Abstract
Human and animal malaria parasites increase their host erythrocyte permeability to a broad range of solutes as mediated by parasite-associated ion channels. Molecular and pharmacological studies have implicated an essential role in parasite nutrient acquisition, but inhibitors suitable for development of antimalarial drugs are missing. Here, we generated a potent and specific drug lead using Plasmodium falciparum, a virulent human pathogen, and derivatives of MBX-2366, a nanomolar affinity pyridazinone inhibitor from a high-throughput screen. As this screening hit lacks the bioavailability and stability needed for in vivo efficacy, we synthesized 315 derivatives to optimize drug-like properties, establish target specificity, and retain potent activity against the parasite-induced permeability. Using a robust, iterative pipeline, we generated MBX-4055, a derivative active against divergent human parasite strains. MBX-4055 has improved oral absorption with acceptable in vivo tolerability and pharmacokinetics. It also has no activity against a battery of 35 human channels and receptors and is refractory to acquired resistance during extended in vitro selection. Single-molecule and single-cell patch-clamp indicate direct action on the plasmodial surface anion channel, a channel linked to parasite-encoded RhopH proteins. These studies identify pyridazinones as novel and tractable antimalarial scaffolds with a defined mechanism of action.
SIGNIFICANCE STATEMENT
Because antimalarial drugs are prone to evolving resistance in the virulent human P. falciparum pathogen, new therapies are needed. This study has now developed a novel drug-like series of pyridazinones that target an unexploited parasite anion channel on the host cell surface, display excellent in vitro and in vivo ADME properties, are refractory to acquired resistance, and demonstrate a well defined mechanism of action.
Introduction
Malaria remains a formidable global health challenge, with Plasmodium falciparum and P. vivax the main human pathogens that caused an estimated 219 million malaria cases and 409,000 deaths in 2019 (PATH, 2011; https://www.who.int/publications/i/item/9789240015791). During the past 20 years, insecticide-impregnated bed nets, vector control, and artemisinin-based combination therapies have reduced morbidity and mortality, but the goals of geographic elimination and global eradication have been thwarted by acquired vector and pathogen resistance (Noedl et al., 2008; Dondorp et al., 2009; Phyo et al., 2012). Chemical screens for small molecules that kill parasites, so-called phenotype screens, have identified many potent antimalarial leads (Gamo et al., 2010; Guiguemde et al., 2010), but are complicated by difficulties of target identification and promiscuous action on multiple parasite activities. The targets of these compounds are typically found through in vitro selection of mutants (Luth et al., 2018). Although these targets are considered validated and druggable, their identification through selection of mutants in small-scale experiments also suggests that acquired resistance may arise quickly when the drug is approved for clinical use. Thus, there is a desperate need to develop new therapies with well defined, but unexploited, targets, particularly those refractory to acquired resistance (Anthony et al., 2012; https://www.who.int/publications/i/item/9789241564694).
To circumvent the limitations of phenotypic screens, we previously used a cell-based assay to find inhibitors of increased erythrocyte permeability after infection, as has been documented for all examined Plasmodium spp. (Kutner et al., 1982; Wagner et al., 2003; Lisk and Desai, 2005). Increased permeability is primarily mediated by the plasmodial surface anion channel (PSAC) linked to the RhopH complex (Nguitragool et al., 2011; Mira-Martinez et al., 2013; Counihan et al., 2017; Ito et al., 2017), but there may also be contributions from upregulated host channels (Staines et al., 2007; Dickerman et al., 2016; Bouyer et al., 2020). Using laboratory strains under in vitro conditions, these permeability changes mediate uptake of essential nutrients and dramatically remodel host erythrocyte cation composition (Pillai et al., 2012, 2013).
