Abstract
OBJECTIVE
With this study, it was aimed to investigate whether the thoracic muscle mass of patients with coronavirus disease 2019 was related to disease severity and disease characteristics and to evaluate whether muscle mass measurement had a predictive effect on predicting disease severity.
MATERIAL AND METHODS
Two hundred twenty-three subjects (patient group = 161 and control = 62) who presented to our coronavirus disease 2019 outpatient clinic between May 2020 and September 2020 were included in the study. The medication, oxygen, and intubation requirements of the patients and their disease duration and hospital stay were also recorded. At the T4 level, thoracic and back (pectoralis, intercostalis, paraspinals, serratus, and latissimus dorsi) muscles and at the T12 level erector spinae muscles were measured in terms of area (cm2).
Results
T4-level muscle cross-sectional area results were found to be negatively correlated with the presence of pneumonia and the requirement of oxygen and intubation. In addition, both T4- and T12-level muscle cross-sectional area results were factors associated with oxygen and intubation requirements. T4-level muscle cross-sectional area results were also associated with the presence of pneumonia.
Conclusion
We predict that there may be a relationship between the decrease in the mass of the accessory respiratory muscles and the severity of the disease.
Keywords: COVID-19, disease severity, erector spinae muscles, thoracic muscle mass
Main Points
Polymerase chain reaction positivity and the presence of pneumonia on computed tomography are associated with age, body mass index, and comorbidities.
Muscle cross-sectional areas at both T4 and T12 levels correlate with oxygen and intubation requirements.
There may be a relationship between the decrease in the mass of the auxiliary respiratory muscles and the severity of the disease.
Introduction
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus, which continues to spread increasingly all over the world.1 This virus emerged in China last year is new for the whole world. Coronavirus disease 2019 can cause a wide range of clinical pictures from a mild flu infection to pneumonia, pulmonary edema, multi-organ failure, and death.2
Studies to understand the disease and to prevent the progression as well as to develop treatment and diagnostic methods for COVID-19 continue all over the world. Despite these prevention methods, the contagiousness of the SARS-CoV-2 virus is high and there are currently over 36 million patients in the world. Although most of the symptomatic infections are mild, patients with initially mild symptoms can quickly cause a marked alveolar inflammatory cell infiltration that progresses to acute respiratory distress syndrome and eventually to multi-organ failure.3
Therefore, disease-related factors and progression should be investigated and preventive methods should be developed to alleviate infections and prevent progression.
Although we learn new things about COVID-19 every day, studies defining risk factors and risk groups are still insufficient and incomplete.4-7 There is no study investigating the relationship between the disease and the thoracic region, including the pulmonary system, which causes high mortality. Considering that respiratory function occurs with the contraction and expansion of the muscles in the thoracic cavity, it can be predicted that the muscle mass in this region will be effective in the progression of the disease. However, this information is still not based on scientific evidence.
Therefore, in this study, it was aimed to investigate whether the thoracic and back muscle mass of patients with COVID-19 was related to disease severity and disease characteristics and to evaluate whether muscle mass measurement had a predictive effect on disease severity.
Our hypothesis in this study was that low thoracic muscle mass might increase the severity of the COVID-19 clinical picture.
Material and Methods
Study Design and Selection of Participants
Two hundred thirty-three participants who presented to our COVID-19 inpatient and outpatient clinics between May 2020 and September 2020 were included in the study.
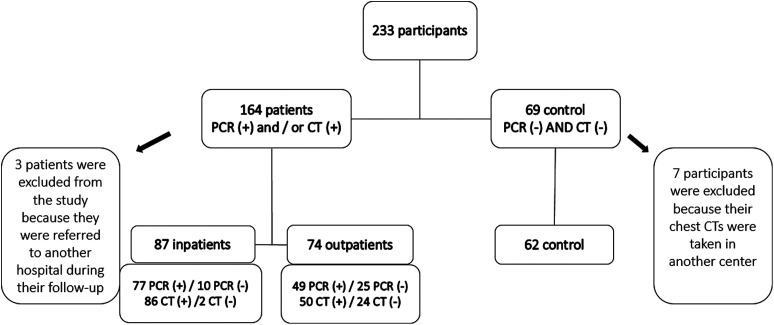
Of these participants, who were followed up and treated according to the national COVID-19 diagnosis and treatment algorithm, 164 patients with positive antigen test [polymerase chain reaction (PCR)] or positive computed tomography (CT) findings or presence of both parameters positive were evaluated as the patient group, and 69 patients with negative test and negative CT findings were evaluated as the control group. Three patients from the patient group were excluded from the study because they were referred to another hospital during their follow-up and 7 participants from the control group were excluded because their chest CTs were taken in another center. The study was completed with 223 participants (patient group n = 161, control group n = 62) (Figure 1).
