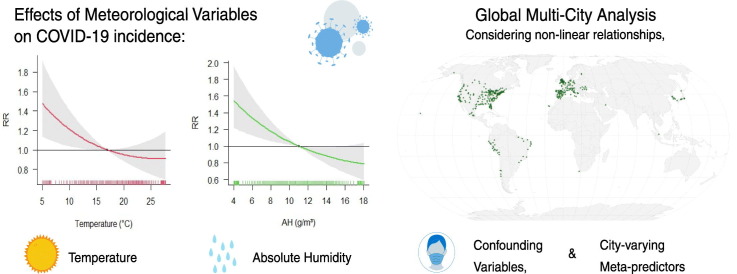
Abstract
Background and aim
The associations between COVID-19 transmission and meteorological factors are scientifically debated. Several studies have been conducted worldwide, with inconsistent findings. However, often these studies had methodological issues, e.g., did not exclude important confounding factors, or had limited geographic or temporal resolution. Our aim was to quantify associations between temporal variations in COVID-19 incidence and meteorological variables globally.
Methods
We analysed data from 455 cities across 20 countries from 3 February to 31 October 2020. We used a time-series analysis that assumes a quasi-Poisson distribution of the cases and incorporates distributed lag non-linear modelling for the exposure associations at the city-level while considering effects of autocorrelation, long-term trends, and day of the week. The confounding by governmental measures was accounted for by incorporating the Oxford Governmental Stringency Index. The effects of daily mean air temperature, relative and absolute humidity, and UV radiation were estimated by applying a meta-regression of local estimates with multi-level random effects for location, country, and climatic zone.
Results
We found that air temperature and absolute humidity influenced the spread of COVID-19 over a lag period of 15 days. Pooling the estimates globally showed that overall low temperatures (7.5 °C compared to 17.0 °C) and low absolute humidity (6.0 g/m3 compared to 11.0 g/m3) were associated with higher COVID-19 incidence (RR temp =1.33 with 95%CI: 1.08; 1.64 and RR AH =1.33 with 95%CI: 1.12; 1.57). RH revealed no significant trend and for UV some evidence of a positive association was found. These results were robust to sensitivity analysis. However, the study results also emphasise the heterogeneity of these associations in different countries.
Conclusion
Globally, our results suggest that comparatively low temperatures and low absolute humidity were associated with increased risks of COVID-19 incidence. However, this study underlines regional heterogeneity of weather-related effects on COVID-19 transmission.
Keywords: Temperature, Humidity, UV radiation, COVID-19, Distributed lag non-linear modelling, Global analysis
Graphical abstract
1. Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic arose in late December 2019 in Wuhan, China. According to the WHO, by 12 July 2021 190 million cases and 4 million deaths had been reported globally due to coronavirus disease 2019 (COVID-19) (WHO, n.d.). Evidence points towards transmission mainly taking place via airborne transmission (respiration of SARS-CoV-2 containing droplets) (Tang et al., 2020). Other modes of transmission, including direct contact through contaminated surfaces, faecal-oral transmission and other body fluids are still under investigation regarding the extent to which they influence the infection dynamics (Gupta et al., 2020; Zhang et al., 2020).
The relationship between COVID-19 incidence and meteorological factors is greatly discussed in the literature and of high public interest. A connection between meteorology and COVID-19 is considered likely as other coronaviruses and respiratory viruses show strong seasonal patterns of disease incidence that can to some extent be explained by meteorological factors in temperate regions (Lowen and Steel, 2014; Anastasiou et al., 2021). There are several ways in which meteorological factors (e.g. air temperature and humidity) could influence COVID-19 incidence. Extreme climatic conditions (e.g., extreme cold and heat) can result in people spending more time indoors, in closed, poorly ventilated spaces, which can increase the transmission of SARS-CoV-2 (Willem et al., 2012; Qian et al., 2020). Moreover, lower temperatures enhance the stability of viral lipid envelopes and lower humidity favours droplet nuclei formation which prolong viability and transmissibility of SARS-CoV-2 (Chan et al., 2011a; Aboubakr et al., 2020; Casanova et al., 2010; Rosti et al., 2020; Shadloo-Jahromi et al., 2020) Also, cold and dry conditions affect the human innate and adaptive immune response in various ways (e.g., in cold nostrils through inhibited mucociliary clearance and a decrease of phago- and leukocyte activity, which changes the likeliness of infection or symptom severity) (Lowen et al., 2007; Kudo et al., 2019; Eccles, 2002; Foxman et al., 2015). Altogether, these mechanisms support the hypothesis that colder and drier conditions would favour SARS-CoV-2 transmission and increase COVID-19 incidence.
Several spatial ecological and time-series studies have investigated the association between meteorological conditions and COVID-19 cases (Carlson et al., 2020; McClymont and Hu, 2021). However, so far the literature remains mainly inconclusive showing positive, negative, and no associations for temperature, humidity (relative and absolute) and UV radiation in different analyses (Mecenas et al., 2020; Briz-Redón and Serrano-Aroca, 2020; Majumder and Ray, 2021; Zheng et al., 2021; Tan and Schultz, 2022; Li et al., 2022; Yao et al., 2020; Carleton et al., 2020a; Sera et al., 2021; Rubin et al., 2020; Xu et al., 2021; Smith et al., 2021; Ma et al., 2021; Fontal et al., 2021). The variation in study results could partially be explained by varying spatial scales of analysis, application of different statistical methods with varying degrees of sophistication, and varying levels of consideration of potential confounding factors. Moreover, according to previous systematic reviews, epidemiologic studies assessing the relationship between weather and COVID-19 incidence could have methodologic limitations that may introduce bias and limit causal inference (Dong et al., 2021; Weaver et al., 2022; Villeneuve and Goldberg, 2020). For example, many studies did not consider the possibility of a non-linear relation and lagged effects of weather and incidence, they did not account for time-varying confounders, and they did not consider location-specific confounders. To address these limitations time-series regression methods could be used. These methods have been used to quantify short-term associations of environmental exposures with health outcomes, notably with infectious diseases (Imai et al., 2015). Time-series regression methods allow seasonality, long-term trends, other time-varying confounding factors, and autocorrelation to be controlled for. It also allows us to explore the association with delayed and non-linear exposure effects (Bhaskaran et al., 2013). With the availability of longer time-series several studies have used time-series methods to evaluate the association between meteorological factors and COVID-19 incidence (Lin et al., 2022; Liu et al., 2022; Qi et al., 2020; Xie and Zhu, 2020; Prata et al., 2020; Yuan et al., 2021a; Ai et al., 2022; Zhou et al., 2022; He et al., 2021; Fong and Smith, 2022; Nottmeyer and Sera, 2021; Donzelli et al., 2022). Among those, three studies were performed on a global scale (Liu et al., 2022; Yuan et al., 2021a), but they considered the country as unit of analysis. City-level studies are more appropriate given the lower measurement error on the outcome and on the exposure. Moreover, they allow accounting for phenomena, like high levels of population density or human mobility, which are only observable on a small scale (Bhaskaran et al., 2013).
The aim of this study is to use city-level time-series models to evaluate the association between meteorological exposures (e.g., temperature, humidity, and UV radiation) and COVID-19 incidence at the global scale.
2. Methods
2.1. Data sources and extraction
The data extraction was performed by members of the Multi-City Multi-Country (MCC) Network, an international research network focused on the study of environmental conditions, climate change, and human health (https://mccstudy.lshtm.ac.uk/). We considered the COVID-19 case time-series data for 455 cities between 3 February and 31 October 2020. Details of the cities and sources can be found in Supplementary Table S1.
We obtained exposure data from the Copernicus ERA5 dataset with a latitude-longitude grid size of 0.25° × 0.25°(roughly 28 × 28 km) (Copernicus, n.d.). We selected temperature and dew temperature in 2 m above the surface as well as the surface downwelling shortwave radiation (solar UV radiation, J/m2). For these variables daily averages were taken from the closest grid cell for each city or small region.
We calculated the relative humidity (RH) from temperature and absolute humidity (AH) using the R “humidity” package (Cai, n.d.). RH measures the percentage of water molecules in the air relative to concentration at full saturation, whereas AH measures the amount of water vapor in a specific volume of air (Babin, 2020). This is the formula of how AH relates to RH and temperature: (Mander, 2020)
The following variables were captured as we expected them to be confounders of the associations between weather variables and COVID-19 incidence. We extracted the Government Stringency Index (GSI) from the Oxford COVID-19 Government Response Tracker (OxCGRT) to control for changing governmental public health measures implemented in response to the pandemic (Hale et al., 2021). The GSI scale ranges from 0 to 100 points with 100 representing the strictest measures implemented to hinder COVID-19 transmission such as closure policies, movement restrictions, income support, and testing policies. For the purpose of sensitivity analysis, we also used residential mobility from the Google Mobility index which measures the change in average duration of time spent at home compared to the median for the same weekday in a pre-pandemic period (3 January to 6 February 2020) (Google LLC, n.d.).
