Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to a global pandemic. Although COVID-19 was initially described as a respiratory disease, there is growing evidence that SARS-CoV-2 is able to invade the brains of COVID-19 patients and cause cognitive impairment. It has been reported that SARS-CoV-2 may have invasive effects on a variety of cranial nerves, including the olfactory, trigeminal, optic, and vagus nerves, and may spread to other brain regions via infected nerve endings, retrograde transport, and transsynaptic transmission. In addition, the blood–brain barrier (BBB), composed of neurovascular units (NVUs) lining the brain microvasculature, acts as a physical barrier between nerve cells and circulating cells of the immune system and is able to regulate the transfer of substances between the blood and brain parenchyma. Therefore, the BBB may be an important structure for the direct and indirect interaction of SARS-CoV-2 with the brain via the blood circulation. In this review, we assessed the potential involvement of neuroinvasion under the SARS-CoV-2 infection, and the potential impact of BBB disorder under SARS-CoV-2 infection on cognitive impairment.
Keywords: COVID-19, SARS-CoV-2, Neuroinvasion, BBB, Cognitive
Introduction
At the end of 2019, a large number of cases of pneumonia were reported, and then the disease spread rapidly and was named COVID-19 [1]. As of June 23, 2022, the COVID-19 pandemic has infected nearly 541 million people worldwide and killed 6.32 million people [2]. Since the outbreak of the COVID-19 pandemic, the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a dramatic impact on global healthcare systems and socioeconomics [3, 4]. With the application of high-efficiency vaccines, the number of COVID-19 survivors has gradually increased, and people have begun to pay attention to the long-term or delayed sequelae that are caused by SARS-CoV-2 infection, commonly known as “long-COVID” syndrome [5, 6]. Although COVID-19 was initially described as a respiratory disease, data suggest that, depending on the severity of COVID-19 symptoms, 30% to 80% of patients develop neurological complications, and some are sufficiently disabling [7–10]. Therefore, understanding the invasion and impact of SARS-CoV-2 on the central nervous system (CNS) is crucial for future studies.
The vasculature of the CNS is the main channel for blood molecules to enter the brain and tightly regulate the movement of ions, molecules and cells between the blood and the brain, known as the blood–brain barrier (BBB) [11–13]. The BBB is mainly composed of brain endothelial cells, vascular basement membrane, pericytes, astrocyte end-foot, microglia and neurons [11, 14]. These structures act as a bridge between the brain parenchyma and the cerebrovascular system and are collectively referred to as the neurovascular units (NVUs) [15, 16]. BBB endothelial cells are sealed by tight junction (TJ) proteins (ZO scaffolding proteins, claudin-5, and occludin) and junctional adhesion molecules to limit the extracellular and transcellular diffusion of molecules in the CNS [17–19]. Thus, in addition to neuroinvasion, disruption of BBB integrity during COVID-19 may expose the brain parenchyma to SARS-CoV-2 in infected blood, which may affect neuronal activity in the CNS.
Although the cause of neuroinvasion and BBB damage in COVID-19 is still unclear, the extent of neurological injury and BBB damage appears to be related to the degree of cognitive impairment and severity of COVID-19 infection. Cranial nerves and the BBB may be important structures for direct and indirect interactions between SARS-CoV-2 and the brain. In this review, we assessed the potential involvement of neuroinvasion and BBB dysfunction in SARS-CoV-2 infection, exploring its impact on COVID-19-related cognitive dysfunction.
Clinical evidence of cognitive impairment associated with COVID-19
The lungs are the most severely affected organ in COVID-19, which manifests as diffuse alveolar damage, hyaline membrane formation, inflammatory cell infiltration, and microvascular damage [20]. Although SARS-CoV-2 infection was initially thought to be limited to the respiratory tract, causing severe respiratory syndrome, it was later found that the virus can invade other organs, including the CNS [10, 21]. After analysing data from 214 patients from 67 studies, Motalvan et al. [22] found that 36.4% of patients with COVID-19 developed neurological symptoms (Table 1). Multiple retrospective cohort studies of COVID-19 survivors found that one-third of the patients developed neurological or psychiatric symptoms 6 months after SARS-CoV-2 infection [23, 24]. In addition, multiple studies have shown a high incidence of cognitive impairment in post-COVID-19 patients, exceeding 50% in all studies reporting prevalence [25–30] (Table 1). Notably, cognitive impairment appears to be more common in critically ill patients. In a cohort study of 1438 COVID-19 survivors, Liu et al. found that 10% of severe COVID-19 survivors had dementia and 26.54% had MCI at 6 months after discharge [31]. At 12 months, the number of dementia patients increased to 15%, while the number of MCI patients remained at 26.15%, higher than nonsevere cases (0.76%) and MCI (5.35%) [31] (Table 1). The presence of abnormal brain magnetic resonance imaging (MRI) findings in COVID-19 patients and the detection of SARS-CoV-2 RNA in cerebrospinal fluid may support the possibility that SARS-CoV-2 has neuroinvasive ability [32–35]. Taken together, SARS-CoV-2 enters the brain parenchyma, which may lead to damage and loss of brain neurons and endothelial cells, thereby causing COVID-19-related neurological symptoms.
Table 1.
Summary of neurological involvement in COVID-19 patients in existing studies
| Number of patients | Incidence of cognitive impairment | Other types of neurological symptoms | References |
|---|---|---|---|
| 214 | – | Headache, disturbance of consciousness, neuralgia, ataxia, acute cerebrovascular disease, seizures | [22] |
| 29 | 59–65% (at 4 months) | Executive dysfunction (33%) | [25] |
| 2103 | 61.5–80% (at 3 months) | – | [26] |
| 53 | 61.5% | Hyposmia (26%), headache (21%), ischaemic stroke (11.1%), coordination deficits (74%), paresis (47%), abnormal reflex status (45%), sensory abnormalities (45%) | [27] |
| 26 | 69.2% | Anosmia (34%), hyposmia (52%), hypogeusia (100%) | [28] |
| 179 | 58.7% (at 2 months) | Impaired immediate verbal memory and learning (38%), delayed verbal memory (11.8%), verbal fluency (34.6%) and working memory (executive function) (6.1%) | [29] |
| 226 | 78% | Impaired executive function (50%), impaired psychomotor coordination (57%) | [30] |
| 1438 | Dementia: 10% (at 6 months)–15% (at 12 months) MCI: 26.54% (at 6 months)–26.1% (at 12 months) | – | [31] |
The table includes some summaries of neurological involvement in COVID-19 patients in existing studies. The incidence of these symptoms varied with sample size and duration of observation. However, cognitive impairment and other neurological symptoms in COVID-19 patients cannot be ignored
Furthermore, in severe cases of COVID-19, SARS-CoV-2 infection can trigger systemic inflammation and cytokine storms [36]. Significantly elevated levels of interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α) were found in the cerebrospinal fluid of patients affected by COVID-19 [37–39]. In vivo and in vitro studies have shown that IL-6 and TNF-⍺ can trigger stress response mechanisms that disrupt synaptic plasticity, memory formation, and hippocampal neurogenesis [40–42]. Viruses can cause brain dysfunction and neuronal damage through direct cytolysis or secondary inflammatory responses (indirect effects) [43]. Regardless of whether the brain is affected by SARS-CoV-2 through primary or secondary pathways, the neurological complications of COVID-19 may be related to the invasive effects of SARS-CoV-2 on brain tissue.
Potential pathways of SARS-CoV-2 invading the CNS
Histopathological studies have shown that SARS-CoV-2 is present in different types of brain parenchyma cells. The underlying neurotropic mechanism of SARS-CoV-2 is not fully understood [44–46]. However, the neurotropic mechanisms previously found in SARS-CoV and other viruses can serve as a reference for evaluating the mechanisms of SARS-CoV-2. According to the current research, there may be two main routes of virus entry into the CNS: neuroinvasive mechanisms and haematogenous transmission routes [47–51].
