Abstract
Background : Nowadays, immunosuppressant drugs are widely used to prevent rejection in organ transplantation and to treat autoimmune diseases. Ototoxicity related to immunosuppressant drugs has been anecdotally reported but scarcely investigated. The aim of this investigation was to systematically review the available data on ototoxicity due to immunosuppressant therapy for transplantation or autoimmune disease.
Methods: A search of electronic databases (PubMed, Web of Science, and Scopus) was performed in order to identify studies concerning otovestibular toxicity due to immunosuppressant therapy for transplantation or autoimmune disease between January 1980 and November 2020.
Results: Eighteen articles were considered eligible for the review. Totally 131 patients experienced ototoxicity related to immunosuppressive treatment. Hearing loss was the most common clinical manifestation (128 cases) and was mainly bilateral. Tinnitus was reported in 52 cases and vertigo in 2. The immunosuppressant drugs most frequently involved in ototoxic manifestations were calcineurin inhibitors (cyclosporine and tacrolimus), often related to their high serum levels.
Conclusion: Immunosuppressant-related ototoxicity is clinically relevant in uncommon but definitely challenging situations. Clinicians should be aware of this and inquire about hearing impairment symptoms during therapy and refer symptomatic patients to an otolaryngologist/audiologist. Further large-scale, prospective investigations are necessary to better characterize the ototoxicity of each class of immunosuppressants.
Keywords: Autoimmune diseases, hearing loss, immunosuppressant drugs, ototoxicity, tinnitus, transplantation, vestibular disorders
Introduction
Immunosuppressant drugs are currently used in a wide spectrum of clinical conditions, ranging from autoimmune diseases to rejection prevention in organ transplantation. Most of these drugs show a narrow therapeutic index, which means that they should be carefully dosed and the patient should be monitored frequently in order to balance therapeutic and adverse effects.1 Categories of immunosuppressant drugs include calcineurin inhibitors (e.g., cyclosporine and tacrolimus), alkylating agents (e.g., cyclophosphamide and chlorambucil), purine synthesis inhibitors (e.g., azathioprine and mycophenolate salts), anti-folic agents (e.g., methotrexate), tumor necrosis factor α blockers (anti-TNFα) (e.g., etanercept and infliximab), and monoclonal antibodies (e.g., muromonab).
Immunosuppressants may have relevant drug–drug interactions among one another when used in combination, as well as significant interactions with other drugs prescribed to treat the side effects and comorbid illnesses that commonly occur in transplanted patients and in those with autoimmune disorders.1
A wide spectrum of toxicities due to immunosuppressive therapy has been reported: (i) increased infection risk (in almost all cases); (ii) myelotoxicity and hematological changes (in particular due to alkylating agents, purine synthesis inhibitors, and anti-TNFα); (iii) carcinogenesis (especially due to alkylating agents and azathioprine in a direct way but also due to other categories as a result of a decreased immune response); (iv) gastrointestinal toxicity (due to alkylating agents, calcineurin inhibitors, and purine synthesis inhibitors); and (v) neurotoxicity (specifically related to alkylating agents, calcineurin inhibitors, anti-TNFα).2-7
Although it has been reported that patients with an autoimmune disease8 or who received organ transplantation frequently complain about hearing impairment,9,10 ototoxicity related to immunosuppressant drugs has scarcely been investigated. In particular, a systematic overview of clinical features, prognosis, and response to drug withdrawal is still lacking for immunosuppressant-related ototoxicity. The aim of this investigation was to systematically review the available data on ototoxicity due to immunosuppressant therapy for transplantation or autoimmune diseases.
Materials and Methods
Electronic Database Search
A search of the English literature from January 1, 1980, to November 30, 2020, was performed on the electronic databases namely Pubmed, Web of Science, and Scopus. The following search terms were used: “immunosuppressant” or “tacrolimus” or “sirolimus” or “azathioprine” or “methotrexate” or “cyclosporine” or “cyclophosphamide” or “etanercept” or “infliximab” or “anakinra” or “mycophenolate” or “muromonab” and “ototoxicity” or “vertigo” or “hearing loss.” MeSH terms and keywords were combined accordingly on the abovementioned databases. The grey literature was also searched on the Opengrey database. The “Related articles” option on the PubMed homepage was also considered. The reference lists of all included articles were carefully checked in order to identify other pertinent studies.
Inclusion and Exclusion Criteria
Studies were included in our investigation when the following general criteria were met: (i) articles were original case series or case reports; (ii) ototoxicity due to immunosuppressant therapy was reported; (iii) detailed information was given about diagnostic workout, drug exposure, audiological evaluation, and outcome; and (iv) English language. Exclusion criteria were (i) animal studies; (ii) concurrent exposure to other known ototoxic drugs; and (iii) studies on immunosuppressant drugs used for cancer treatment.
Data Extraction
The authors accurately screened literature data. Included studies were analyzed to extract all available data and ensure the eligibility of all patients. Demographics, conditions requiring immunosuppressant treatment, otovestibular symptoms, drug exposure, diagnostic procedures, outcomes, and follow-up were analyzed and recorded for all studies. Any disagreements were solved by a discussion among the study team members.
Results
Retrieving Studies
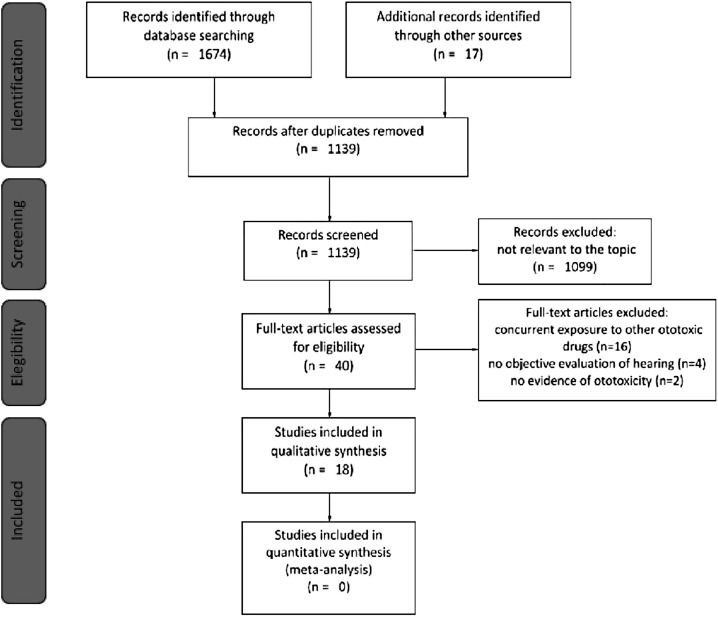
A total of 1691 titles were retrieved from the database search and from cross-references checking, of which 1139 were unique studies (Figure 1; Preferred Reporting Items for Systematic Reviews and Meta-Analysis; the last search was on November 30, 2020). After the selection based on title, abstract, and subsequent full-text screening, 18 articles were considered eligible for the review according to the abovementioned inclusion and exclusion criteria.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow chart. Article screening from identification to inclusion according to PRISMA statements.
