studied. (A) SSD for all species. (B) boxplot of variation in SSD within each family of geomyoids. Dark color represents geomyids. Light color represents heteromyids. Negative values indicate larger males whereas positive values indicate larger females.
Rensch’s rule
A PGLS analysis of SSD and mean GM enables the investigation of Rensch’s rule while accounting for phylogenetic relationships (Figure 6A). Our regression of the entire geomyoid dataset shows a significant relationship between the 2 variables (P = 0.013) with increasingly large male-biased size dimorphism with increasing body size. However, the R2 for this regression is low (0.13). When the relationships between mean GM and SSD are calculated for each of the 2 families, Geomyidae and Heteromyidae, independently, no significant relationship is found (P = 0.33 and 0.68, respectively). The analyses of Rensch’s rule for shape at the superfamily level and the family level do not provide evidence for a significant relationship between species size and shape dimorphism (Figure 6B).
Figure 6.
Phylogenetic Generalized Least Square Regressions for Rensch’s rule. (A) Regression of SSD on mean GM (Rensch’s rule). (B) Regression of ED between mean male and mean female for each taxon on mean GM (Rensch’s rule for shape). Each regression showed is a representative example of the 100 regressions generated by our analyses. The P values are the mean for all 100 trees. Dark color represents geomyids. Light color represents heteromyids. Dashed line represents the regression for the entire Geomyoidea.
Sexual shape dimorphism
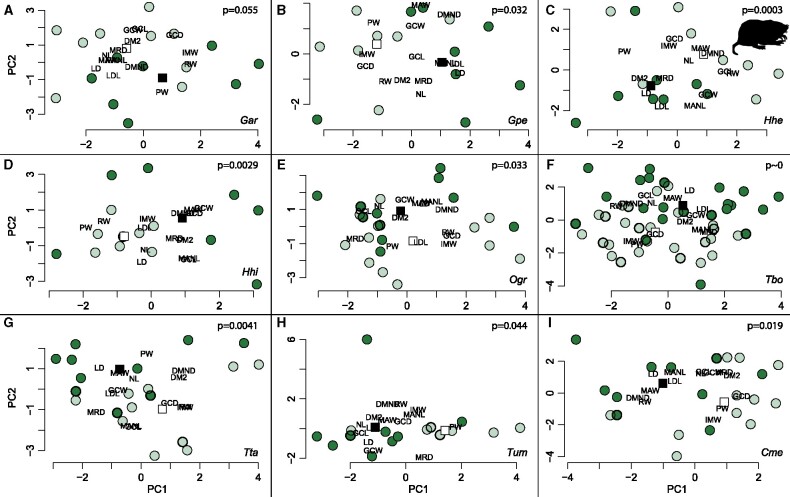
Eight species of Geomyidae display significant differences in shape between sexes (Figure 7 and Table 4). A ninth species shows a marginally significant difference (P = 0.055) and is included in the group of sexually dimorphic species. Pocket gophers sexually dimorphic in shape include Geomys arenarius and G. personatus, both species of Heterogeomys as well as O.grandis, T.bottae, T. talpoides, T. umbrinus, and Cratogeomys merriami. There is no evidence for sexual dimorphism in shape in Pappogeomys and Zygogeomys. In G. arenarius, males are characterized by a wider rostrum, wider maxillary region, and anteroposteriorly shorter skulls than females. In G. personatus, males have generally longer diastemata, longer lower jaws, longer skulls and nasals, wider rostra, and deeper skulls in the rostral and maxillary regions than females. Females have wider skulls in the maxillary and basicranial regions, deeper jaws, and deeper skulls in the auditory region than males. The males of H. heterodus generally display longer diastemata, deeper rostra and maxillae, wider basicrania, and wider anterior portions of the maxilla, as well as longer dentaries than females. Females have generally longer skulls with deeper lower jaws and basicrania, as well as wider rostra and wider zygomatic arches than males. In H. hispidus, males have deeper skulls that are wider in the basicranial region as well as longer and deeper lower jaws than females. Females have proportionately wider rostra and maxillary regions as well as longer diastemata. In O. grandis, males tend to have longer skulls with longer nasals, wider basicranial and zygomatic regions, a deeper maxillary region, and deeper dentaries that are also longer than in females. Females have wider maxillae and broader as well as deeper rostra than males. The basicranium of females is also deeper than that of males. In T. bottae, males have longer diastemata, wider zygomatic arches, longer skulls and nasals, a wider rostrum, and a deeper lower jaw than females; these females have a wider maxillary region and a deeper basicranium than males. Males of T. talpoides have longer diastemata and longer nasals, a wider anterior portion of the maxilla, wider zygomatic arches, a wider basicranium, a deeper maxillary region of the skull and a deeper lower jaw than females. Females of T. talpoides have longer lower jaws, longer skulls, a deeper basicranial region of the skull, a wider rostrum, and a wider posterior portion of the maxilla. Within T. umbrinus, females tend to have a wider anterior portion of the maxilla and a deeper rostrum than males whereas they are smaller than males in all other variables studied. The males of C. merriami have longer diastemata, a longer and deeper lower jaw, wider zygomatic arches, and a wider rostrum than the females; females, in contrast, have wider maxillary regions, and a deeper basicranial region than males.
Figure 7.
Morphospaces for the 9 geomyid species showing significant shape dimorphism. (A) Geomys arenarius, (B) G. personatus, (C) H. heterodus, (D) H. hispidus, (E) O. grandis, (F) T. bottae, (G) T. talpoides, (H) T. umbrinus, (I) C. merriami. Light color denotes females. Dark color denotes males. Dark squares represent mean male shape. Light squares represent mean female shape. The apparent outlier for T. umbrinus is the holotype of T. umbrinus pullus, which displays a distinct rostrum shape. Description of the measurements provided in Table 3. P values are provided for each species in the upper right corner of each graph.