SARS-CoV-2 enters the brain along the olfactory nerve
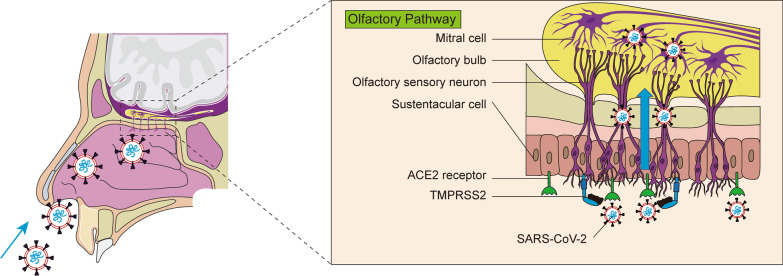
The olfactory nerve is mainly composed of olfactory receptor neurons and directly connects the nasal cavity and the CNS [52]. Some pathogens can infect olfactory sensory neurons and their axons that project into the olfactory bulb, which allows the viruses to bypass the BBB and reach the CNS through the so-called olfactory pathway [53]. In one study, of 38 patients with confirmed COVID-19, 73.7% were reported to have positive RT-PCR tests on nasopharyngeal swabs [54]. In a Spanish COVID-19 cohort, 36% of the patients initially presented with anosmia [55]. In a large European multicentre cohort of mild-to-moderate COVID-19 patients, 85.6% and 88.8%, respectively, had olfactory and taste dysfunction [56].
Each olfactory receptor neuron projects dendrites into the nasal cavity and extends its axons basolaterally through the lamina cribrosa into the olfactory bulb of the brain [52, 57] (Fig. 1). In this pathway, SARS-CoV-2 in the nasal endothelium may attach to motor proteins along sensory and olfactory nerves to travel to the brain [58]. Two days after intranasal administration of SARS-CoV-2 in hamsters, viral antigens were present in the nasal mucosa, bronchial epithelial cells, and areas of lung consolidation, and the virus could infect hamster olfactory sensory neurons [59, 60]. Studies have shown that olfactory epithelial cells express high levels of angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) [61–63], which could provide a plausible explanation for COVID-19-related anosmia (Fig. 1). Early animal studies have shown that SARS-CoV can enter the brains of ACE2-transgenic mice via the olfactory bulb and cause rapid, transneuronal spread to relevant regions of the brain where infected neurons are dysfunctional [64]. Given the highly similar pathophysiological pathways between SARS-CoV and SARS-CoV-2, this may explain the high incidence of anosmia in COVID-19 patients [60, 61, 65, 66].
Fig. 1.
Potential routes of SARS-CoV-2 entry into the brain via the olfactory pathway. Once SARS-CoV-2 is inhaled into the nasal cavity, the virus spreads to the central nervous system along the retrograde axons of the olfactory nerve via the olfactory epithelial receptors ACE2 and TMPRSS2
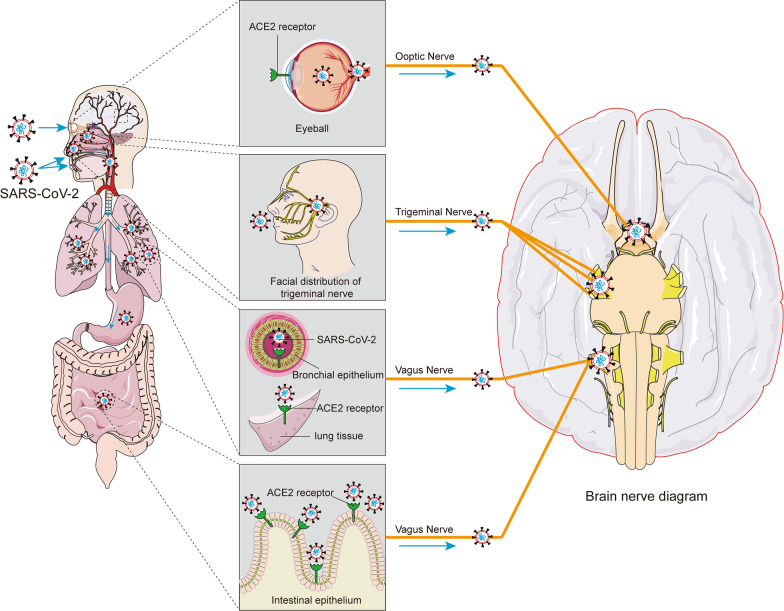
Notably, many research reported that SARS-CoV-2 RNA was detected not only in the olfactory mucosa and olfactory bulb, but also in different branches of the trigeminal nerve [45] (Fig. 2). SARS-CoV-2 may also enter the trigeminal nerve through ACE2 receptor [67]. Afferent fibres from the nasal mucosa travel through the ethmoid nerve to the anterior cranial fossa and travel in the dura to the trigeminal ganglion [68]. Therefore, the strong activation of trigeminal afferents damaged by SARS-CoV-2 may lead to headache and anosmia. The histological changes resulting from intranasal inoculation of neurotropic virus include neuronal and glial necrosis with neutrophil infiltration [69, 70]. Therefore, once SARS-CoV-2 is inhaled into the nasal cavity, the virus may propagate into the CNS along the retrograde axons of the olfactory nerve through the receptors ACE2 and TMPRSS2 on the olfactory mucosa, resulting in neuronal necrosis and dysfunction, thereby causing cognitive impairment due to CNS damage (Fig. 1).
Fig. 2.
Possible pathways by which SARS-CoV-2 enters the brain through other neural invasions. In addition to infection through the olfactory route, SARS-CoV-2 can also be transmitted through direct or indirect contact with the eyes and oral mucosa. SARS-CoV-2 may enter cells through receptors such as ACE2 on the nasal cavity, eyes, respiratory epithelium, lung parenchyma, and gut and in turn affect multiple cranial nerves (including trigeminal, optic, and vagus nerves). SARS-CoV-2 may infect nerve endings, be transported retrogradely, and spread to other brain regions via synapses
SARS-CoV-2 transmission through the ocular route
It has been speculated that this may have something to do with the lack of goggle protection [71]. On January 22, 2020, a member of the National Pneumonia Panel was diagnosed with COVID-19 just days after an episode of red eye [71]. The possibility of eye transmission of SARS-CoV-2 is gradually attracting global attention. If a virus can get into our eyes, the most common target may be our conjunctiva [72]. With the development of the epidemic, many COVID-19 patients have developed conjunctivitis as the first symptom or accompanying symptoms [72, 73]. A clinical study of 172 COVID-19 patients showed that the most common ocular symptom in COVID-19 patients was conjunctivitis (23.3%), manifested as conjunctival hyperaemia, foreign body sensation, and itching [74].
However, whether SARS-CoV-2 can be transmitted through the eyes remains controversial [75, 76]. Meinhardt et al. [45] assessed viral load by RT-qPCR on regionally defined tissue samples and found low levels of viral RNA in the corneal, conjunctival and oral mucosa in addition to SARS-CoV-2 in the olfactory mucosa directly below the sieve plate. In addition, immunohistochemical analysis showed that ACE2 was expressed in the conjunctiva, limbus and cornea [77, 78] (Fig. 2). This provides the molecular basis for the susceptibility of the eye to SARS-CoV-2. Recent studies have shown that SARS-CoV-2 can infect the eyes through droplets with viral particles, and the virus can then spread through the nasolacrimal duct to reach the lungs [79]. Inoculation with TCID50 of SARS-CoV-2 in the conjunctiva and intratracheal and intragastric inoculation of five rhesus monkeys revealed SARS-CoV-2 [80]. Therefore, the eye has the potential to be a potential infection portal for SARS-CoV-2, supporting the possibility of ocular transmission using the conjunctival mucosa as an entry point for SARS-CoV-2 in the setting of insufficient ocular protection (Fig. 2).