We considered the long-term mean temperature, demographic information on population size, density, and age proportion above 65 years in the fixed effects of the meta-regression. Demographic variables were collected from the Organisation for Economic Co-operation and Development (OECD) Global Human Settlement Layer Urban Centre Database unless specified otherwise in the results (OECD, 2016). This data was available at the city-level from the MCC Network.
2.2. Statistical analysis
2.2.1. Descriptive analysis
For the descriptive data analysis, the daily and cumulative COVID-19 cases of the included cities were summed for each country and the cases per 100.000 inhabitants were calculated using total population size of each city (OECD data) (OECD, 2016).
2.2.2. Two-stage design
We used a two-stage design to assess the association between the meteorological factors and COVID-19 incidence. The first stage consists of estimating the city-specific exposure-response association considering time-varying confounding in a time-series regression (TSR). In the second stage, a meta-analytic model is used to combine the city-specific estimates to obtain the pooled exposure-response association curve.
For the first stage of the analysis, independent models for each exposure were formulated for all locations. The city-specific time-series were shortened to start up to 15 days (depending on the considered days of lag) before the first time that 10 cases occurred in that city. This aims to exclude first imported cases. The exposures were modelled using distributed lag non-linear models (DLNMs) (Gasparrini, 2014). The basis function for the exposure dimension (temperature, AH, RH, and UV radiation) was chosen as a 2nd degree polynomial. The lag dimension was modelled with a natural cubic spline containing two equally spaced (at logarithmic scale) internal knots. In the main analysis, a lag of 15 days was considered, since the incubation period was estimated to be around 6 days for COVID-19 (McAloon et al., 2020; Quesada et al., 2021) and there is a delay in testing and reporting. The two bases were then combined to make a bi-dimensional basis called a “cross-basis” (Gasparrini, 2011). The residual variation of case counts was assumed to follow a quasi-Poisson distribution.
Several confounding factors were considered in the main model. Since the reporting, as well as many other factors (e.g., social behaviour and testing capacities), might vary between weekdays, we included a series of dummy day of the week variable (dow). Two other time-varying confounders were considered. The intra-year trend of COVID-19 was considered in the model using a natural spline function of the date with 6 degrees of freedom (df) (NS(date, df = 6)) which equals approximately 1.5 df per month. Changing governmental public health measures were modelled with a linear lag association model of GSI considering up to 15 days of lag dependence (CBGSI). The model was built using the R package “dlnm” (Gasparrini, 2011). An autocorrelation term was included to account for transmission dynamics (Imai et al., 2015). For this purpose, the logarithm of one day lagged cases added to 0.5 was included (AC).
In summary, the basic first stage model for each exposure (temperature, RH, AH, or UV) which was performed for each city looked like this:
| (I) |
For the subsequent second stage meta-analysis, the R package “mixmeta” was used (Sera et al., 2019). The coefficients representing estimated meteorology to COVID-19 associations were cumulated over all lags and their covariance matrices which were obtained at the first step were pooled over all included locations using a random effect meta-analytic model. We used the estimation method of restricted maximum likelihood (REML). In the main model (Model A), we considered groups defined jointly by country and climatic zones as random effects. The same model was used in Model B but only for the subset of locations with complete data in the meta-predictors (GDP, mean temperature, and % of population aged >65 years). In the subset of locations with complete data, we then also fitted the meta-regression model with the meta-predictors as fixed effects (Model C). To evaluate the role of country in explaining the heterogeneity in the association curves, we considered models with city as a random effect and country as a fixed effect (Model D), or random effect (Model E). We then derived country-level Best Linear Unbiased Prediction (BLUP) curves from Model E.
Using the pooled polynomial basis coefficients, we plotted the pooled mean curve (for all included cities) of COVID-19 risk against each exposure (temperature, RH, AH and UV) expressed as relative risk (RR) to the median level which was set as the minimum exposure value.
2.3. Sensitivity analysis
We performed a sensitivity analysis of the observed effect on the days of lags accounted in the first stage model by varying the length of the lag period from 15 to 10 days. The influence of choice of df used to model intra-year trends was also explored by altering from 6 df to 4 df. Furthermore, we evaluated the possible time-varying confounding of air pollution by considering city-level particulate matter (PM10) data in the first stage model using a distributed linear model (DLM) parametrization and up to 15 days of lag. The PM10 data was obtained from the Copernicus Atmosphere Monitoring Service (CAMS) global near-real time service (Christophe, 2019; Morcrette, 2009; Benedetti, 2009). The hourly modelled values of surface PM10 (0.4 × 0.4 arc degrees grid cell resolution) were averaged daily over the observation period and linked to the city using the city centroid coordinates. The statistical analysis was performed using R 4.1.2 statistical software.
3. Results
3.1. Descriptive analysis
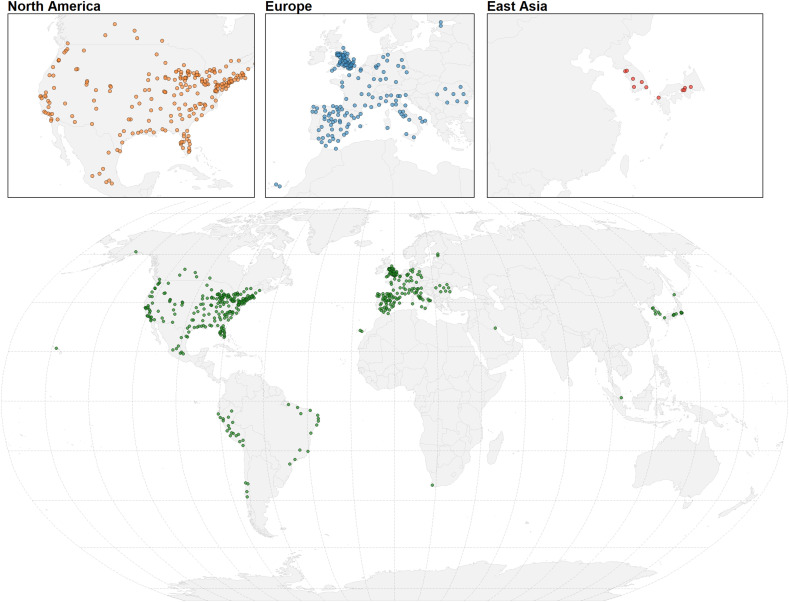
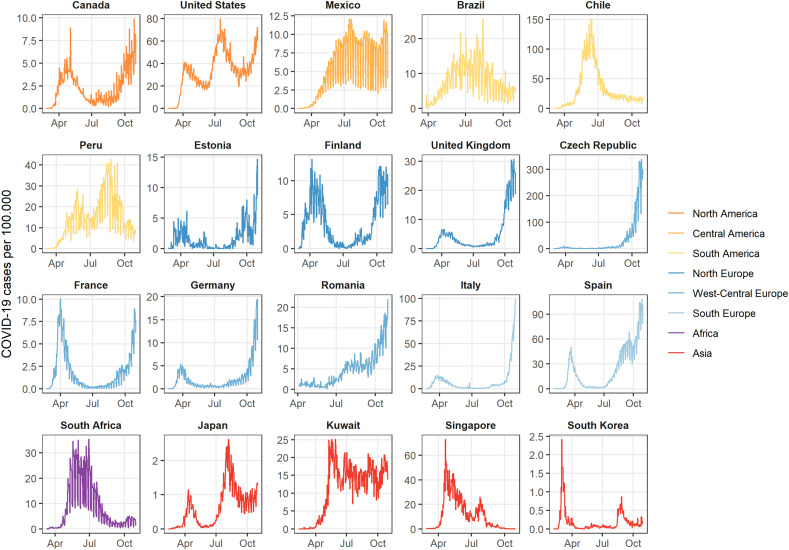
This analysis considered 10.5 million confirmed COVID-19 cases across 455 different cities in 20 countries between 3 February and 31 October 2020. The city locations are shown below as well as the country-wide aggregated time-series of daily reported COVID-19 cases per 100.000, in the included city populations (Fig. 1, Fig. 2 ). In most of the countries in the northern hemisphere we can recognise two waves in late winter or early spring and in autumn, while countries in the southern hemisphere (e.g., Brazil, Chile, Peru, and South Africa) experienced a single wave during the observation period of this study. Table 1 shows the country-wide cumulative incidence per 100,000 inhabitants which varied from 49 in South Korea to 8350 in the USA. The average minimum and maximum recorded exposures per country within the observation period are reported in Table 1. Daily country averages of the meteorological variables over the observation period are represented in Supplementary Figs. S1–4. Countries in a tropical climate or in the southern hemisphere (e.g., Brazil, Chile, Mexico, Peru, Singapore, and South Africa) show less variation of the meteorological variables, especially mean temperature, RH and AH. The correlation between the four exposures is shown in Supplementary Table S4. An overview of the governmental interventions against COVID-19 over time can be seen in Supplementary Fig. S5. Most countries started out with stringent restrictions in the beginning of 2020 and loosened them by the middle of the year. Some tightened them again towards the end of October 2020 (Supplementary Fig. S5). Estonia had the lowest overall level of governmental interventions with an average GSI of 36.9 % during the observation period, whereas Peru ranked highest on governmental stringency with an average GSI at 75.8 % (Table 1).