Figure 1.
Selection of participants.
Inclusion criteria for the study were as follows: patients aged 18-75 years who underwent PCR and CT evaluations within the first 10 days after the onset of symptoms and who were followed up in the COVID-19 outpatient clinic of our pandemic hospital were included in the study.
Patients older than 75 years (to eliminate the possibility of age-related sarcopenia), with a history of surgery and trauma in the thoracic region, with known progressive/non-progressive neurologic disease, with a history of advanced lung disease or malignancy, and those with inflammatory lung diseases were not included in the study.
Before evaluations, the patients were given verbal and written information on the nature of the study. Informed consent forms were signed upon admission to the trial. All procedures were conducted in accordance with the relevant principles of the Helsinki Declaration. Also, approval of the study was obtained from the University of Health Sciences, Diskapi Yildirim Beyazit Training and Research Hospital Ethics Committee (Number: 91/09, Date: July 06, 2020).
The demographic characteristics of all participants including age, sex, job, body mass index (BMI), presence of additional comorbidity, smoking status, and smoking pack/years were recorded. In addition, the medication, oxygen, and intubation requirements of the patients and duration of disease and hospital stay were also recorded. Outpatient participants were followed up for 14 days until the PCR became negative or until the 14-day treatment was completed according to the national COVID-19 diagnosis and treatment algorithm; the control group was followed up for 14 days.
Positivity in PCR and CT was evaluated as disease characteristics. Coronavirus disease 2019 lung involvement was evaluated as suggested by the partnership of Radiological Society of North America, Society of Thoracic Radiology, and American College of Radiology, and it was recorded as having pneumonia or normal.8-10
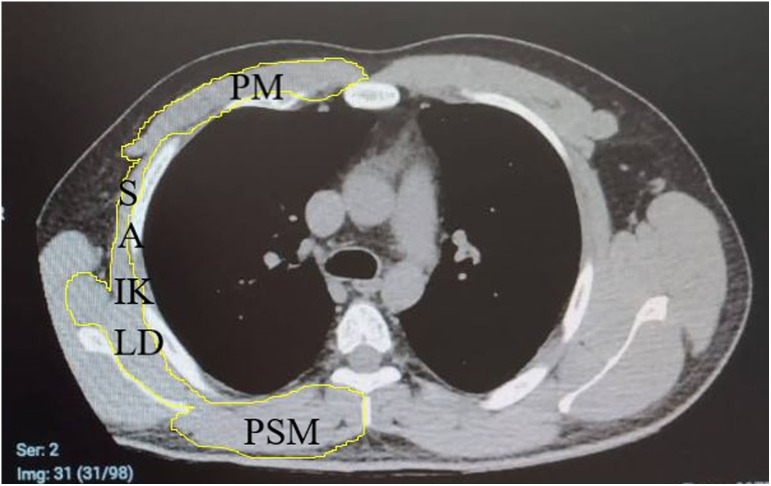
Non-contrast chest CT was performed using a 16-detector CT scanner (Somatom Emotion 16; Siemens Healthcare, Erlangen, Germany) in the radiology department of our hospital. T4 and T12 levels were defined as reported in previous studies.11 The close polygon region of interest tool (NIH-Image J software program) was used to trace the contour of the muscle of interest on T4- and T12-level images for cross-sectional area (CSA) measurements. At the T4 level, thoracic and back (pectoralis, intercostalis, paraspinals, serratus, latissimus dorsi) muscles and at the T12 level erector spinae muscles were measured in terms of area (cm2) (Figure 2).
Figure 2.
T4 level axial computed tomography image.
Study Protocol
The measurements of the CT images were made separately by 2 blind specialists. Each expert took 3 measurements and the 3 measurements were averaged. Then, a single result was generated from the arithmetic mean of the 2 experts’ measurements. To prevent bias, the group allocation of the participants was not shared with the specialists.