Assessing Studies
A quality assessment of the included studies is reported in Table 1. All 18 included articles were identified as having high relevance to the topic. In total, 3 of the studies were retrospective case series,8,11,12 2 were prospective uncontrolled studies,13,14 1 was a case–control study,15 and 12 were case reports.10,15-25
Table 1.
Relevance and Risk of Bias for the Considered Studies
| Relevance | Risk of Bias | |||||||
| First Author (Year) | Design | Patients (a) | Exposure (b) | Cochleovestibular Toxicity (c) | Outcome (d) | Accuracy of Experimental Design (e) | Selective Reporting (f) | Sample Size (g) |
| ||||||||
| Hartnick (2000)11 | RCS | ● | ● | ● | ● | ● | ○ | ○ |
| Fortes (2008)13 | PCS | ● | ● | ● | ○ | ● | ● | ○ |
| Rifai (2012)12 | RCS | ● | ○ | ● | ○ | ○ | ○ | ○ |
| Gulleroglu (2015)14 | PCS | ● | ● | ● | ● | ● | ● | ○ |
| Arinsoy (1993)21 | CR | ● | ● | ● | ● | N/A | N/A | N/A |
| Hartnick (1997)20 | CR | ● | ● | ● | ● | ○ | N/A | N/A |
| Min (1999)16 | CR | ● | ● | ● | ● | ○ | N/A | N/A |
| Marioni (2004)10 | CR | ● | ● | ● | ● | ● | N/A | N/A |
| Norman (2006)18 | CR | ● | ● | ● | ● | ○ | N/A | N/A |
| Rifai (2006)25 | CR | ● | ● | ● | ● | ○ | N/A | N/A |
| Gulleroglu (2013)26 | CR | ● | ● | ● | ● | ○ | N/A | N/A |
| Jenkinson (2014)22 | CR | ○ | ● | ● | ● | ○ | N/A | N/A |
| Lakshmi(2020)24 | CR | ● | ● | ● | ○ | ○ | N/A | N/A |
| ||||||||
| Savastano (2010)8 | RCS | ● | ● | ● | ○ | ○ | ● | ○ |
| Ahmadzadhe (2017)15 | CCS | ● | ○ | ● | ○ | ○ | ● | ○ |
| Porges (1998)19 | CR | ○ | ● | ● | ○ | ○ | N/A | N/A |
| Swale (2005)17 | CR | ○ | ● | ● | ● | N/A | N/A | N/A |
| Tuknayat (2017)23 | CR | ○ | ● | ● | ● | ○ | N/A | N/A |
CCS, case–control study; CR, case report; N/A, not applicable; PCS, prospective cohort study; RCS, retrospective cohort study.
Patients (a): requiring immunosuppressive therapy ● after transplantation; ○ for autoimmune diseases;
Exposure (b): ● reported immunosuppressant drugs with the detailed protocol used or serum level at symptom onset; ○ missing information;
Cochleovestibular toxicity (c): ● tinnitus, vertigo, or HL (confirmed by audiometry) attributed to immunosuppressant regimen; ○ ototoxicity not reported;
Outcome (d): ● hearing recovery/stabilization confirmed by audiometry after therapy discontinuation or dose correction; ○ hearing worsening or outcome not specified;
Selective reporting (e): ● well-defined and adequately described inclusion and exclusion criteria; ○ inadequate;
Accuracy of experimental design (f): ● as ASHA Guidelines for the Audiologic Management of Individuals Receiving Cochleotoxic Drug; ○ inadequate;
Sample size (g): ● sample size adequacy to detect rare adverse events according to Wu Yu-Te et al53; ○ inadequate.
Randomization, concealed allocation, and baseline comparability were not achieved in any of the controlled studies, while such parameters were not applicable to the remaining articles. Incomplete data reporting and lack of outcome standardization were an issue in the majority of the included studies (Table 1).
Study Characteristics
Totally 8 studies regarded patients who had undergone kidney transplantation,10,11,14,20-22,24,26 1 was a kidney-pancreas transplantation case,16 and 4 were case reports regarding liver transplantation.12,13,18,25 The remaining 5 studies included patients with various autoimmune diseases (nephrotic syndrome, rheumatoid arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, and pemphigus vulgaris).8,15,17,19,23 Table 2 summarizes the characteristics of patients included in each study, the immunosuppressive regimens employed, and serum drug concentrations. In each table, data are split into 2 sections: studies on organ transplantation (section a) are followed by studies regarding autoimmune pathologies (section b).
Table 2.
