Abstract
Background : To investigate the possible protective activity of oleuropein compound on noise-induced hearing loss in rats.
Methods: Twenty-eight adult male albino rats were divided into 4 groups. Control normal saline (n = 7) group was kept noise-free. Control oleuropein group (n = 7) group was kept noise-free and was administered with 50 mg/kg/day oleuropein. The experimental normal saline (n = 7) group was subjected to noise. The experimental oleuropein (n = 7) group was subjected to noise and was administered with 50 mg/kg/day oleuropein. The experimental groups were subjected to 4 kHz octave noise with a frequency of 120 dB Sound Pressure Level (SPL) for 4 hours. Hearing level measurements were performed with auditory brainstem response and distortion-product otoacoustic emission tests before and after the 1st, 7th, and 10th day of the noise exposure. On the 10th day, rats were sacrificed. The temporal bones of the rats were removed and the cochlea and spiral ganglion cells were evaluated using hematoxylin–eosin staining under light microscopy.
Results: Better hearing thresholds were achieved in the experimental oleuropein group compared to the experimental normal saline group at 8 kHz, 12 kHz, 16 kHz, and 32 kHz frequencies (P < .05). Although no statistically significant difference was found between the groups, in the experimental normal saline group, the percentage of damaged spiral ganglion cells was higher than the experimental oleuropein group.
Conclusion: Our findings suggest that oleuropein may have a partial protective effect against noise-related hearing loss. However, further research with higher doses is needed to justify this protective effect.
Keywords: Acoustic trauma, animal models, antioxidant, oleuropein, noise-induced hearing loss
Main Points
The antioxidant, anti-inflammatory, antiviral, and anticarcinogenic effects of oleuropein have shown promising results in many studies recently. Our study is the first study in the literature to show that oleuropein is effective on noise-induced hearing loss (NIHL).
Oleuropein could easily be taken orally, so it would provide ease of use and increase patient compliance.
In this study, the effect of oleuropein was demonstrated by objective electrophysiological tests. It was thought that the use of electrophysiological tests would lead to more objective results in determining the effect in similar animal studies.
This partial protective effect detected on NIHL should be supported by further studies and with different doses.
If the results are supported by further research, the clinical use of oleuropein can be made possible and a pharmacological solution can be proposed against NIHL, which is still a significant problem today.
Introduction
Exposure to excessive noise is the most common cause of preventable hearing loss. At least 5%-16% of the global population is at risk of noise-induced hearing loss (NIHL). The World Health Organization reports that one-third of all hearing loss cases are associated with noise.1,2
The most basic strategy for preventing NIHL is to implement regulations to prevent noise from occurring in the first place and to use personal protective equipment, such as earplugs and headphones.3 Although numerous studies have investigated the effect of antioxidant agents in NIHL cases, no widely proven agent has yet been discovered.4
Hearing loss mainly occurs due to mechanical and metabolic damage to the inner ear after exposure to noise. Cellular loss in the inner ear occurs due to apoptotic pathways and necrosis, which are activated as a result of oxidative stress caused by the increase of free oxygen and nitrogen radicals.4
Oleuropein (OLE) can be found in the entire olive tree, but it is mostly isolated from the leaves. Oleuropein’s protective effects have been recently shown against cisplatin-induced ototoxicity, nephrotoxicity, and also cardiotoxicity due to its antioxidant properties.5-7 Moreover, it has antiviral, anti-inflammatory, antiatherogenic, and anticarcinogenic effects.8,9
In this study, rats exposed to noise have been examined by objective audiological and histopathological methods to evaluate the possible protective effects of OLE on NIHL.
Methods
This study was conducted in accordance with the approval of the Local Ethics Committee on Animal Experiments of Dokuz Eylül University University (protocol no: 46/2017, ethics committee decision dated November 14, 2017, numbered 20).
Selection of Animals Used in Research
In this study, 28 Wistar male rats weighing 250-300 g were used. Rats were kept at room temperature, were subjected to 12 hours of light/dark cycles, and were fed standard pellet Wistar rat feed and rested tap water. Water and feed were freely accessible.
Rats with any signs of any external and middle ear pathology were excluded from the study.