All participants were divided into 2 groups, the patient group and the control group, and the parameters were compared between the 2 groups. Later, the patient group was separated as outpatients and inpatients, and the parameters were compared between these groups. Muscle mass results were compared with all parameters to evaluate the factors that might be associated with muscle mass and disease severity.
Statistical Analysis
All statistical analyses were carried out by using Statistical Package for the Social Sciences (version 15.0) for Windows statistical package. The variables were investigated using an analytical method (Kolmogorov‑Smirnov test) to assess whether or not they are normally distributed. Descriptive statistics were demonstrated as median (minimum-maximum) for continuous variables and as a percentage (%) for nominal variables. The difference between 2 groups’ comparison was evaluated using the Mann–Whitney U test. In order to evaluate the relation between T4-T12 levels and evaluation parameters, Spearman’s rho correlation test was performed for continuous variables and univariate analysis for nominal variables. For statistically significant correlations, the age, gender, and comorbidity presence-adjusted model multivariate regression analysis using control group as dependent variable was performed. A P value of .05 or less was considered significant.
Results
Two hundred twenty-three participants (161 (72.2%) patients and 62 (27.8%) controls) were included in the study. The median age of all participants was 46.00 (range, 19.0-75.0) years; 120 (53.8%) were women 103 (46.2%) were men. The distribution of the disease characteristics including the demographic and muscle CSAs at the T4 and T12 levels of the patient and control groups is presented in Table 1.
Table 1.
Distribution and Comparison of Demographic and Disease Characteristics of Patient and Control Groups
| Patients (n = 161) | Controls (n = 62) | P | |
|---|---|---|---|
| Age, med (min-max) | 53.0 (19.0-75.0) | 34.0 (19.0-68.0) | .001 * |
| Sex, n (%) | |||
| Female | 91 (56.5) | 29 (46.8) | .196 |
| Male | 70 (43.5) | 33 (53.2) | |
| BMI (%), med (min-max) | 27.79 (17.83-43.34) | 25.44 (18.42-37.20) | .001 * |
| Job, n (%) | |||
| White collar | 30 (18.6) | 22 (35.5) | .884 |
| Blue collar | 46 (28.6) | 23 (37.1) | |
| Non-working | 85 (52.8) | 17 (27.4) | |
| Additional comorbidity, n (%) | 79 (49.1) | 16 (25.8) | |
| Diabetes mellitus | 3 (1.9) | 1 (1.6) | .004 * |
| Hypertension | 61 (37.9) | 6 (9.7) | |
| Coronary artery disease | 10 (6.2) | 3 (4.8) | |
| Hypothyroidism | 3 (1.9) | 3 (4.8) | |
| Osteoarthritis | 2 (1.2) | 3 (4.8) | |
| Smoking, n (%) | |||
| Active smoking | 23 (14.3) | 21 (33.9) | .106 |
| Not smoking | 125 (77.6) | 34 (54.8) | |
| Stopped smoking | 13 (8.1) | 7 (11.3) | |
| Smoking (pack/years), med (min-max) | 0.0 (0.0-50.0) | 3.0 (1.0-4.0) | .338 |
| PCR positivity, n (%) | 126 (78.3) | 0 | .001 * |
| Pneumonia (CT) positivity, n (%) | 136 (84.5) | 0 | .001 * |
| T4-level muscle CSA (cm2), median (min-max) | 38.39 (10.14-92.34) | 53.17 (21.65-198.70) | .001 * |
| T12-level muscle CSA (cm2), med (min-max) | 19.49 (14.05-77.28) | 32.79 (15.94-79.60) | .001 * |
BMI, body mass index; PCR, polymerase chain reaction; CT, computed tomography; CSA, cross-sectional area; med, median; min, minimum; max, maximum.
* P < .05.
Among the patient and control groups, age (P = .001), BMI (P = .001), and T4- and T12-level muscle CSAs (both for P = .001) were lower in the patient group, and the number of additional comorbidities was higher in this group (P = .004).