Population Characteristics and Immunosuppressive Regimen
| First Author (Year) | No. of Cases | No. of Female | Indication to Immunosuppression | Immunosuppressant Drug | Dose | Duration of Treatment | Serum Level at Symptoms Onset |
| |||||||
| Hartnick (2000)11 | 7 | NR | Renal transplant | OKT3 | 5 mg/day | 7 days | NR |
| Fortes (2008)13 | 42 | 21 | Liver transplant | CS or FK-506 | According to the serum levels | NR | Reported as “Therapeutic” |
| Rifai (2012)12 | 70 | 27 | Liver transplant | CS, FK-506 | NR | NR | NR |
| Gulleroglu (2015)14 | 27 | 16 | Pediatric renal transplant | CS or FK-506 | NR | NR | CS (mean) 1016.2 ± 558.9 ng/mL* |
| Arinsoy (1993)21 | 1 | 0 | Renal transplant | CS | 4 mg/kg/day | 3 months | 200 ng/mL* |
| Hartnick (1997)20 | 1 | 0 | Renal transplant | OKT3 | 20 mg/day | 8 days | NR |
| Min (1999)16 | 1 | 1 | Kidney-pancreas transplant | FK-506 | 13 mg/day | 18 months | 28.3 ng/mL* |
| Marioni (2004)10 | 1 | 0 | Renal transplant | CS | 300 mg/day | 8 years | NR |
| Norman (2006)18 | 1 | 0 | Liver transplant | FK-506 | NR | 2.5 months | 10.9 ng/mL |
| Rifai (2006)25 | 5 | 3 | Liver transplant | CS or FK-506 (+ AZA in 1 case) | NR | NR | CS 343 ng/mL; FK-506 24 ng/mL* |
| Gulleroglu (2013)26 | 2 | 1 | Renal transplant | FK-506 | 0.1 mg/kg/day | 48 months | 22.01 nmol/L; 29.7 nmol/L* |
| Jenkinson (2014)22 | 1 | 1 | Renal transplant | AZA | 75 mg/day | 10 days | NR |
| Lakshmi(2020)24 | 1 | 0 | Renal transplant | FK-506 | 0.05 mg/kg/day | 10 months | 11.0 ng/mL |
| |||||||
| Savastano (2010)8 | 28 | 3 | Ankylosing spondylitis | MTX, ET or IN | 10 mg/week (MTX); 25 mg twice/week(ET); 3 mg/kg every 6 weeks (IN) | 10.7 months (ET/IN group); 21.3 months (ET/IN + MTX group) | NR |
| Ahmadzadhe (2017)15 | 42 | 39 | Rheumatoid arthritis | MTX, AZA, CS, ET | NR | NR | NR |
| Porges (1998)19 | 1 | 0 | Crohn’s disease | CS | 4 mg/kg/day | 5 days | 1290 ng/mL* |
| Swale (2005)17 | 1 | 1 | Psoriasis | MTX | 17.5 mg/week | 21 months | NR |
| Tuknayat (2017)23 | 1 | 1 | Pemphigus vulgaris | Cyclophosphamide | 500 mg/month | 4 months | NR |
*Referred by the authors as high serum level concentration.
AZA, azathioprine; CS, cyclosporine; ET, etanercept; FK-506, tacrolimus; IN, infliximab; MTX, methotrexate; NR, not reported; OKT3, murine monoclonal antibody CD3.
The overall number of patients treated with immunosuppressive drugs included in the considered studies was 233. For 131 of them, reported clinical data allowed to ascertain ototoxicity (Table 2). Only data with adequate quality were included in our count. In a study by Fortes et al.13 the hearing status of 42 patients treated with calcineurin inhibitors was assessed; although the authors reported a significant decrease of hearing threshold in most of them, they did not specify the exact number and the decrease in decibels. Therefore, we did not include these patients in our final tally of 131 ototoxicity cases.
Table 3 summarizes the clinical presentation, evaluation, intervention, and outcome of patients included in each study, both for transplants (Table 3, section a) and for autoimmune pathologies (Table 3, section b).
Table 3.
Cases of Ototoxicity, Intervention, and Outcome for Each Included Study
| First Author (Year) | No Cases | Cochlear-Vestibular Symptoms | Evaluation | Time from Exposure to Evaluation | Intervention | Outcome | Follow-Up Period |
| |||||||
| Hartnick (2000)11 | 7 | SNHL (5) tinnitus (2) | OE, Questionnaire, PTa | 3 days | Interruption of therapy | Full recovery (4/5); No recovery (1/5) | NR |
| Fortes (2008)13 | NR | SNHL | OE, PTa | 636 days (CS); 513 days (FK-506) | PTa monitoring | SNHL at 3000 Hz (CS); SNHL at 4000-8000 Hz (FK-506) | NR |
| Rifai (2012)12 | 65 | SNHL (33), SNHL + tinnitus (32), Bilateral SNHL in 60 patients | OE, Questionnaire; PTa | 75 months | NR | SNHL associated with FK-506 | NR |
| Gulleroglu (2015)14 | 17 | SNHL + tinnitus (1) SNHL (14) SSNHL (2) | OE, PTa, SRT | 21.3 months | Dosage correction | PTA improvement but persistent decrease of SRT | NR |
| Arinsoy (1993)21 | 1 | SSNHL (unilateral) | OE, PTa, SRT, CT | 7 days | Dosage correction (2 mg/kg/day) | Full recovery | 3 months |
| Hartnick (1997)20 | 1 | SSNHL + tinnitus (bilateral) | OE, PTa, SRT | 2 days | Interruption of therapy | Full recovery | NR |
| Min (1999)16 | 1 | SSNHL + tinnitus (bilateral) | OE, PTa | 17 days | Dosage correction (6 mg/day) | Full recovery | 19 months |
| Marioni (2004)10 | 1 | SNHL (progressive bilateral) | OE, PTa, SRT, ABR, VNG, Caloric Test, MRI | 84 months | Dosage correction (200 mg/day) | Stabilization of PTA | 9 months |
| Norman (2006)18 | 1 | SSNHL + tinnitus (bilateral) | OE, PTa, SRT, MRI | 2.5 months | Interruption of therapy | Full recovery | 12 months |
| Rifai (2006)25 | 5 | SSNHL (3, bilateral), SSNHL + tinnitus (1 bilateral; 1 unilateral) |
OE, PTa | NR | Dosage correction Hearing aids | SNHL persistent despite dosage correction | NR |
| Gulleroglu (2013)26 | 2 | SSNHL + tinnitus (bilateral) | OE, PTa, SRT, MRI | 48 months | Dosage correction Hearing aids | Stabilization of PTA | NR |
| Jenkinson (2014)22 | 1 | SNHL + ear fullness (progressive bilateral) | OE, PTa, Tympanometry | 10 days | Interruption of therapy | PTA improvement | 1 month |
| Lakshmi(2020)24 | 1 | SNHL + tinnitus (bilateral) | OE, PTa, Tympanometry, MRI | 10 months | NR | NR | NR |
| |||||||
| Savastano (2010)8 | 18 | SNHL (8), SNHL + tinnitus (8), Tinnitus (2) | OE, PTa, Tympanometry | NR | NR | SNHL associated with treatment time-modality | NR |
| Ahmadzadhe (2017)15 | 9 | SNHL (5), CHL (3), MHL (1) | OE, PTa, Tympanometry, DPOAEs, SRT | NR | NR | SNHL associated with AZA, CS and ET | NR |
| Porges (1998)19 | 1 | SSNHL + vertigo (unilateral) | OE, PTa, Ophthalmologic exams, MRI | 5 days | Interruption of therapy | NR | NR |
| Swale (2005)17 | 1 | Vertigo | OE, MRI | 21 months | Interruption of therapy | Resolution of vertigo | NR |
| Tuknayat (2017)23 | 1 | SNHL (progressive bilateral) | OE, PTa, Tympanometry | 4 months | Interruption of therapy | PTA improvement | 6 months |
ABR, auditory brainstem response; AZA, azathioprine; CHL, conductive hearing loss; CT, computed tomography; DPOAE, distortion product otoacoustic emissions; ET, etanercept; FK-506, tacrolimus; MHL, mixed hearing loss; MRI, magnetic resonance imaging; NR, not reported; OE, otolaryngological evaluation; PTa, pure-tone audiometry; PTA, pure-tone average; SNHL, sensorineural hearing loss; SSNHL, sudden sensorineural hearing loss; SRT, speech recognition test; VNG, videonystagmography.