Only 4 of the 22 species of heteromyids studied show significant shape dimorphism between sexes (Figure 8 and Table 4); 1 of those species shows a marginally significant difference (P = 0.059). These species include D.merriami and three species of Chaetodipus: C. californicus, C. hispidus, and C. intermedius. None of the species of Heteromys, Perognathus, or Microdipodops show evidence of sexual shape dimorphism. Males of D. merriami have deeper rostra and maxillary regions than females; they also have longer skulls and dentaries as well as wider zygomatic arches and wider anterior portions of the maxilla than females. Females have deeper lower jaws, a longer lower diastema, a wider rostrum, and a wider posterior portion of the maxilla than males. Males of C. californicus display a deeper lower jaw, a longer upper diastema, and a wider basicranium than females. Females have deeper rostra, longer skulls, a deeper basicranium, and a wider posterior portion of the maxilla. Within C. hispidus, males display wider rostra and deeper associated with a longer upper diastema and longer nasals than females; this is associated with overall longer skulls and longer lower jaws than in females. The skulls of female C. hispidus are generally deeper than those of males. In general, males of C. intermedius are characterized by wider and deeper skulls in the rostral, maxillary, and basicranial regions as well as longer skulls than females. Females have deeper dentaries, longer diastemata, longer nasals, and wider zygomatic arches than males.
Figure 8.
Morphospaces for the 4 heteromyid species showing significant shape dimorphism. (A) Dipodomys merriami, (B) C. californicus, (C) C. hispidus, and (D) C. intermedius. Light color denotes females. Dark color denotes males. Dark squares represent mean male shape. Light squares represent mean female shape. Description of the measurements provided in Table 3. P values are provided for each species in the upper right corner of each graph.
Evolution of sexual dimorphism
The evolution of SSD on tree shows a complex pattern in which the common ancestor of Geomyoidea displayed a slight male-biased size dimorphism (Figure 9A). This slight male-biased SSD was retained for much of the evolution of the clade; the common ancestor of Heteromyidae as well as that of Geomyidae both display similar SSD as the common ancestor of all geomyoids. In fact, the common ancestors of each geomyoid genus we analyzed except for Microdipodops is reconstructed with a slight male-biased SSD. Female-biased SSD evolved 4 times in geomyoids: once in the common ancestor of the 2 species of Microdipodops, a second time in Perognathus parvus, a third time in C.intermedius, and only once in Geomyidae, in T.townsendii. Male-biased SSD evolved 5 times: once in P.longimembris and 4 times within Geomyidae including once in each of the 2 subgenera of Thomomys (T. umbrinus for Megascapheus and T. talpoides for Thomomys), once in C.merriami, and once in Heterogeomys heterodus. Significant SSD evolved 9 times within Geomyoidea. Parsimonious interpretation of the tree suggests that Dipodomys is ancestrally dimorphic with 2 independent decreases in size dimorphism, once in D. ordii and once in D. heermanni. Significant SSD also evolved in P.longimembris, and C.californicus within Heteromyidae. Within geomyids, significant SSD evolved twice in Thomomys, once in each of the 2 subgenera, C.merriami, the common ancestor of Orthogeomys and Heterogeomys, G.pinetis, and Z.trichopus. The best-fit is found with the OU model (median Akaike weights across all analyses—trend: 9.5 × 10−4, Brownian motion: 1.4 × 10−4, Early burst: 4.4 × 10−5, OU: 0.99).
Figure 9.
Ancestral character state reconstructions of sexual dimorphism. (A) SSD. (B) ED between average males and females for each species in morphospace. Species names in black represent taxa with significant sexual dimorphism.
The analysis of the evolution of SShD on tree shows that the common ancestor of Geomyoidea displayed a small SShD (Figure 9). A similar SShD is reconstructed for the common ancestor of Heteromyidae. Low levels of SShd evolved in the common ancestor of the Dipodomyinae with an even smaller SShD reconstructed for the common ancestor of Dipodomys. Higher levels of SShD than in the common ancestor evolved in Heteromys and Perognathinae. The common ancestor of all Geomyidae show low levels of SShD with increases in ED between the average male and the average female in Thomomys umbrinus, Cratogeomys castanops, Pappogeomys bulleri, H.heterodus, G.pinetis, G. arenarius, and G. personatus. Significant shape dimorphism evolved at least twice within Heteromyidae including once in D.merriami. Within Chaetodipus, significant sexual dimorphism may have evolved once in the common ancestor of the genus and be lost in both C. baileyi and C. penicillatus; it could also have just as parsimoniously evolved independently in all 3 species in which it is present: C. intermedius, C. californicus, and C. hispidus. Within the family Geomyidae, significant sexual dimorphism evolved at least 5 times including once in each of the 2 subgenera of Thomomys, a third time in C.merriami, a fourth time in the common ancestor of Orthogeomys and Heterogeomys, and at least once in Geomys. Within that latter genus, significant sexual dimorphism could have evolved in the common ancestor of G. arenarius, G. bursarius, and G. personatus before to be lost in G. bursarius; it could also have evolved independently in G. arenarius and G. personatus, leading to a sixth instance of the evolution of significant sexual shape dimorphism. The model-fitting supports the OU model as the best fit (median Akaike weights across all analyses—trend: 6.3 × 10−4, Brownian motion: 8.4 × 10−5, Early burst: 2.6 × 10−5, OU: 0.99).