Study Groups
The rats were randomly separated into 4 groups. The control normal saline (NS) (n = 7) group was kept noise-free and was administered with 2 cm3/kg/day NS in the same volume as the active substance solution. The control OLE group (n = 7) group was kept noise-free and was administered with 50 mg/kg/day OLE. The experimental NS (n = 7) group was subjected to noise and was administered with 2 cm3/kg/day NS in the same volume as the active substance solution. Finally, the experimental OLE (n = 7) group was subjected to noise and was administered with 50 mg/kg/day OLE. Oleuropein and NS were administered to all rats by oral gavage.
The researchers who performed the audiological evaluations, the agent administration to the rats, and the histological evaluations were blinded.
Anesthesia Method
Anesthesia before audiological tests was induced by intraperitoneal route via 50 mg/kg 10% ketamine (ketasol 10 mL, 100 mg/mL vial, Richterpharma ag, Wels, Austria) and 5 mg/kg xylazine (xylazinbio 2% 50 mL, 20 mg/mL, Bioveta plc., Ivanovice na Hané, Vyškov,Czechia).
Evaluation of Auditory Functions
Audiological tests were performed under anesthesia and in a room with ambient noise below 40 dB SPL (A). Distortion-product otoacoustic emission (DPOAE) tests were conducted using the “Autodynamics ILO-V6 Cochlear Emission Analyzer,” version 5.61 (Otodynamics, London, UK). For the DPOAE test, the ratio between f2 and f1 frequencies (f2/f1) was set to 1.22. The difference between L1 and L2 levels was kept at 10 dB SPL (L1 = 75 dB SPL, L2 = 65 dB SPL). Distortion-product otoacoustic emission was measured at frequency 2f1−f2. All values with a signal-to-noise ratio above 3 dB were evaluated as a positive response for each frequency. Signal-to-noise ratios at 1 Hz, 1.5 Hz, 2 Hz, 3 Hz, 4 Hz, 6 Hz, and 8 Hz were recorded for the geometric averages of DPOAE, f1, and f2.
Intelligent Hearing Systems (IHS Corp., Miami, Fla, USA) device Smart-EP 10 version was used for auditory brainstem response (ABR) tests. Calibration of the device was conducted by IHS Corp. A tone burst at 37.1/s repetition frequency at 4 kHz, 8 kHz, 12 kHz, 16 kHz, and 32 kHz in alternating polarity with Blackman envelope to further narrow the frequency spectrum of the stimulus and with an up and downtime of 1000 ms was used as the stimulus. The lowest level of intensity at which the second wave was obtained was considered to be the hearing threshold of the rat at that frequency. The active electrode was placed in the vertex, the reference electrode in the test ear, and the ground electrode in the ventrolateral (under the ear) of the opposite ear. Bioelectrical responses collected by electrodes were passed through a 30 Hz-3000 Hz band permeable filter and converted from analog to digital at a sampling rate of 31.3 ms. The waves recorded with 1024× averaging were recorded twice in a row until the last level of intensity at which the threshold was detected. Auditory brainstem response and DPOAE tests were performed on the 1st, 7th, and 10th day of the experiment.
Creating a Noise-Induced Hearing Loss Model
Rats were exposed to 1 octave-band noise centered at 4 kHz at 120 dB SPL for 4 hours in a cage. Speakers (Spekon CT-51AS, Hangzhou, China) by a noise generator and power amplifier (König PRO-2008S, Nedis B.V., Den Bosch, The Netherlands) were used to produce noise. Sound level calibrations were tested at different points of the cage to ensure the stability of the stimulus.
Administration of Agent
The powder form of OLE (Santa Cruz Biotechnology Inc., CAS no:32619-42-4, Dallas, Tex, USA) was prepared by dissolving in distilled water (50 mg/2 cm3). The daily doses of the prepared solution were separately placed in Eppendorf tubes and stored at −18°C. Every day, the agent was dissolved and brought to room temperature immediately before application and then administered to rats via oral gavage.
In the NS group, the same volume of NS (2 cm3/kg/day) as OLE was administered by oral gavage from the 1st day to the 10th day at 24 hours intervals. Control OLE and NS groups were administered with OLE and NS according to the same schedule as the noise groups.
