Abstract
Introduction:
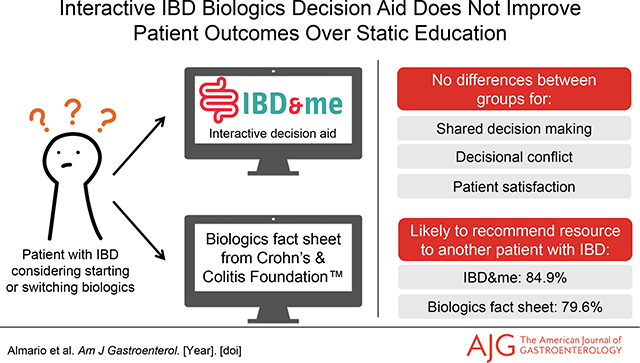
To support shared decision making (SDM) between patients and providers surrounding biologic treatments, we created IBD&me (ibdandme.org)—a freely available, unbranded, interactive decision aid. We performed a multicenter comparative effectiveness trial comparing the impact of IBD&me on SDM vs. a biologics fact sheet developed by the Crohn’s and Colitis Foundation.
Methods:
We enrolled patients with inflammatory bowel disease (IBD) being seen at a clinic within IBD Qorus—a multicenter adult IBD learning health system—between 3/5/2019–5/14/2021. Eligible patients included those with recent IBD-related symptoms who reported that they wanted to discuss biologics with their provider during their upcoming visit. Patients were randomized 1:1 using stratified block randomization and received an email one week before their visit inviting them to review either IBD&me or a fact sheet. The primary outcome was patient perception of SDM as measured by the 9-Item SDM Questionnaire (0–100 scale; higher=better); the Student t-test was used to compare outcomes between arms.
Results:
Overall, 152 patients were randomized (biologics fact sheet—75; IBD&me—77); most patients had Crohn’s disease (66.4%) and were biologic experienced (82.9%). No differences were seen between groups with respect to SDM (fact sheet—72.6±25.6; IBD&me—75.0±20.8; p=0.57). Most patients stated they would be likely to recommend the fact sheet (79.6%) or IBD&me (84.9%; p=0.48) to another patient with IBD.
Conclusion:
No differences in outcomes were seen between IBD&me and the biologics fact sheet in this comparative effectiveness study; patients reported high satisfaction with both resources. Further study, particularly among biologic naïve patients, is needed to determine the utility of interactive components to IBD decision aids.
Graphical Abstract
INTRODUCTION
While biologic medications are effective in treating moderate-to-severe inflammatory bowel disease (IBD), there remains a lack of comparative effectiveness data among biologics, resulting in care pathways that endorse several first-line therapies (1–6). Adding to the complexity is the substantial variation among biologics in mechanism of action, mode of administration, and side effects, among other attributes (7–9). As a result, particularly during brief clinical visits, it is often difficult for patients to navigate the array of treatment options with their physicians and to choose a therapy that aligns with their treatment preferences. Moreover, the decision-making process will become more complex when additional drugs are approved.
We previously conducted a study using conjoint analysis—a technique that can help determine how patients make complex decisions under conditions of uncertainty—that found different approaches to biologic decision making between patients with ulcerative colitis and Crohn’s disease (10). Moreover, across conditions we found divergent individual patient preferences when selecting among biologics. In attempting to identify predictors of individual patient choice, we found that demographic and IBD characteristics were largely unhelpful, which emphasizes the personalized nature of decision making (10).
Because of the highly individualized nature of decision making in IBD, along with healthcare’s increased emphasis on shared decision making (SDM) between patients and providers, it is critical for clinicians to identify what matters most to patients when choosing among therapies (11, 12). Yet, it can be challenging to accurately establish a patient’s unique preferences in the context of a brief clinic visit because no two IBD patients are alike.
To address this gap, we created IBD&me (ibdandme.org)—a freely available, unbranded, interactive, conjoint analysis-based decision aid that aims to enhance SDM between IBD patients and their providers when navigating among the available biologics (10). IBD&me enables patients to learn about the benefits and risks of the different therapies, and then guides them through conjoint analysis exercises to explore and quantify their treatment preferences. Once patients complete the exercises, the website generates a unique personalized report that describes what matters most to them when selecting a biologic, which can subsequently be shared with the doctor. We hypothesized that use of IBD&me and its tailored reports can facilitate a more informed discussion in clinic between patients and clinicians, improve SDM, and better align medical care with patients’ unique preferences. To test this hypothesis, we conducted a pragmatic, multicenter comparative effectiveness study comparing the impact of IBD&me on patient perceptions of SDM vs. a biologics fact sheet developed by the Crohn’s and Colitis Foundation.
METHODS
Trial Design
We conducted a pragmatic, multicenter comparative effectiveness study among patients of member clinics within IBD Qorus—a multicenter adult IBD learning health system (13, 14)—between March 5, 2019 to May 14, 2021. This study was approved by the Cedars-Sinai Institutional Review Board (Pro53056) and Supplementary File 1 includes the study protocol. Consented patients were randomized 1:1 using stratified block randomization and received an email one week before their visit inviting them to review either IBD&me or a biologics fact sheet. The randomization lists were computer-generated with permuted blocks of variable sizes. The random allocation sequence was provided by an independent researcher with no involvement in the study. While physicians and patients could not be blinded due to the study design and nature of the interventions, they were blinded to the specific study outcomes. Moreover, all email invitations, study instructions, and screening and outcomes assessments were fully automated via REDCap (15). Finally, study investigators and the statistician were blinded to the intervention assignments until data collection was complete.
Participants
Eligible study participants included those who met the following criteria: (i) age ≥18 years; (ii) has Crohn’s disease, ulcerative colitis, or indeterminate colitis or IBD unclassified; (iii) had IBD-related symptoms (abdominal pain, bowel incontinence, diarrhea, hematochezia, joint pain, nausea/vomiting, urgency, other symptom) within 30 days of screening; (iv) had a visit with their IBD doctor at least seven days and no later than 60 days following screening; (v) wanted to discuss biologic therapies for controlling their IBD with their provider at the next clinic visit (note: those who stated they were unsure on whether they wanted to discuss biologics with their physician remained eligible for the study).