Fig. 1.
World map showing the included cities colour-coded by region.
Fig. 2.
Time-series of COVID-19 cases per 100,000 inhabitants aggregated by country over the period from 3 February to 31 October 2020.
Table 1.
Summary table of observed COVID-19 cases, meteorological exposures, and governmental stringency index in the different countries.
| Country | Number of included cities | Cumulative cases per day [# per 100.000] | Daily mean temperature [°C] | Daily mean RH [%] | Daily mean AH [g/m3] | Daily mean UV [J/m2] | OxCGRT GSI [%] |
|---|---|---|---|---|---|---|---|
| Brazil | 13 | 1677 | 24.3 | 76.5 | 17.5 | 210.7 | 65.0 |
| (4.7, 31.7) | (30.2, 99.0) | (5.9, 23.6) | (20.3, 353.7) | (51.6, 69.8) | |||
| Canada | 15 | 578 | 12.6 | 67.3 | 8.3 | 207.8 | 60.4 |
| (−21.0, 28.9) | (23.7, 95.8) | (0.7, 20.3) | (10.9, 368.1) | (6.7, 65.1) | |||
| Chile | 4 | 8052 | 11.9 | 74.1 | 7.9 | 169.5 | 68.7 |
| (1.85, 23.7) | (34.8, 96.7) | (4.0, 13.2) | (3.8, 357.6) | (0.0, 78.4) | |||
| Czech Republic | 1 | 7390 | 13.8 | 65.4 | 8.2 | 176.7 | 52.1 |
| (−1.5, 25.7) | (33.6, 93.6) | (2.2, 14.8) | (9.7, 316.0) | (10.9, 80.2) | |||
| Estonia | 1 | 410 | 10.9 | 75.9 | 8.1 | 165.0 | 40.8 |
| (−3.2, 22.5) | (46.0, 96.2) | (2.3, 14.6) | (6.7, 336.1) | (0.0, 63.5) | |||
| Finland | 1 | 915 | 10.9 | 75.2 | 7.90 | 168.6 | 44.4 |
| (−2.6, 23.2) | (45.9, 97.9) | (2.2, 13.9) | (6.4, 341.9) | (16.2, 57.8) | |||
| France | 17 | 477 | 15.9 | 69.0 | 9.6 | 202.0 | 60.0 |
| (0.5, 30.0) | (24.1, 96.9) | (2.4, 20.3) | (11.2, 352.2) | (12.0, 75.0) | |||
| Germany | 12 | 575 | 14.1 | 66.6 | 8.4 | 177.1 | 56.2 |
| (−1.4, 29.2) | (30.8, 97.5) | (2.2, 16.0) | (4.5, 338.7) | (14.6, 69.8) | |||
| Italy | 23 | 2013 | 18.6 | 67.5 | 11.21 | 222.9 | 68.4 |
| (1.0, 30.8) | (26.9, 97.7) | (2.1, 22.0) | (9.6, 345.0) | (49.7, 81.0) | |||
| Japan | 10 | 163 | 19.8 | 74.2 | 14.0 | 182.6 | 43.9 |
| (−5.9, 32.6) | (34.2, 97.8) | (2.4, 25.4) | (11.3, 342.9) | (16.2, 49.0) | |||
| Kuwait | 1 | 2949 (Kuwait Population, 2021) | 30.2 | 40.6 | 12.4 | 274.7 | 58.5 |
| (7.8, 41.3) | (18.6, 84.1) | (2.6, 29.4) | (114.8, 336.0) | (5.2, 79.2) | |||
| Mexico | 8 | 1367 | 20.2 | 59.5 | 10.5 | 269.6 | 51.9 |
| (9.5, 31.1) | (9.8, 96.9) | (2.2, 20.3) | (40.1, 371.4) | (0.0, 62.5) | |||
| Peru | 18 | 3489 (Peru Population, 2021) | 15.0 | 67.0 | 9.8 | 236.7 | 76.3 |
| (1.1, 30.1) | (5.0, 96.3) | (0.5, 24.1) | (36.2, 383.8) | (13.0, 81.8) | |||
| Romania | 8 | 1006 (Romania Population, 2021) | 18.5 | 63.7 | 10.4 | 211.1 | 52.0 |
| (3.3, 30.1) | (22.9, 97.8) | (2.1, 19.2) | (14.6, 338.2) | (42.2, 71.4) | |||
| Singapore | 1 | 2879 | 27.6 | 80.6 | 21.9 | 196.3 | 60.7 |
| (26.0, 29.3) | (71.2, 86.5) | (20.0, 23.6) | (49.1, 304.8) | (31.7, 78.7) | |||
| South Africa | 1 | 1998 (South Africa Population, 2021) | 15.1 | 78.6 | 10.30 | 172.7 | 68.9 |
| (9.9, 19.8) | (56.8, 95.2) | (6.2, 13.5) | (30.4, 350.1) | (14.1, 80.2) | |||
| South Korea | 6 | 49 | 18.5 | 73.3 | 13.0 | 185.1 | 53.3 |
| (−6.0, 29.8) | (24.8, 98.0) | (1.1, 24.7) | (134.8, 332.1) | (22.9, 72.9) | |||
| Spain | 52 | 6210 | 17.9 | 63.9 | 9.9 | 232.2 | 56.8 |
| (0.3, 34.2) | (17.0, 97.3) | (1.7, 22.1) | (12.9, 368.6) | (2.1, 72.9) | |||
| United Kingdom | 54 | 1254 | 13.4 | 75.4 | 9.0 | 170.6 | 65.4 |
| (2.3, 26.2) | (41.9, 99.4) | (3.7, 16.9) | (5.9, 344.0) | (8.3, 71.9) | |||
| United States | 209 | 8350 | 19.4 | 64.4 | 11.7 | 225.7 | 63.0 |
| (−14.1, 41.0) | (5.8, 100.0) | (0.7, 26.0) | (7.1, 384.3) | (8.3, 66.2) |
3.2. Association between COVID-19 cases and temperature
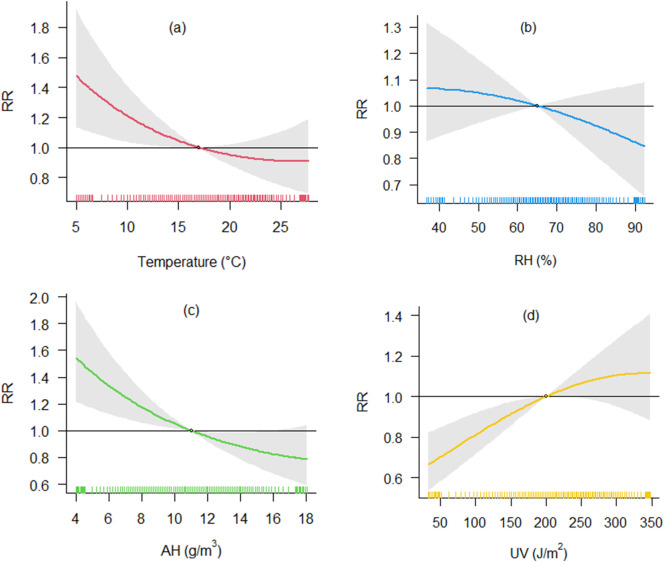
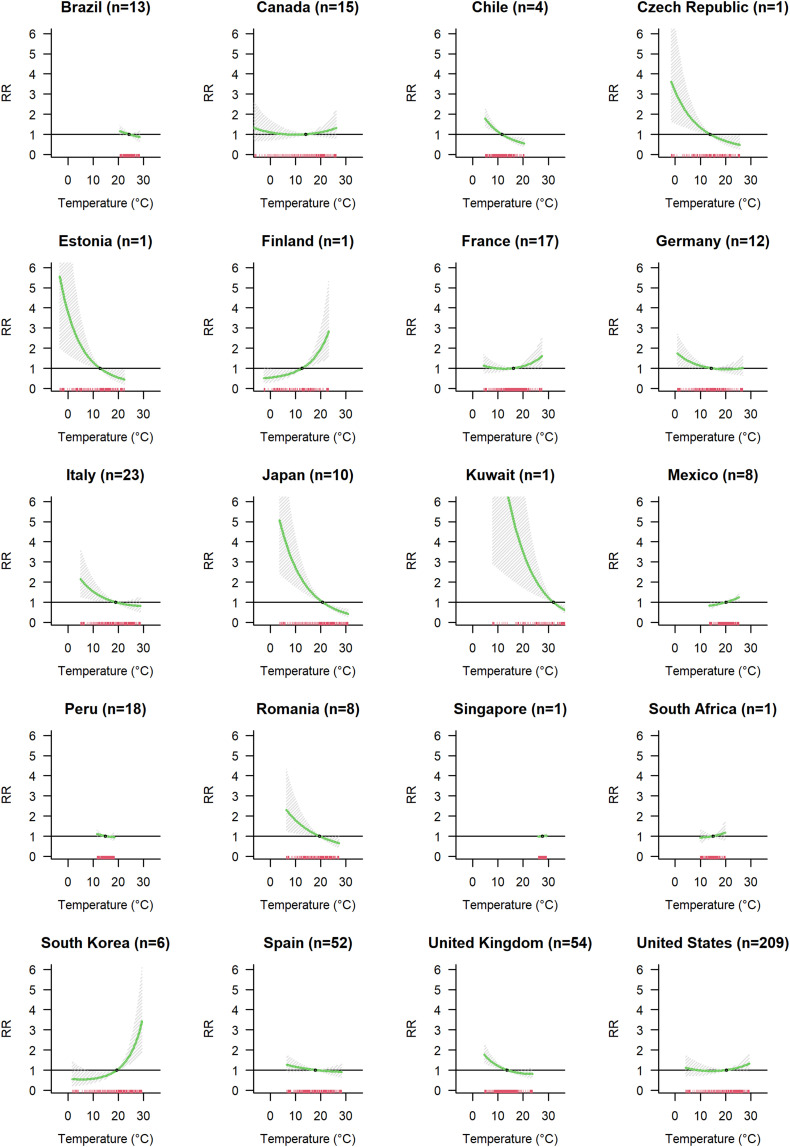