Seventy-four (46%) of the patients were treated as outpatients, and 87 (54%) were treated in the hospital. Inpatients and outpatients were compared in terms of demographic and disease characteristics (Table 2). The median number of days of oxygen need of inpatients was 5.0 (range, 1.0-32.0), the median intubation time was 0.0 (range, 1.0-12.0) days, and the median length of hospital stay was 8.0 (range, 1.0-32.0) days. The age (P = .001), presence of additional comorbidities (P = .022), the number of patients with PCR (P = .003), CT (P = .004) positivity, medication use (P = .022), oxygen (P = .001), and intubation (P = .001) in inpatients was significantly higher. T4- and T12-level muscle CSAs were lower in inpatients (P = .003, P = .004, respectively).
Table 2.
Demographic and Disease Characteristics of Patient Groups
| Inpatient (n = 87) | Outpatient (n = 74) | P | |
|---|---|---|---|
| Age, med (min-max) | 58.0 (19.0-75.0) | 44.50 (19.0-75.0) | .001 * |
| Sex, n (%) | |||
| Female | 45 (51.7) | 46 (62.2) | .184 |
| Male | 42 (48.3) | 28 (37.8) | |
| BMI (%), med (min-max) | 29.04 (17.83-41.79) | 26.54 (20.62-43.34) | .068 |
| Additional comorbidity, n (%) | 60 (69.0) | 33 (44.6) | .022 * |
| Smoking, n (%) | |||
| Active smoking | 9 (10.3) | 14 (18.9) | .206 |
| Not smoking | 8 (9.2) | 55 (74.3) | |
| Stopped smoking | 70 (80.5) | 5 (6.8) | |
| Smoking (pack/years), med (min-max) | 0.0 (0.0-50.0) | 0.0 (0.0-35.0) | .743 |
| PCR positivity, n (%) | 77 (88.5) | 49 (66.2) | .004 * |
| Pneumonia (CT) positivity, n (%) | 86 (98.9) | 50 (67.6) | .003 * |
| Medication, n (%) | |||
| HCQ | 87 (100) | 48 (64.9) | .022 * |
| Anticoagulant | 72 (82.8) | 5 (6.8) | |
| AZT | 71 (81.6) | 19 (25.7) | |
| FVP | 38 (43.7) | 34 (45.9) | |
| Paracetamol | 49 (56.3) | 2 (2.7) | |
| Steroid | 17 (19.5) | 0 | |
| Other (plasma therapy, other antiviral, etc.) | 49 (56.3) | 1 (1.4) | |
| Number of patients in need of oxygen, n (%) | 87 (100) | 0 | .001 * |
| Number of intubated patients, n (%) | 5 (5.7) | 0 | .001 * |
| T4-level muscle CSA (cm2), med (min-max) | 37.07 (10.14-86.90) | 39.43 (19.81-92.34) | .003 * |
| T12 level muscle CSA (cm2), med (min-max) | 19.17 (14.05-31.11) | 21.57 (14.86-67.28) | .005 * |
BMI, body mass index; PCR, polymerase chain reaction; CT, computerized tomography; CSA, cross-sectional area; med, median; min, minimum; max, maximum; HCQ, hydroxychloroquine; AZT, azithromycin; FVP, favipiravir.
* P < .05.
T4- and T12-level CSAs of the patients were negatively correlated with age (Spearman’s rho correlation coefficient (r) = −0.161, P = .042 and r = −0.104, P = .045, respectively). No relationship was found with BMI (r = 0.026, P = .743 and r = 0.061, P = .446, respectively). The univariate regression analysis results of the patients’ T4- and T12-level muscle CSAs, demographic, and disease characteristics are presented in Table 3.
Table 3.