Study sites included member clinics within IBD Qorus (13, 14); while the Cedars-Sinai IBD Center (Los Angeles, CA) is part of IBD Qorus, it was treated as a separate “site” for this study as we had direct access to patient lists for participating physicians. Starting on March 5, 2019, patients scheduled to be seen at Cedars-Sinai were sent an email one week prior to their visit inviting them to participate in the study and to access the screening survey. Patients being seen in the other IBD Qorus sites were sent batched study invitation emails on February 3, 2020 and November 9, 2020 (recruitment was paused for nine months due to the COVID-19 pandemic). Notably, we decided a priori to first start recruitment at Cedars-Sinai to identify any potential implementation issues prior to initiating recruitment at IBD Qorus.
Patients who clicked the screening survey link in the study email invitation were directed to REDCap where they answered questions assessing their eligibility. Afterwards, patients who were deemed eligible were presented with the online consent form and those who agreed to participate provided their digital signature via REDCap for both the study consent and Health Insurance Portability and Accountability Act of 1996 disclosure agreement.
Interventions
Consented patients were randomized 1:1 to IBD&me (interactive online decision aid) or a biologics fact sheet (static education) in PDF form. Our research group previously developed IBD&me based on formative research examining patients’ knowledge, attitudes, and beliefs on biologic therapies (10, 16) and it was iteratively updated based on patient usability testing (17). IBD&me begins with an educational section where patients learn about important concepts related to biologics. Topics that are covered include descriptions of what biologics are, how and when they should be taken, and the potential risks. Afterwards, the site guides patients through adaptive conjoint analysis exercises that present side-by-side comparisons of hypothetical biologic medications and asks respondents to select their preferred options (Figure 1). Once the exercises are completed, IBD&me generates a personalized report with biologic medication attributes rank-ordered by their importance to the patient during the decision-making process (Figure 1). The patient is encouraged to share the report with the doctor to facilitate SDM.
FIGURE 1.
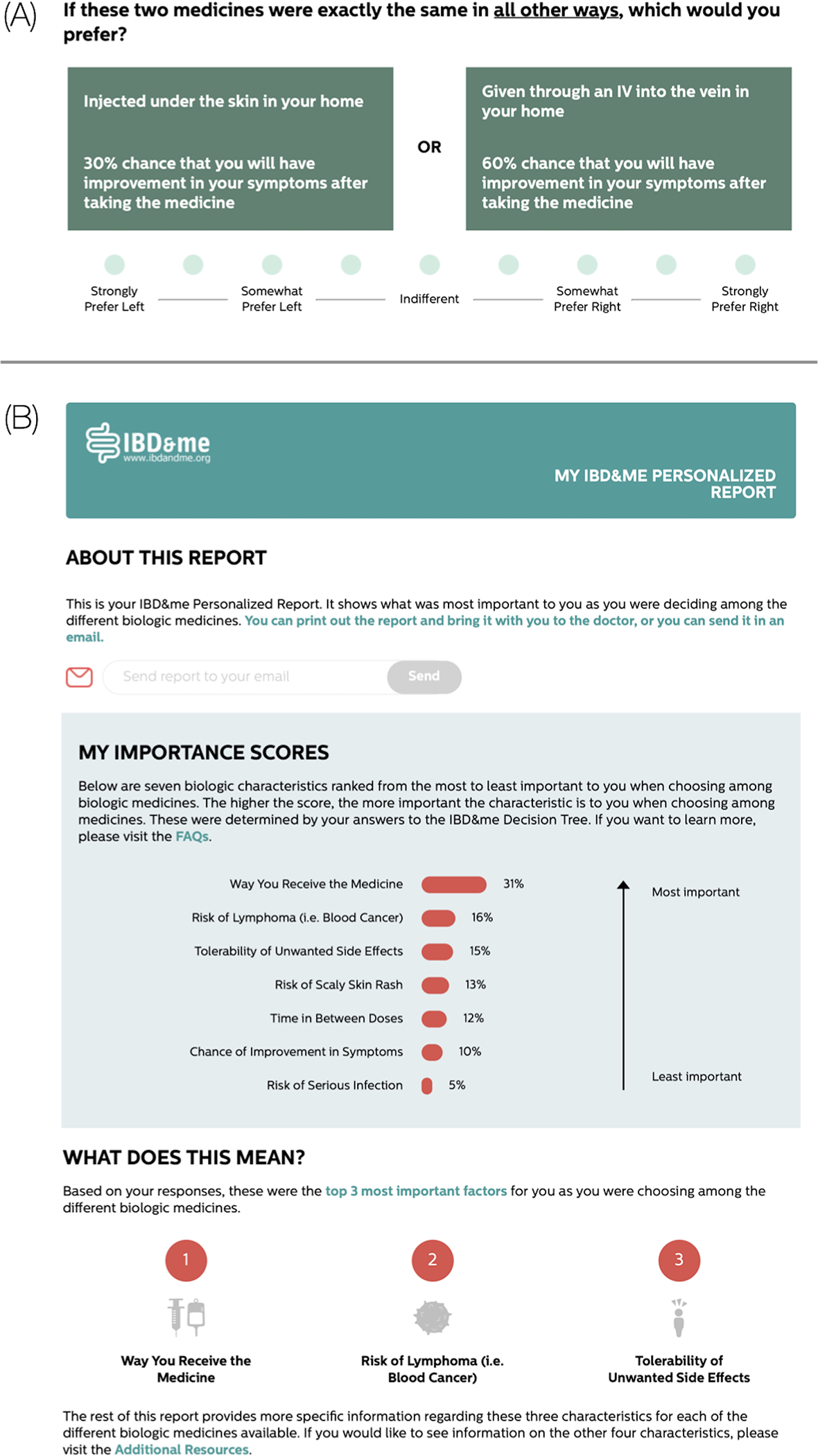
Sample IBD&me conjoint analysis exercise and personalized report. (A) The conjoint analysis exercises show patients side-by-side comparisons of hypothetical biologic medications and asks respondents to select the preferred profile. In the example, a patient weighs how the medicine is given with the chances of symptom improvement with taking the medicine. (B) The personalized report rank orders the biologic medication attributes that were most important to the patient when selecting among the different options in the conjoint analysis exercises. Here, mode of administration was the most important factor in the patient’s decision making.