The pooled association curve, representing overall results across all cities, obtained from the pooled models for temperature exposure (Model A) is represented in Fig. 3a. Low temperatures were associated with higher risk of infection. At 7.5 °C the relative risk of COVID-19 incidence is 1.33-fold higher (CI-95 %: 1.08;1.64) compared to a reference level at 17.0 °C. The exposure-lag association indicated increased RRs with a 3-day lag after temperature exposure, reached a peak at 8–9 days, and decayed by the end of the observed 15 days' lag period (Supplementary Fig. S6a). We observed a substantial heterogeneity in the meta-analytic model (I2 = 67.3 %). Investigating the city-level factors which could explain this heterogeneity (Model C), we found that old population (% population aged >65 years), the average daily mean temperature, and GDP modified the association between temperature and COVID-19 incidence (Supplementary Fig. S7a, Supplementary Table S2). Cities with an older population and lower long-term mean temperature seemed to have a higher impact of lower temperature on COVID-19 spread, but overall, these factors explain only 1.1 % of heterogeneity. We also investigated the role of country on heterogeneity comparing the meta-analytic model with and without country modelled as fixed effect with an I2 decrease equal to 4.3 % (Supplementary Table S2). The Fig. 4 shows the country specific curves obtained using BLUPs prediction from the Model E with country as a random effect. We observed different patterns of the temperature COVID-19 incidence curve with most countries showing curves with higher COVID-19 incidence with cold temperatures (e.g. Chile, Czech Republic, Estonia, Germany, Italy, Japan, Kuwait, Romania, Spain and UK), some with limited exposure variation had a flat curve (Brazil, Peru, Singapore and South Africa), three had no evidence of an association (France, Canada and US), and three showed a tendency of increased COVID-19 risk with higher temperatures (Finland, South Korea, Mexico).
Fig. 3.
Association between meteorological variables and COVID-19 incidence. Association curves were obtained with meta-regression Model A with random effect defined by country and climatic zones.
Fig. 4.
Country specific association between temperature and COVID-19 incidence. For each country the number (n) of cities included in the analysis is indicated.
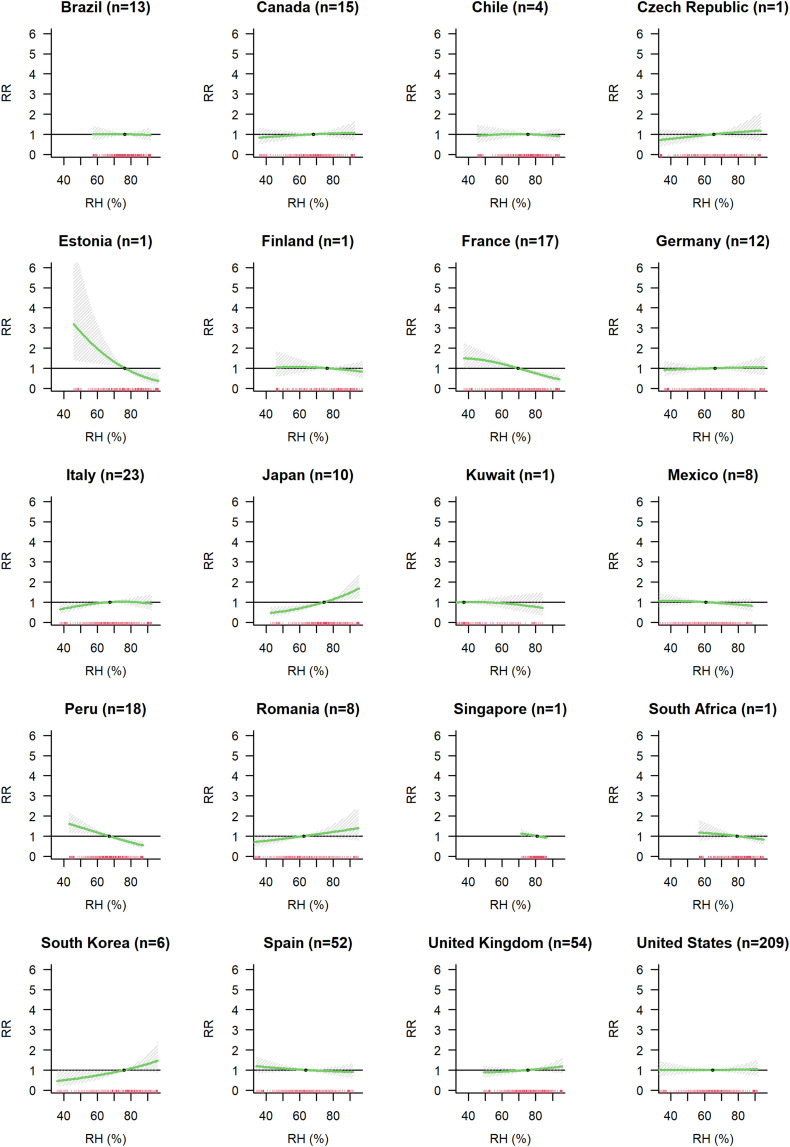
3.3. Association between COVID-19 cases and humidity
Overall, little evidence was found for an association between relative humidity and COVID-19 spread (Model A), with a slight tendency of a lower risk of infection for higher level of RH (Fig. 3b). With respect to a reference level set at 65 % RH, the RR of observing COVID-19 cases was 0.89 at 85 % RH (CI-95 %: 0.75; 1.06). This association did not diverge from RR = 1.00 when considering different lags (Supplementary Fig. S6b). There was substantial heterogeneity in this association (I2 = 68.3 %), but examination of meta-predictors and country specific curves showed no interpretable patterns (Supplementary Fig. S7b). Country modelled as a fixed effect explained 3.6 % of the heterogeneity (Supplementary Table S2). Fig. 5 shows the country specific curves obtained using BLUPs prediction from the model with country as random effect (Model E). Adjusting for daily mean temperature gives a tendency of a protective effect at higher levels of RH (Supplementary Fig. S8).
Fig. 5.
Country specific association between relative humidity and COVID-19 incidence. For each country the number (n) of cities included in the analysis is indicated.