In all studies, ototoxicity was assessed through an ear, nose, and throat evaluation and pure-tone audiometry. Two studies11,12 also used a questionnaire focusing on patient-reported subjective hearing impairment. The most common clinical manifestation of ototoxicity was hearing loss (HL) (128 cases),8,10,11,12,14-16,18-26 retrieved in 101 patients treated for organ transplantation and 27 for autoimmune disorders, respectively. Hearing loss was bilateral in 93 cases (92 of transplanted patients and 1 patient with autoimmune diseases), unilateral in 10 cases (9 and 1 patients, respectively), and not specified in the remaining 25. The HL was almost all sensorineural (SNHL) both in studies about organ transplantation and the ones regarding autoimmune disease (101 and 23 cases, respectively). When reported, SNHL occurred suddenly in 13 cases11,14,16,18,21,25,26 (Table 3), and 3 and 1 cases of conductive and mixed HL, respectively, were described in 1 study regarding hearing status in patients undergoing immunosuppressive therapy for rheumatoid arthritis.15
The degree of HL was reported as mild in 27 patients,8,11,12,20 mild-to-moderate in 27,8,10,14,16,23 moderate in 28,12 moderate-to-severe in 4,22,24,26 and severe or profound in 31.12,18,25 In 9 patients, the degree of HL was not specified. Tinnitus was reported in 52 cases,8,11,12,15,16,18-20,24-26 typically associated to HL, but also isolated in 2 patients treated for ankylosing spondylitis.8 Vertigo was described only in 2 cases.17,19
The immunosuppressant drugs most frequently involved in ototoxic manifestations were calcineurin inhibitors (cyclosporine or tacrolimus), clearly documented in 93 cases and often related to drugs’ high serum levels10,12-14,16,18,19,21,24-26 (Table 2).
Hearing threshold after therapy discontinuation or immunosuppressant drugs dosage correction was evaluated in 3610,11,16,18,20-22,26: 8 patients had full recovery (normal hearing threshold), 19 had a significant hearing improvement, while 9 patients did not present significant changes, resulting in stabilization of HL (Table 3).
Discussion
In the last 3 decades, the number of performed transplantations has constantly increased, and in 2019, more than 43,000 new cases were registered in United States.27 Similarly, the 1-year survival after transplantations has dramatically improved, in line with a reduction of mortality for rejection or graft failure.28 These results have been achieved essentially due to optimized immunosuppressive regimens. The burden of autoimmune diseases has been increasing for years as well: currently, it is estimated that 15 to 30 million people in the USA suffer from autoimmune disease, making it the nation’s largest class of illness.29 Immunomodulatory therapy has revolutionized the treatment of several systemic autoimmune diseases.7,30,31 However, clinicians have to deal with the long-term toxicity of immunosuppressant drugs, which represents an emerging clinical concern.28
Ototoxicity is a relatively frequent side effect of a wide spectrum of drugs and is defined as a set of reversible or irreversible disturbances of the sensory structures of the inner ear (cochlea and vestibule).32 In particular, cochlear toxicity refers to damage affecting the auditory system resulting in sensorineural HL and/or tinnitus, while vestibular toxicity indicates an injury to the vestibular system resulting in dizziness, vertigo, and loss of balance.32 According to the American Speech–Language–Hearing Association (ASHA) Guidelines for the Audiologic Management of Individuals Receiving Cochleotoxic Drug, the ideal management of patients receiving ototoxic treatment should include (i) specific criteria for identification of toxicity, (ii) timely identification of at-risk patients, (iii) pre-treatment counseling regarding potential cochleotoxic effects, (iv) valid baseline measures (pre-treatment or early treatment beginning), (v) monitoring evaluations at sufficient intervals to document the progression of HL or fluctuation in sensitivity, and (vi) follow-up evaluations to determine post-treatment effects.33 Few of the studies included in our review followed the abovementioned criteria and principles.
Ototoxicity seems to be an epiphenomenon of a plethora of organ-specific toxic effects. Among these, nephrotoxicity is known to be one of the most relevant and frequent, especially in case the drug had a renal excretion. The possible nephrotoxic effect of many immunosuppressant drug classes is well known.34 However, the studies included in this review did not allow to find a clear association between nephrotoxicity and ototoxicity. Moreover, some of the included studies found no difference in renal function between patients who developed ototoxicity and those who did not.9,12,25
We herein summarize what emerged from our analysis on ototoxicity related to the main categories of immunosuppressant drugs used in transplantation or autoimmune diseases.
Calcineurin Inhibitors Cyclosporine and Tacrolimus
Considering the results of our review, the majority of reported ototoxicity cases (93 out of 131) occurred during therapy with calcineurin inhibitors.
