Abstract
Kefir, a fermented beverage made from kefir grains, has gained immense popularity around the world due to its potential health-promoting properties. Kefir beverages are both marketed commercially and brewed privately by individuals. Both milk and sugar solutions can be used as substrates with various additives included based on consumer preference. Fermentation occurs via microorganisms including lactic acid bacteria, acetic acid bacteria, and yeasts, which are naturally present in kefir grains. Health-promoting effects of kefir are thought to occur through immune, gastrointestinal, and metabolic regulation. Both clinical trials and mechanistic studies in cell culture and animal models have explored these effects. Studies in vitro and in animals have shown the ability of kefir and kefir components to antagonize pathogens, reduce proinflammatory cytokine production, contribute to cytotoxicity of tumor cell lines and reduce tumor burden, and improve serum glycemic and lipid profiles. However, some data from clinical trials are conflicting, and the precise mechanisms by which kefir promotes well-being are not completely defined. This review summarizes the current body of evidence in both cell culture and animal models that provide insight into the mechanisms by which kefir beverages may protect consumers from enteric infections and improve immune and metabolic health. We believe that readers will gain knowledge helpful for both developing more targeted mechanistic studies and selecting informative outcomes when designing clinical studies.
Keywords: lactic acid bacteria, functional foods, fermentation, fermented beverages, kefir
Introduction and background
Kefir is a fermented beverage that is believed to have beneficial health effects due to its antioxidant, antimicrobial, and anti-inflammatory properties [1]. It is made by adding kefir grains to either milk or a sugar solution and various recipes exist with different additives. Originating from the Caucasus mountains, kefir grains are now produced commercially and used to make products sold in local supermarkets and by individuals who wish to make their own kefir. Grains harbor a complex community of microorganisms including lactic acid bacteria, acetic acid bacteria, and yeasts within a fibrous matrix [2-4]. Within these bacterial groups are many known probiotics, which are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [5]. Clinical trials (reviewed in detail elsewhere) in some cases have demonstrated specific health benefits; however, data are conflicting, and mechanistic studies are still ongoing [6-7]. The known interactions between kefir and the hosts that consume it have been documented using whole kefir products, microorganisms isolated from kefir, and individual biochemical components of kefir. The purpose of this review is to summarize evidence from pre-clinical studies (in vitro and animal models) that suggests potential mechanisms by which kefir contributes to immune health, metabolic health, and protection from pathogens.
PubMed and Google Scholar databases were searched for original research articles using the terms “kefir” AND “inflammation” OR “immune”, “allergy”, “pathogen”, “metabolic”, “lipid”, “cholesterol”, and “clinical trial” without constraints for the year of publication. Titles and abstracts of articles were screened to ensure the inclusion of clinically relevant outcomes versus only chemical and microbial analyses of kefir. Additional articles were identified from reference lists of articles obtained from the initial search. Only full-text articles in English were reviewed. Citations of other reviews are limited and are only used to support broad statements regarding topics beyond the scope of this review. Book chapters, editorials, case reports, case series, technical reports, and non-peer-reviewed literature (e.g., lectures, symposia presentations, magazine and newspaper articles, news reports, press releases, commercial literature such as package inserts and labels, and websites) were excluded.
Review
In vitro models
Immunomodulatory Effects of Kefir Fractions
In vitro studies have focused primarily on microbial isolates and individual biochemical components of kefir. Romanin et al. showed that many different yeast strains isolated from kefir decreased the innate immune response in vitro when cultured with Caco-2 cells stimulated with flagellin, which signals through the toll-like receptor (TLR) 5 pathway [8]. Similarly, several strains of Enterococcus durans isolated from kefir and cultured with Caco-2 cells prevented a flagellin-induced proinflammatory response [9]. Conversely, Probiotics Fermentation Technology (PFT), a freeze-dried mixture of heat-killed Lactobacillus kefiri P-IF, added to cell cultures increased both Th1 cytokine production and interleukin-10 (IL-10, and anti-inflammatory cytokine) production by monocyte-derived dendritic cells isolated from peripheral blood mononucleated cells of healthy individuals [10]. Combined, these results suggest both a stimulatory and anti-inflammatory role of kefir components in immunoregulation.
Cytotoxicity of Kefir Fractions in Cancer Cell Lines
Experiments investigating the effects of kefir on human cancer cell lines have shown positive effects on cell cycle regulation and cancer cell death. Microbial cells isolated from kefir decreased proliferation of malignant T lymphocytes in vitro in a time and dose-dependent manner. Furthermore, the expression of transforming growth factor alpha (TGF-α) decreased while TGF-β1 increased [11]. Cell-free kefir supernatant incubated with HT-29 and Caco-2 cells decreased cell viability and proliferation in a dose and time-dependent manner, and this was thought to occur via cell cycle arrest at the G1 transition checkpoint [12]. Another study using cell-free kefir supernatant incubated with human T-cell lymphotropic virus type 1-infected malignant T cells showed decreased viability and proliferation in a time-dependent manner and inhibition of TGF-α in a dose-dependent manner [13]. Ghoneum et al. showed that PFT added to cultures of human myeloid leukemia cells (HL60/AR) increased the percentage of apoptosis through what may be a hole-piercing mechanism [14]. Another study investigating the activity of natural killer (NK) cells isolated from 12 healthy individuals who consumed kefir demonstrated increased NK cell activity against the K562 cancer cell line [15]. While these data suggest beneficial effects of kefir against various cancer cell lines, the use of kefir in cancer patients should be considered carefully. A clinical trial investigating the effects of kefir on patients undergoing treatment for colorectal cancer showed a decreased quality of life in many domains [16]. Therefore, additional studies should be conducted to determine not only the mechanistic effects against these and other cell lines but also the overall risks and benefits to patients.
