Editor:
The abscopal effect, whereby localized treatment to 1 tumor results in destruction of other untreated sites, is a rare phenomenon, described primarily in case reports. In melanoma, by combining treatments that augment the immune response with interventions (e.g., external-beam radiotherapy) that produce tumor antigens and danger signals from cell death, the abscopal effect can be triggered in as many as 25% of patients (1). In the context of metastatic breast cancer (MBC), abscopal effects have been reported in preclinical studies but not in human patients. Here, we present a patient with MBC who was treated with transarterial radioembolization (TARE) and who had a complete response in liver and extrahepatic metastases. At our institution, case reports are exempt from Institutional Review Board review.
A 44-year-old premenopausal woman with estrogen receptor (ER)-positive, progesterone receptor-positive (PR), and human epidermal growth factor receptor 2 (HER2)-positive metastatic invasive ductal carcinoma metastatic to lymph nodes, lung, bone, brain, and liver presented to the interventional radiology clinic with progression of liver disease. Eight years prior, she had undergone a bilateral nipple-sparing mastectomy for ductal carcinoma in situ. Four years later, she reported abdominal pain, prompting cross-sectional imaging that revealed liver metastasis, which was biopsied and shown to be breast cancer. Breast magnetic resonance imaging then revealed subareolar invasive ductal carcinoma. She underwent partial hepatectomy and thermal ablation for solitary liver lesions, was treated with endocrine therapy (tamoxifen), and 12 months later developed multifocal right-lobe lesions. Repeat biopsy of these liver lesions revealed ER-positive, PR-negative, HER2-positive ductal carcinoma. She was managed primarily with trastuzumab-based therapies and received 2 doses of pembrolizumab and utomilumab on trial, with no effect on her metastatic disease (Fig). Paclitaxel was then started in addition to trastuzumab. She also intermittently received denosumab, which has no antineoplastic effects. Four months after her last pembrolizumab/utomilumab dose, she received 2 doses of the agonistic anti-OX40 monoclonal antibody MEDI6469, a T-cell activator, simultaneously with thermal ablation of a solitary liver metastasis. Nearly 1 month later, a positron emission tomography (PET) scan showed liver disease progression, and she was referred for transarterial therapy. Follow-up 18F-fluorodeoxyglucose (FDG)-PET/computed tomography 2 months after right-lobar TARE with SIR-Spheres (Sirtex Medical Inc., Woburn, Massachusetts), with an administered activity of 39 mCi, showed a complete response in the liver and in her extrahepatic disease, evident as complete resolution of abnormal FDG avidity. This response endured for more than 4 months. No immune function monitoring was performed around the time of TARE, as the patient had come off study. The patient died 23 months after the initial TARE procedure.
Figure.
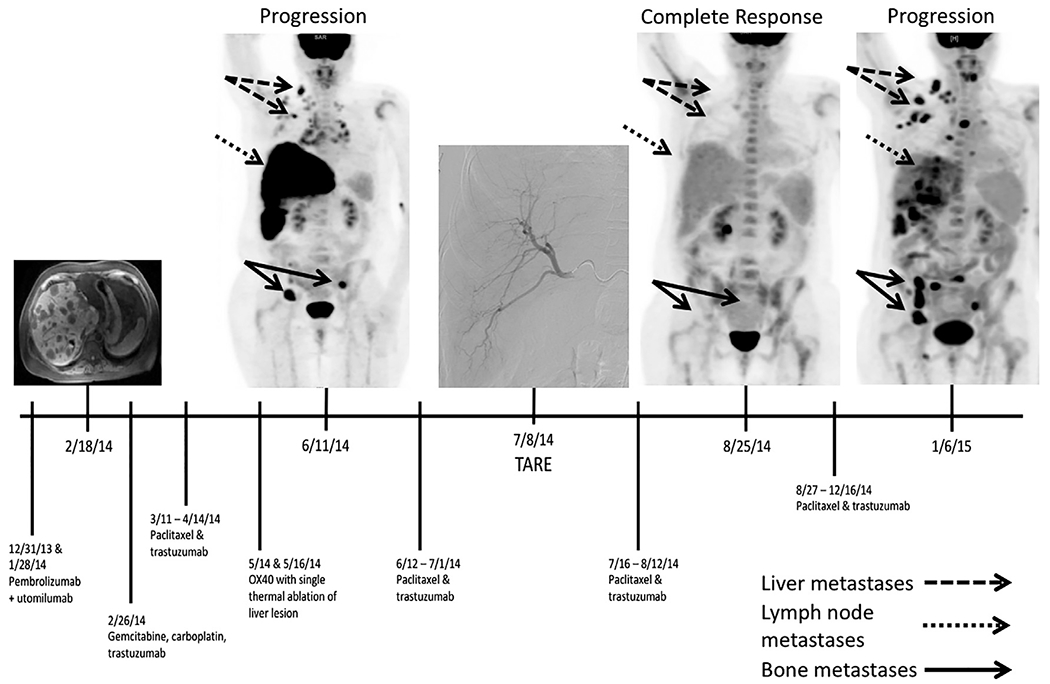
Timeline of treatment and response. TARE = transarterial radioembolization; NED = no evidence of disease; OX40 = agonisticanti-OX40 monoclonal antibody MEDI6469.