A cell-based screen for inhibitors of the parasite-induced permeability identified a potent and tractable pyridazinone scaffold effective against in vitro parasite cultures (Pillai et al., 2012). Using hit compound MBX-2366 (ISG-21, Fig. 1) as a starting point, we have now engaged in a systematic medicinal chemistry campaign to define the structure-activity relationship for PSAC block and in vitro parasite killing. We identified potent derivatives that address the primary liabilities of this scaffold, which included low oral absorption and rapid hepatic metabolism. Single-channel and single-cell electrophysiology studies revealed that our lead compound, MBX-4055 (Fig. 1), acts directly on PSAC to quantitatively abolish parasite-induced permeability changes. MBX-4055 has an encouraging preclinical safety profile; is active against geographically divergent, multi-drug resistant P. falciparum; and is refractory to acquired resistance under extended in vitro selective pressure.
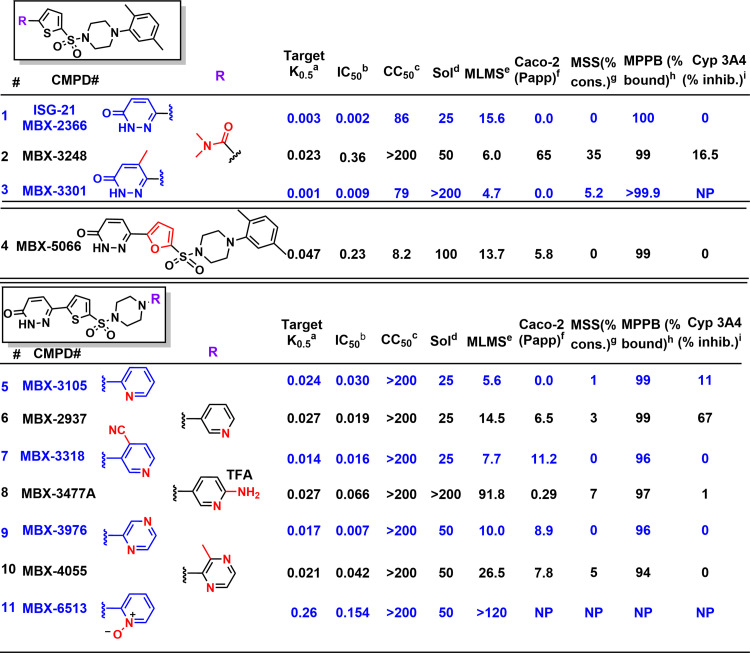
Fig. 1.
In vitro potencies and ADME properties of select analogs of parent compound MBX-2366. (A) Activity in the uptake assay (µM); (B) P. falciparum growth inhibition (µM); (C) cytotoxicity against HeLa cells (µM); (D) solubility in water (µM); (E) murine liver microsome stability (t1/2 in minutes of 1 µM compound in the presence of NADPH at 37°C); (F) Caco-2 permeability, Papp x 10−6 cm/s; (G) stability in the presence of mouse serum (% of compound consumed in 60 minutes); (H) percent protein bound by mouse plasma by equilibrium dialysis; (I) cytochrome P450 3A4 inhibition at 5 µM. NP, not performed.