Discussion
Our analysis of the best sampled species in our dataset does not support the need for very large sample sizes to detect SSD in geomyoids. There is no correlation between sample size and SSD in geomyoids. Additionally, small samples of geomyoids enable the detection of SSD as accurately as large ones. Even estimates of SSD from samples of 4 specimens from each sex are comparable to those obtained with more than 20 specimens per sex. A little more caution is necessary when studying shape dimorphism between sexes. Our results support using samples as small as 3 specimens per sex to assess SShd in 1 of the 4 species we analyzed. The highest number of specimens necessary to accurately estimate shape differences in geomyoid rodents is 8 specimens per sex. Our analyses appear to contradict a previous conclusion that the detection of sexual dimorphism is sample size dependent in heteromyids (Best 1993). This conclusion was partly drawn from qualitative comparisons of SSD across populations. However, sexual dimorphism varies across geomyoid populations (Miller 1964; Hoffmeister and Lee 1967; Schmidly 1971; Baumgardner and Schmidly 1981; Daly and Patton 1986; Best 1987; Robertson et al. 1992). It was also drawn from comparisons of univariate analyses between different sample sizes of Dipodomys. Our multivariate approach shows that a relatively small number of specimens is necessary to overcome population-level morphological variation and reveal species-level sexual dimorphism in several geomyoid taxa. This finding will greatly expand the possibility of studying SSD in Geomyoidea.
The results of our analyses of sexual dimorphism in Geomyidae are generally consistent with published evidence. Of the 17 species we studied, 5 have previously been analyzed. Three were found to display significant SSD (C.merriami, Heterogeomys hispidus, and T.bottae); we also find support for the presence of significant SSD in these species (Tables 1 and 4). Another species previously studied, Geomys bursarius, does not display significant SSD in our analyses. However, prior work showed significant SSD in this species (Hendricksen 1972). This could be an effect of differences in SSD between populations. The specimens studied by Hendricksen (1972) were from Colorado and Kansas whereas our sample includes specimens from North Dakota, South Dakota, Minnesota, Nebraska, Illinois, and Texas. Alternatively, this may indicate a weak SSD relative to geographic differences in morphology. The last species we analyzed that has already been studied, T.umbrinus, shows a complex pattern in which although we did not detect the significant SSD reported previously (Castro-Campilllo and Ramírez-Pulido 2000), we do recover significant sexual shape dimorphism. This pattern hints at the importance of considering size-corrected analyses of shape dimorphism in addition to overall size comparisons. In fact, when considering shape dimorphism in T. umbrinus, males are larger than females in 12 of 14 variables, a pattern not unlike that seen in Castro-Campilllo and Ramírez-Pulido (2000), although we do not find the same size difference for the depth of the rostrum. Finally, our analysis also detects significant SSD in half of the species that had not previously been analyzed. Of the genera including more than 1 species, only Heterogeomys displays SSD in all taxa. Our results also suggest multiple evolutions of SSD within Geomyidae.
SShD is best explored in geomyoids by assessing differential morphospace occupation in species-specific ordinations of morphological data. This is because the tremendous cranial disparity represented by the superfamily Geomyoidea (Hafner and Hafner 1988) leads to difficulty comparing intraspecific variation in an ordination that includes several genera from both families. Among the 9 species of geomyids displaying significant SSD, 7 also display significant SShD. In contrast, only 3 of the 8 species that do not show significant SSD display significant SShD, 1 of them marginally so. A chi-square analysis does not support a significant association between the 2 types of sexual dimorphism (χ2 = 2.95; P = 0.086) but studies of this possible association in additional species will be necessary to conclude definitively. Across species displaying significant SShD, there is no consistent pattern of morphological difference between sexes. The lengths of the diastemata appear to be the only traits we studied that are reliably (but not uniformly) greater in males across taxa; the basicranium is largely deeper in females. The differences in the nature of the shape dimorphism in crania among most species of geomyids suggests that it evolved independently multiple times. Closely related species (H. heterodus, H. hispidus, and O. grandis) that could have inherited SShD from a common ancestor do display greater similarity in the nature of their SShD.
Overall, there is little support for the hypothesis that strong sexual dimorphism is a feature of the family Geomyidae. Although most geomyid taxa we studied display some form of sexual dimorphism, almost half (8 of 17) do not show significant size dimorphism between sexes; the same is true of shape dimorphism. In fact, 5 of the species we studied show neither size nor shape dimorphism. Even within genera, the pattern of sexual dimorphism is complex. Thus, only 1 of the 3 species of Cratogeomys we studied shows dimorphism. Within Geomys, 1 species displays no dimorphism, 1 significant size dimorphism only, and 2 significant shape dimorphism only. A similarly complex pattern is observed within Thomomys with 1 species without dimorphism, 1 with size dimorphism only, 1 with only significant shape dimorphism, and 2 with both size and shape dimorphism. Studies of additional species of Thomomys within both subgenera (Megascapheus and Thomomys) will be necessary to provide a complete picture of the evolution of sexual dimorphism in the genus. Consistent with the varying nature of SShD across taxa, our analysis of SShD on tree suggests the evolution of significant SShD in geomyids 7 times, with 1 instance in the common ancestor of Orthogeomys and Heterogeomys and 6 other instances in individual species across the family. Our ancestral character state reconstruction does not support our hypothesis that significant sexual dimorphism evolved once in the common ancestor of all geomyids. However, the model fittings for both SSD and SShD remain equivocal. Although the support for an OU model is overwhelming in both cases, this support should be interpreted with caution (Cooper et al. 2016). A careful analysis of the α value (α = 2.718 for SSD and 2.198 for SShD) rescaled following the approach of Ives and Garland (2010) suggests that the phylogenetic correlation, although not negligible, is not very strong. A larger sampling of taxa will likely be necessary to definitively assess the mode of evolution of sexual dimorphism in geomyoids, but the presence of multiple optima appears to be a reasonable hypothesis.