In addition to conjunctivitis, SARS-CoV-2 infection has been associated with lesions leading to visual impairment due to retinal vascular obstruction, ischaemic optic neuropathy, chorioretinitis, and optic nerve inflammation [81, 82]. De Figueiredo et al. [83] described viral arrival at the blood‒retinal barrier, expressing ACE2 and CD147 in retinal pigment epithelial cells and vascular endothelial cells. Because CD147 mediates the disruption of the neurovascular barrier, the virus can cross the bloodstream. CD147 mediates the disruption of the neurovascular barrier, and viruses can cross the blood‒retinal barrier and enter the bloodstream [84]. Therefore, in addition to droplet transmission and direct contact transmission of common respiratory viruses, SARS-CoV-2 may be transmitted to the ocular surface through hand–eye contact and aerosol transmission and then to other systems through the nasolacrimal tract and bloodstream transmission. The potential for ocular transmission of SARS-CoV-2 should not be overlooked. Although haematogenous transfer of SARS-CoV-2 through the eye is theoretically possible, more clinical or experimental evidence is needed to confirm this hypothesis. In the current severe outbreak, more evidence is urgently needed to better assess the potential for ocular transmission and the need for protective measures.
Neuroinvasion via the vagus nerve
The vagus nerve is the longest nerve in the body and connects vital organs, including the brain, heart, lungs and intestines. In a study of 200 COVID-19 patients, Aranyó et al. [85] found that symptoms such as dizziness, cough, increased heart rate and gastrointestinal problems were associated with damage to the vagus nerve. Although human data are lacking, the vagus nerve complex is known to express ACE2 in rodents [86]. The nucleus solitarius receives sensory information from mechanoreceptors and chemoreceptors in the lung and respiratory tract, so the vagal nucleus solitarius from the lung may be one of the important pathways for virus transport to the brain [87, 88]. Rangon et al. [89] pointed out that SARS-CoV-2 easily invades from the lung along the vagus nerve to the autonomic nerve centre in the brainstem and is involved in the coupling of cardiovascular and respiratory rhythms (Fig. 2). Netland et al. [64] infected brain slices from ACE2 mice with SARS-CoV and found that the dorsal vagal complex, which is critical for cardiorespiratory function, was infected. As reported by Li et al. [90], SARS-CoV-2 migrates from the lungs to the brain and may cause dysfunction of the pulmonary respiratory centre in the brainstem of patients with COVID-19.
In addition, the possibility of enteral infection of SARS-CoV-2 in patients with COVID-19 has also raised concerns [91]. This may be due to the existence of a large number of ACE2 receptors in intestinal epithelial gland cells in addition to the existence of ACE2 in human respiratory epithelium and lung parenchyma [92, 93], which provide the molecular basis for susceptibility to SARS-CoV-2 (Fig. 2).
Therefore, the dorsal vagus nerve complex of the brainstem can be a target of SARS-CoV-2, and the virus may invade brain tissue along the vagus nerve, which may be the neuroanatomical basis for COVID-19 to affect respiration and related reflexes.
SARS-CoV-2 enters the brain via the BBB
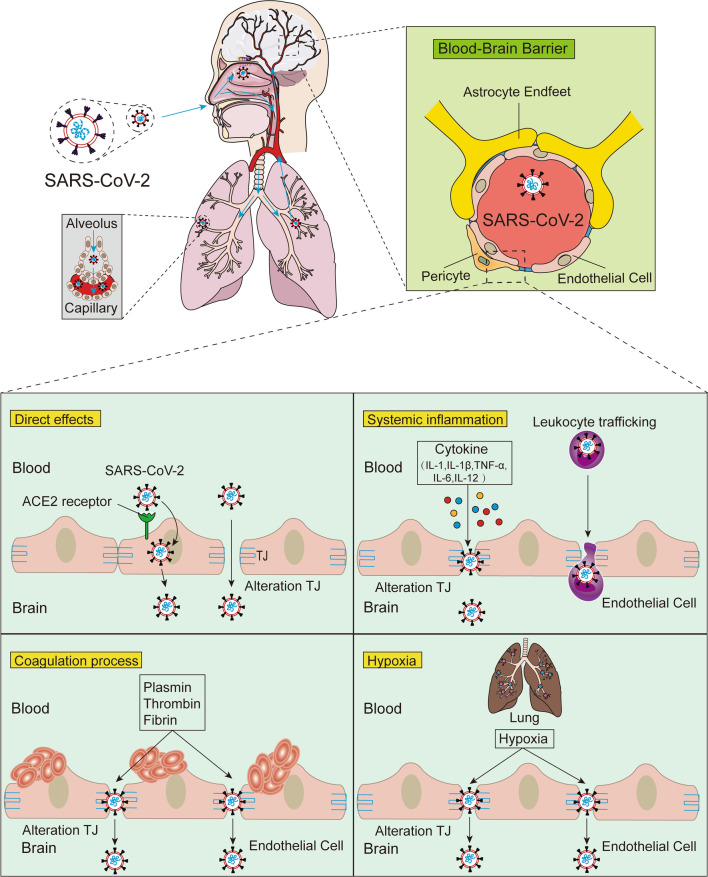
In addition to possibly causing brain infection via the neuroinvasive route, SARS-CoV-2 may also enter the brain via the haematogenous route [49, 94]. Studies have pointed out that SARS-CoV-2 is present in the blood of up to 40% of COVID-19 patients [95, 96]. An autopsy analysis of patients who died from COVID-19 showed that SARS-CoV-2 can infect endothelial cells [97]. Normally, viruses such as SARS-CoV-2 cannot easily enter the brain parenchyma through the endothelial cells that surround the capillaries of the systemic circulatory system due to the unique physiology of the BBB. However, the BBB is not impenetrable. BBB disruption and leakage were reported in 58% of COVID-19 patients in 31 case studies of patients with neurological manifestations [98], and these studies provided the first evidence of SARS-CoV-2-induced BBB dysfunction in humans. Disruption of the BBB allows circulating SARS-CoV-2 to invade the brain parenchyma [93, 99, 100]. A recent study in a BBB-on-a-chip in vitro system suggested that the SARS-CoV-2 spike protein may contribute to BBB dysfunction and loss of integrity [101]. The entry of SARS-CoV-2 into the CNS through the BBB may take many forms, some through direct infection and others through secondary mechanisms, such as systemic inflammatory responses and ischaemic and hypoxic changes associated with intravascular coagulation disorders (Fig. 3).
Fig. 3.
Possible mechanism of SARS-CoV-2 infection of the brain via the haematogenous route. Pulmonary infection with SARS-CoV-2 leads to vascular endothelial damage and increase capillary permeability, allowing virus transfer from the lungs to the pulmonary microcirculation. After reaching the BBB, SARS-CoV-2 may enter the CNS through direct interaction with ACE2 receptors or by altering tight junction proteins formed by BBB endothelial cells. Infection with SARS-CoV-2 increases circulating concentrations of proinflammatory cytokines (IL-1, IL-1β, TNF-α, IL-6, IL-12, etc.), thrombin, fibrinogen, and plasmin, and induced hypoxia to disrupt the BBB may lead to paracellular passage of SARS-CoV-2 as a means of entry into the CNS. In addition, infected leukocytes can carry SARS-CoV-2 across the BBB to infect the CNS through a “Trojan horse” mechanism
SARS-CoV-2 directly interacts with components of the BBB
Examination of brain tissue from postmortem SARS-CoV-2-infected patients revealed the presence of viral particles in brain capillaries, endothelial cells, pericytes, and neurons [97, 102, 103]. Studies have shown that SARS-CoV-2 enters cells through the activities of the spike protein, which binds to the ACE2 receptor, and the spike protein is a protein that allows viral RNA to enter healthy cells, allowing the virus to replicate through a complex series of steps [101, 104–106]. The neural transmission of SARS-CoV-2 through the BBB occurs via the infection of vascular endothelial cells due to the presence of ACE2 receptors in endothelial cells [102, 107, 108] (Fig. 3). Once the neurotrophic virus passes through vascular endothelial cells, SARS-CoV-2 invades brain cells through the binding of the S1 subunit of its S protein to ACE2 receptors, which triggers a conformational change in the S2 subunit to achieve membrane fusion with the host cell [109]. The function and overactivity of the ACE2 receptor may affect these target cells and organs, increasing the patient’s susceptibility to infection [110]. The possibility of entry into the cerebrovascular system via other SARS-CoV-2 receptors, such as neuropilin-1 (NRP1) [111] and TMPRSS2 [106], cannot be ruled out. Notably, ACE2 and TMPRSS2 are also expressed in human choroid plexus cells [112]. The choroid plexus has a more permeable blood–cerebrospinal fluid barrier than the tightly regulated BBB and may be a potential site for viral entry into the CNS. Taken together, these data suggest that binding of SARS-CoV-2 with ACE2 receptors on the cerebral vascular endothelium may lead to SARS-CoV-2 crossing the BBB into the brain parenchyma.