Histological Examination
Hematoxylin–Eosin Staining
The right and left temporal bones of all rats were dissected as a whole under ether anesthesia after audiological examinations on the 10th day. Temporal bones were immediately placed in 10% formalin solution 10 times their volume. They were fixed for 48 hours at room temperature. Tissue samples were decalcified in 5% glacial acetic acid solution for 5 days. When decalcified, samples were checked by cross-sectioning, washed under tap water for 1 hour, and placed in cassettes for routine paraffin tissue sampling. Five-micron sections taken from the prepared paraffin blocks were stained with hematoxylin–eosin (H&E) staining and histopathologically evaluated. After staining, preparations were recorded and digitally evaluated by an image analysis system comprising Olympus BX-50 light microscope (Olympus DP Controller ver. 3.1.1.267, Olympus Corp, Shinjuku, Tokyo, Japan) and camcorder (Samsung SCC-101BP, 520, Samsung Corp., Seocho, Seoul, South Korea). Structure and sequence of cells forming Corti organ in cochlea samples, the number of picnotic changes in the nucleus of spiral ganglia cells, the presence of chromatolysis, and the presence of vacuolization in the stria vascularis were evaluated and compared with the control group. All of the cells in spiral ganglia were counted and picnotic cell number was calculated as a percentage.
Evaluation of Data and Statistical Analysis
Statistical analysis was performed at P < .05 significance level with Statistical Package for the Social Sciences software for Windows version 24.0 (IBM SPSS Corp.; Armonk, NY, USA). All data were presented as mean ± standard deviation. Non-parametric tests were performed as the Shapiro–Wilk test revealed that the data were not within normal distribution limits. Kruskal–Wallis variance analysis was used to analyze descriptive statistics and intergroup differences. Mann–Whitney U test was used to determine which group the difference originated from. Intra-group measurements were evaluated with Friedman variance analysis and Wilcoxon signed-rank test.
Results
Auditory Assessments
Auditory Brainstem Response
No significant difference was present between groups in baseline hearing evaluation (P > .05). Moreover, no significant difference was observed in ABR thresholds performed before and on days 1, 7, and 10 after agent administration at all frequencies of the control NS and OLE groups (P > .05).
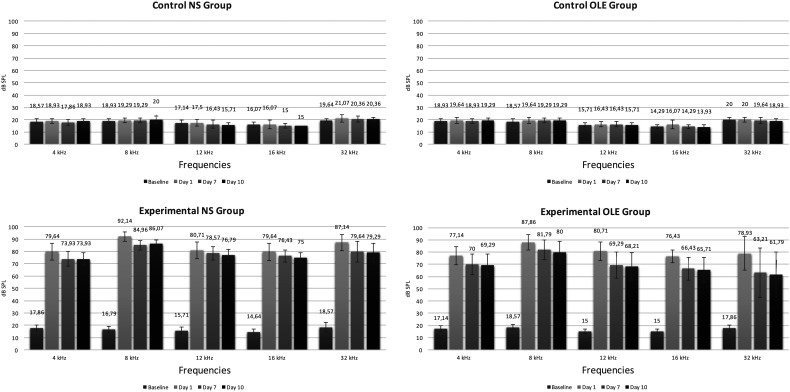
A statistically significant loss was observed between baseline and day 1 hearing measurements of experimental NS and OLE groups at all frequencies (P < .01). The frequency with the most significant loss after the noise was determined to be 8 kHz (Figure 1).
Figure 1.
Comparison of ABR thresholds ± standard deviation values of all groups on baseline and on days 1, 7, and 10 after noise.
In the experimental NS group, a significant difference was present in hearing loss between days 1 and 7 and days 1 and 10 after noise at 4 kHz, 8 kHz, and 32 kHz ABR thresholds and between days 1 and 10 at 12 kHz and 16 kHz ABR thresholds; but no significant difference was observed between days 7 and 10 measurements (P > .05).
In the experimental OLE group, a statistically significant difference was observed between days 1 and 7 and 1 and 10 thresholds after noise at all frequencies, whereas no significant difference was observed between days 7 and 10 measurements (P > .05).