As in melanoma, it is not clear why only certain patients develop an abscopal effect after local therapies. In this patient, her metastatic disease was progressing despite systemic therapy, including immunotherapy. After locoregional therapy to the hepatic metastases, complete response of all sites of disease was noted despite no change in systemic therapy. This abscopal effect occurred following immunotherapy with an agonistic anti-OX40 monoclonal antibody, which has a half-life of 3–4 weeks. Anti-OX40 agents induce proliferation of effector T lymphocytes. Thus, it may be that anti-OX40 played a role in enabling the abscopal effect, rather than the pembrolizumab, which was given months earlier and has a half-life of 26 days. The abscopal effect occurred immediately following TARE. It is possible that combining anti-OX40 therapy with thermal ablation had a delayed effect, or that the combination of ablation and TARE allowed for an abscopal effect. Notably, progression of disease was seen after ablation, before TARE was performed. This is the second reported case of the abscopal effect after TARE (2) but the first report of it affecting extrahepatic disease or in a patient with MBC.
MBC is the second leading cause of cancer death for women, with a 2-year overall survival limited by inevitable resistance to available therapies. Immunotherapy is a personalized approach using each tumor’s biology to overcome treatment resistance. Unfortunately, in the context of breast cancers, checkpoint inhibitors (CTLA-4, PD1, and PDL1-blocking antibodies) alone have not led to significant clinical responses (3). One hypothesis is that breast cancers do not present neoantigens effectively. Combining locoregional therapy with immunotherapy may increase tumor antigen release (4). Specifically, delivering high doses of radiation to multiple liver lesions via TARE may be most effective, as it results in constant irradiation over a period of roughly 2 weeks, with ongoing cell death. This may lead to prolonged tumor antigen liberation, optimally directing the immune system to target tumor cells. Prospective studies combining TARE with immunotherapy can assess the possibility of transforming liver-directed therapy into a systemic treatment.
Footnotes
None of the authors have identified a conflict of interest.
Contributor Information
Amy R. Deipolyi, Interventional Radiology Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065.
Jacqueline F. Bromberg, Breast Medicine Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065.
Joseph P. Erinjeri, Interventional Radiology Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065.
Stephen B. Solomon, Interventional Radiology Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065.
Lynn A. Brody, Interventional Radiology Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065.
Christopher C. Riedl, Molecular Imaging and Therapy Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065.
REFERENCES
- 1.Chandra RA, Wilhite TJ, Balboni TA, et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 2015; 4:e1046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghodadra A, Bhatt S, Camacho JC, Kim HS. Abscopal effects and yttrium-90 radioembolization. Cardiovasc Intervent Radiol 2016; 39:1076–1080. [DOI] [PubMed] [Google Scholar]
- 3.Nanda R, Chow L, Dees E, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol 2016; 34:2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McArthur H, Diab A, Page D, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin Cancer Res 2016; 22:5729–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]