Pathogen Antagonism
In vitro studies using whole kefir, kefir isolates, and kefir fractions have demonstrated antagonizing effects against various bacterial pathogens. Viable counts of two Salmonella enterica subspecies (Arizonae and Typhimurium) were rapidly reduced when cultured in kefir fermented milk versus standard milk [17]. Similarly, supernatants from various kefir grain milk cultures inhibited the growth of enteric pathogens in the culture [18]. In vitro experiments in the Caco-2 cell line demonstrated the ability of the cell-free fraction of kefir preincubated with Salmonella enterica serovar Enteritidis to reduce invasion of epithelial cells [19]. Golowczyc et al. demonstrated similar results using a strain of L. kefiri added directly to the cell culture [20]. Supernatants of kefir isolates demonstrated variable ability to inhibit the cytotoxicity of Clostridium difficile spent culture supernatant on Vero cells [21]. In the same cell line, S-layer proteins isolated from L. kefiri inhibited C. difficile toxin cell damage as measured by cell detachment [22]. Some Lactobacilli species (and their cell membrane components) isolated from kefir demonstrated the ability to increase cell viability when cultured with Vero cells in the presence of E. coli O157:H7 culture supernatants [23]. In another study by Bolla et al., a mixture of yeasts and bacteria isolated from kefir, when pre-incubated with Caco-2 and HT-29 cells, decreased Shigella flexneri invasion and proinflammatory cytokine production in culture [24]. These results demonstrate a myriad of pathogen antagonistic effects involving different kefir preparations and isolates on a variety of enteric bacteria.
Antioxidant Properties
Antioxidant properties of kefir have been demonstrated with various biochemical assays [25-26]. These properties are dependent on fermentation time and storage time [27]. Other studies have investigated the effects of individual components and fractions of kefir. Exopolysaccharides from milk kefir fermentation demonstrated the ability to reduce oxidative protein damage in a concentration-dependent manner [28]. A study using HT-29 cells incubated with genotoxic fecal water from several individuals demonstrated the ability of kefir supernatant to reduce the amount of DNA damage. Furthermore, the supernatant displayed greater antioxidant capacity versus milk (control); however, these effects were not consistent among all subjects studied [29].
Animal models
Immune Modulation in Healthy Rodents
The immunomodulating effects of kefir in animal models have been primarily described as stimulation of phagocyte activity, induction of immunoglobin A (IgA), and Th1 cytokine production with some evidence of anti-inflammatory properties via upregulation of IL-10. Mice fed raw or pasteurized kefir ad libitum displayed increases in numbers of IgA-producing cells and macrophage phagocytic activity in both intestinal and bronchial tissue, which suggests the immunological effects of kefir may be systemic [30]. L. kefiri CIDCA 8348 given over 21 days increased fecal sIgA concentrations and increased IL-10 gene expression in the ileum and mesenteric lymph nodes of healthy Swiss mice [31]. Mice consuming kefiran, an exopolysaccharide extracted from kefir grains, ad libitum (300 mg/L) for two days displayed an increase in the number of lamina propria IgA+ cells and macrophages. Furthermore, there was an upward trend in the number of macrophages and both total and activated dendritic cells in Peyer’s patches after seven days of treatment. Peritoneal macrophage counts were also increased after seven days [32]. Another study in which mice received kefiran for seven days displayed increased numbers of IgA+ cells and Th1 cytokine production in the small intestine, while both IgA+ and IgG+ cell numbers and both Th1 and Th2 cytokine concentrations increased in the large intestine [33]. Another study by the same group using kefir protein given to mice for seven days displayed a peak, typically on day two, of inflammatory cytokine production by peritoneal macrophages and both inflammatory and anti-inflammatory cytokine production in cells derived from Peyer’s patches [34]. The associated proinflammatory cytokine release in peritoneal macrophages is thought to occur through a TLR-2-mediated pathway [35].
Immunomodulation in Inflammatory Models
The beneficial effects of kefir on inflammation have further been demonstrated in dextran sodium sulfate-induced colitis models. Kefir fed to rats reduced histologic colitis scores and prevented an increase of TNF-α concentrations in colonic tissue [36]. Similar effects have been shown with Lactobacillus kefiranofaciens M1, a well-studied isolate of kefir. L. kefiranofaciens M1 decreased proinflammatory cytokines (TNF-α and IL-1β), increased colonic concentrations of IL-10, and improved histological and bleeding scores in mice [37]. In a study involving germ-free mice, L. kefiranofaciens M1 reduced cecal enlargement and histological scores after eight weeks of treatment versus placebo. L. kefiranofaciens M1 was not detected in stools after seven days post-inoculation, which suggests that it did not colonize the gastrointestinal tract [38].
Immunomodulation in Allergic Models
Kefir fed to ovalbumin sensitized mice has been shown to have implications in mitigating allergic responses. Lee et al. showed decreased eosinophils and IgE concentrations in bronchiolar lavage fluid [39]. Another study on mice receiving milk or soymilk kefir revealed lower concentrations of serum IgE and IgG1 (but not IgG2a) and increased fecal counts of Lactobacillus and bifidobacteria versus placebo [40]. Similar effects have been shown with L. kefiranofaciens. Heat-inactivated L. kefiranofaciens M1 given to mice for 28 days decreased serum IgE concentrations and increased the production of Th1 cytokines in splenocytes [41]. In another study, L. kefiranofaciens M1 treatment for 32 days decreased proinflammatory cytokines in splenocytes in a dose-dependent manner during only the sensitization and challenge periods; however, this effect was seen in bronchiolar lavage fluid throughout the entire study period, which suggests such immunomodulating effects of kefir are both systemic and site-specific. Additionally, serum OVA-specific IgE concentrations were decreased at the end of the study period [42].