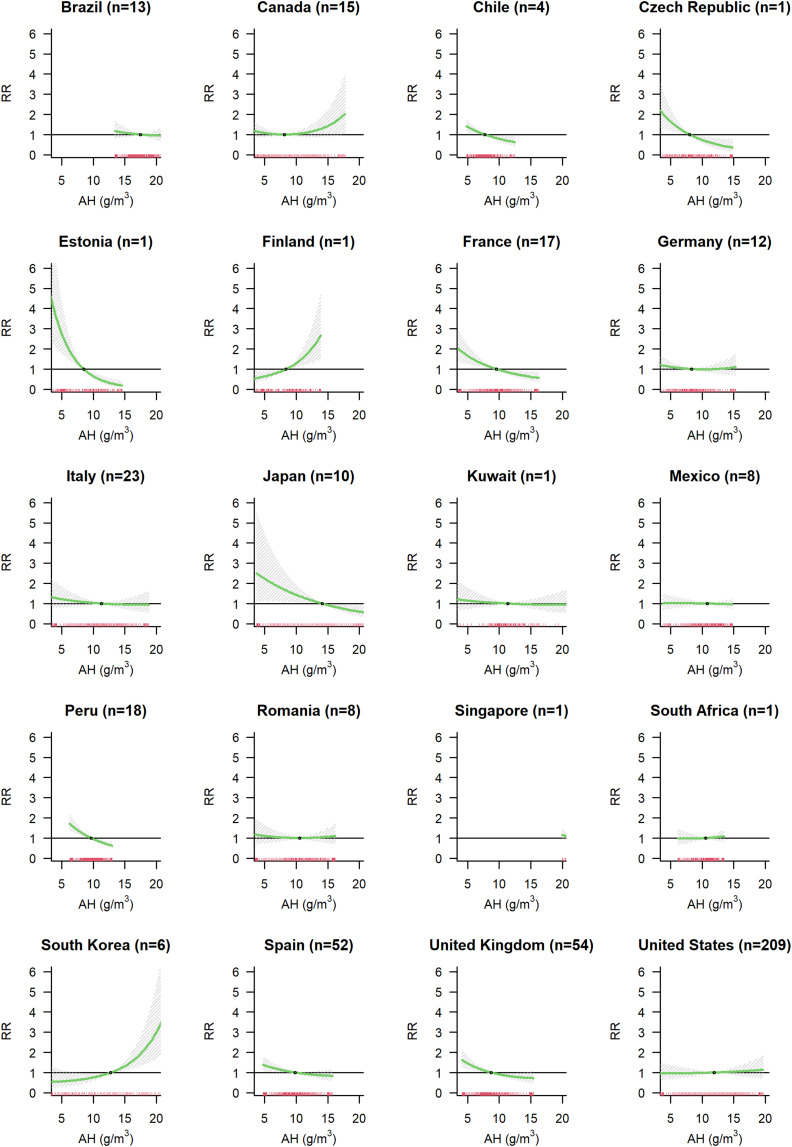
For AH, we observed an inverse association (Model A in Fig. 3c). Compared to the median value of 11.0 g/m3 there was a 1.33-fold increased RR at the AH of 6.0 g/m3 (95%-CI: 1.12; 1.57). The RRs were observed to be increased (RR > 1.00) between 3 and 15 days of lag (Supplementary Fig. S6c). The meta-predictors old population, long-term mean temperature and GDP explained 3.7 % of the heterogeneity (Supplementary Fig. S7c, Supplementary Table S2). Cities with higher long-term mean temperature show a lower risk of COVID-19 infection associated with high levels of AH. Country modelled as fixed effect explained 4.7 % of the heterogeneity. Country BLUPs estimates are presented in Fig. 6 (Model E). As observed for temperature, we found different patterns of the association between AH and COVID-19 incidence in different countries. There are countries with higher COVID-19 incidence with low AH (e.g., Chile, Czech Republic, Estonia, France, Japan, Spain and UK), countries with no evidence of an association (Brazil, Kuwait, Mexico, Italy, Romania, Singapore, South Africa and US), and countries showing a tendency of increased COVID-19 risk with higher AH (Canada, Finland, and South Korea).
Fig. 6.
Country specific association between absolute humidity and COVID-19 incidence. For each country the number (n) of cities included in the analysis is indicated.
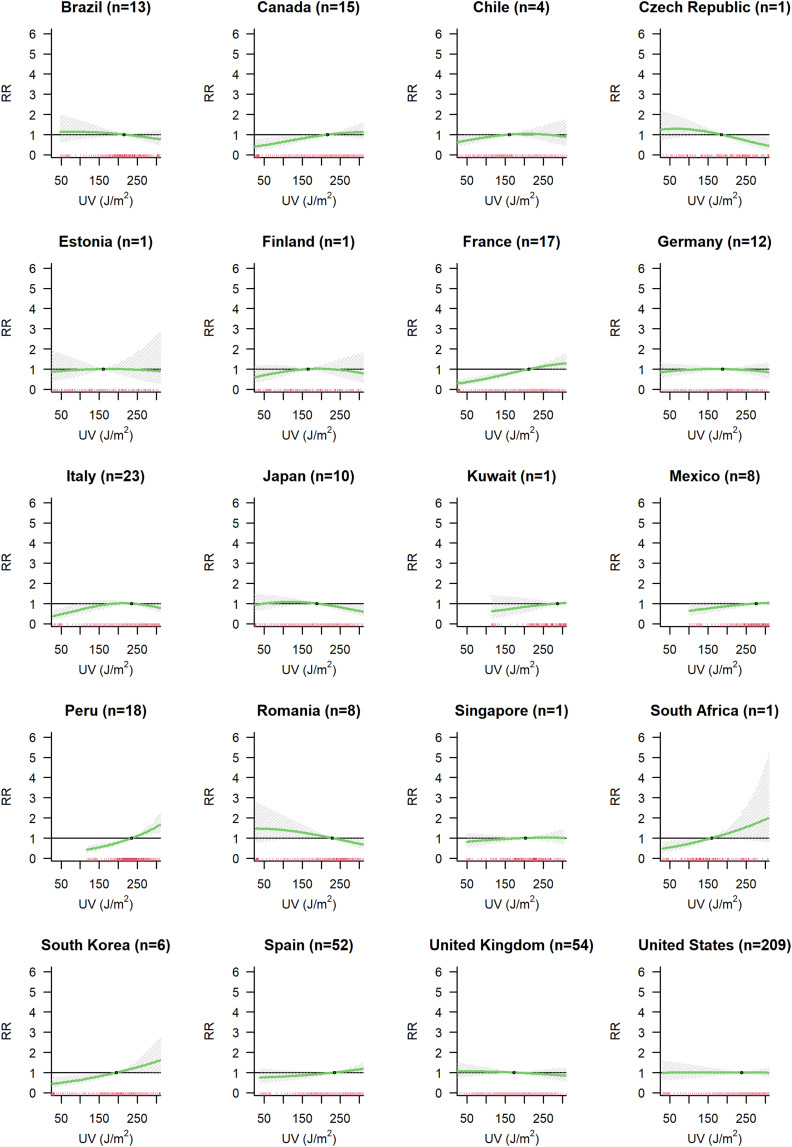
3.4. Association between COVID-19 cases and UV
We found some evidence of an association between UV exposure and COVID-19 spread (Model A in Fig. 3d). Meta-predictors have little influence on the association curve explaining only 1.2 % of the I2 (Supplementary Fig. S6d, Supplementary Table S2). Country modelled as fixed effect explained 3.4 % of the heterogeneity. Country BLUPs estimates are presented in Fig. 7 , with some countries (Canada, Finland, Kuwait, Mexico, Spain and US) showing lower COVID-19 incidence with lower levels of UV radiation.
Fig. 7.
Country specific association between UV radiation and COVID-19 incidence. For each country the number (n) of cities included in the analysis is indicated.
3.5. Sensitivity analysis
A sensitivity analysis was conducted to assess the robustness of estimates of the previously described models. A decrease to 10 days of lag or lower degrees of freedom of long-term trend (4 df instead of 6 df) in general led to similar association curves (Supplementary Fig. S10 and S11). Also, the inclusion of PM10 into the first stage model resulted in no major change of the exposure to COVID-19 associations (Supplementary Fig. S12). Stratifying the analysis according to climatic zone, for air temperature all curves show a decreasing trend. For RH tropical cities show a higher COVID-19 spread in dry conditions. More variability was observed for AH and UV radiation (Supplementary Fig. S13).
4. Discussion
4.1. Main findings
Overall, this study supports previous findings that temperature and absolute humidity are environmental factors that potentially influence the spread of COVID-19. Globally, low temperatures and low absolute humidity were associated with higher COVID-19 incidences, but for RH no evidence of an association was found. There was substantial heterogeneity in the associations of the respective environmental exposures and COVID-19 risk between countries.