Univariate Analysis of T4- and T12-Level Muscle CSAs and Demographic and Disease Characteristics of Patients
| β | SE | P | 95% CI (LL/UL) | |
|---|---|---|---|---|
| T4-level CSA | ||||
| Sex | 0.605 | 0.793 | .447 | 0.068/0.959 |
| Additional comorbidity | −2.400 | 0.827 | .059 | −4.039/−0.762 |
| Active smoking | −2.388 | 2.365 | .316 | −0.792/−1.316 |
| PCR positivity | 4.062 | 2.970 | .173 | −1.791/9.915 |
| Pneumonia presence | −15.08 | 2.70 | .001 * | −20.422/−9.749 |
| Need of oxygen | −16.095 | 2.929 | .001 * | −22.767/−11.222 |
| Need of intubation | −17.859 | 6.949 | .012 * | −31.683/−4.035 |
| T12-level CSA | ||||
| Sex | −1.879 | 0.914 | .071 | −3.681/−0.077 |
| Additional comorbidity | 0.211 | 0.146 | .150 | −0.078/0.501 |
| Active smoking | −0.519 | 0.490 | .214 | −1.491/0.453 |
| PCR positivity | −2.623 | 0.830 | .068 | −4.259/−0.986 |
| Pneumonia presence | −3.387 | 0.265 | .001 * | −5.193/−1.580 |
| Need of oxygen | −4.313 | 0.892 | .001 * | −6.072/−2.555 |
| Need of intubation | −3.913 | 1.382 | .006 * | −6.660/−1.166 |
SE, standard error; LL, lower level; UL, upper level; PCR, polymerase chain reaction; CSA, cross-sectional area.
* P < .05.
T4-level muscle CSAs were found to be negatively correlated with the presence of pneumonia and the requirement of oxygen and intubation.
For significant correlations, as a result of age-, sex- and comorbidity-adjusted multivariate regression analysis, both T4- and T12-level muscle CSAs were factors associated with oxygen and intubation requirement. In addition, T4-level muscle CSAs were also associated with the presence of pneumonia (Table 4).
Table 4.
Multivariate Regression Analysis for Significantly Correlations
| β | SE | P | 95% CI (LL/UL) | |
|---|---|---|---|---|
| T4-level CSA | ||||
| Pneumonia presence | −16.232 | 3.471 | .006 * | −23.073/−9.391 |
| Need of oxygen | −10.718 | 4.613 | .021 * | −19.810/−1.627 |
| Need of intubation | −16.714 | 6.554 | .013 * | −29.749/−3.678 |
| T12-level CSA | ||||
| Pneumonia presence | −2.996 | 0.925 | .309 | −8.813/−2.281 |
| Need of oxygen | −7.509 | 2.926 | .014 * | −13.226/−2.667 |
| Need of intubation | −4.040 | 1.340 | .003 * | −6.704/−1.376 |
SE, standard error; LL, lower level; UL, upper level; CSA, cross-sectional area.
* P < .05.
Discussion
In this study, it was aimed to investigate the relationship between T4- and T12-level muscle mass CSAs in patients with COVID-19 and the demographic and disease characteristics of the patients. As a result of the study, it was found that patients who were PCR positive and with the presence of pneumonia on CT were older, had higher BMI, and had more comorbidities compared with the control group. Also, it was found that patients with advanced age and comorbidities had the presence of pneumonia on CT and needed hospitalization. T4- and T12-level muscle CSAs were found lower in the patient group than in the control group, as well as lower in inpatients compared to outpatients. It was found that these levels of muscle CSAs were associated with the need for oxygen and intubation and that the T4-level CSA was also associated with the presence of pneumonia.
The SARS-CoV-2 virus appears as a simple respiratory tract infection at levels ranging from mild disease to sepsis. In its clinical classification, the World Health Organization divides COVID-19 as mild symptomatic disease, pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and advanced stage with septic shock.12 According to the September 2020 data of the European Centers for Disease Prevention and Control, it was reported that most patients with COVID-19 had an asymptomatic or mild course of the disease, 21-25% needed hospitalization, and 9% required intensive care treatment.13 In our study, the rate of inpatients was 39% when all symptomatic patients (regardless of PCR and CT results) were considered. This situation is probably related to our health system because this study was conducted in a pandemic hospital. In other words, it is a place where patients are sent to an upper center from clinics that provide primary health care. This is not the first place where most patients are admitted. Therefore, patients with more pronounced symptoms and thought to be progressive may have applied to us or been referred to a primary health care center.
The need for hospitalization and intensive care increases both morbidity and mortality in individuals and socioeconomic burden in countries. Studies for this purpose have been conducted to determine in which patient the condition will be aggravated and risk factors for the severe course of the disease. These studies were found that disease and demographic characteristics such as advanced age, presence of additional comorbidities, and smoking have been defined as risk factors for severe disease.4-7 In our study, age was significantly higher and additional comorbidities were higher in the patient group compared with the controls as well as in inpatients compared with outpatients. These results confirm that COVID-19 pneumonia will be more prevalent in patients with advanced age and additional comorbidities because all of our inpatients had severe pneumonia requiring oxygen support and these patients were older and had more comorbidities.