Immunomodulation in Other Models of Injury
The physiological milieu appears to affect the way kefir potentiates immune responses. Some evidence suggests kefir may reduce inflammation-mediated organ damage after ischemic events. Kefir fed to rats for 30 days via oral gavage decreased serum urea, creatinine, and tumor necrosis factor-alpha (TNF-α) concentrations after aortic ischemia and reperfusion [43]. However, in an oncogenic model, the immune modulating effects of kefir were variable. Mice inoculated with 4T1 breast cancer cells that received water kefir treatment displayed increased serum Th1 cytokines concentrations but decreased IL-10 concentrations. Furthermore, the kefir-fed mice exhibited decreased tumor size, volume, and number of metastatic tumor cells in lung tissue compared to controls [44]. Conversely, another study in rats using a 1,2-dimethylhydrazine colorectal cancer model showed that kefir versus placebo decreased tissue concentrations of IL-1, IL-6, and TNF-α, but the rats similarly displayed fewer numbers of tumors. These differences were associated with changes in gut microbiota community composition [45]. A notable difference between the two studies was the site of cytokine analysis (serum versus tissue). These results suggest the effects of kefir on cytokine production may be dependent upon multiple factors including anatomical site, interactions with gut microbiota, and the physiological environment. However, data from these studies appear to consistently lead to favorable clinical outcomes.
Pathogen Antagonism
Animal studies using whole kefir and organisms isolated from kefir have shown antagonistic effects against various intestinal parasites and bacterial organisms similar to in vitro studies. Kefir fed to mice infected with Entamoeba histolytica for seven days decreased the number of parasites in feces and increased intestinal sIgA concentrations [46]. In another study using mice infected with Giardia intestinalis, kefir decreased the amount of viable luminal trophozoites and increased the number of IgA-positive lamina propria cells in the small intestines [47]. L. kefiranofaciens M1 fed to mice daily for seven days with concurrent enterotoxic hemorrhagic Escherichia coli (EHEC) inoculation reduced histological inflammation scores and EHEC translocation into visceral organs. Furthermore, the mice displayed increased EHEC-specific fecal sIgA concentrations [48]. Lactobacillus plantarum C4, an isolate from kefir with less recognition in the kefir literature but whose species is often studied for its probiotic properties, decreased Yersinia enterocolitica colonization in Peyer’s patches and increased cecal sIgA concentrations in mice fed a typical probiotic dose of 109 colony-forming units/day [49]. A study by Huseini et al. in rats with burn injuries inoculated with Pseudomonas aeruginosa displayed quicker wound healing with a kefir gel treatment versus silver sulfadiazine, a standard treatment for burn injuries [50]. Data from studies examining rodents infected with C. difficile are conflicting regarding the benefits and harms of kefir and kefir constituents. A mixture of kefir-isolated organisms given to hamsters in drinking water decreased the biological activity of toxins after infection with C. difficile [51]. However, one study showed decreased survival in mice receiving kefir [52]. These discordant results may be due to differing animal models, study designs, outcome measures, and specific interventions.
Metabolic Effects
Studies that have examined outcomes related to metabolic syndrome, particularly serum glucose, have involved both whole kefir and kefir isolates. Kefir fed to male hyperglycemic Wistar rats decreased serum glucose and increased serum IL-10 concentrations [53]. The blood glucose-lowering effects of kefir in rats were also shown by Urdanteta et al. [54]. This effect has also been demonstrated after an oral glucose tolerance test. In that study, the kefir-fed rats displayed decreased glycogen concentrations in renal tubules and superoxide ion concentrations in the renal cortex [55]. An experiment in vitro using rat skeletal muscle cells demonstrated increased glucose uptake in cells incubated with the supernatant of a kefir product both in the presence and absence of insulin [56]. The particular type of substrate fermented with kefir may affect its ability to modulate not only blood glucose but also inflammatory markers. Diabetic rats fed goat or soy milk kefir via oral gavage (2mL/d) for four weeks displayed lower concentrations of serum IL-6, but only goat milk kefir was able to decrease serum C-reactive protein (CRP) and glucose concentrations [57].
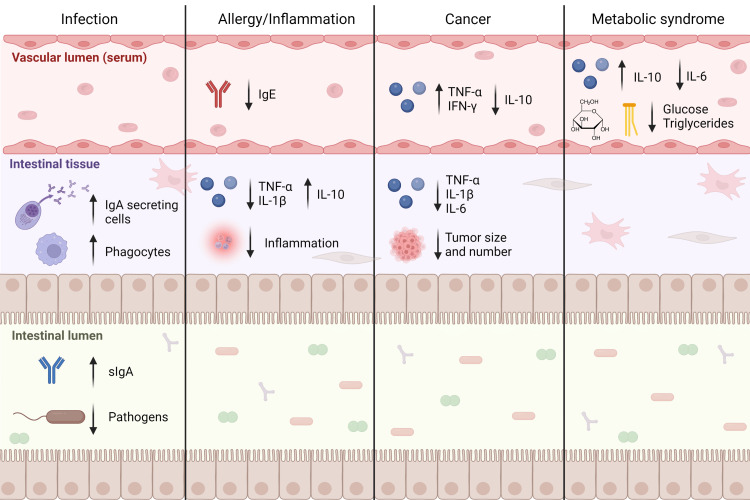
Additional studies focused on other aspects of metabolic syndrome, including blood lipids and obesity, have demonstrated modulating effects of kefir and kefir isolates. Obese mice that received 140 mg/mg/day kefir for four weeks had lower body weights and increased basal metabolic rates and energy expenditure compared to obese mice that received a control diet [58]. Apolipoprotein E knockout mice receiving water kefir versus plain drinking water for four weeks displayed lower serum concentrations of triglycerides and higher concentrations of high-density lipoprotein. However, fatty streaking of the aorta was not different between groups [59]. Kefir containing several strains of lactobacilli reduced body weight gain, insulin resistance, and hepatic steatosis in mice fed a high-fat diet [60]. Complementary results were seen in an experiment in which three different isolates (Lactobacillus acidophilus LA15. L. plantarum B23, and L. kefiri D17) were studied. L. plantarum B23 fed to rats for four weeks lowered serum triglycerides, whereas L. kefiri D17 lowered both serum triglycerides and low-density lipoprotein (LDL). All three intervention groups displayed lower hepatic and higher fecal total cholesterol concentrations [61]. Studies in which kefir components were used revealed similar findings. Kefir peptides fed to mice in 30% fructose solution decreased hepatic fat accumulation and the number of inflammatory foci in hepatic tissue versus fructose alone [62]. Surface layer proteins from bacteria isolated from kefir grains, when added to a high-fat diet, decreased body weight, adipose tissue weight, and serum triglycerides in mice [63]. These results suggest that the effects of kefir on lipids and metabolic outcomes are multifactorial as demonstrated by the ability of different components (microbial organisms and biochemical fractions) to produce similar effects when given as isolates. The effects of kefir on both immune and metabolic physiology in rodents are summarized in Figure 1.