Materials and Methods
Chemical Synthesis
All commercially obtained reagents and solvents were used as received. 1H and 13C NMR spectra were recorded on a Bruker 300 MHz instrument. Chemical shifts are given in δ values referenced to the internal standard tetramethylsilane. Liquid chromatography/Mass spectrometry (LC/MS) analyses were performed on a Thermo-Finnigan Surveyor LC unit connected to a Thermo LTQ Fleet MS unit. High-performance liquid chromtaography purification was performed on a Gilson Unipoint instrument equipped with a 00G-4252-P0-AX C18, 10 µm, 150 mm or 250 mm × 21.2 mm column from Phenomonex. Silica gel column purification was performed on Isco brand Combi-flash Rf liquid chromatography system using 50 µm silica Luknova SuperSep columns. Melting points were taken on EZ-Melt automated melting point apparatus (Stanford Research Systems, Inc.) in manual mode, and are uncorrected. Thin-layer chromatography was performed on silica gel (Gypsum Hard Layer Fluorescent) plates from Analtech (Newark, DE), and the chromatograms were visualized under UV light at 254 nm. Chlorosulfonic acid and dimethylamine were purchased from Sigma-Aldrich (St. Louis, MO); 4-oxo-4-(thiophen-2-yl)butanoic acid was purchased from Combi blocks (San Diego, CA); Sodium 3-nitrobenzenesulfonate hydrate was purchased from Asta Tech (Bristol, PA); Phosphorous pentachloride was purchased from Strem Chemicals (Newburyport, MA); piperazine derivatives were purchased from Enamine (Kyiv, Ukraine); and N, N-Diisopropylethyl amine and all solvents were purchased from Millipore-Sigma (St. Louis, MO). As shown in Supplemental Scheme 1, synthesis of the pyridazinones (MBX-4055) was initiated by condensation of readily available 4-oxo-4-(thiophen-2-yl)butanoic acid 1, and hydrazine hydrate followed by dehydrogenation of compound 2 using sodium 3-nitrobenzenesulfonate to form the corresponding pyridazinone 3 (Fang et al., 2010). Upon treatment of 3 with chlorosulfonic acid, the resulting sulfonyl chloride 4 was then reacted with selected amine 5, providing the sulfonamide, MBX-4055 [Supplemental Scheme 1 (Fang et al., 2010; Sengupta et al., 2015)].
Synthesis of Sulfonyl Chloride Intermediates
Formation of 5-(6-oxo-1,6-Dihydropyridazin-3-yl)Thiophene-2-Sulfonyl Chloride (Compound I)
Step 1: To a flask containing 4-oxo-4-(thiophen-2-yl)butanoic acid (110.0g, 597 mmol, 1.0 eq) and hydrazine hydrate (152.5 g, 299 mmol, 5.0 eq) was added EtOH (660 ml). The mixture was heated at 100°C reflux for 7 hours with stirring and the yellowish reaction product precipitated during the reaction. The mixture was cooled and filtered under reduced pressure and rinsed thoroughly with water. The crude product was recrystallized with EtOAc (400 ml) at ice-water bath to give 6-(thiophen-2-yl)-4,5-dihydropyridazin-3(2H)-one as yellow solid (65.2 g, 361 mmol, 61% yield1H NMR (DMSO-d6): δ 10.85 (s, NH), 7.61 (d, 1H), 7.44 (d, 1H), 7.10 (m, 1H), 2.97 (t, 2H), 2.46 (t, 2H); MS: 181.0 (M+1).
Step 2: To a flask containing 6-(thiophen-2-yl)-4,5-dihydropyridazin-3(2H)-one (70.0 g, 388 mmol, 1 eq) was added H2O (8 ml), sodium 3-nitrobenzenesulfonate hydrate (6.12 g, 271 mmol, 0.7 eq) and NaOH (38.8 g, 971 mmol, 2.5 eq) at 25°C. The mixture was heated at 100°C for 2 hours with stirring. The resultant reaction mixture was filtered and adjusted to pH = 5–6 with 1 M HCl solution. The residue was purified by column chromatography on silica gel with petroleum ether/ethyl acetate (10:1–1:3) to give 6-(thiophen-2-yl)pyridazin-3(2H)-one (36.5 g, 204 mmol, 53% yield) as light yellow liquid; MS: 179.0 (M+1).