The biologics fact sheet, developed by the Crohn’s and Colitis Foundation, is an evidence-based and clearly presented overview of IBD biologic therapies, but without an interactive, personalized component (see Supplementary File 2 for PDF used in the trial; the latest fact sheet can be found here at https://www.crohnscolitisfoundation.org/sites/default/files/2021-06/Biologics 6.2021.pdf). The fact sheet includes information on the different biologics, their mechanisms of action, and frequency of dosing. It also describes the risks and special considerations for such therapies.
Seven days prior to patients’ clinic visits, they were sent automated emails via REDCap with instructions directing them to go through their assigned resource (IBD&me or fact sheet). Emails were also sent to patients the day before their visit reminding them to navigate through their respective resource as well as to print and bring the IBD&me personalized report or fact sheet to their visit if they thought it may be helpful for facilitating discussions with their doctor.
Outcomes
Outcome survey questionnaires were sent to patients via REDCap the day after their clinic visit and two months later. Our primary outcome was patient perceptions of SDM as measured by the validated 9-Item Shared Decision-Making Questionnaire (SDM-Q-9) one day after the clinic visit (18). The SDM-Q-9 assesses patients’ level of agreement with nine statements related to decision making during the visit: disclosure that a decision needs to be made; formulation of equality of partners; presentation of treatment options; informing on the benefits and risks of the options; investigation of patient’s understanding and expectations; identification of both parties’ preferences; negotiation; reaching a shared decision; arrangement of follow-up (18). See Supplementary File 1 for the actual SDM-Q-9 items. Secondary outcomes assessed the day after the visit included patient perceptions of decisional conflict (informed and values clarity subscales of the Decisional Conflict Scale [DCS]) (19) and patient satisfaction (Patient Satisfaction Questionnaire Short Form [PSQ-18] domains relating to communication, general satisfaction, interpersonal manner, and time spent with the doctor) (20).
Secondary outcomes assessed two months after the visit included change compared to baseline in IBD disease control and quality of life as assessed by the IBD-Control-8 and IBD-Control-VAS (21). We also determined the proportion of patients who started or switched biologics, had an IBD-related emergency department visit, or had surgery for their IBD since their initial visit. To maximize response rates, patients received an honorarium after completing the following steps: $10—consenting to participate in the study; $30—completing the follow-up questionnaire sent one day after the clinic visit; $10—completing the 2-month follow-up questionnaire.
Covariates
We collected baseline data on sociodemographics including age, sex, race/ethnicity, marital status, educational attainment, total annual household income, employment status, and health insurance coverage. We also measured IBD clinical characteristics including duration of IBD, history of prior intestinal surgery for IBD, and IBD medication use.
Sample Size
While the SDM-Q-9 is a widely used, validated measure, we are unaware of data measuring the minimally clinically important difference on the scale. Therefore, the sample size was calculated to achieve a moderate effect size of 0.5 (a half standard deviation difference, which generally correlates with the minimally clinically important difference) in mean SDM-Q-9 scores between groups (22, 23). Assuming a two-tailed 5% significance level with 80% power, the minimum sample size needed to show an effect size of 0.5 is 64 patients per group. Using an estimated 15% dropout rate, we aimed for a sample size of 152 patients, or 76 patients per arm.
Statistical Analyses
Statistical analyses were performed using R (version 4.0.1; R Core Team, 2020) and a two-tailed p-value <.05 was considered statistically significant. Our primary analysis was performed from the intention-to-treat (ITT) perspective. We also performed the analyses using the modified ITT (patient acknowledged that they received the email with instructions to review the fact sheet or IBD&me before their clinic visit) and per protocol (patient stated that they reviewed the fact sheet or IBD&me prior to the visit) perspectives. Patients’ characteristics and outcomes were summarized by study arms using frequencies and percentages for the categorical variables and means and standard deviations for the continuous variables. Bivariate associations between the study arms and outcomes were compared using the X2 test (or Fisher’s exact test when needed) and Student t-test for proportions and means, respectively.
RESULTS
Participants
Recruitment and data collection for the multicenter comparative effectiveness study occurred between March 5, 2019 to May 14, 2021. Figure 2 presents the CONSORT flow diagram, and 152 patients were randomized (fact sheet—75; IBD&me—77). Table 1 presents the demographics of the study cohort; no differences were seen between arms (all p>.05). Most enrolled patients were female, non-Hispanic White, college educated, and employed/student. In Table 2, we depict the IBD clinical characteristics and medication use at the time of randomization; no differences were seen between groups (all p>.05). Approximately two-thirds of participants had Crohn’s disease. About half of patients were diagnosed with IBD more than 10 years ago and more than four-fifths were biologic experienced. Slightly more than half of patients received care from the Cedars-Sinai IBD Center while the other half were seen at other IBD Qorus sites; patients from 24 out of 30 IBD Qorus sites participated.
FIGURE 2.