4.2. Possible biological and behavioural mechanisms
Our results can be viewed in light of previous studies investigating the mechanistic principles behind associations between meteorological variables and COVID-19. The observation that low temperatures lead to higher transmission rates of viral disease has been made in many previous studies. Biophysical theory and laboratory results suggest that lower temperatures support the stability and viability of viral particles (Polozov et al., 2008; Chan et al., 2011b). Additionally, animal experiments hint towards a connection with lower blood circulation and consequent local impairment of adaptive immunity at low temperatures, thereby affecting the host's immune system's ability to fight respiratory viruses (Lowen et al., 2007; Tang, 2009).
The association between lower levels of humidity and higher levels of infections could be explained by virus-containing droplets having short ballistic settling characteristics under wet conditions. In contrast at dry conditions, droplets evaporate forming dry nuclei that are able to maintain floating over longer durations of time (Rosti et al., 2020; Wei et al., 2022). Influenza-related studies also hinted at an impaired immune response under dry conditions (e.g., through impaired mucociliary clearance and other innate responses) (Kudo et al., 2019). A US study found that outdoor AH is a good predictor for indoor AH while this is not true for RH (Nguyen and Dockery, 2016). Hence, it could be that AH is a more useful predictor for COVID-19 incidence than RH. Previous studies came to that conclusion regarding AH as predictor for influenza transmission rates as well (Metz and Finn, 2015). However, the high correlation between AH and temperature (r = 0.88, average of all cities in our dataset) implies that it is difficult to disentangle effects of the two exposures, with one of the associations possibly merely reflecting confounding by the other.
There was some evidence of a positive association between COVID-19 cases and UV radiation. This was unexpected, as one hypothesis is that UV light could cause inactivation of viruses in the air and on surfaces (Carleton et al., 2020). Also, there is a theory that more solar radiation could lead to less vitamin D deficiency (contributing to a better functioning immune system).
4.3. Comparison to other modelling studies
Due to the extensively growing literature in this field, the state of scientific knowledge on this topic is constantly evolving. A review from late 2020 reporting on about 60 studies on associations between COVID-19 and weather identified a variety of findings for temperature and humidity (Briz-Redón and Serrano-Aroca, 2020). The included studies that reported a linear trend mostly showed a negative association between COVID-19 cases and temperature as well as humidity (33 vs. 6 studies and 13 vs. 3, respectively). Global analyses support these local findings for temperature and humidity. Using different methodologies Sarkodie et al., Wu et al., Yuan et al., and Zhang et al. all found a negative association between temperature and RH with COVID-19 case rates in 20 countries, 166 countries, 127 countries, and 1236 regions globally with data until April, March, August, and May 2020, respectively (Sarkodie and Owusu, 2020; Wu et al., 2020; Yuan et al., 2021b; Zhang et al., 2021). The study from Yuan et al. also widened their analysis to include 188 countries with data through December 2020, and also analysed the non-linear associations, showing similar exposure response associations as found in our study using generalized additive model and as well DLNM methods (Yuan et al., 2021a; Yuan et al., 2021b). The temperature of minimum COVID-19 risk in both Yuan et al. studies was around 20 °C and for RH the risk was highest at humidity around 70 %. Another global study using DLNM from Guo et al. including 190 countries showed a similar association for temperature (highest RR at 5 °C and lowest at 20 °C) but exhibited a different exposure-risk association for RH (risk maximum at 72 % RH) (Guo et al., 2021). Two studies that also used DLNM models on US counties only also found elevated infection risks (increased Rt levels) at lower temperatures and one of them as well for lower specific humidities (Rubin et al., 2020; Ma et al., 2021). Fontal et al. analysed the transitory associations of temperature and AH until October 2020 in 10 world regions and obtained negative associations for both (Fontal et al., 2021). One global study did find only a small effect of temperature in 3739 global locations (Xu et al.) and two global studies did not find a statistically relevant effect for temperature (Carleton et al., Islam et al.) and RH (Islam et al.) in 206 countries and 3235 regions, respectively (Carleton et al., 2020a; Xu et al., 2021; Islam et al., 2021). However, Guo et al., Xu et al., Carleton et al., and Islam et al. all had a comparatively short study period reaching until April 2020 (Carleton et al., 2020a; Xu et al., 2021; Guo et al., 2021; Islam et al., 2021).
We recently performed a different global city-level analysis of meteorological factors and SARS-CoV-2 transmission (Majumder and Ray, 2021). This used an ecological approach comparing effective reproduction number (Re) and meteorological variables between cities in the early phase of the pandemic, and identified a non-linear (though primarily downward) association between mean temperature, and absolute humidity with Re, and a tendency of a negative association between RH and Re. Non-pharmaceutical interventions had a greater effect on Re. The results of the current study complement our previous results that showed higher Re at lower mean temperature, lower absolute humidity, and a negative association between RH and Re.
Regarding UV exposure, out of the 60 studies analysed in the previously mentioned systematic review, only six analysed solar radiation and among those there was no consensus of whether there is an association and if so what type of association (Briz-Redón and Serrano-Aroca, 2020). Two of the previously mentioned global analyses also included UV variables. Carleton et al. reported in contrast to our study that higher UV radiations were associated with lower COVID-19 growth rates, whereas Islam et al. concluded the relationship to be inconclusive within the same time period (Carleton et al., 2020a; Islam et al., 2021).
4.4. Strength and weaknesses
Our study has several important strengths. It considered a multitude of locations globally with smaller spatial units of analysis and longer observation periods than most published studies. Lagged effects of exposure were considered, as were potential non-linear relationships of the exposure with COVID-19 incidence. Ecological and time-varying confounders were analysed and incorporated.
Possible short comings of this study are that the case definitions differed from country to country, that GSI might not adjust sufficiently for changes in governmental measures over time, and that the distribution of cities included is not equally distributed around the globe, with some regions underrepresented and only few locations close to the equator. Thus, while this study is one of the most detailed global analyses to date, the pooled estimates provide insights into the associations in the included cities but are not fully representative for everywhere around the globe. Also, a global estimate itself might be of limited use due to the heterogeneity among locations that was encountered. We considered factors explaining this heterogeneity and we found that long-term mean temperature (a proxy of the city climate) and the percentage of the population older than 65 years modify the association found. There was a tendency in cities with lower long-term temperature and older population to have higher COVID-19 incidence in colder and drier conditions, but these factors explain only a small amount of the observed heterogeneity leading to some difference among countries. These differences could be due to limited sample size in some countries (e.g., Estonia, Finland, South Africa), and different and narrower ranges of exposure experience in countries (e.g. Brazil, Mexico, Chile, Peru and Singapore). Moreover, the observed differences could also be due to different adaptation of local populations to various weather conditions.
5. Conclusion
This study indicates that there is a tendency of a higher risk of COVID-19 cases at low temperature or absolute humidity levels, which aligns to an extent with available mechanistic explanations and previous literature basis. The between country heterogeneity of weather-related effects on COVID-19 when applying our uniform modelling framework in a global analysis shows the importance of determining location specific estimates of meteorological effects on COVID-19 spread. As more data accumulates, studies using longer observational periods will help elucidate weather-sensitivity and seasonal patterns of COVID-19 transmission.
Funding
R.L. was supported by a Royal Society Dorothy Hodgkin Fellowship.
S.A. and S.M. were funded by the Wellcome Trust (grant 210758/Z/18/Z210758/Z/18/Z).
D.R. was supported by a postdoctoral research fellowship of the Xunta de Galicia (Spain).
A.T. supported by MCIN/AEI/10.13039/501100011033 (grant CEX2018-000794-S).
A.G. was funded by the Medical Research Council-UK (Grant IDs: MR/R013349/1 and MR/V034162/1), the Natural Environment Research Council UK (Grant ID: NE/R009384/1) and the European Union's Horizon 2020 Project Exhaustion (Grant ID: 820655).
J.K. and A.U. were supported by the Czech Science Foundation, project 22-24920S.
H.K. was supported by the National Research Foundation of Korea (BK21 Center for Integrative Response to Health Disasters, Graduate School of Public Health, Seoul National University).
A.S., was funded by the European Union's Horizon 2020 Project Exhaustion (Grant ID: 820655).
N.S., was supported by the NIEHS-funded HERCULES Center (P30ES019776).