Among the 22 species of Heteromyidae we analyzed, our results are consistent with published evidence in 15 and contradict previous findings in only 3. Alike Best (1993); Hall (1941); and Schitoskey (1968), we find no evidence for significant SSD in Microdipodops. We also do not recover any evidence for significant SSD in Heteromys. This result is broadly consistent with the literature including Best (1993); Engstrom et al. (1987); and Anderson and Gutiérrez (2009). Although Genoways (1973) recovered significant SSD for several measurements of the skull and body of H. pictus, Best (1993) could not find such dimorphism in any of the 19 variables he analyzed. Our results are consistent with Best (1993). It is worth noting that the sample studied by Genoways (1973) was limited to the state of Jalisco. Our sample covers 4 other states (Colima, Guerrero, Oaxaca, and Chiapas). The population-level sexual dimorphism recovered by Genoways (1973) is overcome by morphological differences among populations. Genoways (1973) also analyzed SSD in a sample of H. irroratus from the state of Jalisco and found males to be larger than females in 7 of the 13 measurements he studied. Best (1993) recovered significant SSD in 4 of the 19 measurements he analyzed for this species. We do not find significant SSD in this taxon when analyzing our sample of specimens from Tlaxcala, Chihuahua, San Luis Potosi, Oaxaca, Puebla, and Texas. When sexual dimorphism is present in Heteromys, it represents a smaller effect on morphological variation than geographic variation.
Best (1993) found mixed support for the presence of SSD in Chaetodipus; our results are consistent with this conclusion. Like Best (1993), we do not recover a significant SSD in C. baileyi, C. hispidus, and C. penicillatus. These findings are also consistent with those of Glass (1947) as well as Andersen and Light (2012) for C. hispidus and Hall (1946) for C. penicillatus. Wilkins and Schmidly (1979) detected significant SSD for several measurements in a Trans-Pecos population of C. penicillatus. Another study found that SSD is quite variable across populations (Hoffmeister and Lee 1967). Our analysis of a sample including specimens from Arizona, California, Texas, and Utah shows that SSD is dwarfed by geographic variation in morphology in C. penicillatus. On the contrary, our analysis of a C. californicus sample spanning 12 counties and representing much of the geographic range of the species shows significant SSD (unlike Best 1993), suggesting a strong dimorphism that can overwhelm geographic variation. Past analyses of C. intermedius in New Mexico (Weckerly and Best 1985) and Texas (Wilkins and Schmidly 1979) found significant SSD for a minority of the cranial measurements studied as well as some external measurements although the 2 studies differ in the nature of some of the measurements that are dimorphic. We do not find evidence for significant SSD in our sample of C. intermedius dominated by specimens from Arizona (27 of 28 specimens with locality information). Sexual dimorphism in this species, alike several other heteromyids, is population specific.
Within the genus Perognathus, the lack of significant SSD for P. flavescens and P. flavus is consistent with Baker (1954); Williams (1978); Williams and Genoways (1979); Reed and Choate (1986); Best (1993); and Best and Skupski (1994). Despite previous research that found no SSD in P. longimembris (Hall 1946; Best 1993), we recover significant dimorphism in this species. The sample of Hall (1946) was restricted to Nevada; there is no published information on the sample of Best (1993). Our sample is dominated by specimens from 5 counties in California, but includes material from Arizona, Nevada, and Utah. Our results also differ from published findings with regards to P. parvus. We do not find significant SSD in this species unlike Best (1993) and Hall (1946). Our sample does not include any specimen from Nevada, unlike Hall (1946); instead, it is composed of specimens from Utah, Montana, California, Oregon, and Arizona. Together with our data from the genera Heteromys and Chaetodipus, these data show that SSD is best assessed using samples that cover a wide portion of the geographic range of the species and include populations with varying degrees of dimorphism.
Our analyses of 6 species of the genus Dipodomys yield results identical to Hall (1946); Lidicker (1960); Nader (1978); and Best (1993) for D. deserti and D. merriami. Nader (1978) and Best (1993) found contradictory evidence for D. spectabilis; our findings are consistent with those of Best (1993). Nader (1978) studied 2 populations from Arizona; our sample includes specimens from Arizona, New Mexico, Texas, and 3 states within Mexico (Sonora, Chihuahua, and Aguascalientes). Support for SSD in D. ordii is equivocal. Some populations from west Texas show strong sexual dimorphism (Desha 1967; Schmidly 1971; Robertson et al. 1992) whereas populations from the southern part of the state and Mexico do not (Schmidly and Hendricks 1976; Baumgardner and Schmidly 1981). An analysis across the entire geographic range of the species (Kennedy and Schnell 1978) recovered significant SSD using univariate analyses. Our multivariate approach of a large sample dominated by west Texas specimens does not support the presence of significant SSD in D. ordii, providing evidence that sexual dimorphism in this species is subtle and highly variable. We find support for a significant SSD in D. ingens alike Best (1993). Best (1993) recovered only weak evidence of SSD in D. heermanni (2 of 19 measurements significantly different between sexes). Our multivariate analysis broadly agrees; we do not find support for significant SSD in D. heermanni.