Cyclosporine, since its first use in kidney transplantation in 1978, has revolutionized transplant medicine and dramatically improved the 1-year graft survival rate.35 The mechanisms by which cyclosporine can induce HL or vestibular disorders are still not clearly known.10,36 However, microscopic thromboembolic events, hypomagnesemia, impaired molecular diffusion through the blood–inner ear barrier,36 and alteration of the P-glycoprotein multidrug extrusion pump of the inner ear plasma membrane37 have been advocated as possible mechanisms. In particular, the latter effect seems to result in a significant accumulation of ototoxic molecules in the inner ear.37
Nevertheless, it has been also reported that cyclosporine therapy is not responsible for hearing impairment.38 Cyclosporine-associated ototoxicity may be dependent on the drug’s widely variable serum concentrations and pharmacokinetics, thus related to its dosage and duration of therapy.
Tacrolimus, a macrolide calcineurin inhibitor, is mostly used in liver transplanted patients. According to quite recent studies,9,13 it was more frequently associated with hearing disorders than cyclosporine. Rifai et al9 investigated hearing disorders in a large cohort of 695 patients undergoing long-term immunosuppressive therapy after liver transplantation, using an appropriate questionnaire. Hearing loss and/or tinnitus were found in 141 patients with a significant positive association to tacrolimus therapy in uni- and multivariate analyses. Such results were consistent with those by Fortes et al.13 The exact pathogenetic mechanism is unknown. A neurotoxic mechanism related to the inhibition of calcineurin activity (highly involved in many neuronal functions) has been mainly hypothesized9,16 although vasculopathy or a disturbance of the blood–inner ear barrier cannot be ruled out.9 Because of its chemical structure, tacrolimus may act in the same way as macrolides,39,40 decreasing the endo-cochlear potential and inducing HL.16,18
Anti-TNFα Etanercept and Infliximab
Etanercept is a human fusion protein with a dimeric structure composed of the human Fc portion of immunoglobulin (Ig) G1 and extracellular ligand-binding domain of the TNF p75 receptor.31 Infliximab is a chimeric IgG1 monoclonal antibody constituting approximately 75% of human-derived amino acids and the remaining 25% of murine-derived amino acids.31
Recently, Savastano et al8 reported the occurrence of sensorineural HL in 100% of patients treated with TNFα blockers (etanercept or infliximab) plus methotrexate for ankylosing spondylitis and in 43% of patients treated with TNFα blockers alone, with a time-dependent association. A drug-induced vasculitis of the inner ear arteries as a possible mechanism of hearing dysfunction in these patients has been hypothesized. Anti-TNFα agents may cause a type III hypersensitivity reaction forming immunocomplexes with TNFα or may induce autoantibody production and subsequent blood vessel reaction.8 Nevertheless, it is difficult to clearly distinguish between SNHL TNFα blockers induced and HL due to autoimmune processes related to the basal disease, as these drugs are typically prescribed for patients with more severe disease refractory to first-line treatment.15 In the reported rare cases of conductive HL, this differentiation is clearer, as they are theoretically due to the arthritis process at the inter-ossicular joints of the middle ear.
Only 2 studies reported ototoxicity occurring during the TNFα regimen.8,15 On the contrary, Toktas et al.41 considering 22 patients with rheumatoid arthritis and ankylosing spondylitis, did not find any significant changes in pure-tone audiometry 2 and 6 months after starting infliximab. It should be noted that in animal models, TNFα inhibitors were shown to be effective in inducing and maintaining remission in immune-mediated inner ear disease.15,42,43 Furthermore, infliximab is increasingly a potential first-line therapy in conjunction with corticosteroids in Cogan’s syndrome.44
Anti-Folic Agents Methotrexate
Methotrexate is a folic acid analog, an antimetabolite that binds to dihydrofolate reductase enzyme with high selectivity and affinity and prevents the synthesis of the active form of B9 vitamin, the tetrahydrofolate. This has an anti-proliferative effect because DNA, RNA, and protein synthesis are blocked. Moreover, methotrexate enhances extracellular adenosine release which contributes to immunosuppressive activity.45 It is widely used for the treatment of malignancies and autoimmune diseases such as rheumatoid arthritis and psoriasis.
Neurotoxicity and leukoencephalopathy have been described with a high-dose methotrexate regimen.46 Adenosine dysregulation could affect endolymph ion homeostasis but reported cochleovestibular toxicity associated with methotrexate is extremely rare; in fact, only Swale et al17 and Savastano et al8 reported such cases. Animal studies have evaluated the effects of intratympanic administration of methotrexate and found no ototoxicity.47 Methotrexate is used for refractory autoimmune inner ear disease.48
Muromonab-CD3 (OKT3)
OKT3 is used for steroid-resistant acute rejection after transplantation and acts by binding T-cell receptors and inducing apoptosis of T cell.49 Few cases of HL after OKT3 have been reported. Considering the studies included in our review, Hartnick et al11 found high-frequency sensorineural HL 72 hours after OKT3 therapy in 5 patients who had shown a normal hearing level in a previous evaluation. An ototoxic mechanism analog to that of kanamycin or amikacin causing selective destruction of the outer hair cells at the basal turn of the cochlea has been hypothesized.
Purine Synthesis Inhibitors Azathioprine
Purine synthesis inhibitors interrupt the de novo pathway of purine synthesis. Azathioprine has been the most widely used antimetabolite anti-rejection drug since the mid-1990s. Possible azathioprine ototoxicity has been reported in 4 patients taking immunosuppressant therapy for rheumatoid arthritis15 and in 1 patient after renal transplantation.22 However, an acceptable explanation for this side effect is still lacking. Azathioprine has also been used to treat autoimmune inner ear disease resistant to steroid therapy.48
Alkylating Agents Cyclophosphamide
Alkylating agents contribute to alkyl groups that bind to the nitrogen and oxygen atoms of guanine, inducing aberrant couplets in the DNA strand and leading to cell mutagenesis and apoptosis. These drugs are used as antineoplastic agents, but they may also exert immunosuppressive activity due to cytotoxic and anti-proliferative effects on B-lymphocytes.1
Tuknayat et al23 reported a case of ototoxicity in a patient receiving cyclophosphamide for pemphigus; the patient’s hearing improved after therapy discontinuation. Cyclophosphamide-related ototoxicity due to the formation of reactive oxygen species and nitric oxide that may have damaged the outer hair cells of the organ of Corti was hypothesized. Other possible ototoxic mechanisms regard cyclophosphamide interaction with type 1 neurons of spiral ganglion causing myelin sheath apoptosis or injury to the stria vascularis.