Furthermore, viral invasion of the BBB may be associated with disruption of endothelial TJs, leading to BBB dysfunction and enhanced permeability [113, 114]. Using a 3D tissue model of the BBB, the SARS-CoV-2 spike protein was shown to damage endothelial cells and increase the permeability of the BBB [101]. The SARS-CoV-2 spike protein triggers a proinflammatory response in brain endothelial cells, which may lead to alterations in the BBB functional status. Subsequent studies found that the SARS-CoV-2 spike protein led to disruption of the BBB by downregulating TJ proteins in human brain microvascular endothelial cells, resulting in viral entry into the CNS via a paracellular pathway [115, 116] (Fig. 3).
SARS-CoV-2 infection triggers systemic inflammation and promotes BBB leakage
In severe cases of COVID-19, SARS-CoV-2 infection can trigger systemic inflammation and cytokine storms [36]. Krasemann et al. [117] used an in vitro BCEC model to show intrinsic inflammatory signatures following exposure to SARS-CoV-2. Indirect effects of a hyperinflammatory state may be the mechanism involved in the BBB disruption associated with COVID-19 [118]. Elevated levels of proinflammatory factors are closely related to changes in TJ function and BBB disruption. For example, elevated levels of IL-1 can lead to impaired BBB integrity [119]. IL-1β also activates extracellular signal-regulated kinases, upregulates matrix metalloproteinase (MMP)-9 and disrupts TJ proteins [120, 121]. In addition, cytokines such as TNF-α, IL-6, and IL-12 can lead to the degradation of TJ proteins (occludin, claudin-5, ZO proteins), resulting in impaired BBB permeability [122, 123]. Concomitant with inflammatory damage to the BBB, the extravasation of immune cells through the BBB increases, resulting in increased SARS-CoV-2 viral particles, as well as proinflammatory cytokines, in the brain parenchyma. Exposure of astrocytes to viral particles and proinflammatory mediators triggers the activation of cytokines and the production of vascular endothelial growth factor (VEGF) in these cells [124, 125]. VEGF in brain capillary endothelial cells breaks down TJ proteins by activating the phosphoinositide 3 (PI3)-kinase and AKT signalling pathways and by upregulating MMP-9 protein levels, resulting in BBB leakage [126–128]. Increased secretion of proinflammatory cytokines associated with COVID-19 impairs BBB integrity and accelerates SARS-CoV-2 entry into the brain parenchyma [99, 122, 129] (Fig. 3).
In addition, virus-infected leukocytes spread into the blood circulation and extravasate into the brain parenchyma with other immune cells, and this may be another route for the virus to enter the CNS. Infected leukocytes with neurotropic viruses, such as human immunodeficiency virus (HIV) and West Nile virus (WNV), can infiltrate the brain through the vasculature, meninges, and choroid plexus, and this mechanism is known as the “Trojan horse” [130, 131]. SARS-CoV-2 likely also uses this mechanism to invade the CNS by infecting ACE2-expressing leukocytes. This evidence, combined with the systemic inflammatory and hypoxic conditions in COVID-19, shows that there is increased leukocyte infiltration through the BBB during infection [118, 132], which reinforces this pathway for SARS-CoV-2 to invade nerves (Fig. 3). Experience from cohort observations suggests that persistent systemic inflammation during COVID-19 infection is associated with subsequent cognitive decline [133] and leads to persistent electroencephalography (EEG) changes and hippocampal atrophy [134].
Therefore, in cognitive impairment related to COVID-19, attention should be given to the impact of proinflammatory factors on the body. SARS-CoV-2 infection causes proinflammatory cytokines to activate specific signalling cascades and increase BBB leakage by impairing TJ proteins assembly and expression levels, which in turn allows SARS-CoV-2, peripheral immune cells and other molecules to enter the CNS, thereby exacerbating brain damage.
SARS-CoV-2 infection leads to coagulopathy, promotes TJ disruption and increases BBB permeability
Acute viral infections, including SARS-CoV-2, have been reported to increase the risk of ischaemic stroke [135, 136]. The severity of COVID-19 is positively correlated with the risk of stroke [51]. Coagulation is frequently impaired in COVID-19 patients, resulting in a common hypercoagulable state in patients, and may be related to the incidence of stroke [137–139]. In two COVID-19 cohorts, the incidence of ischaemic stroke secondary to thromboembolic complications was 1.6% [140] and 2.5% [141]. Critically ill patients infected with SARS-CoV-2 often exhibit elevated d-dimer levels and severe thrombocytopenia, which may increase the probability of cerebrovascular events [95, 142]. Han et al. [143] conducted a prospective retrospective analysis of coagulation data from 94 patients with confirmed COVID-19 and found that d-dimer, fibrin/fibrinogen degradation products and fibrinogen levels were significantly higher in all SARS-CoV-2-infected cases than in the healthy control group.
Using an adult rat model of intraventricular haemorrhage, Liu et al. [144] found that thrombin can disrupt the BBB through a molecule that activates the phosphorylation of Src kinase by its protease-activated receptor. Src family members can increase BBB permeability by phosphorylating MMPs and TJ proteins [145, 146] and upregulating VEGF [147]. In addition, it was shown in vascular endothelial experiments that fibrinogen can damage endothelial cell integrity by disrupting TJ proteins bound to actin filaments [148]. Increased actin formation may lead to cellular stiffness, retraction of actin filaments, and widening of interendothelial junctions, thereby disrupting endothelial cell integrity [149, 150]. By the intraventricular injection of endogenous tissue plasminogen activator (tPA), Yepes et al. [151] found a rapid dose-dependent increase in vascular permeability. Consistent with the features of thrombotic microangiopathy, coagulation factors were elevated in COVID-19 patients with abnormal mental status [138]. Computed tomography (CT) images of a patient with COVID-19 and necrotizing haemorrhagic encephalitis showed symmetrical hypodensity in the bilateral medial thalamus, and MRI confirmed haemorrhagic lesions in the bilateral thalamus, subinsula, and medial temporal lobes [152]. These studies suggest that ischaemia-induced increases in endothelial permeability involve a cascade of events in which the thrombin, fibrinogen and plasmin systems are major players.
In summary, abnormalities in the coagulation system caused by SARS-CoV-2 infection may increase the permeability of the BBB and increase the entry of SARS-CoV-2 into the brain parenchyma by disrupting TJ proteins (Fig. 3). Invasion of vascular endothelial cells by SARS-CoV-2 activates a thrombotic and inflammatory cascade leading to capillary occlusion. For example, brain structures that manage cognition, such as the hippocampus, temporal lobe, and thalamus, are involved, resulting in ischaemia and hypoxia damage to nerve cells nourished by these capillaries, which can promote the occurrence of vascular cognitive impairment.
Lung injury caused by SARS-CoV-2 leads to hypoxia increasing BBB permeability
Patients with COVID-19 frequently experience severe hypoxia due to respiratory distress, putting them at risk for hypoxia-related encephalopathy [153, 154]. An autopsy analysis of the brains of COVID-19 subjects revealed a very high incidence of acute hypoxic injury [155]. Respiratory failure from lung damage can lead to severe hypoxia in the brain [156]. Neurons rely on blood vessels to provide oxygen and nutrients. When brain tissue is continuously hypoxic, it will eventually lead to irreversible damage to neurons [157]. Consistent with hypoxic brain injury, postmortem studies of COVID-19 have demonstrated neuronal damage in regions of the neocortex, hippocampus, and cerebellum [155, 158, 159]. Autopsy studies have shown that hypoxia can lead to oligodendrocyte death and extensive gliosis [160]. Numerous in vitro and in vivo studies have shown that hypoxia leads to BBB disruption, which may be a trigger for subsequent CNS disease [161].