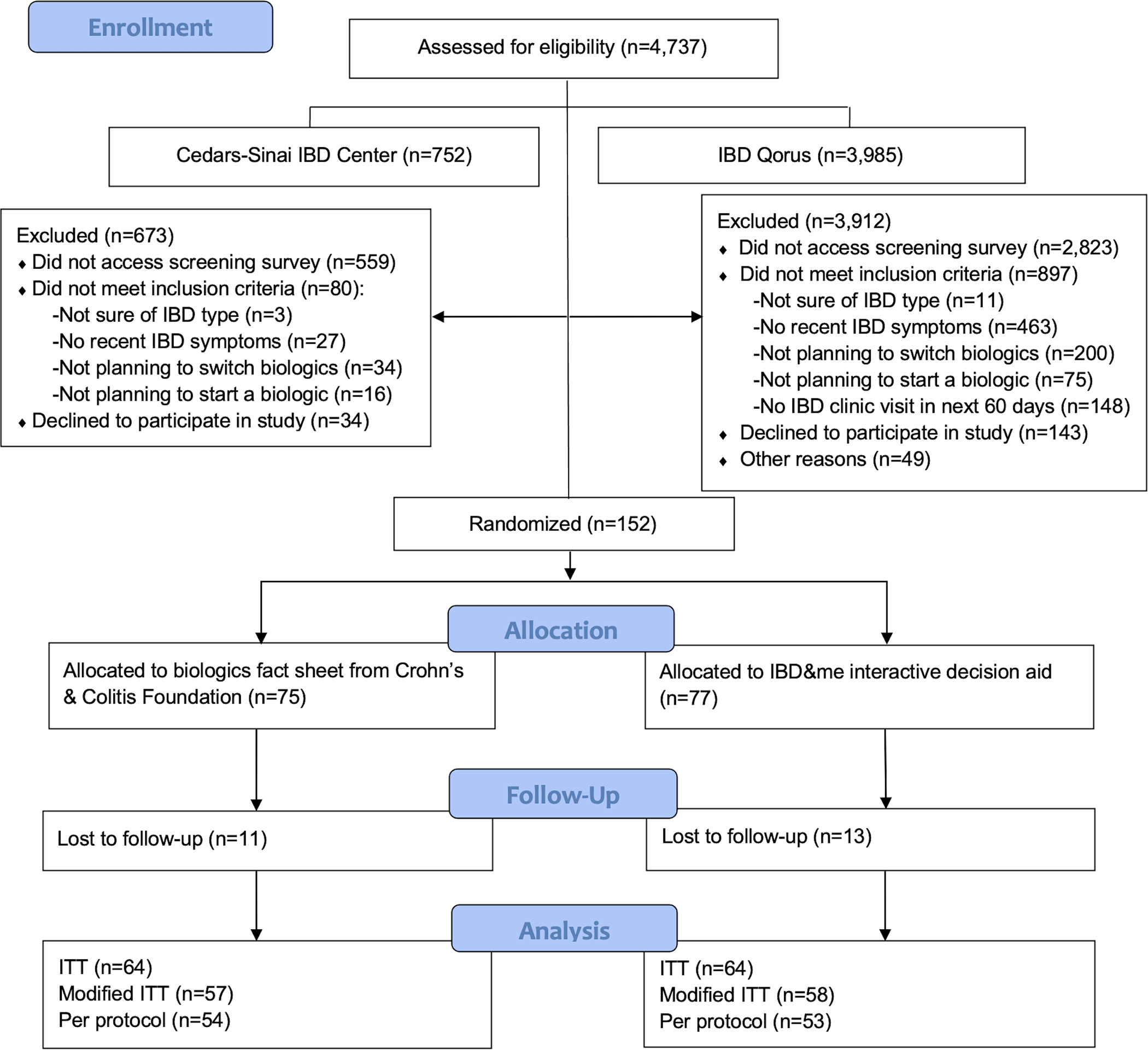
CONSORT flow diagram. IBD, inflammatory bowel disease; ITT, intention-to-treat.
TABLE 1.
Demographics of the study cohort (N=152).
| Variable | Biologics fact sheet (n=75) |
IBD&me interactive decision aid (n=77) |
| Age: | ||
| 18–30y | 26 (34.7%) | 18 (23.4%) |
| 31–40y | 16 (21.3%) | 26 (33.8%) |
| ≥41y | 33 (44.0%) | 33 (42.9%) |
| Sex: | ||
| Male | 23 (30.7%) | 22 (28.6%) |
| Female | 52 (69.3%) | 55 (71.4%) |
| Race/ethnicity: | ||
| Non-Hispanic White only | 65 (86.7%) | 68 (88.3%) |
| Non-Hispanic Black only | 3 (4.0%) | 1 (1.3%) |
| Hispanic | 2 (2.7%) | 5 (6.5%) |
| Non-Hispanic Asian only | 1 (1.3%) | 0 (0.0%) |
| Other/multiracial | 4 (5.3%) | 3 (3.9%) |
| Marital status: | ||
| Single, widowed, divorced, or separated | 33 (44.0%) | 41 (53.2%) |
| Married, domestic partnership, or long-term relationship | 42 (56.0%) | 36 (46.8%) |
| Educational attainment: | ||
| High school degree or less | 3 (4.0%) | 6 (7.8%) |
| Some college education | 23 (30.7%) | 14 (18.2%) |
| College degree | 49 (65.3%) | 57 (74.0%) |
| Total annual household income, $: | ||
| ≤50,000 | 20 (26.7%) | 20 (26.0%) |
| 50,001–100,000 | 18 (24.0%) | 27 (35.1%) |
| ≥100,001 | 27 (36.0%) | 22 (28.6%) |
| Prefer not to say | 10 (13.3%) | 8 (10.4%) |
| Employment status: | ||
| Unemployed, on disability, on leave of absence from work, retired, homemaker, or other | 21 (28.0%) | 19 (24.7%) |
| Employed or student | 54 (72.0%) | 58 (75.3%) |
| Has health insurance | 75 (100.0%) | 77 (100.0%) |
Data are presented as n (% of column).
TABLE 2.
IBD clinical characteristics at time of randomization (N=152).
| Variable | Biologics fact sheet (n=75) | IBD&me interactive decision aid (n=77) |
|---|---|---|
| Site of care: | ||
| Cedars-Sinai IBD Center | 40 (53.3%) | 39 (50.6%) |
| IBD Qorus site | 35 (46.7%) | 38 (49.4%) |
| Type of IBD: | ||
| Crohn’s disease | 52 (69.3%) | 49 (63.6%) |
| Ulcerative colitis | 22 (29.3%) | 24 (31.2%) |
| Indeterminate colitis | 1 (1.3%) | 4 (5.2%) |
| IBD duration: | ||
| <1 year | 5 (6.7%) | 5 (6.5%) |
| 1–5 years | 16 (21.3%) | 19 (24.7%) |
| 5–10 years | 20 (26.7%) | 11 (14.3%) |
| >10 years | 34 (45.3%) | 42 (54.5%) |
| Had prior intestinal surgery for IBD | 29 (38.7%) | 24 (31.2%) |
| Current IBD medication use: | ||
| Rectal mesalamines or steroids | 4 (5.3%) | 8 (10.4%) |
| Oral mesalamines or sulfasalazine | 13 (17.3%) | 9 (11.7%) |
| Oral steroid or budesonide | 13 (17.3%) | 13 (16.9%) |
| Azathioprine or 6-mercaptopurine | 11 (14.7%) | 7 (9.1%) |
| Tofacitinib | 1 (1.3%) | 1 (1.3%) |
| Biologic | 53 (70.7%) | 52 (67.5%) |
| Other | 9 (12.0%) | 16 (20.8%) |
| Not currently taking a medicine for IBD | 8 (10.7%) | 8 (10.4%) |
| Biologic experience: | ||
| Biologic naïve | 12 (16.0%) | 14 (18.2%) |
| Prior use of biologic but not currently using one | 10 (13.3%) | 11 (14.3%) |
| Currently using biologic | 53 (70.7%) | 52 (67.5%) |
| IBD-Control-8 score (0–16; higher=better control) | 7.7 ± 5.3 | 7.9 ± 4.5 |
| IBD-Control-VAS score (0–100; higher=better control) | 54.1 ± 24.0 | 54.8 ± 23.6 |
IBD, inflammatory bowel disease; VAS, visual analogue scale.