CRediT authorship contribution statement
Luise Nottmeyer: Data curation, Formal analysis, Writing – original draft. Ben Armstrong: Methodology, Writing – review & editing. Rachel Lowe: Writing – review & editing. Sam Abbott: Writing – review & editing. Sophie Meakin: Writing – review & editing. Kathleen M. O'Reilly: Writing – review & editing. Rosa von Borries: Writing – review & editing. Rochelle Schneider: Data curation, Writing – review & editing. Dominic Royé: Visualization, Writing – review & editing. Masahiro Hashizume: Data curation, Writing – review & editing. Mathilde Pascal: Data curation, Writing – review & editing. Aurelio Tobias: Data curation, Writing – review & editing. Ana Maria Vicedo-Cabrera: Writing – review & editing. Eric Lavigne: Data curation, Writing – review & editing. Patricia Matus Correa: Data curation, Writing – review & editing. Nicolás Valdés Ortega: Data curation, Writing – review & editing. Jan Kynčl: Data curation, Writing – review & editing. Aleš Urban: Data curation, Writing – review & editing. Hans Orru: Data curation, Writing – review & editing. Niilo Ryti: Writing – review & editing. Jouni Jaakkola: Data curation, Writing – review & editing. Marco Dallavalle: Data curation, Writing – review & editing. Alexandra Schneider: Writing – review & editing. Yasushi Honda: Writing – review & editing. Chris Fook Sheng Ng: Data curation, Writing – review & editing. Barrak Alahmad: Data curation, Writing – review & editing. Gabriel Carrasco-Escobar: Data curation, Writing – review & editing. Iulian Horia Holobâc: Data curation, Writing – review & editing. Ho Kim: Writing – review & editing. Whanhee Lee: Data curation, Writing – review & editing. Carmen Íñiguez: Data curation, Writing – review & editing. Michelle L. Bell: Writing – review & editing. Antonella Zanobetti: Writing – review & editing. Joel Schwartz: Writing – review & editing. Noah Scovronick: Data curation, Writing – review & editing. Micheline de Sousa Zanotti Stagliorio Coélho: Data curation, Writing – review & editing. Paulo Hilario Nascimento Saldiva: Data curation, Writing – review & editing. Magali Hurtado Diaz: Data curation, Writing – review & editing. Antonio Gasparrini: Methodology, Writing – review & editing. Francesco Sera: Supervision, Conceptualization, Methodology, Data curation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This paper used Copernicus Climate Change Service (C3S) and Copernicus Atmosphere Monitoring Service (CAMS) information. Hence, the authors would like to thank the European Centre for Medium-Range Weather Forecasts (ECMWF) for its data generation on behalf of the European Union.
Editor: Scott Sheridan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2022.158636.
Appendix A. Supplementary data
All supplementary tables and figures can be found in the Supplementary Material.
Data availability
The authors do not have permission to share data.
References
- Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transbound. Emerg. Dis. 2020;00:1–17. doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai H., Nie R., Wang X. Evaluation of the effects of meteorological factors on COVID-19 prevalence by the distributed lag nonlinear model. J. Transl. Med. 2022;20:1–9. doi: 10.1186/s12967-022-03371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiou O.E., et al. Seasonality of non-SARS, non-MERS coronaviruses and the impact of meteorological factors. Pathogens. 2021;10:1–12. doi: 10.3390/pathogens10020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babin S. Use of weather variables in SARS-CoV-2 transmission studies. Int. J. Infect. Dis. 2020;100:333–336. doi: 10.1016/j.ijid.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A. Aerosol analysis and forecast in the European Centre for medium-range weather forecasts integrated forecast system: 2. Data assimilation. J. Geophys. Res. Atmos. 2009;114:1–18. [Google Scholar]
- Bhaskaran K., Gasparrini A., Hajat S., Smeeth L., Armstrong B. Time series regression studies in environmental epidemiology. Int. J. Epidemiol. 2013;42:1187–1195. doi: 10.1093/ije/dyt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz-Redón Á., Serrano-Aroca Á. The effect of climate on the spread of the COVID-19 pandemic: a review of findings, and statistical and modelling techniques. Prog. Phys. Geogr. Earth Environ. 2020;44:591–604. [Google Scholar]
- Cai, J. Humidity: Calculate Water Vapor Measures from Temperature and Dew Point. CRAN.
- Carleton T., Cornetet J., Huybers P., Meng K.C., Proctor J. Global evidence for ultraviolet radiation decreasing COVID-19 growth rates. Proc. Natl. Acad. Sci. U. S. A. 2020;118:1–9. doi: 10.1073/pnas.2012370118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C.J., Gomez A.C.R., Bansal S., Ryan S.J. Misconceptions about weather and seasonality must not misguide COVID-19 response. Nat.Commun. 2020;11:1–4. doi: 10.1038/s41467-020-18150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L.M., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010;76:2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., et al. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011;1–7 doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., et al. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011;2011:1–7. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe Y. Validation report of the CAMS near-real-time global atmospheric composition service: period March–May 2019. Copernicus Atmos. Monit. Serv. Rep. 2019:1–150. [Google Scholar]
- Copernicus ERA5-Land hourly data from 1981 to present. https://cds.climate.copernicus.eu/cdsapp#!/dataset/reanalysis-era5-single-levels?tab=form
- Dong Z., et al. Data-related and methodological obstacles to determining associations between temperature and COVID-19 transmission. Environ. Res. Lett. 2021;16:1–9. [Google Scholar]
- Donzelli G., Biggeri A., Tobias A., Nottmeyer L.N., Sera F. Role of meteorological factors on SARS-CoV-2 infection incidence in Italy and Spain before the vaccination campaign. A multi-city time series study. Environ. Res. 2022;211:1–7. doi: 10.1016/j.envres.2022.113134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol. 2002;122:183–191. doi: 10.1080/00016480252814207. [DOI] [PubMed] [Google Scholar]
- Fong F.C., Smith D.R. Exposure-lag response of air temperature on COVID-19 incidence in twelve Italian cities: a meta-analysis. Environ. Res. 2022;212:1–6. doi: 10.1016/j.envres.2022.113099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontal A., et al. Climatic signatures in the different COVID-19 pandemic waves across both hemispheres. Nat. Comput. Sci. 2021;1:655–665. doi: 10.1038/s43588-021-00136-6. [DOI] [PubMed] [Google Scholar]
- Foxman E.F., et al. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc. Natl. Acad. Sci. U. S. A. 2015;112:827–832. doi: 10.1073/pnas.1411030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. Distributed lag linear and non-linear models in R: the package dlnm. J. Stat. Softw. 2011;43:1–20. [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. Modeling exposure–lag–response associations with distributed lag non-linear models. Stat. Med. 2014;33:881–899. doi: 10.1002/sim.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Google LLC Mobility report for the corona crisis. https://www.google.com/covid19/mobility/
- Guo C., et al. Meteorological factors and COVID-19 incidence in 190 countries: an observational study. Sci. Total Environ. 2021;757:1–8. doi: 10.1016/j.scitotenv.2020.143783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Raghuwanshi G.S., Chanda A. Effect of weather on COVID-19 spread in the US: a prediction model for India in 2020. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T., et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker) Nat. Hum. Behav. 2021;5:529–538. doi: 10.1038/s41562-021-01079-8. [DOI] [PubMed] [Google Scholar]
- He Z., et al. The influence of average temperature and relative humidity on new cases of COVID-19: time-series analysis. JMIR Public Health Surveill. 2021;7:1–14. doi: 10.2196/20495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai C., Armstrong B., Chalabi Z., Mangtani P., Hashizume M. Time series regression model for infectious disease and weather. Environ. Res. 2015;142:319–327. doi: 10.1016/j.envres.2015.06.040. [DOI] [PubMed] [Google Scholar]
- Islam N., et al. COVID-19 and climatic factors: a global analysis. Environ. Res. 2021;193:1–5. doi: 10.1016/j.envres.2020.110355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo E., et al. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc. Natl. Acad. Sci. U. S. A. 2019;166:10905–10910. doi: 10.1073/pnas.1902840116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwait Population Worldometer. 2021. https://www.worldometers.info/world-population/kuwait-population/