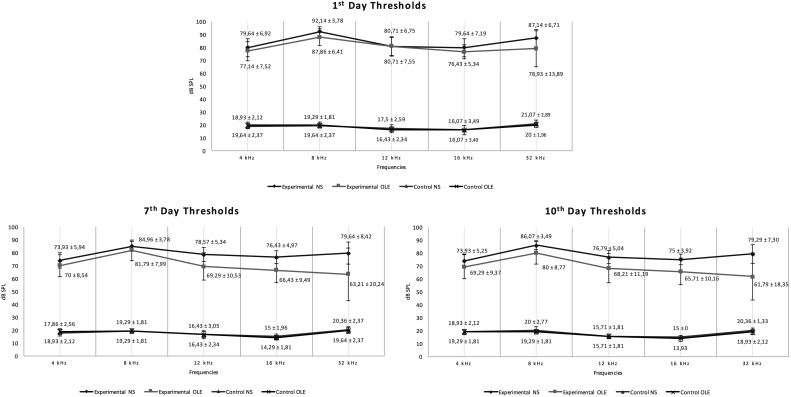
Comparison of the audiological measurements between experimental OLE and NS groups revealed that hearing thresholds in the experimental OLE group were significantly better than the experimental NS group at 8 kHz, 12 kHz, 16 kHz, and 32 kHz frequencies (P < .05) at both 7th- and 10th-day measurements (Figure 2).
Figure 2.
ABR thresholds and standard deviation values of all groups on the 1st, 7th, and 10th day after noise.
No statistical difference was observed within the groups between the right and left ears on baseline and days 1, 7, and 10 hearing thresholds of the experimental and control groups.
Distortion-Product Otoacoustic Emission No statistically significant difference was observed between baseline DPOAE amplitude averages of all groups. Furthermore, no statistically significant difference was observed between days 1, 7, and 10 DPOAE amplitude averages of control NS and OLE groups (P > .05).
Statistical analysis could not be performed because DPOAE responses were completely lost on days 1, 7, and 10 in the experimental NS and OLE groups.
Histopathological Imaging Results
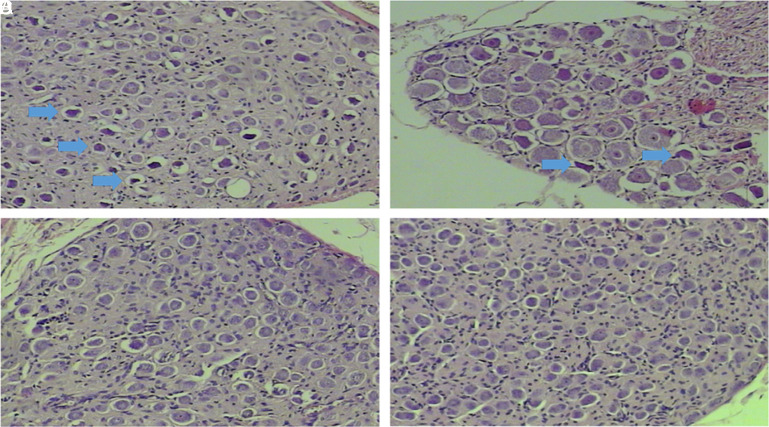
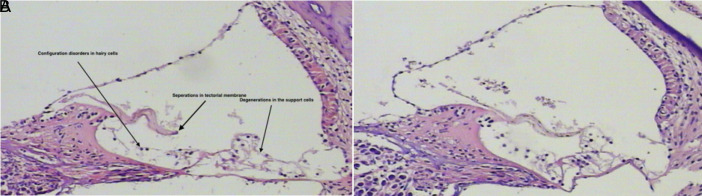
In histopathological examinations, H&E staining of experimental groups revealed marked picnotic changes, especially in spiral ganglion cells, reduction in cell count, Schwann cell proliferation, and cell morphology disturbances compared to the control group (Figure 3). Examinations of the Corti organ revealed deteriorations in cell configuration (Figure 4).
Figure 3. a-d.
H&E staining of spiral ganglion cross-section of all groups. (a) Experimental NS group 100×, (b) experimental OLE group 200×, (c) Control NS group 100×, (d) Control OLE group 100×. Prominent picnotic cells (blue thick arrows) in the experimental OLE group (b) are lesser than the experimental NS group (a), while no picnosis was observed in the control NS and OLE group (c and d). H&E, hematoxylin–eosin; OLE, oleuropein; NS, normal saline.
Figure 4. a,b.
H&E staining of Corti organ section belonging to the experimental OLE and NS group in 100× magnification. Morphological deteriorations in cells are shown by the arrows experimental NS group (a) and experimental OLE group (b). H&E, hematoxylin–eosin; OLE, oleuropein; NS, normal saline.
In light of microscopic examination, picnotic cell percentages in rat spiral ganglia were individually evaluated for each rat in all groups. Consequently, the percentage of picnotic cells in the experimental OLE group was found to be lower than that in the experimental NS group (Table 1).