Figure 1. Effects of kefir administration on immune and metabolic physiology in rodent models.
Ig: immunoglobulin; TNF: tumor necrosis factor; IL: interleukin
Clinical trials
Clinical trials have revealed conflicting data regarding the beneficial health effects of kefir. While several trials have demonstrated positive effects of kefir, others have shown no effect. Of the trials that demonstrate positive clinical effects, measured outcomes compared to pre-clinical studies, and those in pre-clinical studies are too heterogenous to effectively translate the results from pre-clinical studies into the clinical sphere. Furthermore, heterogeneity between clinical study designs and outcome measures contributes to the lack of reproducibility between clinical studies and further limits the adoption of clinical practices involving kefir.
Kefir given to patients with inflammatory bowel disease decreased serum markers of inflammation, namely erythrocyte sedimentation rate and CRP, and increased the quality of life in the setting of altered gut microbial community composition [64]. However, another study by Praznikar et al. showed no changes in serum inflammatory marker concentrations (CRP and adiponectin) with kefir administration despite a decrease in serum zonulin (a marker of intestinal permeability) concentrations [65]. Bellikci-Koyu et al. showed that patients with metabolic syndrome experienced a decrease in serum concentrations of proinflammatory cytokines (TNF-α, IL-6, and interferon-gamma), but also experienced a decrease in IL-10 with kefir administration. The same study showed increases in serum apolipoprotein A1 concentrations versus placebo but showed no differences in serum apolipoprotein B and LDL concentrations [66]. In another study by the same group, both kefir and the placebo lowered serum concentrations of both insulin and the same cytokines; thus, these decreases cannot be attributed to kefir administration [67]. Furthermore, in a study by Topez et al., patients with cancer undergoing chemotherapy were given kefir versus placebo and showed no changes in serum cytokine concentrations or incidence of mucositis [68]. Together, these results suggest that the potential anti-inflammatory effects of kefir are likely modulated by other physiological factors such as gut microbial community composition, disease status, and concurrent medication use.
A study examining serum glucose concentrations showed that kefir led to smaller increases versus high glycemic index solutions, but hemoglobin A1c and both insulinemic and satiety indices were unaffected [69]. Another study similarly showed that kefir reduced serum concentrations of glucose, but hemoglobin A1c also decreased significantly. Serum lipids were unaffected [70]. St-Onge et al. also showed no changes in serum lipid profiles with kefir administration in a small male cohort [71]. However, Ghizi et al. showed a decrease in serum LDL and triglycerides with kefir versus placebo in patients with metabolic syndrome, and an increase in serum high-density lipoprotein (HDL) was only observed in females [72]. Similarly, in a study including both patients with dyslipidemia and patients with normal lipid profiles, only patients with dyslipidemia displayed decreases in serum lipid concentrations, but this study did not involve a placebo group [73]. Comparable to the reported effects of kefir on immune outcomes in clinical studies, metabolic outcomes appear to be affected by host characteristics such as disease status and gender. Of note, all aforementioned clinical studies had relatively small sample sizes (n=<100) compared with many phase 3 and 4 clinical trials (often, n=>1000). Therefore, there is a need for both additional small clinical trials with reproducible results as well as larger confirmatory trials.
Conclusions
Kefir has been shown to have potential health-promoting effects in various models; however, study designs to date have been confoundingly heterogeneous. The variation in study design stems from each of the kefir grains chosen, the fraction of the kefir product used, the in vitro or animal model employed, and the outcomes measured. Recipes for various fermented kefir products differ, including their fermentation substrates, and the products studied include whole kefir beverages, individual or a consortium of bacterial isolates, or structural components. Further variability is introduced by the variation of microbial community composition between various grains. Lastly, experimental models and outcome measures are inconsistent between studies as exemplified above. Therefore, further mechanistic studies are needed to close existing knowledge gaps and demonstrate a consistency of results among similar studies. Additionally, current clinical trial data are conflicting, and more clinical trials in humans are needed to translate these mechanistic findings into relevant clinical outcomes with reproducibility. The pre-clinical studies reviewed herein, in the context of current clinical trial data, should guide clinical researchers’ choices of interventions and outcome measures when designing further clinical and translational research studies.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared financial relationships, which are detailed in the next section.
Tyler Culpepper declare(s) employment from EcoConvergence Group. I was employed as a consultant by this company during part of the time the literature review was conducted, but the company did not use any of these findings for product development, advertisement, or any sort of economical gain. Neither the company nor its consituents directed me on how to conduct the reviwew. The company did not participate in manuscript preparation, revision, edititing, nor did it (or any of its employees) provide and advisement on content. The company did not provide funding for publicaiton.