Step 3: PCl5 (29.2 g, 140 mmol, 1 eq) and chlorosulfonic acid (40.9 g, 350 mmol, 23.4 ml, 2.5 eq) were added to a 500 ml three-necked round bottom flask equipped with a magnetic stirrer at 0°C. Then 6-(thiophen-2-yl)pyridazin-3(2H)-one (25.0 g, 140 mmol, 1 eq) in CHCl3 (175 ml) was slowly added to the above mixture at 0°C over 30 minutes, and the mixture was warmed to 25°C and stirred for 5 hours. The reaction mixture was carefully poured into ice water; the resulting yellow solid was collected by filtration, rinsed with water, and dried in vacuum to provide crude product. The crude product was recrystallized with MTBE/petroleum ether (1:1) to give 5-(6-oxo-1,6-dihydropyridazin-3-yl)thiophene-2-sulfonyl chloride (I) (13.8 g, 44%, 95% purity) as a light yellow solid; 1H NMR (DMSO-d6): δ 14.51 (brs, NH), 7.99 (d, 1H), 7.45 (d, 1H), 7.07 (d, 1H), 6.93 (d, 1H); MS: 277.2 (M+1).
Formation of 5-(4-Methyl-6-Oxo-1, 6-Dihydropyridazin-3-yl)Thiophene-2-Sulfonyl Chloride (Compound II)
Step 1: A flask containing glyoxylic acid monohydrate (4.17 g, 45.3 mmol) and 1-(thiophen-2-yl)propan-1-one (19.0 g, 135 mmol) in water (20 ml) was heated at 130°C for 16 hours. The mixture was cooled to room temperature and neutralized by adding a mixture of water (20 ml) and NH4OH (3 ml, 28%–30% aqueous solution). The excess remaining 1-(thiophen-2-yl)ethenone was then removed by extraction with CH2Cl2 (10 ml x 2). Hydrazine hydrate (2.2 ml) was added to the resulted aqueous layer, and the mixture was heated at reflux for 16 hours. After cooling to room temperature, the liquid was decanted off and methanol (3 ml) was added to the remaining waxy solid. After filtering the resulting suspension, the solid was rinsed with methanol and dried in vacuo to yield 5-methyl-6-(thiophen-2-yl)pyridazin-3(2H)-one as a yellow solid (2.55 g, 29%); 1H NMR (DMSO-d6): δ 13.14 (br s, 1H), 7.64 (d, 1H), 7.48 (d, 1H), 7.50 (dd, 1H), 6.83 (s, 1H), 2.37 (s, 3H); MS: 193.0 (M+1).
Step 2: To a flask containing CHCl3 (10 ml), cooled in an ice bath, was slowly added ClSO3H (1 ml). After 10 minutes, 5-methyl-6-(thiophen-2-yl)pyridazin-3(2H)-one (0.69 g, 3.6 mmol) was added in portions over 30 minutes. The reaction mixture was allowed to warm to room temperature. After 8 hours, the reaction mixture was poured onto ice. The resultant yellow solid was collected by filtration, rinsed with water, and dried in vacuo to yield 5-(4-methyl-6-oxo-1, 6-dihydropyridazin-3-yl)thiophene-2-sulfonyl chloride as a yellow solid (0.7 g, 67%, >98% purity); 1H NMR (DMSO-d6): δ 13.09 (br s, NH), 7.26 (d, 1H), 7.09 (d, 1H), 6.83 (s, 1H), 2.36 (s, 3H); MS: 291.1 (M+1).
Method A: General Synthesis of sulfonamides
N,N-diisopropylethylamine (1.5 eq) was added to a flask containing the corresponding sulfonyl chloride (1.0 eq) and piperazine (1.0 eq) in Dimethylformamide (DMF) (0.13 M). The reaction was stirred at room temperature under argon atmosphere for 2–12 hours. Upon completion, the reaction mixture was added to water, and the resulting precipitate was filtered and dried. Impure precipitates were purified with High-performance liquid chromatography (5%–95% MeCN/H2O + 0.1% trifluoroacetic acid).