Table 1.
Mean ± Standard Deviation and Minimum/Maximum Values of Picnotic Cell Percentages in Spiral Ganglia in Light Microscopic Examination Performed with H&E Staining in All Groups
| Picnotic Cell % Groups | Mean ± Standard Deviation % | Min/Max, % |
| Control NS | 0.86 ± 0.90 | 0/2 |
| Control OLE | 0.86 ± 0.90 | 0/2 |
| Experimental NS | 20 ± 3.21 | 16/25 |
| Experimental OLE | 16.29 ± 4.71 | 11/24 |
OLE, oleuropein; NS, normal saline.
According to the Mann–Whitney U test, the difference between the experimental NS and the experimental OLE group was not statistically significant (P > .05).
Discussion
The most commonly used substances for protection from NIHL in animal models and clinical trials are antioxidant group agents. These agents act by suppressing oxidative stress in NIHL. In the present study, the possible protective effects of OLE, which is considered to have antioxidant properties, against NIHL were investigated by audiological and histopathological methods. This study is important as, to the best of our knowledge, it is the first study in English literature investigating the effect of OLE on NIHL.
To investigate the protective effect of OLE in cisplatin ototoxicity, Çelik et al5 applied OLE to 24 female rats for 15 days at a dose of 50 mg/kg/day via oral gavage and performed DPOAE measurements. Day 15 DPOAE measurements of the group receiving cisplatin and OLE were found to be significantly better than those of the group given only cisplatin. In the present study, the protective effect of OLE was also shown in the high-frequency ABR test.
Many studies have stated that OLE has antioxidant, anti-inflammatory, antiviral, and anticancer effects. Zhang et al6 administered 20 mg/kg/day OLE via oral gavage for 4 weeks in an experimental autoimmune myocarditis model created in rats. They found that OLE reduced inflammatory cell infiltration in rat myocardial tissue and decreased proinflammatory cytokines levels and T-lymphocyte proliferation.
In the cisplatin-induced acute renal insufficiency model created by Potocnjak et al7 in mice, 5, 10, and 20 mg/kg/day OLE was given via oral gavage for 2 days 48 hours after cisplatin administration. Urea and creatinine levels were lower in the OLE administered group in comparison to the non-administered group, the expression of CYP2E1, an oxidative enzyme; 4-hydroxinonenal (4-HNE) and 3-nitrotyrosine (3-NT), the end products of lipid peroxidation, decreased and the level of proinflammatory cytokines, such as tumor necrosis factor-alpha, decreased. In addition, p53, Bax, and Bcl-2 were analyzed by Western blot technique, and the terminal deoxynucleotidyl transferase dUTP nick-end labeling method concluded that renal apoptosis was suppressed. They noted that the most effective dose was 20 mg/kg/day.7 Similarly, Geyikoğlu et al10 intraperitoneally administered 50, 100, and 200 mg/kg/day OLE for 3 days in the cisplatin-induced acute renal insufficiency model. Oxidative damage was demonstrated by measuring 8-hydroxy-2-deoxyguanosine (8-OHdG) levels, which is a reliable indicator of oxidative DNA damage, and malondialdehyde (MDA) levels, an indicator of lipid peroxidation. Levels of 8-OHdG and MDA similarly decreased in groups receiving 100 and 200 mg/kg/day OLE. This was interpreted as evidence for the strong antioxidant effects of OLE at a dose of 100 mg/kg/day and its protective effects against cisplatin-induced renal cell damage.
In the NIHL model created in our study, the use of OLE was preferred to benefit from its effects suppressing apoptosis and decreasing proinflammatory cytokines and lipid peroxidation end products. A review of past studies shows that OLE has been administered in different doses, by different routes, as well as in different models. However, no study has used OLE in the NIHL model. Based on earlier administration of 50 mg/kg/day OLE in cisplatin ototoxicity studies and other studies reporting that the effective dose range is 20-100 mg/kg/day, OLE dose was chosen as 50 mg/kg/day.