References
- 1.Microbiological, biochemical, and functional aspects of sugary kefir fermentation - a review. Fiorda FA, de Melo Pereira GV, Thomaz-Soccol V, Rakshit SK, Pagnoncelli MG, Vandenberghe LP, Soccol CR. Food Microbiol. 2017;66:86–95. doi: 10.1016/j.fm.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 2.The microbial diversity of water kefir. Gulitz A, Stadie J, Wenning M, Ehrmann MA, Vogel RF. Int J Food Microbiol. 2011;151:284–288. doi: 10.1016/j.ijfoodmicro.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Microbial species diversity, community dynamics, and metabolite kinetics of water kefir fermentation. Laureys D, De Vuyst L. Appl Environ Microbiol. 2014;80:2564–2572. doi: 10.1128/AEM.03978-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Identification of bacteria and yeast communities in Thai sugary kefir by polymerase chain reaction-denaturing gradient gel electrophoresis. Boonyarattanakalin S, Sarikkha P, Nitisoravut R, Poljungreed I. http://www.ojs.kmutnb.ac.th/index.php/joindtech/article/viewFile/4215/3014 Journal Ind Technol. 2015;11:25–39. [Google Scholar]
- 5.Probiotics: definition, scope and mechanisms of action. Reid G. Best Pract Res Clin Gastroenterol. 2016;30:17–25. doi: 10.1016/j.bpg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Effect of kefir beverage consumption on glycemic control: a systematic review and meta-analysis of randomized controlled clinical trials. Salari A, Ghodrat S, Gheflati A, Jarahi L, Hashemi M, Afshari A. Complement Ther Clin Pract. 2021;44:101443. doi: 10.1016/j.ctcp.2021.101443. [DOI] [PubMed] [Google Scholar]
- 7.Kefir in the prevention and treatment of obesity and metabolic disorders. Bourrie BC, Richard C, Willing BP. Curr Nutr Rep. 2020;9:184–192. doi: 10.1007/s13668-020-00315-3. [DOI] [PubMed] [Google Scholar]
- 8.Down-regulation of intestinal epithelial innate response by probiotic yeasts isolated from kefir. Romanin D, Serradell M, González Maciel D, Lausada N, Garrote GL, Rumbo M. Int J Food Microbiol. 2010;140:102–108. doi: 10.1016/j.ijfoodmicro.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Safety and potential beneficial properties of Entercoccus strains isolated from kefir. Carasi P, Jacquot C, Romanin DE, Elie AM, De Antoni GL, Urdaci MC, Serradell MA. Int Dairy J. 2014;39:193–200. [Google Scholar]
- 10.A novel kefir product (PFT) activates dendritic cells to induce CD4+T and CD8+T cell responses in vitro. Ghoneum M, Felo N, Agrawal S, Agrawal A. Int J Immunopathol Pharmacol. 2015;28:488–496. doi: 10.1177/0394632015599447. [DOI] [PubMed] [Google Scholar]
- 11.Kefir induces cell-cycle arrest and apoptosis in HTLV-1-negative malignant T-lymphocytes. Maalouf K, Baydoun E, Rizk S. Cancer Manag Res. 2011;3:39–47. doi: 10.2147/CMR.S15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kefir exhibits anti‑proliferative and pro‑apoptotic effects on colon adenocarcinoma cells with no significant effects on cell migration and invasion. Khoury N, El-Hayek S, Tarras O, El-Sabban M, El-Sibai M, Rizk S. Int J Oncol. 2014;45:2117–2127. doi: 10.3892/ijo.2014.2635. [DOI] [PubMed] [Google Scholar]
- 13.The antiproliferative effect of kefir cell-free fraction on HuT-102 malignant T lymphocytes. Rizk S, Maalouf K, Baydoun E. Clin Lymphoma Myeloma. 2009;9:0–203. doi: 10.3816/CLM.2009.s.012. [DOI] [PubMed] [Google Scholar]
- 14.Apoptotic effect of a novel kefir product, PFT, on multidrug-resistant myeloid leukemia cells via a hole-piercing mechanism. Ghoneum M, Gimzewski J. Int J Oncol. 2014;44:830–837. doi: 10.3892/ijo.2014.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anticancer activities of kefir against LMS and K562 cell lines by flow cytometry analysis. Toliopoulos IK, Simos Y, Verginadis I, Papandreou D, Oikonomidis S, Evangelou A. Nutri Food Sci. 2012;42:261–270. [Google Scholar]
- 16.Effect of kefir on the quality of life of patients being treated for colorectal cancer. Can G, Topuz E, Derin D, Durna Z, Aydiner A. https://www.proquest.com/docview/223112824. Oncol Nurs Forum. 2009;36:0–42. doi: 10.1188/09.ONF.E335-E342. [DOI] [PubMed] [Google Scholar]
- 17.Antimicrobial properties of traditional kefir: an in vitro screening for antagonistic effect on Salmonella Typhimurium and Salmonella Arizonae. Gut AM, Vasiljevic T, Yeager T, Donkor ON. Int Dairy J. 2022;124:105180. [Google Scholar]
- 18.Antimicrobial activity of kefir against various food pathogens and spoilage bacteria. Kim DH, Jeong D, Kim H, Kang IB, Chon JW, Song KY, Seo KH. Korean J Food Sci Anim Resour. 2016;36:787–790. doi: 10.5851/kosfa.2016.36.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biological activity of the non-microbial fraction of kefir: antagonism against intestinal pathogens. Iraporda C, Abatemarco Júnior M, Neumann E, Nunes ÁC, Nicoli JR, Abraham AG, Garrote GL. J Dairy Res. 2017;84:339–345. doi: 10.1017/S0022029917000358. [DOI] [PubMed] [Google Scholar]