The interesting point of the study was that more than half of the outpatients who were followed up had lung involvement findings of COVID-19 (67.6%). Although the outpatient’s median age value was lower than the inpatient group, there was no significant difference between patient groups. In other words, although outpatients had lung involvement, they were not at a level that required oxygen support. In previous studies, it has been reported that some individuals with very severe COVID-19 disease have severe lung damage and decrease in diffusion capacity with lung fibrosis.14 It has been shown that some of these patients can recover completely, whereas others experience serious morbidity or even mortality. We think that the reason for this is related to low muscle mass in the thoracic region, which we demonstrated in our study. The respiratory system consists of the lung which provides the exchange of respiratory gases and the chest wall and respiratory muscles which pump out exhaled gases (expiration).15 Moreover, lung ventilation is a mechanical process in which the respiratory muscles act together to allow air in and out of the lungs. Any change in the performance of the respiratory muscles can reduce the effectiveness of ventilation.15 The inspiratory muscles that help the thoracic cavity to expand are the diaphragm, intercostal, and parasternal muscles (e.g., pectoralis, serratus, and latissimus), and the expiratory muscles that provide compression of the thoracic cavity are the abdominal muscles and paraspinal muscles. In respiratory function, the primary inspiratory muscle is the diaphragm, and the expiratory muscles are the abdominal muscles. Other muscles, namely accessory muscles come into play when dysfunction in the respiratory system occurs and in the increase of metabolic need such as exercise.16 Studies conducted on patients in intensive care with COVID-19 disease have shown that an extreme hypermetabolic process occurs and that this may be a major contributing factor to the extraordinary ventilatory and oxygenation demands in patients with COVID‐19.17,18 Based on this hypothesis, we measured the CSA of accessory respiratory muscles at the T4 and T12 levels.
As a result of the study, we found that there was a significant decrease in muscle mass in the patient group compared with the control group as well as in inpatients compared with outpatients. Moreover, we found that the muscle mass CSAs at the T4 and T12 levels were associated with oxygen and intubation requirements as independent of age, sex, and the presence of comorbidities. There is little information in the literature evaluating the relationship between disease severity and thoracic muscle mass in patients with COVID-19. In fact, as far as we know, this study is the first to examine both thoracic and back muscles mass in patients with COVID-19. Some studies in the literature were performed in patients with chronic obstructive pulmonary disease and pulmonary fibrosis, including measurements of the same levels and muscles similar to our study. These studies have shown that muscle CSAs were associated with symptoms, severe airflow limitation, emphysema severity, prognosis, and mortality.19-22 We think that these results show that people with high muscle mass CSAs can compensate the respiratory problems caused by COVID-19 without the need for oxygen or even hospitalization and those with low muscle mass CSAs can fall into a decompensation that may need external support.
The most important limitation of our study is that we could not include healthy individuals without symptoms. The participants we received as a control group were individuals who showed symptoms of COVID-19 but were not diagnosed as having COVID-19 according to the international COVID-19 criteria. To avoid an unnecessary radiation load, we could not recruit healthy individuals for the study. In addition, we could not include the diaphragm and abdominal muscles, which play a primary role in respiratory function. We think that large-scale studies that include these muscles will make our results more understandable.
Conclusion
We still know very little about COVID-19. In this study, it has been shown that decreased mass of the muscles defined as accessory respiratory muscles at the T4 and T12 levels is associated with the need for oxygen and intubation. We think that if the mass of these accessory muscles decreases, it can be predicted that the disease will progress seriously and that the respiratory load cannot be compensated.
Footnotes
Ethics Committee Approval: This study was approved by Ethics committee of University of Health Sciences, Dışkapı Yıldırım Beyazıt Training and Research Hospital (Aproval No: 91/09).