In this study, OLE was administered through oral gavage as it can be easily taken via this route by people exposed to noise. Oleuropein reaches its maximum plasma concentration after 30 minutes to 2 hours following oral intake and it is absorbed from the stomach and jejunum. Depending on the dose and route of administration, its absorption and excretion change. It reportedly conjugates faster in males.11,12
In the literature on NIHL, one can notice that investigated agents were given before and/or after the noise exposure. Le Prell4 claims that antioxidant treatment started in the first 24 hours after noise exposure is as effective as those started before but should not be delayed by more than 3 days. Yamashita et al13 stated that the production of free oxygen radicals and free nitrogen radicals begins immediately after the noise and continues for 7-10 days, and antioxidant therapy administered within this period will be effective, and they also reported that after 10 days, the outer hair cell loss and ABR thresholds stabilized.13 Thus, OLE treatment was started 1 hour after the noise and applied for 10 days at intervals of 24 hours. Since we cannot always predict when the noise will be encountered, and in terms of ease of use, we planned to investigate the effects of oral use of OLE after trauma.
No universal standard was used in the creation of the NIHL model. In the study conducted by Kashani et al14 using the same model as that in the present study, N-acetyl cysteine was used and the highest ABR threshold was found at 50 dB levels on day 1. In other studies where the noise was centered at 4 kHz octave band and 120 dB SPL intensity for 5 h, 4 kHz, 8 kHz, and 16 kHz ABR thresholds on day 1 were observed around 50 dB and 60 dB.15,16 In the model of Ogurlu et al17 using thymoquinone in rats, the noise was centered at 4 kHz octave band with 120 dB SPL intensity for 4 hours and the heads of the rats were placed 5-7 cm away from the speakers. On the first day after trauma, ABR measurements revealed 45-50 dB thresholds at frequencies of 2 kHz and 4 kHz.17 In the present study, hearing thresholds at day 1 after noise trauma measured by ABR were the highest at 92 dB for 8 kHz frequency and the lowest at 76 dB for 16 kHz frequency. Compared to other studies in literature, hearing thresholds obtained in the present study are higher.
Le Prell et al15 applied noise centered at 4 kHz octave band with 120 dB SPL intensity for 5 hours on guinea pigs. They intraperitoneally administered vitamins A, C, and E and magnesium 1 hour before the noise and up to 5 days after. They found hearing thresholds of 40 dB at 4 kHz and 50 dB at 8 kHz and 16 kHz in ABR after noise, and they achieved about 30 dB hearing gain after treatment. In addition to this study, the hearing gain detected with ABR in many studies using antioxidants, such as glutathione, d-methionine, resveratrol, coenzyme Q, and ginkgo biloba, varies between 10 dB and 30 dB.2,18-20 In the present study, better thresholds of 8-17 dB were achieved at all frequencies compared to day 1 in the experimental OLE group. Day 10 ABR thresholds of the experimental OLE group were 4.6-17.5 dB better at all frequencies compared to the thresholds of the experimental NS group. In terms of the healing effect, a statistically significant difference was observed at frequencies of 8 kHz, 12 kHz, 16 kHz, and 32 kHz (P < .05). These findings were evaluated to be lower than other averages in the literature. However, the hearing loss generated in most of the models mentioned above is moderate or moderate-advanced, and the protective effect of agents is investigated in these models. In the present study, a low average hearing gain can be associated with advanced and very advanced hearing loss after noise.
In the studies of Attias et al21 and Balatsouras et al.22 DPOAE was reported to be a useful test for detecting the initial effects of noise in the NIHL models and evaluating responses to antioxidants. Another study reported that DPOAE responses may be lost in threshold changes above 40 dB, and this test typically yields information about the auditory function and external hair cell damage up to 30-35 dB NIHL hearing loss, whereas no clear information about hair cell function or the state of the inner ear can be obtained at 35-100 dB NIHL hearing loss. Distortion-product otoacoustic emission is also unable to provide information about inner hair cells and the auditory nerve.23 In the present study, DPOAE responses were lost after the noise and statistical evaluation could not be made. Therefore, we believe that it will be appropriate to use electrophysiological tests to assess auditory functions in experimental studies.
In the histopathological evaluation in our study, H&E staining was performed on 5-micron sections prepared from the inner ears of rats in all groups and evaluated under light microscopy. In many samples belonging to the experimental NS and experimental OLE groups, configuration disorders in hairy cells in the Corti organ, separations in the tectorial membrane, and degenerations in the support cells were observed. However, in the sections where spiral ganglia were imaged, the picnotic cell ratio in the experimental OLE group was lower than the experimental NS group (16.29% vs. 20%), although this difference was not reflected in statistical analyses. In literature, it is known that the mechanisms of permanent threshold change include external and internal hairy cell losses, followed by degeneration of spiral ganglion and afferent nerve fibers.24-27 In our study, the above described histological findings were observed in the Corti organ and spiral ganglion cells in accordance with the literature.