- 20.Cellular injuries of spray-dried Lactobacillus spp. isolated from kefir and their impact on probiotic properties. Golowczyc MA, Silva J, Teixeira P, De Antoni GL, Abraham AG. Int J Food Microbiol. 2011;144:556–560. doi: 10.1016/j.ijfoodmicro.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Kefir-isolated Lactococcus lactis subsp. lactis inhibits the cytotoxic effect of Clostridium difficile in vitro. Bolla PA, Carasi P, Serradell Mde L, De Antoni GL. J Dairy Res. 2013;80:96–102. doi: 10.1017/S0022029912000623. [DOI] [PubMed] [Google Scholar]
- 22.Surface proteins from Lactobacillus kefir antagonize in vitro cytotoxic effect of Clostridium difficile toxins. Carasi P, Trejo FM, Pérez PF, De Antoni GL, Serradell Mde L. Anaerobe. 2012;18:135–142. doi: 10.1016/j.anaerobe.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Lactobacillus plantarum isolated from kefir protects vero cells from cytotoxicity by type-II shiga toxin from Escherichia coli O157:H7. Kakisu E, Abraham AG, Farinati CT, Ibarra C, De Antoni GL. J Dairy Res. 2013;80:64–71. doi: 10.1017/S0022029912000659. [DOI] [PubMed] [Google Scholar]
- 24.Kefir-isolated bacteria and yeasts inhibit Shigella flexneri invasion and modulate pro-inflammatory response on intestinal epithelial cells. Bolla PA, Abraham AG, Pérez PF, de Los Angeles Serradell M. Benef Microbes. 2016;7:103–110. doi: 10.3920/BM2015.0061. [DOI] [PubMed] [Google Scholar]
- 25.Antioxidant activity, biochemical components and sub-chronic toxicity of different brown rice kefir powders. Chunchom S, Talubmook C, Deeseenthum S. Pharmacogn J. 2017;9:388–394. [Google Scholar]
- 26.Antioxidant activities of kefir. Liu JR, Lin YY, Chen MJ, Chen LJ, Lin CW. Asian-Australas J Anim Sci. 2005;18:567–573. [Google Scholar]
- 27.The antioxidative capacity of kefir produced from goat milk. Yilmaz-Ersan L, Ozcan T, Akpinar-Bayizit A, Şahin S. https://d1wqtxts1xzle7.cloudfront.net/71516006/0ab0dabe65a06a6122a5b6fb02162d386c04-with-cover-page-v2.pdf?Expires=1658853224&Signature=RxSLCc80Np8ts1ylapNWC9BSrqGXpvXbMz2yYqvC-Eex5cJUvwwhTato2EmI49DTLz8NuGixFS6jDmgngw89RBazG51bzhMeajDmt8ZFVK6AXQSjsdanD2pagZS~l-iprGGr9z2wWlMf2PZBhsNxRDEG8HKXmwYVk7M0Hkz~AkXFSKDfWtf-5g1R2MNvxH9K6ioD3uTU4USSAGyuHiTyEk5jWusI4ncQd24x9O8-g5AMlAZJTpfeMH8QHqB5BsTaMCrjYJnyi97g0lGpFZBHIV-SYOyFDJSdNTUbeR0q6u4UyFvgNiff1c5dXSNyfG5Slg~AcK-gzhn7yTEgYHBazw__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA Int J Chem Eng Appl. 2016;7:22–26. [Google Scholar]
- 28.Chemical and physical characteristics and antioxidant activities of the exopolysaccharide produced by Tibetan kefir grains during milk fermentation. Chen Z, Shi J, Yang X, Nan B, Liu Y, Wang Z. Int Dairy J. 2015;43:15–21. [Google Scholar]
- 29.Antigenotoxic effect of kefir and ayran supernatants on fecal water-induced DNA damage in human colon cells. Grishina A, Kulikova I, Alieva L, Dodson A, Rowland I, Jin J. Nutr Cancer. 2011;63:73–79. doi: 10.1080/01635581.2010.516873. [DOI] [PubMed] [Google Scholar]
- 30.Distal mucosal site stimulation by kefir and duration of the immune response. Vinderola CG, Duarte J, Thangavel D, Perdigon G, Farnworth E, Matar C. Eur J Inflamm. 2005;3:63–73. [Google Scholar]
- 31.Impact of kefir derived Lactobacillus kefiri on the mucosal immune response and gut microbiota. Carasi P, Racedo SM, Jacquot C, Romanin DE, Serradell MA, Urdaci MC. J Immunol Res. 2015;2015:361604. doi: 10.1155/2015/361604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oral administration of kefiran induces changes in the balance of immune cells in a murine model. Medrano M, Racedo SM, Rolny IS, Abraham AG, Pérez PF. J Agric Food Chem. 2011;59:5299–5304. doi: 10.1021/jf1049968. [DOI] [PubMed] [Google Scholar]
- 33.Effects of the oral administration of the exopolysaccharide produced by Lactobacillus kefiranofaciens on the gut mucosal immunity. Vinderola G, Perdigón G, Duarte J, Farnworth E, Matar C. Cytokine. 2006;36:254–260. doi: 10.1016/j.cyto.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Effects of kefir fractions on innate immunity. Vinderola G, Perdigon G, Duarte J, Thangavel D, Farnworth E, Matar C. Immunobiology. 2006;211:149–156. doi: 10.1016/j.imbio.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Effects of kefir supernatant and lactic acid bacteria isolated from kefir grain on cytokine production by macrophage. Hong WS, Chen HC, Chen YP, Chen MJ. Int Dairy J. 2009;19:244–251. [Google Scholar]
- 36.Kefir treatment ameliorates dextran sulfate sodium-induced colitis in rats. Senol A, Isler M, Sutcu R, Akin M, Cakir E, Ceyhan BM, Kockar MC. World J Gastroenterol. 2015;21:13020–13029. doi: 10.3748/wjg.v21.i46.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lactobacillus kefiranofaciens M1 isolated from milk kefir grains ameliorates experimental colitis in vitro and in vivo. Chen YP, Hsiao PJ, Hong WS, Dai TY, Chen MJ. J Dairy Sci. 2012;95:63–74. doi: 10.3168/jds.2011-4696. [DOI] [PubMed] [Google Scholar]