Data are presented as n (% of column) or mean ± SD.
Uptake of the Interventions
Table 3 presents data on use of the fact sheet and IBD&me interactive decision aid; uptake was not statistically different between groups. Most patients acknowledged that they received the email with instructions to review their assigned resource. Of those who confirmed receiving the email, the vast majority reviewed the fact sheet or IBD&me prior to their clinic visit. Most patients who reviewed the fact sheet (79.6%) or IBD&me (84.9%) were likely to recommend it to another patient with IBD.
TABLE 3.
Patient use of biologics fact sheet or IBD&me interactive decision aid prior to clinic visit (n=128a).
| Variable | Biologics fact sheet | IBD&me interactive decision aid | P-value |
|---|---|---|---|
| Patient acknowledged receiving email with instructions to review biologics fact sheet or IBD&me | 57/64 (89.1%) | 58/64 (90.6%) | 0.77 |
| Patient reviewed biologics fact sheetb or IBD&mec prior to clinic visit | 54/57 (94.7%) | 53/58 (91.4%) | 0.72 |
| Patient brought biologics fact sheet or IBD&me personalized report to clinic visit | 31/54 (57.4%) | 21/53 (39.6%) | 0.07 |
| Likely to recommend the biologics fact sheet or IBD&me to another patient with IBD | 43/54 (79.6%) | 45/53 (84.9%) | 0.48 |
Data are presented as n (%). The X2 test or Fisher’s exact test were used to compare groups.
Twenty-four people who were randomized did not complete the outcome assessments one day after the clinic visit.
Determined by a “Yes” response to “Did you read this fact sheet about biologics before your visit with your doctor?”
Determined by a “Yes” response to “Did you look at the IBD&me website before your visit with your doctor?”
Among individuals who reviewed their resource, 57.4% and 39.6% reported that they brought the fact sheet or IBD&me personalized report, respectively, to their visit with the doctor. Patients in the fact sheet group (n=31) reported using it in the following manner: showed fact sheet to doctor and they discussed it during the visit—8 (25.8%); showed fact sheet to doctor but they did not discuss it during the visit—2 (6.5%); did not show fact sheet to doctor during visit—13 (41.9%); other—8 (25.8%). Those in the IBD&me arm (n=21) stated the following: showed report to doctor and they discussed it during the visit—5 (23.8%); showed report to doctor but they did not discuss it during the visit—4 (19.0%); did not show report to doctor during visit—6 (28.6%); other—6 (28.6%).
Among the 32 patients who reviewed IBD&me but did not bring the personalized report to their visit, they reported the following reasons: did not want to share report with doctor—14 (43.8%); forgot to bring report to visit—11 (34.4%); verbally relayed report results to doctor during visit—2 (6.3%); could not share report with doctor due to virtual visit logistics—2 (6.3%); emailed report to doctor before visit—1 (3.1%); IBD&me site did not email report to patient—1 (3.1%); visited IBD&me but left before receiving the report—1 (3.1%).
Outcomes Assessed One Day After Clinic Visit
Among the 152 randomized patients, 128 (fact sheet—64; IBD&me—64) individuals completed the outcomes assessment surveys administered one day after the visit. All patients from Cedars-Sinai (n=68) were seen before the COVID-19 pandemic while 47 (78.3%) of the 60 patients from the other IBD Qorus sites were seen during the pandemic.
For the primary outcome of patient perception of SDM, no differences in SDM-Q-9 scores were seen between the fact sheet and IBD&me arms (Table 4). Even when analyzing the data from the modified ITT (patient acknowledged receiving the email with instructions to review the fact sheet or IBD&me before their clinic visit) and per protocol (patient confirmed that they reviewed their assigned resource prior to the clinic visit) perspectives, no differences were seen in SDM-Q-9 scores between groups. Similarly, we did not observe differences between groups with respect to decisional conflict (DCS) or patient satisfaction (PSQ-18) scores when examining the data from the ITT, modified ITT, and per protocol perspectives.
TABLE 4.
Outcomes assessed one day after the clinic visit.