Conclusion
Our findings suggest that OLE has a partial protective effect against NIHL as reflected by audiological results. We also think that more prominent protection may be achieved with higher doses of OLE in terms of both audiological and histopathological results. Further studies with different doses of OLE are needed to reveal the mechanism of this protection.
Footnotes
Ethics Committee Approval: This study was conducted in accordance with the approval of the Local Ethics Committee on Animal Experiments of Dokuz Eylül University (protocol no: 46/2017, ethics committee decision dated 14/11/2017, numbered 20).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – Ö.K., Y.O., S.M.D., S.S., S.A., G.K. ; Design – Ö.K., S.M.D., Y.O., S.S., S.A., G.K.; Supervision – G.K., S.S., S.A.; Resources – Ö.K., S.S.; Materials – Ö.K., S.S.; Data Collection and/or Processing – Ö.K., S.M.D., Y.O., S.A., S.S., G.K.; Analysis and/or Interpretation – Ö.K., S.M.D., S.A.; Literature Search – Ö.K., S.M.D., Y.O.; Writing Manuscript – Ö.K., Y.O., S.M.D.; Critical Review – Y.O., S.M.D., G.K., S.A., S.S.
Acknowledgments: The authors would like to thank Meryem Çalişir* for her valuable contributions in animal experiments.
*Department of Laboratory Of Animal Science, Dokuz Eylül University Faculty of Medicine, İzmir, Turkey.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: This research was financially supported by Dokuz Eylul University Department of Scientific Research Projects with the project number: 2018.KB.SAG.037.
References
- 1. Le TN, Straatman LV, Lea J, Westerberg B. Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J Otolaryngol Head Neck Surg. 2017;46(1):41. 10.1186/s40463-017-0219-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sha SH, Schacht J. Emerging therapeutic interventions against noise-induced hearing loss. Expert Opin Investig Drugs. 2017;26(1):85–96.. 10.1080/13543784.2017.1269171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oishi N, Schacht J. Emerging treatments for noise-induced hearing loss. Expert Opin Emerg Drugs. 2011;16(2):235–245.. 10.1517/14728214.2011.552427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. 2007;226(1-2):22–43.. 10.1016/j.heares.2006.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Çelik M. Ratlarda Cisplatin Ototoksisitesinde Oleuropein Kullanımının Protektif Etkinliği [Unpublished Master’s thesis]. Kahramanmaraş: , Turkey: Kahramanmaraş Sütçü Imam University; 2016. [Google Scholar]
- 6. Zhang JY, Yang Z, Fang K, Shi ZL, Ren DH, Sun J. Oleuropein prevents the development of experimental autoimmune myocarditis in rats. Int Immunopharmacol. 2017;48:187–195.. 10.1016/j.intimp.2017.05.013) [DOI] [PubMed] [Google Scholar]
- 7. Potočnjak I, Škoda M, Pernjak-Pugel E, Peršić MP, Domitrović R. Oral administration of oleuropein attenuates cisplatin-induced acute renal injury in mice through inhibition of ERK signaling. Mol Nutr Food Res. 2016;60(3):530–541.. 10.1002/mnfr.201500409) [DOI] [PubMed] [Google Scholar]
- 8. Umeno A, Takashima M, Murotomi K, et al. Radical-scavenging activity and antioxidative effects of olive leaf components oleuropein and hydroxytyrosol in comparison with homovanillic alcohol. J Oleo Sci. 2015;64(7):793–800.. 10.5650/jos.ess15042) [DOI] [PubMed] [Google Scholar]
- 9. Beauchamp GK, Keast RSJ, Morel D, et al. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature. 2005;437(7055):45-46. 10.1038/437045a) [DOI] [PubMed] [Google Scholar]