Chen et al. [132] established an in vitro BBB model by coculturing mouse brain microvascular endothelial cells and astrocytes and found that hypoxia reduces ZO protein expression and induces ZO protein phosphorylation. Furthermore, using primary bovine brain microvascular endothelial cells, Mark et al. [162] found that hypoxia resulted in a 2.6-fold increase in actin rearrangement and the paracellular permeability marker [14C] sucrose. These findings are consistent with previous reports showing increased permeability of brain capillary endothelial cells after 2 h to 48 h of hypoxia treatment [163, 164]. Notably, the expression of occludin and ZO proteins increased, while the protein expression or localization of claudin-1 was almost unchanged after hypoxia or reoxygenation [162]. This suggests that claudin may not be involved in the hypoxia-induced changes in paracellular permeability. The hypoxia-induced increase in paracellular permeability of brain capillary endothelial cells may be associated with altered actin distribution and the loss of TJ proteins (Fig. 3). Conditions such as hypoxia, encephalitis, and stroke are known to produce long-term or even permanent neurocognitive impairment [165]. Therefore, some patients with COVID-19 are expected to develop long-term neurocognitive sequelae after the acute disease has resolved. In general, the chronic cognitive sequelae of ischaemia and hypoxia can range from mild attention and memory impairment to general cognitive decline and dementia and even coma.
Conclusions
Growing evidence suggested that survivors of COVID-19 suffer from neurological involvement. The brain is undoubtedly one of the targets of COVID-19. The exact pathophysiology of CNS infection is currently still speculative but appears to be related to a range of processes, including neuroinvasion and the effects of systemic infection consequences, both of which trigger BBB dysfunction, neuroinflammation, ischaemia and hypoxia and thus lead to secondary infections and brain dysfunction. The infection mechanism of COVID-19 in the brain may be related to the high-density expression of ACE2 receptors in the brain and other organ tissues and the entry of the virus into the brain through the olfactory nerve, trigeminal nerve, optic nerve and vague nerve pathways. Another blood-borne route is also possible, which involves viruses crossing the BBB. Mechanisms by which SARS-CoV-2 interacts with the BBB may lead to the neurological dysfunction that is associated with SARS-CoV-2-induced COVID-19. Recent information suggests that SARS-CoV-2 is able to infect CNS cells, especially the brain microvascular endothelial cells of the BBB. The effects of SARS-CoV-2 on the CNS may cause acute and long-term changes in the nervous system or could exacerbate existing neurological diseases or symptoms. Therefore, neuroinvasion and BBB dysfunction may be potential pathways that promote SARS-CoV-2 entry into the CNS and may contribute to the cognitive impairment observed during disease progression.
Notably, it is important for SARS-CoV-2 models to reliably test the molecular and functional consequences of infection and drug treatment strategies via the establishment of a high paracellular tightness in vitro that is comparable to physiological conditions in vivo. Currently, the link between BBB leakage and cognitive decline is poorly understood, and more research is needed to further define this link. Furthermore, COVID-19 will continue to affect the health of the body long after the epidemic ends, so continuous assessment of an individual's susceptibility to cognitive decline and dementia in the future will be important to improve patients’ quality of life later in life. Although it is too early to elucidate the long-term side effects of SARS-CoV-2 infection, growing evidence pointed that SARS-CoV-2 may lead to permanent sequelae of the CNS, including cognitive decline. However, whether SARS-CoV-2 could enter the brain and replicate in the brain parenchyma, whether it has neuroinvasive capabilities, should be explored in future. In summary, with the advent of the post-epidemic era, the subsequent brain damage caused by SARS-CoV-2 will become a clinical symptom and social problem that cannot be ignored. The exploration of the mechanism on cognitive impairment in patients with COVID-19 and the early intervention will improve patient's life quality in future.
Acknowledgements
Not applicable.
Abbreviations
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- BBB
Blood–brain barrier
- NVUs
Neurovascular units
- CNS
Central nervous system
- TJ
Tight junction
- JAMs
Junctional adhesion molecules
- MRI
Magnetic resonance imaging
- Aβ
Amyloid-beta
- IL-6
Interleukin-6
- TNF-α
Tumour necrosis factor-α
- ACE2
Angiotensin-converting enzyme 2
- TMPRSS2
Transmembrane protease serine 2
- NRP1
Neuropilin-1
- MMP
Matrix metalloproteinase
- VEGF
Vascular endothelial growth factor
- NO
Nitric oxide
- PI3
Phosphoinositide 3
- HIV
Human immunodeficiency virus
- WNV
West Nile virus
- EEG
Electroencephalography
- tPA
Tissue plasminogen activator
- CT
Computed tomography
Author contributions
YC wrote the review and designed the figures. WY critically revised the manuscript. FC instructed the manuscript and designed the figures. LC formulated the original idea, revised and approved the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82071190 and No. 82101269) to Lili Cui.
Availability of data and materials
This review does not contain any analysable data. All authors cited in this paper are publicly available.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanting Chen and Wenren Yang contributed equally to this work.
References
- 1.Tadj A, Lahbib SSM. Our overall current knowledge of Covid 19: an overview. Microbes Infect Chemother. 2021;1:e1262. doi: 10.54034/mic.e1262. [DOI] [Google Scholar]
- 2.COVID-19 Map. Johns Hopkins coronavirus resource center. 2022. https://coronavirus.jhu.edu/map.html. Accessed 23 June 2022.
- 3.Kucharski AJ, Russell TW, Diamond C, Liu Y, Edmunds J, Funk S, Eggo RM. Centre for mathematical modelling of infectious diseases C-wg: early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis. 2020;20:553–558. doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 6.Sivan M, Taylor S. NICE guideline on long covid. BMJ. 2020;371:m4938. doi: 10.1136/bmj.m4938. [DOI] [PubMed] [Google Scholar]
- 7.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77:1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pleasure SJ, Green AJ, Josephson SA. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection: neurologists move to the frontlines. JAMA Neurol. 2020;77:679–680. doi: 10.1001/jamaneurol.2020.1065. [DOI] [PubMed] [Google Scholar]
- 9.Chou SH, Beghi E, Helbok R, Moro E, Sampson J, Altamirano V, Mainali S, Bassetti C, Suarez JI, McNett M, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19—a report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw Open. 2021;4:e2112131. doi: 10.1001/jamanetworkopen.2021.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langen UH, Ayloo S, Gu C. Development and cell biology of the blood–brain barrier. Annu Rev Cell Dev Biol. 2019;35:591–613. doi: 10.1146/annurev-cellbio-100617-062608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daneman R, Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott NJ. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood–brain barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segarra M, Aburto MR, Hefendehl J, Acker-Palmer A. Neurovascular interactions in the nervous system. Annu Rev Cell Dev Biol. 2019;35:615–635. doi: 10.1146/annurev-cellbio-100818-125142. [DOI] [PubMed] [Google Scholar]
- 16.Paredes I, Himmels P, Ruiz de Almodovar C. Neurovascular communication during CNS development. Dev Cell. 2018;45:10–32. doi: 10.1016/j.devcel.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Greene C, Campbell M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers. 2016;4:e1138017. doi: 10.1080/21688370.2015.1138017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zlokovic BV. The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 20.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsivgoulis G, Palaiodimou L, Katsanos AH, Caso V, Kohrmann M, Molina C, Cordonnier C, Fischer U, Kelly P, Sharma VK, et al. Neurological manifestations and implications of COVID-19 pandemic. Ther Adv Neurol Disord. 2020;13:1756286420932036. doi: 10.1177/1756286420932036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahase E. Covid-19: one in three has neurological or psychiatric condition diagnosed after Covid infection, study finds. BMJ. 2021;373:n908. doi: 10.1136/bmj.n908. [DOI] [PubMed] [Google Scholar]