| Variable | Biologics fact sheet | IBD&me interactive decision aid | P-value |
|---|---|---|---|
| ITT—patient randomized to biologics fact sheet or IBD&me group | n=64 | n=64 | |
| Shared decision making, SDM-Q-9 (0–100; higher=better) | 72.6 ± 25.6 | 75.0 ± 20.8 | 0.57 |
| Decisional conflict, DCS (0–100; lower=less decisional conflict) | |||
| Informed subscale | 21.4 ± 17.2 | 28.3 ± 26.1 | 0.08 |
| Values clarity subscale | 23.3 ± 21.5 | 25.1 ± 26.3 | 0.67 |
| Patient satisfaction, PSQ-18 (20–100; higher=better) | |||
| General satisfaction subscale | 85.2 ± 17.7 | 82.5 ± 16.5 | 0.38 |
| Interpersonal manner subscale | 91.4 ± 12.2 | 93.3 ± 10.5 | 0.35 |
| Communication subscale | 88.9 ± 15.3 | 88.4 ± 14.2 | 0.86 |
| Time spent with doctor subscale | 83.4 ± 17.0 | 83.9 ± 15.5 | 0.87 |
| Modified ITT—patient acknowledged receiving email with instructions to review biologics fact sheet or IBD&me | n=57 | n=58 | |
| Shared decision making, SDM-Q-9 (0–100; higher=better) | 75.2 ± 23.7 | 76.2 ± 21.2 | 0.80 |
| Decisional conflict, DCS (0–100; lower= less decisional conflict) | |||
| Informed subscale | 20.9 ± 17.3 | 28.0 ± 26.6 | 0.09 |
| Values clarity subscale | 23.0 ± 21.3 | 23.6 ± 26.0 | 0.89 |
| Patient satisfaction, PSQ-18 (20–100; higher=better) | |||
| General satisfaction subscale | 87.2 ± 15.6 | 82.9 ± 16.4 | 0.16 |
| Interpersonal manner subscale | 93.2 ± 10.4 | 94.0 ± 9.5 | 0.66 |
| Communication subscale | 90.5 ± 13.0 | 89.5 ± 13.3 | 0.67 |
| Time spent with doctor subscale | 84.9 ± 15.3 | 84.0 ± 15.7 | 0.74 |
| Per protocol—patient reviewed biologics fact sheet or IBD&me prior to clinic visit | n=54 | n=53 | |
| Shared decision making, SDM-Q-9 (0–100; higher=better) | 74.4 ± 24.0 | 76.3 ± 22.0 | 0.67 |
| Decisional conflict, DCS (0–100; lower=better=less decisional conflict) | |||
| Informed subscale | 20.2 ± 17.3 | 27.2 ± 27.5 | 0.12 |
| Values clarity subscale | 22.8 ± 21.9 | 21.1 ± 25.4 | 0.70 |
| Patient satisfaction, PSQ-18 (20–100; higher=better) | |||
| General satisfaction subscale | 86.9 ± 15.8 | 83.0 ± 16.7 | 0.23 |
| Interpersonal manner subscale | 92.8 ± 10.5 | 93.6 ± 9.8 | 0.68 |
| Communication subscale | 90.4 ± 13.2 | 89.2 ± 13.7 | 0.67 |
| Time spent with doctor subscale | 84.8 ± 15.5 | 84.0 ± 16.2 | 0.78 |
DCS, Decisional Conflict Scale; ITT, intention-to-treat; PSQ-18, Patient Satisfaction Questionnaire Short Form; SDM-Q-9, 9-Item Shared Decision-Making Questionnaire.
Data are presented as mean ± SD. The Student t-test was used to compare groups.
Subgroup Analyses
Supplementary Table 1 shows data for those who brought the fact sheet (n=31) or IBD&me personalized report (n=21) to their clinic visit; no differences were seen between groups with respect to SDM, decisional conflict, or patient satisfaction scores. In Supplementary Table 2, we present data among biologic naïve patients (fact sheet—9; IBD&me—11). No differences in SDM or patient satisfaction scores were seen between groups. However, from the ITT and modified ITT perspectives, individuals assigned to the fact sheet had less decisional conflict when compared to those in the IBD&me arm.
We also performed subgroup analyses among patients seen at Cedars-Sinai and those receiving care at other IBD Qorus sites; patients from the former were all seen before the pandemic while most patients from the latter were seen during the pandemic. Among individuals seen at Cedars-Sinai (Supplementary Table 3), those assigned to the fact sheet had less decisional conflict when compared to those in the IBD&me arm in ITT analysis. No differences were seen among the remaining outcomes. For patients from the other IBD Qorus sites (Supplementary Table 4), those in the IBD&me group had higher ratings for their physicians’ interpersonal manner vs. the fact sheet group when using the ITT perspective; no differences were seen for the other outcomes.
Outcomes Assessed Two Months After Clinic Visit
Supplementary Table 5 shows outcomes assessed two months after the clinic visit. From the ITT view, no differences were seen between the fact sheet and IBD&me arms in change in IBD-Control-8 and IBD-Control-VAS scores compared to baseline. Moreover, no statistical differences were seen between groups with respect to the proportion of patients who started or switched biologics, had an IBD-related emergency department visit, or had IBD-related surgery since their initial visit. Findings were similar when analyzing the data from the modified ITT and per protocol perspectives.
DISCUSSION
In this multicenter comparative effectiveness study testing an interactive online decision aid vs. static educational material, we largely found no differences in SDM, decisional conflict, or patient satisfaction between groups. Our data suggest that interactive components to IBD decision aids, although often well received by patients, may not be necessary to enhance SDM and that the resources needed to develop and maintain such components may yield diminishing returns. While there are no comparable studies in IBD, these findings are similar to those seen in the colorectal cancer screening literature. Jimbo and colleagues performed a randomized trial comparing a web-based decision aid that interactively assessed and clarified patients’ preferences for colorectal cancer screening tests to a decision aid with the same content but no interactive tools (24). While both arms improved screening uptake over baseline, the interactive decision aid did not increase screening over the non-interactive version (24). Similarly, a meta-analysis by Volk et al. found comparable screening rates between groups assigned to a decision aid vs. general colorectal cancer screening information (25). Thus, while decision aids increase participants’ knowledge, decrease decisional conflict, and improve patient-clinician communication (26), dedicating substantial resources to incorporate and maintain interactive components may be low yield; high-quality yet static educational material may be sufficient.
While additional research is needed to determine the utility of interactive decision aids in IBD, there are several other potential explanations for our “negative” findings. First, the COVID-19 pandemic occurred during the study and some patients recruited during this period may have had virtual visits with their providers rather than in-person encounters. It is possible that the IBD&me personalized report was less impactful during virtual visits and did not allow for the expected in-depth, patient-centered discussions. Second, due to logistical considerations, we randomized individuals rather than providers, which could have led to contamination. In other words, providers exposed to IBD&me personalized reports may have been more apt to engage in enhanced SDM discussions with all their patients, including those in the comparator group. Future trials could consider either a cluster randomized trial where physicians are the unit of randomization or a stepped wedge design to prevent contamination bias. Third, due to the pragmatic study design and diverse practice settings, IBD&me was not integrated into sites’ electronic health record systems and instead relied on patients to bring their personalized reports to the clinic visit. Only 39.6% of patients who reviewed IBD&me brought their report to the visit, thereby potentially muting the impact of the intervention; interestingly, 57.4% of patients who reviewed the fact sheet brought it to their visit which may relate to its branding from the Crohn’s & Colitis Foundation—a well-recognized and trusted source of IBD information. Yet, even if patients did not bring the personalized report or fact sheet to their visit, patients’ review of the content could have affected how they approached treatment discussions with their doctor in clinic. Nonetheless, future studies should connect decision aids to electronic health record systems in a manner that allows individual patients to seamlessly and efficiently send the report to their physician if so desired. Fourth, most patients enrolled in our study were biologic experienced and may have already been familiar with the content in the fact sheet and IBD&me. When restricting the analysis to only biologic naïve patients (n=20), no differences in SDM and patient satisfaction were seen between groups. However, patients in the fact sheet arm had lower decisional conflict scores vs. the IBD&me arm; this finding may be spurious given the very small sample size and perhaps related to multiple comparisons. Regardless, future studies assessing the impact of interactive decision aids over general information should consider focusing on biologic naïve patients. Finally, all providers were from centers with high levels of expertise in treating IBD and likely had considerable experience discussing the pros and cons of the biologic options with patients. While such experience does not necessarily translate to better SDM, we nevertheless may have encountered a “ceiling effect”; use of decisions aids among clinicians already adept at discussing IBD therapeutic options with patients may not offer incremental benefits. Additional research examining the impact of decision aids among providers with a broader range of experience in managing moderate-to-severe IBD is warranted.