Sexual shape dimorphism is rare in heteromyids. Only 1 species of Dipodomys and 3 of the 5 species of Chaetodipus studied display significant shape dimorphism. There is no published analysis of sexual shape dimorphism in heteromyids to compare to these results. There is no apparent association between the presence of SSD and SShD. Dipodomys merriami and C. californicus are the only 2 species to display both size and shape dimorphism. Additionally, the nature of the variables involved in the SShD varies across species, particularly within the genus Chaetodipus. This supports a scenario whereby sexual dimorphism evolved independently within each species rather than in their common ancestor. Eight of the 22 species of heteromyids we studied display some form of significant sexual dimorphism, in size, shape, or both. Half of those are species within the genus Dipodomys; they represent two-third of the species studied from this genus. Four species of perognathines also display sexual dimorphism. These data support the hypothesis that sexual dimorphism is limited within Heteromyidae and that, when present, it appears to be mostly within Dipodomys as well as perognathines (see also Best 1993; Garcia-Navas 2017).
Our analysis of Rensch’s rule show a very weak relationship between species size and SSD at the superfamily level and no relationships at the family level. None of the analyses of Rensch’s rule for shape show any significant relationship. These results support our hypothesis that there is no evidence for Rensch’s rule in geomyoids, consistent with the findings in heteromyids of Garcia-Navas (2017). Other studies have also failed to support Rensch’s rule in ground squirrels (Matějů and Kratochvíl 2013), the burrowing rodent Ctenomys (Martínez and Bidau 2016), the bat Myotis (Stevens and Platt 2015), didelphimorph marsupials (Astúa 2010), and canids (Johnson et al. 2017). Prior analyses have showed support for an intraspecific form of Rensch’s rule in some, but not all, burrowing rodents, including the gopher C.castanops (Martínez and Bidau 2016). Our data provide additional support for the conclusion that Rensch’s rule is a rare pattern mostly observed at the intraspecific level.
Supplementary Material
Acknowledgments
Meg Daly and Grant Terrell (OSUM), John Wible and Suzanne McLaren (CM), Darrin Lunde (USNM), Marisa Surovy (AMNH), Roberta Muelheim (CMNH), Jim Dines (LACM), Verity Mathis (UF), and Charles Kilpatrick (UVM) provided access to specimens and records. We thank the University of California Berkeley Museum of Vertebrate Zoology (MVZ) for use of their specimen images and CalPhotos for access and use of their digital resources. We thank the USNM and UF for access and use of specimen photos. Space at the OSUM was provided to the Calede lab by Rachelle Adams and Bryan Carstens. Sam Hopkins and Sam Price provided their tree file. Erica Scarpitti, Anas Tantash, Lily Noftz, and Madeline Ball assisted with data collection. The silhouette of the pocket gopher used in the figures is from phylopic (Public Domain Dedication 1.0 licence). We thank 4 anonymous reviewers for their feedback on earlier versions of the manuscript.
Funding
This research was funded by a Paleontological Society Norman Newel Award, a College of Arts and Sciences Regional Campus Research and Creative Activity Grant from the Ohio State University, a research grant from the Ohio State University at Marion, and startup funds from the Ohio State University to J.C. Some of the ideas for this project were developed while J.C. was supported by a Meaningful Inquiry grant from the Ohio State University library system.
Authors’ Contributions
A.B. and J.C. developed the data collection protocol and collected data, JC analyzed the data and wrote the manuscript, A.B. and J.C. edited and approved the manuscript.
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
Conflict of Interest Statement
The authors declare no conflict of interest.
Contributor Information
Jonathan J M Calede, Department of Evolution, Ecology, and Organismal Biology, The Ohio State University, 318 W. 12th Ave., 300 Aronoff Laboratory, Columbus, OH 43210, USA and; The Ohio State University at Marion, 1459 Mount Vernon Avenue, Marion, OH 43302, USA.
Andrew Brown, Department of Evolution, Ecology, and Organismal Biology, The Ohio State University, 318 W. 12th Ave., 300 Aronoff Laboratory, Columbus, OH 43210, USA and.
References
- Abouheif E, Fairbairn D, 1997. A comparative analysis of allometry for sexual size dimorphism: assessing Rensch’s rule. Am Nat 149:540–562. [Google Scholar]
- Andersen JJ, Light JE, 2012. Phylogeography and subspecies revision of the hispid pocket mouse Chaetodipus hispidus (Rodentia: Heteromyidae). J Mammal 93:1195–1215. [Google Scholar]
- Anderson RP, 2003. Taxonomy, distribution, and natural history of the genus Heteromys (Rodentia: heteromyidae) in western Venezuela, with the description of a dwarf species from the Península de Paraguaná. Am Mus Novit 3396:1–43. [Google Scholar]