- 25.Miskowiak KW, Johnsen S, Sattler SM, Nielsen S, Kunalan K, Rungby J, Lapperre T, Porsberg CM. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crivelli L, Palmer K, Calandri I, Guekht A, Beghi E, Carroll W, Frontera J, Garcia-Azorin D, Westenberg E, Winkler AS, et al. Changes in cognitive functioning after COVID-19: a systematic review and meta-analysis. Alzheimers Dement. 2022;18:1047–1066. doi: 10.1002/alz.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ermis U, Rust MI, Bungenberg J, Costa A, Dreher M, Balfanz P, Marx G, Wiesmann M, Reetz K, Tauber SC, Schulz JB. Neurological symptoms in COVID-19: a cross-sectional monocentric study of hospitalized patients. Neurol Res Pract. 2021;3:17. doi: 10.1186/s42466-021-00116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosp JA, Dressing A, Blazhenets G, Bormann T, Rau A, Schwabenland M, Thurow J, Wagner D, Waller C, Niesen WD, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144:1263–1276. doi: 10.1093/brain/awab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendez R, Balanza-Martinez V, Luperdi SC, Estrada I, Latorre A, Gonzalez-Jimenez P, Feced L, Bouzas L, Yepez K, Ferrando A, et al. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J Intern Med. 2021;290:621–631. doi: 10.1111/joim.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazza MG, Palladini M, De Lorenzo R, Magnaghi C, Poletti S, Furlan R, Ciceri F, Rovere-Querini P, Benedetti F, COVID-19 BioB Outpatient Clinic Study group Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu YH, Chen Y, Wang QH, Wang LR, Jiang L, Yang Y, Chen X, Li Y, Cen Y, Xu C, et al. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol. 2022;79:509–517. doi: 10.1001/jamaneurol.2022.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kremer S, Lersy F, de Seze J, Ferre JC, Maamar A, Carsin-Nicol B, Collange O, Bonneville F, Adam G, Martin-Blondel G, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297:E242–E251. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Espindola OM, Brandao CO, Gomes YCP, Siqueira M, Soares CN, Lima M, Leite A, Torezani G, Araujo AQC, Silva MTT. Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development. Int J Infect Dis. 2021;102:155–162. doi: 10.1016/j.ijid.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang HY, Li XL, Yan ZR, Sun XP, Han J, Zhang BW. Potential neurological symptoms of COVID-19. Ther Adv Neurol Disord. 2020;13:1756286420917830. doi: 10.1177/1756286420917830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naughton SX, Raval U, Pasinetti GM. Potential novel role of COVID-19 in Alzheimer’s disease and preventative mitigation strategies. J Alzheimers Dis. 2020;76:21–25. doi: 10.3233/JAD-200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benameur K, Agarwal A, Auld SC, Butters MP, Webster AS, Ozturk T, Howell JC, Bassit LC, Velasquez A, Schinazi RF, et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis. 2020;26:2016–2021. doi: 10.3201/eid2609.202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, Men D, Huang Q, Liu Y, Yang B, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farhadian S, Glick LR, Vogels CBF, Thomas J, Chiarella J, Casanovas-Massana A, Zhou J, Odio C, Vijayakumar P, Geng B, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. Res Sq. 2020 doi: 10.21203/rs.3.rs-28583/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tancredi V, D'Arcangelo G, Grassi F, Tarroni P, Palmieri G, Santoni A, Eusebi F. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci Lett. 1992;146:176–178. doi: 10.1016/0304-3940(92)90071-e. [DOI] [PubMed] [Google Scholar]
- 41.Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. FASEB J. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- 42.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 43.van den Pol AN. Viral infections in the developing and mature brain. Trends Neurosci. 2006;29:398–406. doi: 10.1016/j.tins.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Matschke J, Lutgehetmann M, Hagel C, Sperhake JP, Schroder AS, Edler C, Mushumba H, Fitzek A, Allweiss L, Dandri M, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brunink S, Greuel S, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 46.Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, Lu P, Weizman OE, Liu F, Dai Y, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. bioRxiv. 2020 doi: 10.1101/2020.06.25.169946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrauwen EJ, Herfst S, Leijten LM, van Run P, Bestebroer TM, Linster M, Bodewes R, Kreijtz JH, Rimmelzwaan GF, Osterhaus AD, et al. The multibasic cleavage site in H5N1 virus is critical for systemic spread along the olfactory and hematogenous routes in ferrets. J Virol. 2012;86:3975–3984. doi: 10.1128/JVI.06828-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada M, Bingham J, Payne J, Rookes J, Lowther S, Haining J, Robinson R, Johnson D, Middleton D. Multiple routes of invasion of wild-type Clade 1 highly pathogenic avian influenza H5N1 virus into the central nervous system (CNS) after intranasal exposure in ferrets. Acta Neuropathol. 2012;124:505–516. doi: 10.1007/s00401-012-1010-8. [DOI] [PubMed] [Google Scholar]
- 49.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dube M, Talbot PJ. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019 doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lima M, Siokas V, Aloizou AM, Liampas I, Mentis AA, Tsouris Z, Papadimitriou A, Mitsias PD, Tsatsakis A, Bogdanos DP, et al. Unraveling the possible routes of SARS-COV-2 invasion into the central nervous system. Curr Treat Options Neurol. 2020;22:37. doi: 10.1007/s11940-020-00647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deana C, Bagatto D. Severe stroke in patients admitted to intensive care unit after COVID-19 infection: pictorial essay of a case series. Brain Hemorrhages. 2022;3:29–35. doi: 10.1016/j.hest.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235:277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 53.Bryche B, Fretaud M, Saint-Albin Deliot A, Galloux M, Sedano L, Langevin C, Descamps D, Rameix-Welti MA, Eleouet JF, Le Goffic R, Meunier N. Respiratory syncytial virus tropism for olfactory sensory neurons in mice. J Neurochem. 2020;155:137–153. doi: 10.1111/jnc.14936. [DOI] [PubMed] [Google Scholar]
- 54.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, Wu K. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand. 2020;142:14–22. doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vilensky JA. The neglected cranial nerve: nervus terminalis (cranial nerve N) Clin Anat. 2014;27:46–53. doi: 10.1002/ca.22130. [DOI] [PubMed] [Google Scholar]
- 58.Briguglio M, Bona A, Porta M, Dell'Osso B, Pregliasco FE, Banfi G. Disentangling the hypothesis of host dysosmia and SARS-CoV-2: the bait symptom that hides neglected neurophysiological routes. Front Physiol. 2020;11:671. doi: 10.3389/fphys.2020.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sia SF, Yan LM, Chin AWH, Fung K, Choy KT, Wong AYL, Kaewpreedee P, Perera R, Poon LLM, Nicholls JM, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bryche B, St Albin A, Murri S, Lacote S, Pulido C, ArGouilh M, Lesellier S, Servat A, Wasniewski M, Picard-Meyer E, et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun. 2020;89:579–586. doi: 10.1016/j.bbi.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci. 2020;11:1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanageswaran N, Demond M, Nagel M, Schreiner BS, Baumgart S, Scholz P, Altmuller J, Becker C, Doerner JF, Conrad H, et al. Deep sequencing of the murine olfactory receptor neuron transcriptome. PLoS ONE. 2015;10:e0113170. doi: 10.1371/journal.pone.0113170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, Chance R, Macaulay IC, Chou HJ, Fletcher RB, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020 doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bilinska K, Butowt R. Anosmia in COVID-19: a bumpy road to establishing a cellular mechanism. ACS Chem Neurosci. 2020;11:2152–2155. doi: 10.1021/acschemneuro.0c00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gupta K, Mohanty SK, Mittal A, Kalra S, Kumar S, Mishra T, Ahuja J, Sengupta D, Ahuja G. The cellular basis of loss of smell in 2019-nCoV-infected individuals. Brief Bioinform. 2021;22:873–881. doi: 10.1093/bib/bbaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Satarker S, Nampoothiri M. Involvement of the nervous system in COVID-19: the bell should toll in the brain. Life Sci. 2020;262:118568. doi: 10.1016/j.lfs.2020.118568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finger TE, Bottger B. Peripheral peptidergic fibers of the trigeminal nerve in the olfactory bulb of the rat. J Comp Neurol. 1993;334:117–124. doi: 10.1002/cne.903340110. [DOI] [PubMed] [Google Scholar]