- Li H.L., et al. A meta-analysis result: uneven influences of season, geo-spatial scale and latitude on relationship between meteorological factors and the COVID-19 transmission. Environ. Res. 2022;212:1–12. doi: 10.1016/j.envres.2022.113297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Wang X., Huang J. The influence of weather conditions on the COVID-19 epidemic: evidence from 279 prefecture-level panel data in China. Environ. Res. 2022;206:1–11. doi: 10.1016/j.envres.2021.112272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., et al. Association between temperature and COVID-19 transmission in 153 countries. Environ. Sci. Pollut. Res. Int. 2022;29:16017–16027. doi: 10.1007/s11356-021-16666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen A.C., Steel J. Roles of humidity and temperature in shaping influenza seasonality. J. Virol. 2014;88:7692–7695. doi: 10.1128/JVI.03544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen A.C., Mubareka S., Steel J., Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Pei S., Shaman J., Dubrow R., Chen K. Role of meteorological factors in the transmission of SARS-CoV-2 in the United States. Nat. Commun. 2021;12:1–9. doi: 10.1038/s41467-021-23866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder P., Ray P.P. A systematic review and meta-analysis on correlation of weather with COVID-19. Sci. Rep. 2021;11:1–10. doi: 10.1038/s41598-021-90300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander P. CARNOTCYCLE - Class. Blog Thermodyn. 2020. How to convert relative humidity to absolute humidity. [Google Scholar]
- McAloon C., et al. Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research. BMJ Open. 2020;10:1–9. doi: 10.1136/bmjopen-2020-039652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClymont H., Hu W. Weather variability and COVID-19 transmission: a review of recent research. Int. J. Environ. Res. Public Health. 2021;18:1–19. doi: 10.3390/ijerph18020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecenas P., Bastos R., Vallinoto A., Normando D. Effects of temperature and humidity on the spread of COVID-19: a systematic review. PLoS One. 2020:1–21. doi: 10.1371/journal.pone.0238339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz J.A., Finn A. Influenza and humidity – why a bit more damp may be good for you! J. Infect. 2015;71:54–58. doi: 10.1016/j.jinf.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Morcrette J.-J. Aerosol analysis and forecast in the European Centre for medium-range weather forecasts integrated forecast system: forward modeling. J. Geophys. Res. Atmos. 2009;114:1–17. [Google Scholar]
- Nguyen J.L., Dockery D.W. Daily indoor-to-outdoor temperature and humidity relationships: a sample across seasons and diverse climatic regions. Int. J. Biometeorol. 2016;60:221–229. doi: 10.1007/s00484-015-1019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottmeyer L.N., Sera F. Influence of temperature, and of relative and absolute humidity on COVID-19 incidence in England - a multi-city time-series study. Environ. Res. 2021;196:1–8. doi: 10.1016/j.envres.2021.110977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD Metropolitan areas - regions at a glance. 2016. https://stats.oecd.org/Index.aspx?Datasetcode=CITIES
- Peru: Administrative Division (Regions and Provinces) - population statistics, charts and map. https://www.citypopulation.de/en/peru/admin/
- Polozov I.V., Bezrukov L., Gawrisch K., Zimmerberg J. Progressive ordering with decreasing temperature of the phospholipids of influenza virus. Nat. Chem. Biol. 2008;4:248–255. doi: 10.1038/nchembio.77. [DOI] [PubMed] [Google Scholar]
- Prata D.N., Rodrigues W., Bermejo P.H. Temperature significantly changes COVID-19 transmission in (sub)tropical cities of Brazil. Sci. Total Environ. 2020;729:1–7. doi: 10.1016/j.scitotenv.2020.138862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H., et al. COVID-19 transmission in mainland China is associated with temperature and humidity: a time-series analysis. Sci. Total Environ. 2020;728:1–6. doi: 10.1016/j.scitotenv.2020.138778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., et al. Indoor transmission of SARS-CoV-2. Indoor Air. 2020;Vol. 00:1–7. doi: 10.1111/ina.12766. [DOI] [PubMed] [Google Scholar]
- Quesada J.A., et al. Incubation period of COVID-19: a systematic review and meta-analysis. Rev. Clin. Esp. 2021;221:109–117. doi: 10.1016/j.rceng.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romania Population Worldometer. 2021. https://www.worldometers.info/world-population/romania-population/
- Rosti M.E., Olivieri S., Cavaiola M., Seminara A., Mazzino A. Fluid dynamics of COVID-19 airborne infection suggests urgent data for a scientific design of social distancing. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-80078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D., et al. Association of social distancing, population density, and temperature with the instantaneous reproduction number of SARS-CoV-2 in counties across the United States. JAMA Netw. Open. 2020;3:1–12. doi: 10.1001/jamanetworkopen.2020.16099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkodie S.A., Owusu P.A. Impact of meteorological factors on COVID-19 pandemic: evidence from top 20 countries with confirmed cases. Environ. Res. 2020;191:1–7. doi: 10.1016/j.envres.2020.110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sera F., Armstrong B., Blangiardo M., Gasparrini A. An extended mixed-effects framework for meta-analysis. Stat. Med. 2019;38:5429–5444. doi: 10.1002/sim.8362. [DOI] [PubMed] [Google Scholar]
- Sera F., et al. A cross-sectional analysis of meteorological factors and SARS-CoV-2 transmission in 409 cities across 26 countries. Nat. Commun. 2021;12:1–11. doi: 10.1038/s41467-021-25914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadloo-Jahromi A., Bavi O., Hossein Heydari M., Kharati-Koopaee M., Avazzadeh Z. Dynamics of respiratory droplets carrying SARS-CoV-2 virus in closed atmosphere. Results Phys. 2020;19:1–5. doi: 10.1016/j.rinp.2020.103482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.P., et al. Temperature and population density influence SARS-CoV-2 transmission in the absence of nonpharmaceutical interventions. Proc. Natl. Acad. Sci. U. S. A. 2021;118:1–8. doi: 10.1073/pnas.2019284118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South Africa Population Worldometer. 2021. https://www.worldometers.info/world-population/south-africa-population/
- Tan L., Schultz D.M. How is COVID-19 affected by weather? Metaregression of 158 studies and recommendations for best practices in future research. Weather. Clim. Soc. 2022;14:237–255. [Google Scholar]
- Tang J.W. The effect of environmental parameters on the survival of airborne infectious agents. J. R. Soc. Interface. 2009;6:737–746. doi: 10.1098/rsif.2009.0227.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 2020;144 doi: 10.1016/j.envint.2020.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve P.J., Goldberg M.S. Methodological considerations for epidemiological studies of air pollution and the SARS and COVID-19 coronavirus outbreaks. Environ. Health Perspect. 2020;128:095001-1–095001-13. doi: 10.1289/EHP7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A.K., Head J.R., Gould C.F., Carlton E.J., Remais J.V. Environmental factors influencing COVID-19 incidence and severity. Annu. Rev. Public Health. 2022;43:271–291. doi: 10.1146/annurev-publhealth-052120-101420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., et al. A narrative review on the role of temperature and humidity in COVID-19: transmission, persistence, and epidemiological evidence. Eco-Environ. Health. 2022;1:73–85. doi: 10.1016/j.eehl.2022.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO Coronavirus Disease (COVID-19) Dashboard. WHO Homepage. https://covid19.who.int/?gclid=CjwKCAiA6aSABhApEiwA6Cbm_x4hKWdM2Gp8wJGifRwWNUoL0Bg-PDBLjw5tE76umDPhlk1PSuIjZRoCa3MQAvD_BwE
- Willem L., Van Kerckhove K., Chao D.L., Hens N., Beutels P. A Nice day for an Infection? Weather conditions and social contact patterns relevant to influenza transmission. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., et al. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Sci. Total Environ. 2020;729:1–7. doi: 10.1016/j.scitotenv.2020.139051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Zhu Y. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724:1–5. doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., et al. Weather, air pollution, and SARS-CoV-2 transmission: a global analysis. Lancet Planet. Health. 2021;5:e671–e680. doi: 10.1016/S2542-5196(21)00202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., et al. No association of COVID-19 transmission with temperature or UV radiation in Chinese cities. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00517-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Wu Y., Jing W., Liu J., Du M., Wang Y., Liu M. Association between meteorological factors and daily new cases of COVID-19 in 188 countries: a time series analysis. Sci. Total Environ. 2021;780:1–10. doi: 10.1016/j.scitotenv.2021.146538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., et al. Non-linear correlation between daily new cases of COVID-19 and meteorological factors in 127 countries. Environ. Res. 2021;193:1–8. doi: 10.1016/j.envres.2020.110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., et al. The role of weather conditions in COVID-19 transmission: a study of a global panel of 1236 regions. J. Clean. Prod. 2021;292:1–13. doi: 10.1016/j.jclepro.2021.125987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H.-L., et al. Effects of climate variables on the transmission of COVID-19: a systematic review of 62 ecological studies. Environ. Sci. Pollut. Res. Int. 2021:1–18. doi: 10.1007/s11356-021-15929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Dai H.Y., Zha W.T., Lv Y. The impact of meteorological factors and PM2.5 on COVID-19 transmission. Epidemiol. Infect. 2022;150:1–7. doi: 10.1017/S0950268821002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All supplementary tables and figures can be found in the Supplementary Material.
Data Availability Statement
The authors do not have permission to share data.