Overall the impact of ototoxicity related to immunosuppressive therapy for organ transplantation and autoimmune disorders is limited if we consider the relatively few cases reported in the international literature compared to the huge use of the abovementioned substances. However, the scarce knowledge that clinicians have about this adverse reaction could possibly have led to underestimating ototoxicity related to immunosuppressive therapy, and we should remember that most cases of sudden sensorineural HL involving this type of patients are probably classified as idiopathic.50
It should be remarked that it is difficult to differentiate between SNHL caused by immunosuppressant therapy and SNHL due to the basal disease, especially in autoimmune disorders. Sensorineural hearing loss of immune-mediated origin may occur both as a sign associated with several systemic autoimmune diseases and as primary autoimmune inner ear disease (AIED). Although AIED is typically progressive and bilateral, in some cases, it can manifest as a sudden unilateral SNHL,51 as in some cases of ototoxicity found by our analysis. Finally, it is important to notice that immunosuppressive agents have also been used in cases of sudden SNHL that did not improve with steroid treatment. The rationale for this is the likely role of autoimmunity in the pathogenesis of sudden SNHL. However, conclusive results on the efficacy of this treatment are still inconsistent.52
The main strength of this literature review is the well-defined method of searching for available cases. All cases of ototoxicity manifested selectively in patients treated for transplantations and autoimmune diseases were analyzed, this may contribute to supporting the reported result. However, the characteristics of the available literature, mainly composed of case series and case reports, intrinsically affect the evidence about this emerging topic.
Furthermore, comparing outcomes of the reported ototoxicity cases may be difficult due to the following reasons: (i) data on the duration of immunosuppressive treatment were not homogeneous and often lacking; (ii) the design of the studies was mainly retrospective; (iii) the management of ototoxicity varied from complete discontinuation to optimization of the immunosuppressant dose; and (iv) the follow-up modalities were not homogeneous.
In conclusion, calcineurin inhibitors were the immunosuppressant drugs most frequently involved in ototoxic manifestations, generally due to their high serum level concentration. Dosage correction or discontinuation of therapy very often led to hearing improvement or even to full recovery. Ototoxicity during therapy with other immunosuppressants is almost anecdotal and the potential etiopathogenetic mechanisms are speculative; it follows that certainties on this adverse reaction are far from being acquired.
From our viewpoint, even with the intrinsic limitations due to the characteristics of the available studies, a systematic review of the literature on ototoxicity from immunosuppressive treatment can be clinically relevant in a rather uncommon but definitely challenging situation. Clinicians who manage patients treated with these drugs must be aware of this possible harmful effect, monitor the patients by questioning their hearing status, and refer to an ENT specialist if any audiological symptoms are reported. A baseline pure-tone audiometry should be considered in patients with a previous history of audiovestibular symptoms and/or other known risk factors for HL. Checking the immunosuppressant serum level and eventually adjusting it, as well as avoiding the concomitant use of other likely ototoxic medications, if possible, is also essential.
However, to better define and characterize the ototoxicity of each class of immunosuppressants, further large-scale prospective investigations are required, and the setting of these studies should follow the ASHA Guidelines for the Audiologic Management of Individuals Receiving Cochleotoxic Drugs.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – G.M.; Design – L.F., A.L., G.M.; Supervision – A.L.; C.d.F., G.M.; Resources – G.M.; Data Collection and/or Processing – L.F., A.F., D.P.; Analysis and/or Interpretation – L.F., A.F., D.P., A.L., G.M.; Literature Search – L.F., A.F., D.P.; Writing Manuscript – L.F., A.F., D.P., A.L., G.M.; Critical Review – C.d.F., G.M.
Acknowledgments: The authors would like to thank Mrs. Alison Garside for correcting the English version of the paper.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: This study was supported in part by grant no. DOR2090593/20 (G. Marioni) from the University of Padova, Italy.
References
- 1. Ponticelli C, Glassock RJ. Prevention of complications from use of conventional immunosuppressants: a critical review. J Nephrol. 2019;32(6):851–870.. 10.1007/s40620-019-00602-5) [DOI] [PubMed] [Google Scholar]
- 2. Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34(8):1081–1085.. 10.1136/gut.34.8.1081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moreso F, Serón D, Morales JM, et al. Incidence of leukopenia and cytomegalovirus disease in kidney transplants treated with mycophenolate mofetil combined with low cyclosporine and steroid doses. Clin Transplant. 1998;12(3):198–205.. [PubMed] [Google Scholar]
- 4. Latta K, von Schnakenburg C, Ehrich JH. A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol. 2001;16(3):271–282.. 10.1007/s004670000523) [DOI] [PubMed] [Google Scholar]