- 69.Iwasaki T, Itamura S, Nishimura H, Sato Y, Tashiro M, Hashikawa T, Kurata T. Productive infection in the murine central nervous system with avian influenza virus A (H5N1) after intranasal inoculation. Acta Neuropathol. 2004;108:485–492. doi: 10.1007/s00401-004-0909-0. [DOI] [PubMed] [Google Scholar]
- 70.Plourde JR, Pyles JA, Layton RC, Vaughan SE, Tipper JL, Harrod KS. Neurovirulence of H5N1 infection in ferrets is mediated by multifocal replication in distinct permissive neuronal cell regions. PLoS ONE. 2012;7:e46605. doi: 10.1371/journal.pone.0046605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395:e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olofsson S, Kumlin U, Dimock K, Arnberg N. Avian influenza and sialic acid receptors: more than meets the eye? Lancet Infect Dis. 2005;5:184–188. doi: 10.1016/S1473-3099(05)01311-3. [DOI] [PubMed] [Google Scholar]
- 73.Casalino G, Monaco G, Di Sarro PP, David A, Scialdone A. Coronavirus disease 2019 presenting with conjunctivitis as the first symptom. Eye (Lond) 2020;34:1235–1236. doi: 10.1038/s41433-020-0909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cavalleri M, Brambati M, Starace V, Capone L, Nadin F, Pederzolli M, Gorgoni F, Di Biase C, Corbelli E, Battista M, et al. Ocular features and associated systemic findings in SARS-CoV-2 infection. Ocul Immunol Inflamm. 2020;28:916–921. doi: 10.1080/09273948.2020.1781198. [DOI] [PubMed] [Google Scholar]
- 75.Hui KP, Peiris M, Nicholls JM, Chan MC. SARS-CoV-2 infection in conjunctival tissue—Authors’ reply. Lancet Respir Med. 2020;8:e58. doi: 10.1016/S2213-2600(20)30273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu YC, Ang M, Ong HS, Wong TY, Mehta JS. SARS-CoV-2 infection in conjunctival tissue. Lancet Respir Med. 2020;8:e57. doi: 10.1016/S2213-2600(20)30272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf. 2020;18:537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roehrich H, Yuan C, Hou JH. Immunohistochemical study of SARS-CoV-2 viral entry factors in the cornea and ocular surface. Cornea. 2020;39:1556–1562. doi: 10.1097/ICO.0000000000002509. [DOI] [PubMed] [Google Scholar]
- 79.Qing H, Li Z, Yang Z, Shi M, Huang Z, Song J, Song Z. The possibility of COVID-19 transmission from eye to nose. Acta Ophthalmol. 2020;98:e388. doi: 10.1111/aos.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deng W, Bao L, Gao H, Xiang Z, Qu Y, Song Z, Gong S, Liu J, Liu J, Yu P, et al. Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques. Nat Commun. 2020;11:4400. doi: 10.1038/s41467-020-18149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gregorczyk M, Roskal-Walek J. Ocular symptoms in SARS-CoV-2 infection. Pol Merkur Lekarski. 2022;50:86–93. [PubMed] [Google Scholar]
- 82.Finsterer J, Scorza FA, Scorza CA, Fiorini AC. SARS-CoV-2 impairs vision. J Neuroophthalmol. 2021;41:166–169. doi: 10.1097/WNO.0000000000001273. [DOI] [PubMed] [Google Scholar]
- 83.de Figueiredo CS, Raony I, Giestal-de-Araujo E. SARS-CoV-2 targeting the retina: host–virus interaction and possible mechanisms of viral tropism. Ocul Immunol Inflamm. 2020;28:1301–1304. doi: 10.1080/09273948.2020.1799037. [DOI] [PubMed] [Google Scholar]
- 84.Arima M, Cui D, Kimura T, Sonoda KH, Ishibashi T, Matsuda S, Ikeda E. Basigin can be a therapeutic target to restore the retinal vascular barrier function in the mouse model of diabetic retinopathy. Sci Rep. 2016;6:38445. doi: 10.1038/srep38445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aranyo J, Bazan V, Llados G, Dominguez MJ, Bisbal F, Massanella M, Sarrias A, Adelino R, Riverola A, Paredes R, et al. Inappropriate sinus tachycardia in post-COVID-19 syndrome. Sci Rep. 2022;12:298. doi: 10.1038/s41598-021-03831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruchaya PJ, Speretta GF, Blanch GT, Li H, Sumners C, Menani JV, Colombari E, Colombari DS. Overexpression of AT2R in the solitary-vagal complex improves baroreflex in the spontaneously hypertensive rat. Neuropeptides. 2016;60:29–36. doi: 10.1016/j.npep.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 87.Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- 88.Hadziefendic S, Haxhiu MA. CNS innervation of vagal preganglionic neurons controlling peripheral airways: a transneuronal labeling study using pseudorabies virus. J Auton Nerv Syst. 1999;76:135–145. doi: 10.1016/s0165-1838(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 89.Rangon CM, Krantic S, Moyse E, Fougere B. The vagal autonomic pathway of COVID-19 at the crossroad of Alzheimer’s disease and aging: a review of knowledge. J Alzheimers Dis Rep. 2020;4:537–551. doi: 10.3233/ADR-200273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H, Wang Y, Ji M, Pei F, Zhao Q, Zhou Y, Hong Y, Han S, Wang J, Wang Q, et al. Transmission routes analysis of SARS-CoV-2: a systematic review and case report. Front Cell Dev Biol. 2020;8:618. doi: 10.3389/fcell.2020.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dahiya DS, Kichloo A, Albosta M, Pagad S, Wani F. Gastrointestinal implications in COVID-19. J Investig Med. 2020;68:1397–1401. doi: 10.1136/jim-2020-001559. [DOI] [PubMed] [Google Scholar]
- 93.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baig AM. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther. 2020;26:499–501. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, Xie G, Lin S, Wang R, Yang X, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bellon M, Schweblin C, Lambeng N, Cherpillod P, Vazquez J, Lalive PH, Schibler M, Deffert C. Cerebrospinal fluid features in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcription polymerase chain reaction (RT-PCR) positive patients. Clin Infect Dis. 2021;73:e3102–e3105. doi: 10.1093/cid/ciaa1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haidar MA, Jourdi H, Haj Hassan Z, Ashekyan O, Fardoun M, Wehbe Z, Maaliki D, Wehbe M, Mondello S, Abdelhady S, et al. Neurological and neuropsychological changes associated with SARS-CoV-2 infection: new observations. New Mech Neurosci. 2021 doi: 10.1177/1073858420984106. [DOI] [PubMed] [Google Scholar]
- 100.McQuaid C, Brady M, Deane R. SARS-CoV-2: is there neuroinvasion? Fluids Barriers CNS. 2021;18:32. doi: 10.1186/s12987-021-00267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, Bullock TA, McGary HM, Khan JA, Razmpour R, Hale JF, Galie PA, Potula R, et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol Dis. 2020;146:105131. doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nath A, Smith B. Neurological complications of COVID-19: from bridesmaid to bride. Arq Neuropsiquiatr. 2020;78:459–460. doi: 10.1590/0004-282X20200121. [DOI] [PubMed] [Google Scholar]