- 10. Geyikoglu F, Emir M, Colak S, et al. Effect of oleuropein against chemotherapy drug-induced histological changes, oxidative stress, and DNA damages in rat kidney injury. J Food Drug Anal. 2017;25(2):447–459.. 10.1016/j.jfda.2016.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Bock M, Thorstensen EB, Derraik JGB, Henderson HV, Hofman PL, Cutfield WS. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol Nutr Food Res. 2013;57(11):2079–2085.. 10.1002/mnfr.201200795) [DOI] [PubMed] [Google Scholar]
- 12. Rubió L, Valls RM, Macià A, et al. Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chem. 2012;135(4):2922–2929.. 10.1016/j.foodchem.2012.07.085) [DOI] [PubMed] [Google Scholar]
- 13. Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019(1-2):201–209.. 10.1016/j.brainres.2004.05.104) [DOI] [PubMed] [Google Scholar]
- 14. Motalebi Kashani M, Saberi H, Hannani M. Prevention of acoustic trauma-induced hearing loss by N-acetylcysteine administration in rabbits. Arch Trauma Res. 2013;1(4):145–150.. 10.5812/atr.7839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic Biol Med. 2007;42(9):1454–1463.. 10.1016/j.freeradbiomed.2007.02.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Minami SB, Yamashita D, Schacht J, Miller JM. Calcineurin activation contributes to noise-induced hearing loss. J Neurosci Res. 2004;78(3):383–392.. 10.1002/jnr.20267) [DOI] [PubMed] [Google Scholar]
- 17. Ogurlu M, Celebi Erdivanli O, Tumkaya L, et al. The therapeutic effect of thymoquinone on acoustic trauma-induced hearing loss in rats. Eur Arch Otorhinolaryngol. 2017;274(2):743–749.. 10.1007/s00405-016-4319-4) [DOI] [PubMed] [Google Scholar]
- 18. Olgun Y, Kırkım G, Kolatan E, et al. Friend or foe? Effect of oral resveratrol on cisplatin ototoxicity. Laryngoscope. 2014;124(3):760–766.. 10.1002/lary.24323) [DOI] [PubMed] [Google Scholar]
- 19. Claussen AD, Fox DJ, Yu XC, et al. D-methionine pre-loading reduces both noise-induced permanent threshold shift and outer hair cell loss in the chinchilla. Int J Audiol. 2013;52(12):801–807.. 10.3109/14992027.2013.840933) [DOI] [PubMed] [Google Scholar]
- 20. Sjostrand AP, Dogan R, Kocyigit A, Karatas E, Budak BB, Ozturan O. Therapeutic efficacy of Ginkgo biloba for early-period noise-induced hearing loss: an experimental animal study. Am J Otolaryngol. 2016;37(5):416–424.. 10.1016/j.amjoto.2016.05.004) [DOI] [PubMed] [Google Scholar]
- 21. Attias J, Bresloff I, Reshef I, Horowitz G, Furman V. Evaluating noise induced hearing loss with distortion product otoacoustic emissions. Br J Audiol. 1998;32(1):39–46.. 10.3109/03005364000000049) [DOI] [PubMed] [Google Scholar]
- 22. Balatsouras DG, Tsimpiris N, Korres S, Karapantzos I, Papadimitriou N, Danielidis V. The effect of impulse noise on distortion product otoacoustic emissions. Int J Audiol. 2005;44(9):540–549.. 10.1080/14992020500190201) [DOI] [PubMed] [Google Scholar]
- 23. Salvi R, Boettcher FA. Animal models of noise-induced hearing loss. In: Conn PM.ed. Sourcebook of Models for Biomedical Research. Totowa, NJ: Humana Press; 2008:289–301.. [Google Scholar]
- 24. Lonsbury Martin BL, Martin GK. Noise-induced hearing loss. In: Flint PW, Haughey BH, Lund V, et al., eds. Cummings Otolaryngology. 6th ed. Canada: Elsevier Inc.; 2015:2345–2358.e3.. [Google Scholar]
- 25. Liberman MC. Noise-induced hearing loss: permanent versus temporary threshold shifts and the effects of hair cell versus neuronal degeneration. Adv Exp Med Biol. 2016;875:1–7.. 10.1007/978-1-4939-2981-8_1) [DOI] [PubMed] [Google Scholar]
- 26. Kurabi A, Keithley EM, Housley GD, Ryan AF, Wong AC-Y. Cellular mechanisms of noise-induced hearing loss. Hear Res. 2017;349:129–137.. 10.1016/j.heares.2016.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng HW, Chen J, Sha SH. Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death Dis. 2014;5:e1262. 10.1038/cddis.2014.177) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a