Informed Consent: Informed consent forms were signed upon admission to the trial.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – Z.AY., E.KU., G.A., M.ÇE.; Design – Z.AY., E.KU., G.A., M.ÇE.; Supervision – Z.AY., E.KU.; Resources – Z.AY., E.KU., G.A., M.ÇE.; Materials – Z.AY., E.KU., G.A., M.ÇE.; Data Collection and/or Processing – Z.AY., E.KU., G.A., M.ÇE.; Analysis and/or Interpretation – Z.AY., E.KU., G.A., M.ÇE.; Literature Search – Z.AY., E.KU., G.A., M.ÇE.; Writing Manuscript – Z.AY., E.KU., G.A., M.ÇE.; Critical Review – Z.AY., E.KU., G.A., M.ÇE.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91 98. 10.1016/j.jare.2020.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133(11):1261 1267. 10.1097/CM9.0000000000000824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyd S, Martin-Loeches I. The incidence of venous thromboembolism in critically ill patients with COVID-19 compared with critically ill non-COVID patients. Ir J Med Sci. 2021;190(4):1 4. 10.1007/s11845-020-02503-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054 1062. 10.1016/S0140-6736(20)30566-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Y, Sun W, Li J, et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. MedRxiv. 2020. 10.1101/2020.02.17.20024166) [DOI] [Google Scholar]
- 6. Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1):e35. [PMC free article] [PubMed] [Google Scholar]
- 7. Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133(9):1032 1038. 10.1097/CM9.0000000000000775). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145-151. 10.3760/cma.j.issn.0254-6450.2020.02.003) [DOI] [PubMed] [Google Scholar]
- 9. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32 E40. 10.1148/radiol.2020200642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American College of Radiology (ACR). ACR Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. 2020. Available at: https://www.acr.org/. [Google Scholar]
- 11. Moon SW, Choi JS, Lee SH, et al. Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir Res. 2019;20(1):35. 10.1186/s12931-019-1001-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao X, Li T, He Z, et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):411 417. 10.3760/cma.j.cn112151-20200312-00193) [DOI] [PubMed] [Google Scholar]
- 13. European Centre for Disease Prevention and Control. 2020. Available at: https://covid19-surveillance-report.ecdc.europa.eu./. [Google Scholar]
- 14. Lazzeri M, Lanza A, Bellini R, et al. Respiratory physiotherapy in patients with COVID-19 infection in acute setting: a Position Paper of the Italian Association of Respiratory Physiotherapists (ARIR). Monaldi Arch Chest Dis. 2020;90(1). 10.4081/monaldi.2020.1285) [DOI] [PubMed] [Google Scholar]
- 15. Ratnovsky A, Elad D, Halpern P. Mechanics of respiratory muscles. Respir Physiol Neurobiol. 2008;163(1-3):82 89. 10.1016/j.resp.2008.04.019) [DOI] [PubMed] [Google Scholar]
- 16. Hill NS. Noninvasive ventilation for chronic obstructive pulmonary disease. Respir Care. 2004;49(1):72 87; discussion 87. [PubMed] [Google Scholar]
- 17. Yu PJ, Cassiere H, DeRosa S, Bocchieri K, Yar S, Hartman A. Hypermetabolism and coronavirus disease 2019. JPEN J Parenter Enter Nutr. 2020;44(7):1234 1236. 10.1002/jpen.1948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whittle J, Molinger J, MacLeod D, Haines K, Wischmeyer PE. LEEP-COVID Study Group. Persistent hypermetabolism and longitudinal energy expenditure in critically ill patients with COVID-19. Crit Care. 2020;24(1):581. 10.1186/s13054-020-03286-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanimura K, Sato S, Fuseya Y, et al. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease: novel chest computed tomography–derived index for prognosis. Ann Am Thorac Soc. 2016;13(3):334 341. 10.1513/AnnalsATS.201507-446OC) [DOI] [PubMed] [Google Scholar]
- 20. Mathur S, Rodrigues N, Mendes P, Rozenberg D, Singer LG. Computed tomography–derived thoracic muscle size as an indicator of sarcopenia in people with advanced lung disease. Cardiopulm Phys Ther J. 2017;28(3):99 105. 10.1097/CPT.0000000000000054) [DOI] [Google Scholar]
- 21. McDonald ML, Diaz AA, Ross JC, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease: a cross-sectional study. Ann Am Thorac Soc. 2014;11(3):326 334. 10.1513/AnnalsATS.201307-229OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rozenberg D, Mathur S, Herridge M, et al. Thoracic muscle cross‐sectional area is associated with hospital length of stay post lung transplantation: a retrospective cohort study. Transpl Int. 2017;30(7):713 724. 10.1111/tri.12961) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a