- 104.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(271–280):e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen R, Wang K, Yu J, Howard D, French L, Chen Z, Wen C, Xu Z. the spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol. 2020;11:573095. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol. 2020;16:636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya T, Anastasina M, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deffner F, Scharr M, Klingenstein S, Klingenstein M, Milazzo A, Scherer S, Wagner A, Hirt B, Mack AF, Neckel PH. Histological evidence for the enteric nervous system and the choroid plexus as alternative routes of neuroinvasion by SARS-CoV2. Front Neuroanat. 2020;14:596439. doi: 10.3389/fnana.2020.596439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bleau C, Filliol A, Samson M, Lamontagne L. Brain invasion by mouse hepatitis virus depends on impairment of tight junctions and beta interferon production in brain microvascular endothelial cells. J Virol. 2015;89:9896–9908. doi: 10.1128/JVI.01501-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robinson CP, Busl KM. Neurologic manifestations of severe respiratory viral contagions. Crit Care Explor. 2020;2:e0107. doi: 10.1097/CCE.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reynolds JL, Mahajan SD. SARS-COV2 alters blood brain barrier integrity contributing to neuro-inflammation. J Neuroimmune Pharmacol. 2021;16:4–6. doi: 10.1007/s11481-020-09975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9:23. doi: 10.1186/2045-8118-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krasemann S, Haferkamp U, Pfefferle S, Woo MS, Heinrich F, Schweizer M, Appelt-Menzel A, Cubukova A, Barenberg J, Leu J, et al. The blood–brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Rep. 2022;17:307–320. doi: 10.1016/j.stemcr.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alquisiras-Burgos I, Peralta-Arrieta I, Alonso-Palomares LA, Zacapala-Gomez AE, Salmeron-Barcenas EG, Aguilera P. Neurological complications associated with the blood–brain barrier damage induced by the inflammatory response during SARS-CoV-2 infection. Mol Neurobiol. 2021;58:520–535. doi: 10.1007/s12035-020-02134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Almutairi MM, Gong C, Xu YG, Chang Y, Shi H. Factors controlling permeability of the blood–brain barrier. Cell Mol Life Sci. 2016;73:57–77. doi: 10.1007/s00018-015-2050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.RalayRanaivo H, Zunich SM, Choi N, Hodge JN, Wainwright MS. Mild stretch-induced injury increases susceptibility to interleukin-1beta-induced release of matrix metalloproteinase-9 from astrocytes. J Neurotrauma. 2011;28:1757–1766. doi: 10.1089/neu.2011.1799. [DOI] [PubMed] [Google Scholar]
- 121.Yang F, Zhao K, Zhang X, Zhang J, Xu B. ATP induces disruption of tight junction proteins via IL-1 beta-dependent MMP-9 activation of human blood–brain barrier in vitro. Neural Plast. 2016;2016:8928530. doi: 10.1155/2016/8928530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Erickson MA, Rhea EM, Knopp RC, Banks WA. Interactions of SARS-CoV-2 with the blood–brain barrier. Int J Mol Sci. 2021 doi: 10.3390/ijms22052681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang X, Hussain B, Chang J. Peripheral inflammation and blood–brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 2021;27:36–47. doi: 10.1111/cns.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Erickson MA, Banks WA. Neuroimmune axes of the blood–brain barriers and blood–brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol Rev. 2018;70:278–314. doi: 10.1124/pr.117.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tremblay ME, Madore C, Bordeleau M, Tian L, Verkhratsky A. Neuropathobiology of COVID-19: the role for glia. Front Cell Neurosci. 2020;14:592214. doi: 10.3389/fncel.2020.592214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kilic E, Kilic U, Wang Y, Bassetti CL, Marti HH, Hermann DM. The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF's neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB J. 2006;20:1185–1187. doi: 10.1096/fj.05-4829fje. [DOI] [PubMed] [Google Scholar]
- 127.Miao Z, Dong Y, Fang W, Shang D, Liu D, Zhang K, Li B, Chen YH. VEGF increases paracellular permeability in brain endothelial cells via upregulation of EphA2. Anat Rec (Hoboken) 2014;297:964–972. doi: 10.1002/ar.22878. [DOI] [PubMed] [Google Scholar]
- 128.Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, Divoux D, Mackenzie ET, Bernaudin M, Roussel S, Petit E. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005;25:1491–1504. doi: 10.1038/sj.jcbfm.9600148. [DOI] [PubMed] [Google Scholar]
- 129.Alam SB, Willows S, Kulka M, Sandhu JK. Severe acute respiratory syndrome coronavirus 2 may be an underappreciated pathogen of the central nervous system. Eur J Neurol. 2020;27:2348–2360. doi: 10.1111/ene.14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev. 2013;254:102–113. doi: 10.1111/imr.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Paul AM, Acharya D, Duty L, Thompson EA, Le L, Stokic DS, Leis AA, Bai F. Osteopontin facilitates West Nile virus neuroinvasion via neutrophil “Trojan horse” transport. Sci Rep. 2017;7:4722. doi: 10.1038/s41598-017-04839-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen W, Ju XZ, Lu Y, Ding XW, Miao CH, Chen JW. Propofol improved hypoxia-impaired integrity of blood–brain barrier via modulating the expression and phosphorylation of zonula occludens-1. CNS Neurosci Ther. 2019;25:704–713. doi: 10.1111/cns.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Semmler A, Widmann CN, Okulla T, Urbach H, Kaiser M, Widman G, Mormann F, Weide J, Fliessbach K, Hoeft A, et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry. 2013;84:62–69. doi: 10.1136/jnnp-2012-302883. [DOI] [PubMed] [Google Scholar]
- 135.Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, Schenck EJ, Goyal P, Bruce SS, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nicholson P, Alshafai L, Krings T. Neuroimaging findings in patients with COVID-19. AJNR Am J Neuroradiol. 2020;41:1380–1383. doi: 10.3174/ajnr.A6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, Humphries F, Jager HR, Losseff NA, Perry RJ, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Han H, Yang L, Liu R, Liu F, Wu KL, Li J, Liu XH, Zhu CL. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 144.Liu DZ, Ander BP, Xu H, Shen Y, Kaur P, Deng W, Sharp FR. Blood–brain barrier breakdown and repair by Src after thrombin-induced injury. Ann Neurol. 2010;67:526–533. doi: 10.1002/ana.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guerrero J, Santibanez JF, Gonzalez A, Martinez J. EGF receptor transactivation by urokinase receptor stimulus through a mechanism involving Src and matrix metalloproteinases. Exp Cell Res. 2004;292:201–208. doi: 10.1016/j.yexcr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 146.Kale G, Naren AP, Sheth P, Rao RK. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem Biophys Res Commun. 2003;302:324–329. doi: 10.1016/s0006-291x(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 147.Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med. 2001;7:222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- 148.Tyagi N, Roberts AM, Dean WL, Tyagi SC, Lominadze D. Fibrinogen induces endothelial cell permeability. Mol Cell Biochem. 2008;307:13–22. doi: 10.1007/s11010-007-9579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ehringer WD, Yamany S, Steier K, Farag A, Roisen FJ, Dozier A, Miller FN. Quantitative image analysis of F-actin in endothelial cells. Microcirculation. 1999;6:291–303. doi: 10.1111/j.1549-8719.1999.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 150.Trepat X, Grabulosa M, Buscemi L, Rico F, Farre R, Navajas D. Thrombin and histamine induce stiffening of alveolar epithelial cells. J Appl Physiol. 1985;2005(98):1567–1574. doi: 10.1152/japplphysiol.00925.2004. [DOI] [PubMed] [Google Scholar]
- 151.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood–brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ladopoulos T, Zand R, Shahjouei S, Chang JJ, Motte J, Charles James J, Katsanos AH, Kerro A, Farahmand G, Vaghefi Far A, et al. COVID-19: Neuroimaging features of a pandemic. J Neuroimaging. 2021;31:228–243. doi: 10.1111/jon.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9:e016219. doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF, Jr, Sabeti P. Neuropathological features of Covid-19. N Engl J Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kantonen J, Mahzabin S, Mayranpaa MI, Tynninen O, Paetau A, Andersson N, Sajantila A, Vapalahti O, Carpen O, Kekalainen E, et al. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020;30:1012–1016. doi: 10.1111/bpa.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 161.Yang Y, Rosenberg GA. Blood–brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H1485–1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol. 1999;276:C812–820. doi: 10.1152/ajpcell.1999.276.4.C812. [DOI] [PubMed] [Google Scholar]
- 164.Abbruscato TJ, Davis TP. Combination of hypoxia/aglycemia compromises in vitro blood–brain barrier integrity. J Pharmacol Exp Ther. 1999;289:668–675. [PubMed] [Google Scholar]
- 165.Li J, Long X, Zhang Q, Fang X, Fang F, Lv X, Zhang D, Sun Y, Li N, Hu S, et al. Emerging evidence for neuropsycho-consequences of COVID-19. Curr Neuropharmacol. 2021;19:92–96. doi: 10.2174/1570159X18666200507085335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review does not contain any analysable data. All authors cited in this paper are publicly available.