- Anderson RP, Gutiérrez EE, 2009. Taxonomy, distribution, and natural history of the genus Heteromys (Rodentia: Heteromyidae) in central and eastern Venezuela, with the description of a new species from the Cordillera de la Costa. In: Voss RS, Carleton MD, editors. Systematic Mammalogy: Contributions in Honor of Guy G. Musser. New York City, NY: Bulletin of the American Museum of Natural History. 331: 33–93. [Google Scholar]
- Anderson RP, Jarrin VP, 2002. A new species of spiny pocket mouse (Heteromyidae: Heteromys) endemic to western Ecuador. Am Mus Novit 3382:1–26. [Google Scholar]
- Astúa D, 2010. Cranial sexual dimorphism in New World marsupials and a test of Rensch’s rule in Didelphidae. J Mammal 91:1011–1024. [Google Scholar]
- Baker RH, 1954. The silky pocket mouse Perognathus flavus of Mexico. Mus Nat Hist 7:339–347. [Google Scholar]
- Bartholomew GA Jr, Cary GR, 1954. Locomotion in pocket mice. J Mammal 35:386–392. [Google Scholar]
- Bartholomew GA Jr, Caswell HH Jr, 1951. Locomotion in kangaroo rats and its adaptive significance. J Mammal 32:155–169. [Google Scholar]
- Baumgardner GD, Schmidly DJ, 1981. Systematics of the southern races of two species of Kangaroo rats (Dipodomys compactus and D. ordii). The Museum. Museum of Texas Tech University, TX, USA, no. 73. 1–27. [Google Scholar]
- Bertrand OC, Schillaci MA, Silcox MT, 2016. Cranial dimensions as estimators of body mass and locomotor habits in extant and fossil rodents. J Vertebr Paleontol 36:e1014905. [Google Scholar]
- Best TL, 1978. Variation in kangaroo rats (genus Dipodomys) of the Heermanni group in Baja California, Mexico. J Mammal 59:160–175. [Google Scholar]
- Best TL, 1983a. Intraspecific variation in the agile kangaroo rat Dipodomys agilis. J Mammal 64:426–436. [Google Scholar]
- Best TL, 1983b. Morphologic variation in the San Quintin kangaroo rat (Dipodomys gravipes Huey 1925). Am Midl Nat 109:409–413. [Google Scholar]
- Best TL, 1987. Sexual dimorphism and morphometric variation in the Texas kangaroo rat (Dipodomys elator Merriam 1894). Southwest Nat 32:53–59. [Google Scholar]
- Best TL, 1993. Patterns of morphologic and morphometric variation in heteromyid rodents. In: Genoways HH, Brown JH, editors. Biology of the Heteromyidae. Special Publication, The American Society of Mammalogists. 10:197–235. [Google Scholar]
- Best TL, Skupski MP, 1994. Perognathus flavus. Mamm Species 471:1–10. [Google Scholar]
- Best TL, Sullivan RM, Cook JA, Yates TL, 1986. Chromosomal, genic, and morphologic variation in the agile kangaroo rat, Dipodomys agilis (Rodentia: Heteromyidae). Syst Zool 35:311–324. [Google Scholar]
- Bidau CJ, Medina AI, 2013. Sexual size dimorphism and testis size allometry in tuco-tucos (Rodentia: Ctenomyidae). Mammalia 77:81–93. [Google Scholar]
- Bradley RD, Davis SK, Baker RJ, 1991. Genetic control of premating-isolating behavior: Kaneshiro’s hypothesis and asymmetrical sexual selection in pocket gophers. J Hered 82:192–196. [Google Scholar]
- Calede JJM, Rasmussen DLR, 2020. New gophers (Rodentia: Geomyidae) from the Cabbage Patch beds of Montana (Renova Formation) and the phylogenetic relationships within Entoptychinae. Ann Carnegie Mus 86:107–167. [Google Scholar]
- Calede JJM, Samuels JX, Chen M, 2019. Locomotory adaptations in entoptychine gophers (Rodentia: Geomyidae) and the mosaic evolution of fossoriality. J Morphol 280:879–907. [DOI] [PubMed] [Google Scholar]
- Cassini MH, 2020. A mixed model of the evolution of polygyny and sexual size dimorphism in mammals. Mammal Rev 50:112–120. [Google Scholar]
- Castro-Campilllo A, Ramírez-Pulido J, 2000. Systematics of the smooth-toothed pocket gopher Thomomys umbrinus in the Mexican transvolcanic belt. Am Mus Novit 3297:1–37. [Google Scholar]
- Cooper N, Thomas GH, Venditti C, Meade A, Freckleton RP, 2016. A cautionary note on the use of Ornstein Uhlenbeck models in macroevolutionary studies. Biol J Linn Soc 118:64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csuti BA, 1979. Patterns of adaptation and variation in the Great Basin kangaroo rat Dipodomys microps. Univ Calif Publ Zool 111:1–69. [Google Scholar]
- Dale FH, 1939. Variability and environmental responses of the kangaroo rat Dipodomys heermanni saxatilis. Am Midl Nat 22:703–731. [Google Scholar]
- Daly JC, Patton JL, 1986. Growth, reproduction, and sexual dimorphism in Thomomys bottae pocket gophers. J Mammal 67:256–265. [Google Scholar]
- Desha PG, 1967. Variation in a population of kangaroo rats Dipodomys ordii medius (Rodentia: Heteromyidae) from the high plains of Texas. Southwest Nat 12:275–289. [Google Scholar]
- Djawdan M, 1993. Locomotor performance of bipedal and quadrupedal heteromyid rodents. Funct Ecol 7:195–202. [Google Scholar]
- Dunmire WW, 1955. Sex dimorphism in the pelvis of rodents. J Mammal 36:356–361. [Google Scholar]
- Engstrom MD, Genoways HH, Tucker PK, 1987. Morphological variation, karyology, and systematic relationships of Heteromys gaumeri (Rodentia: Heteromyidae). Fieldiana. Zool 39:289–303. [Google Scholar]
- Fabre PH, Hautier L, Dimitrov D, Douzery Ejp, 2012. A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evol Biol 12: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famoso NA, Davis EB, Feranec RS, Hopkins SSB, Price SA, 2016. Are hypsodonty and occlusal enamel complexity evolutionarily correlated in ungulates? J Mammal Evol 23:43–47. [Google Scholar]