Our study has strengths. First, we performed a multicenter comparative effectiveness study testing IBD&me vs. static education among a diverse number of sites across the US. Of the few IBD decision aids described in the literature, most have only undergone pilot testing and/or focused on specific situations (e.g., pregnancy and IBD, surgery and ulcerative colitis) (27–30). Further efforts to develop and validate effective IBD decision aids are sorely needed as the treatment of IBD will become more complex when additional drugs are approved. Second, we employed a pragmatic approach by not mandating how patients and providers should use the fact sheet and IBD&me personalized report. While we could have tested the efficacy of the interventions in a tightly controlled setting by mandating and monitoring how they were used, we instead opted to test the effectiveness of both resources in settings that resembled the “real world.”
This study also has limitations. First, due to budget constraints, we could not include a second control group without intervention; the incremental benefit on outcomes related to use of the fact sheet and IBD&me over usual care remains unclear and additional research is warranted. Second, we did not systematically assess what treatment decisions, if any, were made during the clinic visit; we instead focused on actual changes in treatments made within two months of the visit. Mixed-methods research is needed to assess how IBD decision aids affect treatment decision making at the point of care. Third, recruitment and delivery of the educational resources were only conducted via the Internet; our findings may not generalize to patients with IBD who are uncomfortable using computers or do not have Internet access. Fourth, all enrolled patients in our trial were insured and most were college graduates. It is unclear whether our results would extend to uninsured individuals receiving care at federally qualified health centers or those with lower educational attainment; this is worthy of further study. Finally, due to technical considerations and the multicenter study’s pragmatic design, we could not objectively track some process outcomes (e.g., opening of study email, clicking links to assigned resources, completion of certain components of the decision aid); we instead relied on patient self-report regarding uptake of the interventions. When feasible, future studies testing digital tools should incorporate methods to track such metrics for measuring and supporting implementation.
In summary, while patients reported high satisfaction with both IBD&me and the biologics fact sheet from the Crohn’s and Colitis Foundation, there were no differences in outcomes between groups. While our data suggest that interactive components to IBD decision aids may not be necessary to enhance SDM, further study is needed—particularly among biologic naïve patients—to determine the utility of interactive IBD decision aids.
Supplementary Material
STUDY HIGHLIGHTS.
WHAT IS KNOWN:
For patients with moderate-to-severe inflammatory bowel disease (IBD), care pathways endorse several first-line therapy options.
It can be difficult for patients to navigate the array of treatment options with their physicians and to choose a therapy that aligns with their unique treatment preferences.
To support shared decision making (SDM) between patients and providers surrounding biologic treatments, we created an online, interactive decision aid called IBD&me (ibdandme.org).
WHAT IS NEW HERE:
In a multicenter randomized comparative effectiveness study comparing IBD&me vs. a biologics fact sheet developed by the Crohn’s and Colitis Foundation, no difference was seen in patient perception of SDM between groups.
Further study is needed to determine the utility of interactive components to IBD decision aids.
ACKNOWLEDGMENTS
We thank the Crohn’s & Colitis Foundation’s IBD Qorus Learning Health System, and in particular Alandra S. Weaver and Ridhima Oberai, for facilitating patient recruitment through IBD Qorus.
FINANCIAL SUPPORT:
This study was funded by a grant from the American Gastroenterological Association Institute, Crohn’s & Colitis Foundation, and Pfizer Independent Grants for Learning & Change Program; the funders were not involved in the design or conduct of the study, collection, management, analysis or interpretation of the data, preparation, review, or approval of the manuscript, or in the decision to submit the manuscript for publication. IBD Qorus is an initiative of the Crohn’s & Colitis Foundation. IBD Qorus is made possible in part by the support of AbbVie, AMAG Pharmaceuticals, Eli Lilly, Helmsley Charitable Trust, Janssen Biotech, Inc., Luitpold Pharmaceuticals, Inc., Nephroceuticals LLC, Nestle Health Sciences, Pfizer, Inc., Takeda Pharmaceuticals U.S.A., Inc., and UCB/Ferring; these supporters had no involvement in the design or conduct of the study, collection, management, analysis or interpretation of the data, preparation, review, or approval of the manuscript, or in the decision to submit the manuscript for publication, nor did they provide direct funding to investigators for any aspect of this study. Dr. Almario was supported by an NIH Loan Repayment Program Award L30DK106734. Drs. Almario and Spiegel are supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
ABBREVIATIONS:
- DCS
Decisional Conflict Scale
- IBD
inflammatory bowel disease
- ITT
intention-to-treat
- PSQ-18
Patient Satisfaction Questionnaire Short Form
- SDM
shared decision making
- SDM-Q-9
9-Item Shared Decision-Making Questionnaire
Footnotes
POTENTIAL COMPETING INTERESTS:
• Christopher V. Almario, MD, MSHPM—Consultant: Arena Pharmaceuticals. Research support: funding from the Crohn’s & Colitis Foundation for work related to the IBD Qorus™ Learning Health System.
• Welmoed K. van Deen, PhD, MD—Consultant: Crohn’s and Colitis Foundation. Research support: funding from the Crohn’s & Colitis Foundation for work related to the IBD Qorus™ Learning Health System.