- 5. Shang W, Ning Y, Xu X, et al. Incidence of cancer in ANCA-associated vasculitis: a meta-analysis of observational studies. PLoS One. 2015;10(5):e0126016. 10.1371/journal.pone.0126016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hardinger KL, Brennan DC, Lowell J, Schnitzler MA. Long-term outcome of gastrointestinal complications in renal transplant patients treated with mycophenolate mofetil. Transpl Int. 2004;17(10):609–616.. 10.1007/s00147-004-0768-6) [DOI] [PubMed] [Google Scholar]
- 7. Subedi S, Gong Y, Chen Y, Shi Y. Infliximab and biosimilar infliximab in psoriasis: efficacy, loss of efficacy, and adverse events. Drug Des Devel Ther. 2019;13:2491–2502.. 10.2147/DDDT.S200147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Savastano M, Marioni G, Giacomelli L, Ramonda R, Ferraro SM, Punzi L. Sensorineural hearing loss in ankylosing spondylitis treated with TNF blockers. B-ENT. 2010;6(3):183–188.. [PubMed] [Google Scholar]
- 9. Rifai K, Kirchner GI, Bahr MJ, et al. A new side effect of immunosuppression: high incidence of hearing impairment after liver transplantation. Liver Transpl. 2006;12(3):411–415.. 10.1002/lt.20610) [DOI] [PubMed] [Google Scholar]
- 10. Marioni G, Perin N, Tregnaghi A, Bellemo B, Staffieri A, de Filippis C. Progressive bilateral sensorineural hearing loss probably induced by chronic cyclosporin A treatment after renal transplantation for focal glomerulosclerosis. Acta Otolaryngol. 2004;124(5):603–607.. 10.1080/00016480410016225) [DOI] [PubMed] [Google Scholar]
- 11. Hartnick CJ, Smith RV, Tellis V, Greenstein S, Ruben RJ. Reversible sensorineural hearing loss following administration of muromonab-CD3 (OKT3) for cadaveric renal transplant immunosuppression. Ann Otol Rhinol Laryngol. 2000;109(1):45–47.. 10.1177/000348940010900108) [DOI] [PubMed] [Google Scholar]
- 12. Rifai K, Pischke S, Agne C, Rosenau J, Klempnauer JL, Manns MP. High rate of unperceived hearing loss in patients after liver transplantation. Clin Transplant. 2012;26(4):577–580.. 10.1111/j.1399-0012.2011.01592.x) [DOI] [PubMed] [Google Scholar]
- 13. Fortes ME, Marroni CA, Coser PL, de los Santos CA. Audiometric changes in patients undergoing liver transplantation using distinct immunosuppressive protocols. Liver Transpl. 2008;14(4):509–511.. 10.1002/lt.21385). [DOI] [PubMed] [Google Scholar]
- 14. Gulleroglu K, Baskin E, Aydin E, Ozluoglu L, Moray G, Haberal M. Hearing status in pediatric renal transplant recipients. Exp Clin Transplant. 2015;13(4):324–328.. 10.6002/ect.2014.0158) [DOI] [PubMed] [Google Scholar]
- 15. Ahmadzadeh A, Daraei M, Jalessi M, et al. Hearing status in patients with rheumatoid arthritis. J Laryngol Otol. 2017;131(10):895–899.. 10.1017/S0022215117001670) [DOI] [PubMed] [Google Scholar]
- 16. Min DI, Ku YM, Rayhill S, Corwin C, Wu YM, Hunsicker LG. Sudden hearing loss associated with tacrolimus in a kidney-pancreas allograft recipient. Pharmacotherapy. 1999;19(7):891–893.. 10.1592/phco.19.10.891.31562) [DOI] [PubMed] [Google Scholar]
- 17. Swale VJ, Sahota A. Severe vertigo requiring hospitalization whilst taking low-dose oral methotrexate for psoriasis. Clin Exp Dermatol. 2005;30(3):295–296.. 10.1111/j.1365-2230.2005.01742.x) [DOI] [PubMed] [Google Scholar]
- 18. Norman K, Bonatti H, Dickson RC, Aranda-Michel J. Sudden hearing loss associated with tacrolimus in a liver transplant recipient. Transpl Int. 2006;19(7):601–603.. 10.1111/j.1432-2277.2006.00317.x) [DOI] [PubMed] [Google Scholar]
- 19. Porges Y, Blumen S, Fireman Z, Sternberg A, Zamir D. Cyclosporine-induced optic neuropathy, ophthalmoplegia, and nystagmus in a patient with Crohn disease. Am J Ophthalmol. 1998;126(4):607–609.. 10.1016/s0002-9394(98)00137-8) [DOI] [PubMed] [Google Scholar]
- 20. Hartnick CJ, Cohen AF, Smith RV. Reversible sensorineural hearing loss after renal transplant immunosuppression with OKT3 (muromonab-CD3). Ann Otol Rhinol Laryngol. 1997;106(8):640–642.. 10.1177/000348949710600804) [DOI] [PubMed] [Google Scholar]
- 21. Arinsoy T, Akpolat T, Ataman M, et al. Sudden hearing loss in a cyclosporin-treated renal transplantation patient. Nephron. 1993;63(1):116–117.. 10.1159/000187158) [DOI] [PubMed] [Google Scholar]
- 22. Jenkinson PW, Syed MI, Mcclymont L. Progressive, reversible sensorineural hearing loss caused by azathioprine. J Laryngol Otol. 2014;128(9):838–840.. 10.1017/S0022215114001807) [DOI] [PubMed] [Google Scholar]
- 23. Tuknayat A, Thami GP, Gogia P, Bhutani M. Cyclophosphamide-induced hearing loss: reversibility and preventive strategies. Am J Ther. 2018;25(6):e692–e695.. 10.1097/MJT.0000000000000701) [DOI] [PubMed] [Google Scholar]
- 24. Lakshmi BS, Vidya B, Reddy MHK, Kumar ACV, Ram R, Kumar VS. Sensorineural deafness following tacrolimus use. Exp Clin Transplant. 2020;18(1):110–111.. 10.6002/ect.2017.0114) [DOI] [PubMed] [Google Scholar]
- 25. Rifai K, Klempnauer J, Manns MP, Strassburg CP. Sudden hearing loss associated with high levels of calcineurin inhibitors after liver transplantation. Transplantationsmedizin. 2006;18:S33–S35.. [Google Scholar]
- 26. Gulleroglu K, Baskin E, Bayrakci U, et al. Sudden hearing loss associated with tacrolimus after pediatric renal transplant. Exp Clin Transplant. 2013;11(6):562–564.. 10.6002/ect.2012.0241) [DOI] [PubMed] [Google Scholar]
- 27. U.S. Department of Health & Human Services. National Data-OPTN. 2021. Available at: https://optn.transplant.hrsa.gov/. [Google Scholar]
- 28. Tasdogan BE, Ma M, Simsek C, Saberi B, Gurakar A. Update on immunosuppression in liver transplantation. Euroasian J Hepatogastroenterol. 2019;9(2):96–101.. 10.5005/jp-journals-10018-1301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abend A, Gilbert A, Craig M, Sparks A. The ARI Online Database. Autoimmune Registry Inc. www.autoimmuneregistry.org. Accessed February 1, 2021 https://www.autoimmuneregistry.org/. [Google Scholar]