- 38.Effects of Lactobacillus kefiranofaciens M1 isolated from kefir grains on germ-free mice. Chen YP, Chen MJ. PLoS One. 2013;8:0. doi: 10.1371/journal.pone.0078789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anti-inflammatory and anti-allergic effects of kefir in a mouse asthma model. Lee MY, Ahn KS, Kwon OK, et al. Immunobiology. 2007;212:647–654. doi: 10.1016/j.imbio.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 40.The anti-allergic properties of milk kefir and soymilk kefir and their beneficial effects on the intestinal microflora. Liu JR, Wang SY, Chen MJ, Yueh PY, Lin CW. J Sci Food Agric. 2006;86:2527–2533. [Google Scholar]
- 41.The antiallergic effect of kefir Lactobacilli. Hong WS, Chen YP, Chen MJ. J Food Sci. 2010;75:0–53. doi: 10.1111/j.1750-3841.2010.01787.x. [DOI] [PubMed] [Google Scholar]
- 42.Effect of heat-inactivated kefir-isolated Lactobacillus kefiranofaciens M1 on preventing an allergic airway response in mice. Hong WS, Chen YP, Dai TY, Huang IN, Chen MJ. J Agric Food Chem. 2011;59:9022–9031. doi: 10.1021/jf201913x. [DOI] [PubMed] [Google Scholar]
- 43.Effects of kefir on ischemia-reperfusion injury. Yener AU, Sehitoglu MH, Ozkan MT, et al. http://www.europeanreview.org/wp/wp-content/uploads/887-896.pdf. Eur Rev Med Pharmacol Sci. 2015;19:887–896. [PubMed] [Google Scholar]
- 44.The antimetastatic and antiangiogenesis effects of kefir water on murine breast cancer cells. Zamberi NR, Abu N, Mohamed NE, et al. Integr Cancer Ther. 2016;15:0–66. doi: 10.1177/1534735416642862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kefir modulates gut microbiota and reduces DMH-associated colorectal cancer via regulation of intestinal inflammation in adulthood offsprings programmed by neonatal overfeeding. Guiomar de Almeida Brasiel P, Cristina Potente Dutra Luquetti S, Dutra Medeiros J, et al. Food Res Int. 2022;152:110708. doi: 10.1016/j.foodres.2021.110708. [DOI] [PubMed] [Google Scholar]
- 46.Role of kefir milk on the pathogenesis of Entamoeba histolytica. Mohammed ST. https://www.iasj.net/iasj/download/4430c9b340c1f990 Iraqi J Sci. 2016;57:1116–1124. [Google Scholar]
- 47.Administration of kefir-fermented milk protects mice against Giardia intestinalis infection. Franco MC, Golowczyc MA, De Antoni GL, Pérez PF, Humen M, Serradell ML. J Med Microbiol. 2013;62:1815–1822. doi: 10.1099/jmm.0.068064-0. [DOI] [PubMed] [Google Scholar]
- 48.Effects of Lactobacillus kefiranofaciens M1 isolated from kefir grains on enterohemorrhagic Escherichia coli infection using mouse and intestinal cell models. Chen YP, Lee TY, Hong WS, Hsieh HH, Chen MJ. J Dairy Sci. 2013;96:7467–7477. doi: 10.3168/jds.2013-7015. [DOI] [PubMed] [Google Scholar]
- 49.A Lactobacillus plantarum strain isolated from kefir protects against intestinal infection with Yersinia enterocolitica O9 and modulates immunity in mice. De Montijo-Prieto S, Moreno E, Bergillos-Meca T, Lasserrot A, Ruiz-López MD, Ruiz-Bravo A, Jiménez-Valera M. Res Microbiol. 2015;166:626–632. doi: 10.1016/j.resmic.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Evaluation of wound healing activities of kefir products. Huseini HF, Rahimzadeh G, Fazeli MR, Mehrazma M, Salehi M. Burns. 2012;38:719–723. doi: 10.1016/j.burns.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Protective effect of a mixture of kefir-isolated lactic acid bacteria and yeasts in a hamster model of Clostridium difficile infection. Bolla PA, Carasi P, Bolla Mde L, De Antoni GL, Serradell Mde L. Anaerobe. 2013;21:28–33. doi: 10.1016/j.anaerobe.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Administration of probiotic kefir to mice with Clostridium difficile infection exacerbates disease. Spinler JK, Brown A, Ross CL, Boonma P, Conner ME, Savidge TC. Anaerobe. 2016;40:54–57. doi: 10.1016/j.anaerobe.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The effects of oral plain kefir supplementation on proinflammatory cytokine properties of the hyperglycemia Wistar rats induced by streptozotocin. Hadisaputro S, Djokomoeljanto RR, Judiono Judiono, Soesatyo MH. http://www.inaactamedica.org/archives/2012/22745139.pdf. Acta Med Indones. 2012;44:100–104. [PubMed] [Google Scholar]
- 54.Intestinal beneficial effects of kefir-supplemented diet in rats. Urdanteta E, Barrenetxe J, Aranguren P, Irigoyen A, Marzo F, Ibáñez FC. Nutri Res. 2007;27:653–658. [Google Scholar]