- Fernández JA, Hafner MS, Hafner DJ, Cervantes FA, 2014. Conservation status of rodents of the families Geomyidae and Heteromyidae of Mexico. Rev Mex Biodivers 85:576–588. [Google Scholar]
- García-Navas V, 2017. Lack of evolution of sexual size dimorphism in Heteromyidae (Rodentia): the influence of resource defense and the trade-off between pre- and post-copulatory trait investment. Evol Biol 44:56–68. [Google Scholar]
- Genoways HH, 1973. Systematics and Evolutionary Relationships of Spiny Pocket Mice, Genus Liomys. Special Publications, Texas Tech University, NE, USA. 5:1–368. [Google Scholar]
- Genoways HH, Jones JK Jr, 1971. Systematics of southern banner-tailed kangaroo rats of the Dipodomys phillipsii group. J Mammal 52:265–287. [Google Scholar]
- Glass BP, 1947. Geographic variation in Perognathus hispidus. J Mammal 28:174–179. [Google Scholar]
- Grafen A, 1989. The phylogenetic regression. Philos Trans R Soc Lond B Biol Sci 326: 119–157. [DOI] [PubMed] [Google Scholar]
- Hafner JC, Hafner MS, 1988. Heterochrony in rodents. In: McKinney ML, editor. Heterochrony in Evolution. New York, NY: Plenum Publishing Corporation. 217–235. [Google Scholar]
- Hafner MS, Spradling TA, Light JE, Hafner DJ, Demboski JR, 2004. Systematic revision of pocket gophers of the Cratogeomys gymnurus species group. J Mammal 85:1170–1183. [Google Scholar]
- Hafner MS, Light JE, Hafner DJ, Brant SV, Spradling TA. et al. 2005. Cryptic species in the Mexican pocket gopher Cratogeomys merriami. J Mammal 86:1095–1108. [Google Scholar]
- Hafner MS, Hafner DJ, Gonzáles EE, Demastes JW, Spradling TS. et al. 2014. Rediscovery of the pocket gopher Orthogeomys lanius (Rodentia: Geomyidae) in Veracruz, Mexico. J Mammal 95:792–802. [Google Scholar]
- Hall ER, 1941. Revision of the rodent genus Microdipodops. Field Mus Nat Hist Zool Ser 27:233–277. [Google Scholar]
- Hall ER, 1946. Mammals of Nevada. Berkeley, CA: University of California Press. [Google Scholar]
- Hall ER, Dale FH, 1939. Geographic races of the kangaroo rat Dipodomys microps. Occas Pap Mus Zool La State Univ 4:47–63. [Google Scholar]
- Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W, 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24:129–131. [DOI] [PubMed] [Google Scholar]
- Hendricksen RL, 1972. Variation in the plains pocket gopher Geomys bursarius along a transect across Kansas and eastern Colorado. Trans Kans Acad Sci 75:322–368. [Google Scholar]
- Hoffmeister DF, Lee MR, 1967. Revision of the pocket mice, Perognathus penicillatus. J Mammal 48:361–380. [Google Scholar]
- Isaac JL, 2005. Potential causes and life-history consequences of sexual size dimorphism in mammals. Mammal Rev 35:101–115. [Google Scholar]
- Ives AR, Garland T Jr, 2010. Phylogenetic logistic regression for binary dependent variables. Syst Biol 59:9–26. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Noonan MJ, Kitchener AC, Harrington LA, Newman C. et al. 2017. Rensching cats and dogs: feeding ecology and fecundity trends explain variation in the allometry of sexual size dimorphism. R Soc Open Sci 4:170453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WT, 1993. The social systems of heteromyid rodents. In: Genoways HH, Brown JH eds. Biology of the Heteromyidae. Special Publication, The American Society of Mammalogists. 10:575–595. [Google Scholar]
- Jungers WL, Falsetti AB, Wall CE, 1995. Shape, relative size, and size-adjustments in morphometrics. Yearb Phys Anthropol 38:137–161. [Google Scholar]
- Kennedy ML, Schnell GD, 1978. Geographic variation and sexual dimorphism in Ord’s kangaroo rat, Dipodomys ordii. J Mammal 59:45–59. [Google Scholar]
- Legendre P, 2018. lmodel2: Model II Regression. R package version 1.7-3. https://cran.r-project.org/package=lmodel2.
- Lidicker WZ Jr, 1960. An analysis of intraspecific variation in the kangaroo rat Dipodomys merriami. Univ Calif Publ Zool 67:125–218. [Google Scholar]
- Lukas D, Clutton-Brock TH, 2013. The evolution of social monogamy in mammals. Science 341:526–530. [DOI] [PubMed] [Google Scholar]
- Madar SI, Rose MD, Kelley J, MacLatchy L, Pilbeam D, 2002. New Sivapithecus postcranial specimens from the Siwaliks of Pakistan. J Hum Evol 42:705–752. [DOI] [PubMed] [Google Scholar]
- Matějů J, Kratochvíl L, 2013. Sexual size dimorphism in ground squirrels (Rodentia: Sciuridae: Marmotini) does not correlate with body size and sociality. Front Zool 10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk CL, Houck MA, Bradley RD, 1999. Morphometric analysis of seven species of pocket gophers (Geomys). J Mammal 80:499–511. [Google Scholar]
- Mammal Diversity Database, 2020. Mammal Diversity Database (Version 1.31). Zenodo. 10.5281/zenodo.4139818. [DOI]
- Martínez PA, Bidau CJ, 2016. A re-assessment of Rensch’s rule in tuco-tucos (Rodentia: Ctenomyidae: Ctenomys) using a phylogenetic approach. Mamm Biol 81:66–72. [Google Scholar]
- McGarigal K, 2015. Biostats: http://www.umass.edu/landeco/teaching/ecodata/labs/biostats.R. Published date February 25, 2015.