- 30. Meyer KC, Decker C, Baughman R. Toxicity and monitoring of immunosuppressive therapy used in systemic autoimmune diseases. Clin Chest Med. 2010;31(3):565–588.. 10.1016/j.ccm.2010.05.006) [DOI] [PubMed] [Google Scholar]
- 31. Zhao S, Mysler E, Moots RJ. Etanercept for the treatment of rheumatoid arthritis. Immunotherapy. 2018;10(6):433–445.. 10.2217/imt-2017-0155) [DOI] [PubMed] [Google Scholar]
- 32. Favrelière S, Delaunay P, Lebreton JP, et al. Drug-induced hearing loss: a case/non-case study in the French pharmacovigilance database. Fundam Clin Pharmacol. 2020;34(3):397–407.. 10.1111/fcp.12533) [DOI] [PubMed] [Google Scholar]
- 33. Ganesan P, Schmiedge J, Manchaiah V, Swapna S, Dhandayutham S, Kothandaraman PP. Ototoxicity: a challenge in diagnosis and treatment. J Audiol Otol. 2018;22(2):59–68.. 10.7874/jao.2017.00360). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El Hennawy HM, Faifi ASA, El Nazer W, et al. Calcineurin inhibitors nephrotoxicity prevention strategies with stress on Belatacept-based rescue immunotherapy: a review of the current evidence. Transplant Proc. 2021;53(5):1532–1540.. 10.1016/j.transproceed.2021.03.028) [DOI] [PubMed] [Google Scholar]
- 35. Margreiter R. Impact of cyclosporine on organ transplantation. Transplant Proc. 1991;23(4):2180–2182.. [PubMed] [Google Scholar]
- 36. Waissbluth S. Is cyclosporine ototoxic? Front Neurol. 2020;11:593917. 10.3389/fneur.2020.593917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saito T, Zhang ZJ, Tokuriki M, et al. Cyclosporin A inhibits the extrusion pump function of p-glycoprotein in the inner ear of mice treated with vinblastine and doxorubicin. Brain Res. 2001;901(1-2):265–270.. 10.1016/s0006-8993(01)02321-6) [DOI] [PubMed] [Google Scholar]
- 38. Kasap-Demir B, Özmen D, Kırkım G, et al. Cyclosporine causes no hearing defect in paediatric patients with nephrotic syndrome. Int J Audiol. 2017;56(9):701–705.. 10.1080/14992027.2017.1329556) [DOI] [PubMed] [Google Scholar]
- 39. Vasquez EM, Maddux MS, Sanchez J, Pollak R. Clinically significant hearing loss in renal allograft recipients treated with intravenous erythromycin. Arch Intern Med. 1993;153(7):879–882.. 10.1001/archinte.153.7.879) [DOI] [PubMed] [Google Scholar]
- 40. Kobayashi T, Rong Y, Chiba T, Marcus DC, Ohyama K, Takasaka T. Ototoxic effect of erythromycin on cochlear potentials in the guinea pig. Ann Otol Rhinol Laryngol. 1997;106(7 Pt 1):599–603.. 10.1177/000348949710600713) [DOI] [PubMed] [Google Scholar]
- 41. Toktas H, Okur E, Dundar U, Dikici A, Kahveci OK. Infliximab has no apparent effect in the inner ear hearing function of patients with rheumatoid arthritis and ankylosing spondylitis. Clin Rheumatol. 2014;33(10):1481–1487.. 10.1007/s10067-014-2625-z) [DOI] [PubMed] [Google Scholar]
- 42. Ihler F, Sharaf K, Bertlich M, et al. Etanercept prevents decrease of cochlear blood flow dose-dependently caused by tumor necrosis factor alpha. Ann Otol Rhinol Laryngol. 2013;122(7):468–473.. 10.1177/000348941312200711) [DOI] [PubMed] [Google Scholar]
- 43. Wang X, Truong T, Billings PB, Harris JP, Keithley EM. Blockage of immune-mediated inner ear damage by etanercept. Otol Neurotol. 2003;24(1):52–57.. 10.1097/00129492-200301000-00012) [DOI] [PubMed] [Google Scholar]
- 44. Espinoza GM, Wheeler J, Temprano KK, Keller AP. Cogan’s syndrome: clinical presentations and update on treatment. Curr Allergy Asthma Rep. 2020;20(9):46. 10.1007/s11882-020-00945-1) [DOI] [PubMed] [Google Scholar]
- 45. Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65(3):168–173.. [PubMed] [Google Scholar]
- 46. Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21(12):1471–1482.. 10.1634/theoncologist.2015-0164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eren SB, Dogan R, Yenigun A, et al. Evaluation of ototoxicity of intratympanic administration of methotrexate in rats. Int J Pediatr Otorhinolaryngol. 2017;100:132–136.. 10.1016/j.ijporl.2017.06.035) [DOI] [PubMed] [Google Scholar]
- 48. Breslin NK, Varadarajan VV, Sobel ES, Haberman RS. Autoimmune inner ear disease: a systematic review of management. Laryngoscope Investig Otolaryngol. 2020;5(6):1217–1226.. 10.1002/lio2.508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ueda D, Hori T, Nguyen JH, Uemoto S. Muromonab-CD3 therapy for refractory rejections after liver transplantation: a single-center experience during two decades in Japan. J Hepatobiliary Pancreat Sci. 2010;17(6):885–891.. 10.1007/s00534-010-0288-y) [DOI] [PubMed] [Google Scholar]
- 50. Chandrasekhar SS, Tsai Do BS, Schwartz SR, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2019;161(suppl 1):S1–S45.. 10.1177/0194599819859885). [DOI] [PubMed] [Google Scholar]
- 51. Ciorba A, Corazzi V, Bianchini C, et al. Autoimmune inner ear disease (AIED): a diagnostic challenge. Int J Immunopathol Pharmacol. 2018;32:2058738418808680. 10.1177/2058738418808680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li G, You D, Ma J, Li W, Li H, Sun S. The role of autoimmunity in the pathogenesis of sudden sensorineural hearing loss. Neural Plast. 2018;2018:7691473. 10.1155/2018/7691473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu Y, Makuch RW. Detecting rare adverse events in postmarketing studies: sample size considerations. Drug Inf J. 2006;40(1):89–98.. 10.1177/009286150604000111) [DOI] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a