- 55.Kefir administration reduced progression of renal injury in STZ-diabetic rats by lowering oxidative stress. Punaro GR, Maciel FR, Rodrigues AM, et al. Nitric Oxide. 2014;37:53–60. doi: 10.1016/j.niox.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 56.Fermented milk, Kefram-Kefir enhances glucose uptake into insulin-responsive muscle cells. Teruya K, Yamashita M, Tominaga R, et al. Cytotechnology. 2002;40:107–116. doi: 10.1023/A:1023926407877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The influence of goat milk and soybean milk kefir on IL-6 and CRP levels in diabetic rats. Sunarti S, Nurliyani N, Tyas ASA, Kristian SD, Prasetyastuti P. http://www.rjdnmd.org/index.php/RJDNMD/article/view/133 Romanian Journal of Diabetes Nutrition& Metabolic diseases. 2015;22:261–267. [Google Scholar]
- 58.Kefir improves fatty liver syndrome by inhibiting the lipogenesis pathway in leptin-deficient ob/ob knockout mice. Chen HL, Tung YT, Tsai CL, et al. Int J Obes (Lond) 2014;38:1172–1179. doi: 10.1038/ijo.2013.236. [DOI] [PubMed] [Google Scholar]
- 59.Kefir supplementation improves lipid profile and oxidative stress but does not reduce atherosclerotic lesion in apoE deficient mice. Jascolka TL, Aguilar EC, Teixeira LG, Lages PC, Raimundo IDC. https://www.scitechnol.com/peer-review/kefir-supplementation-improves-lipid-profile-and-oxidative-stress-but-does-not-reduce-atherosclerotic-lesion-in-apoe-deficient-mic-9Qgw.php J Food Nutr Disor. 2013;2:1–7. [Google Scholar]
- 60.AB-Kefir reduced body weight and ameliorated inflammation in adipose tissue of obese mice fed a high-fat diet, but not a high sucrose diet. Chen YT, Hsu AH, Chiou SY, Lin YC, Lin JS. Nutrients. 2021;13:3–7. doi: 10.3390/nu13072182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Probiotic properties of Lactobacillus strains isolated from Tibetan kefir grains. Zheng Y, Lu Y, Wang J, Yang L, Pan C, Huang Y. PLoS One. 2013;8:0. doi: 10.1371/journal.pone.0069868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kefir peptides prevent high-fructose corn syrup-induced non-alcoholic fatty liver disease in a murine model by modulation of inflammation and the JAK2 signaling pathway. Chen HL, Tsai TC, Tsai YC, Liao JW, Yen CC, Chen CM. Nutr Diabetes. 2016;6:0. doi: 10.1038/nutd.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Effect of surface layer proteins derived from paraprobiotic kefir lactic acid bacteria on inflammation and high-fat diet-induced obesity. Kim E, Lee HG, Han S, Seo KH, Kim H. J Agric Food Chem. 2021;69:15157–15164. doi: 10.1021/acs.jafc.1c05037. [DOI] [PubMed] [Google Scholar]
- 64.Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: a randomized controlled trial. Yılmaz İ, Dolar ME, Özpınar H. Turk J Gastroenterol. 2019;30:242–253. doi: 10.5152/tjg.2018.18227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Effects of kefir or milk supplementation on zonulin in overweight subjects. Pražnikar ZJ, Kenig S, Vardjan T, Bizjak MČ, Petelin A. J Dairy Sci. 2020;103:3961–3970. doi: 10.3168/jds.2019-17696. [DOI] [PubMed] [Google Scholar]
- 66.Probiotic kefir consumption improves serum apolipoprotein A1 levels in metabolic syndrome patients: a randomized controlled clinical trial. Bellikci-Koyu E, Sarer-Yurekli BP, Karagozlu C, Aydin-Kose F, Ozgen AG, Buyuktuncer Z. Nutr Res. 2022;102:59–70. doi: 10.1016/j.nutres.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Effects of regular kefir consumption on gut microbiota in patients with metabolic syndrome: a parallel-group, randomized, controlled study. Bellikci-Koyu E, Sarer-Yurekli BP, Akyon Y, et al. Nutrients. 2019;11:2089. doi: 10.3390/nu11092089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Effect of oral administration of kefir on serum proinflammatory cytokines on 5-FU induced oral mucositis in patients with colorectal cancer. Topuz E, Derin D, Can G, et al. Invest New Drugs. 2008;26:567–572. doi: 10.1007/s10637-008-9171-y. [DOI] [PubMed] [Google Scholar]
- 69.Glycemic index, insulinemic index, and satiety index of kefir. Kong KL, Hendrich S. J Am Coll Nutr. 2012;31:280–287. doi: 10.1080/07315724.2012.10720435. [DOI] [PubMed] [Google Scholar]
- 70.Effect of probiotic milk (kefir) on gylcemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Ostadrahimi A, Taghizadeh A, Mobasseri M, Farrin N, Payahoo L, Gheshlaghi ZB, Vahedjabbari M. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4401881/pdf/IJPH-44-228.pdf. Iran J Public Health. 2015;44:228–237. [PMC free article] [PubMed] [Google Scholar]
- 71.Kefir consumption does not alter plasma lipid levels or cholesterol fractional synthesis rates relative to milk in hyperlipidemic men: a randomized controlled trial [ISRCTN10820810] St-Onge MP, Farnworth ER, Savard T, Chabot D, Mafu A, Jones PJ. BMC Complement Altern Med. 2002;2:1. doi: 10.1186/1472-6882-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kefir improves blood paramaeters and reduces cardiovascular risks in patients with metabolic syndrome. Ghizi ACS, Silva MA, Moraes FSA, et al. PharmaNutrition. 2021;16:100266. [Google Scholar]
- 73.The effect of kefir consumption on the lipid profile for individuals with normal and dyslipidemic properties: a randomized controlled trial. Yilmaz I, Arslan B. Rev Nutr. 2022;35:210098. [Google Scholar]