• Michelle Chen, MPH—No relevant disclosures to report.
• Rebecca Gale, MPH—No relevant disclosures to report.
• Stéphanie Sidorkiewicz, MD, PhD—No relevant disclosures to report.
• So Yung Choi, MS—No relevant disclosures to report.
• Nirupama Bonthala, MD—No relevant disclosures to report.
• Christina Ha, MD—Advisory board: Abbvie, Bristol Myers Squibb, Genentech, Lilly, InDex Pharmaceuticals, Janssen, Pfizer, Takeda; Consultant: Abbvie, Genentech, Janssen; Speakers bureau: Abbvie; Research support: Pfizer.
• Gaurav Syal, MD, MHDS—Research support: Pfizer Independent Grants for Learning & Change Program.
• Taylor Dupuy, BS—No relevant disclosures to report.
• Xiaoyu Liu, MPH—No relevant disclosures to report.
• Gil Y. Melmed, MD, MS—Consultant: Abbvie, Arena Pharmaceuticals, Boehringer-Ingelheim, Bristol-Meyers-Squibb/Celgene, Entasis, Janssen, Medtronic, Pfizer, Samsung Bioepis, Takeda, Techlab. Research support: Pfizer Independent Grants for Learning & Change Program.
• Brennan M.R. Spiegel, MD, MSHS—Research support: Pfizer Independent Grants for Learning & Change Program.
CLINICALTRIALS.GOV IDENTIFIER: NCT03695783
REFERENCES
- 1.Dassopoulos T, Cohen RD, Scherl EJ, et al. Ulcerative colitis care pathway. Gastroenterology 2015;149:238–45. [DOI] [PubMed] [Google Scholar]
- 2.Sandborn WJ. Crohn’s disease evaluation and treatment: clinical decision tool. Gastroenterology 2014;147:702–5. [DOI] [PubMed] [Google Scholar]
- 3.Feuerstein JD, Ho EY, Shmidt E, et al. AGA clinical practice guidelines on the medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease. Gastroenterology 2021;160:2496–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology 2020;158:1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 6.Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 7.Danese S, Vuitton L, Peyrin-Biroulet L. Biologic agents for IBD: practical insights. Nat Rev Gastroenterol Hepatol 2015;12:537–45. [DOI] [PubMed] [Google Scholar]
- 8.Dulai PS, Sandborn WJ. Next-Generation therapeutics for inflammatory bowel disease. Curr Gastroenterol Rep 2016;18:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 10.Almario CV, Keller MS, Chen M, et al. Optimizing selection of biologics in inflammatory bowel disease: development of an online patient decision aid using conjoint analysis. Am J Gastroenterol 2018;113:58–71. [DOI] [PubMed] [Google Scholar]
- 11.Siegel CA, Lofland JH, Naim A, et al. Novel statistical approach to determine inflammatory bowel disease: patients’ perspectives on shared decision making. Patient 2016;9:79–89. [DOI] [PubMed] [Google Scholar]
- 12.Siegel CA, Lofland JH, Naim A, et al. Gastroenterologists’ views of shared decision making for patients with inflammatory bowel disease. Dig Dis Sci 2015;60:2636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson LC, Melmed GY, Nelson EC, et al. Fostering collaboration through creation of an IBD learning health system. Am J Gastroenterol 2017;112:406–408. [DOI] [PubMed] [Google Scholar]
- 14.Melmed GY, Oliver B, Hou JK, et al. Quality of care program reduces unplanned health care utilization in patients with inflammatory bowel disease. Am J Gastroenterol 2021;116:2410–2418. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez B, Dailey F, Almario CV, et al. Patient understanding of the risks and benefits of biologic therapies in inflammatory bowel disease: insights from a large-scale analysis of social media platforms. Inflamm Bowel Dis 2017;23:1057–1064. [DOI] [PubMed] [Google Scholar]
- 17.Chen MS, Sidorkiewicz S, Conovitz S, et al. P010 Qualitative evaluation of patient perspectives regarding IBD&me (ibdandme.org), a novel online biologic decision aid for patients with inflammatory bowel disease. Inflam Bowel Dis 2019;25:S7–S7. [Google Scholar]
- 18.Kriston L, Scholl I, Holzel L, et al. The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ and Couns 2010;80:94–99. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making 1995;15:25–30. [DOI] [PubMed] [Google Scholar]
- 20.Marshall GN, Hays RD. The patient satisfaction questionnaire short-form (PSQ-18). In: RAND; Santa Monica, CA; 1994. [Google Scholar]
- 21.Bodger K, Ormerod C, Shackcloth D, et al. Development and validation of a rapid, generic measure of disease control from the patient’s perspective: the IBD-control questionnaire. Gut 2014;63:1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J A power primer. Psychological bulletin 1992;112:155. [DOI] [PubMed] [Google Scholar]
- 23.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41:582–92. [DOI] [PubMed] [Google Scholar]
- 24.Jimbo M, Sen A, Plegue MA, et al. Interactivity in a decision aid: findings from a decision aid to technologically enhance shared decision making RCT. Am J Prev Med 2019;57:77–86. [DOI] [PubMed] [Google Scholar]
- 25.Volk RJ, Linder SK, Lopez-Olivo MA, et al. Patient decision aids for colorectal cancer screening: a systematic review and meta-analysis. Am J Prev Med 2016;51:779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4:Cd001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker DM, Lee MJ, Folan AM, et al. Development and evaluation of a patient decision aid for patients considering ongoing medical or surgical treatment options for ulcerative colitis using a mixed-methods approach: protocol for DISCUSS study. BMJ Open 2020;10:e031845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohan JN, Ozanne EM, Sewell JL, et al. A novel decision aid for surgical patients with ulcerative colitis: results of a pilot study. Dis Colon Rectum 2016;59:520–8. [DOI] [PubMed] [Google Scholar]
- 29.Kim AH, Girgis A, De Cruz P, et al. Development and feasibility of a web-based decision aid for patients with ulcerative colitis: qualitative pilot study. J Med Internet Res 2021;23:e15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams AJ, Karimi N, Chari R, et al. Shared decision making in pregnancy in inflammatory bowel disease: design of a patient orientated decision aid. BMC Gastroenterol 2021;21:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