- Miller RS, 1964. Ecology and distribution of pocket gophers (Geomyidae) in Colorado. Ecology 45:256–272. [Google Scholar]
- Millien V, Bovy H, 2010. When teeth and bones disagree: body mass estimation of a giant extinct rodent. J Mammal 91:11–18. [Google Scholar]
- Nader JA, 1978. Kangaroo rats: intraspecific variation in Dipodomys spectabilis Merriam and Dipodomys deserti Stephens. Ill Biol Monogr 49:1–116. [Google Scholar]
- Orme D, 2018. The caper package: comparative analysis of phylogenetics and evolution in R. https://CRAN.R-project.org/package=caper.
- Paradis E, Claude J, Strimmer K, 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. [DOI] [PubMed] [Google Scholar]
- Patton JL, Brylski PV, 1987. Pocket gophers in alfalfa fields: causes and consequences of habitat-related body size variation. Am Nat 130:493–506. [Google Scholar]
- Patton JL, Smith MF, 1993. Molecular evidence for mating asymmetry and female choice in a pocket gopher (Thomomys) hybrid zone. Mol Ecol 2:3–8. [DOI] [PubMed] [Google Scholar]
- Pérez-Barbería FJ, Gordon IJ, Pagel M, 2002. The origins of sexual dimorphism in body size in ungulates. Evolution 56:1276–1285. [DOI] [PubMed] [Google Scholar]
- Price SA, Hopkins SSB, 2015. The macroevolutionary relationship between diet and body mass across mammals. Biol J Linn Soc 115:173–184. [Google Scholar]
- R, Core Team 2019. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Reed KM, Choate JR, 1986. Geographic variation in the plains pocket mouse Perognathus flavescens on the Great Plains. Tex J Sci 38:227–240. [Google Scholar]
- Revell LJ, 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. [Google Scholar]
- Revell LJ, 2013. Ancestral character estimation under the threshold model from quantitative genetics. Evolution 68:743–759. [DOI] [PubMed] [Google Scholar]
- Ritz C, Spiess A-N, 2008. qpcr: an R package for sigmoidal model selection in quantitative real-time polymerase chain reaction analysis. Bioinformatics 24:1549–1551. [DOI] [PubMed] [Google Scholar]
- Roberts HR, Wilkins KT, Flores J, Thompson-Gorozpe A, 1997. Burrowing ecology of pocket gophers (Rodentia: Geomyidae) in Jalisco, Mexico. Southwest Nat 42:323–327. [Google Scholar]
- Robertson RN, Hollander RRK, Jones J Jr, 1992. Secondary Sexual Dimorphism and Geographic Variation in Ord’s Kangaroo rat Dipodomys ordii on the Llano Estacado and in Adjacent Areas of Texas. Occasional Papers, Museum Texas Tech University, Lubbock, TX, USA. 150:1–22. [Google Scholar]
- Schitoskey F Jr, 1968. Notes on morphological variation in the dark kangaroo mouse. Southwest Nat 13:243–248. [Google Scholar]
- Schmidly DJ, 1971. Population variation in Dipodomys ordii from western Texas. J Mammal 52:108–120. [PubMed] [Google Scholar]
- Schmidly DJ, Hendricks FS, 1976. Systematics of the southern races of Ord’s kangaroo rat Dipodomys ordii. Bull South Calif Acad Sci 75:225–237. [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW, 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer HW, 1949. Subspeciation in the Kangaroo Rat Dipodomys Ordii. University of Kansas Publication, Museum of Natural History. 1:473–573. [Google Scholar]
- Smith MF, Patton JL, 1988. Subspecies of pocket gophers: causal baes for geographic differentiation in Thomomys bottae. Syst Zool 37:163–178. [Google Scholar]
- Spradling TA, Demastes JW, Hafner DJ, Milbach PL, Cervantes FA. et al. , 2016. Systematic revision of the pocket gopher genus Orthogeomys. J Mammal 97:405–423. [Google Scholar]
- Stevens RD, Platt RN, 2015. Patterns of secondary sexual size dimorphism in new world Myotis and a test of Rensch’s rule. J Mammal 96:1128–1134. [Google Scholar]
- Straney DO, Patton JL, 1980. Phylogenetic and environmental determinants of geographic variation of the pocket mouse Perognathus goldmani Osgood. Evolution 34:888–903. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Best TL, 1997. Effects of environment on phenotypic variation and sexual dimorphism in Dipodomys simulans (Rodentia: Heteromyidae). J Mammal 78:798–810. [Google Scholar]
- Webb TJ, Freckleton RP, 2007. Only half right: species with female-biased sexual size dimorphism consistently break Rensch’s rule. PLoS One 2:e897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster WMD, Jones JK Jr, 1985. Nongeographic variation, reproduction, and demography in the Texas kangaroo rat, Dipodomys elator (Rodentia: Heteromyidae). Tex J Sci 37:51–61. [Google Scholar]
- Weckerly FW, Best TL, 1985. Morphologic variation among rock pocket mice Chaetodipus intermedius from New Mexico lava fields. Southwest Nat 30:491–501. [Google Scholar]
- Wilkins KT, Roberts HR, 2007. Comparative analysis of burrow systems of seven species of pocket gophers (Rodentia: Geomyidae). Southwest Nat 52:83–88. [Google Scholar]
- Wilkins KT, Schmidly DJ, 1979. Identification and distribution of three species of pocket mice (genus Perognathus) in Trans-Pecos Texas. Southwest Nat 24:17–31. [Google Scholar]
- Williams DF, 1978. Systematics and ecogeographic variation of the Apache pocket mouse (Rodentia: Heteromyidae). Bull Carnegie Mus Nat Hist 10:1–57. [Google Scholar]
- Williams DF, Genoways HH, 1979. A systematic review of the olive-backed pocket mouse Perognathus fasciatus (Rodentia, Heteromyidae). Ann Carnegie Mus 48:73–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.