Synthesis of 5-((4-(2,5-Dimethylphenyl)Piperazin-1-Yl)Sulfonyl)-N,N-Dimethylthiophene-2-Carboxamide (MBX-3248)
Step 1: Triethylamine (0.120 ml, 1.3 eq) was added to a flask containing methyl 5-(chlorosulfonyl)thiophene-2-carboxylate (150 mg, 0.67 mmol, 1.0 eq) and 1-(2,5-dimethylphenyl)piperazine (153 mg, 0.80 mmol, 1.2 eq) in dichloromethane (4.0 ml); this reaction was stirred at room temperature for 18 hours. The mixture was then diluted with water (10 ml) before extracting the aqueous layer with CH2Cl2 (2 × 10 ml). The combined organics were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure and dried under vacuum to give desired product quantitatively as a yellow solid. The resultant compound (295 mg, 0.67 mmol, 1.0 equiv.) was dissolved in THF/H2O (1:1, 0.04 M), and to it was added 21.0 ml of 1 N LiOH(aq) and stirred at room temperature for 18 hours. The volatiles were removed and the resultant aqueous layer was acidified to pH∼2 using 2 N HCl. The formed precipitate was filtered and washed with hexanes (3 x 5 ml) and dried in vacuo to yield 0.306 g of product, which was used for the next reaction without further purification; MS: 381 (M+1). 1H NMR (DMSO-d6): δ 7.84 (d, 1H), 7.70 (d, 1H), 7.00 (d, 1H), 6.89 (s, 1H), 6.78 (d, 1H), 3.34 (s, 4H), 2.93 (s, 4H), 2.23 (s, 3H), 2.10 (s, 3H).
Step 2: To a solution of 5-((4-(2,5-dimethylphenyl)piperazin-1-yl)sulfonyl)thiophene-2-carboxylic acid (306 mg, 0.80 mmol, 1.0 eq) in CH2Cl2 (0.15 M) was added Dimethylformamide (0.50 ml, 3.0 eq) followed by cooling in an ice-water bath. Oxalyl chloride (0.3 ml, 2.0 eq) was then added. After warming to room temperature and stirring for 1 hour, the reaction mixture was bubbled with Me2NH gas for a few minutes and stirred at room temperature from 1–5 hours. The reaction was monitored by thin layer chromatography. After the reaction was complete, water (10 ml) was added and the organics were separated. The aqueous layer was extracted with CH2Cl2 (2 × 10 ml). All the organics were combined, dried over anhydrous Na2SO4, filtered, and concentrated to give crude product, which was purified using a silica gel column with EtOAc/CH2Cl2 0%–40% gradient). Desired fractions were combined and concentrated under reduced pressure and dried in vacuo to yield pure desired product as a white solid (225 mg, 69%). 1H NMR (300 MHz, CDCl3) δ 7.49 (d, 1H), 7.36 (d, 1H), 7.06 (d, 1H), 6.84–6.81 (m, 2H), 3.30–3.20 (m, 4H), 3.20–3.15 (m, 6H), 3.02–2.99 (m, 4H), 2.30 (s, 3H), 2.18 (s, 3H); MS 408.0 (M + 1); Rf: 0.50 (3% MeOH/CH2Cl2).
MBX-2366: Purchased from CreaGen, Inc. 1H NMR (DMSO-d6) δ 8.11 (d, 1H), 7.85 (d, 1H), 7.68 (d, 1H), 7.03 (d, 1H), 6.98 (d, 1H), 6.82 (s, 1H), 6.75 (d, 1H), 3.11 (m, 4H), 2.91 (m, 4H), 2.21 (s, 3H), 2.07 (s, 3H); MS: 431.2 (M + 1); mp: 260–263°C (decomp.); Rf: 0.75 (86:13:1 CHCl3:MeOH:NH3).
MBX-3301: Prepared according to Method A using Compound II and (2,5-dimethylphenyl)piperazine. 25.0 mg (48%); white solid; 1H NMR (DMSO-d6) δ 13.32 (br s, 1H), 7.68 (m, 2H), 7.02 (d, 1H), 6.91 (s, 1H), 6.84 (s, 1H), 6.78 (d, 1H), 3.13 (m, 4H), 2.94 (m, 4H), 2.43 (s, 3H), 2.30 (s, 3H), 2.10 (s, 3H); MS: 445.1 (M + 1); Rf: 0.62 (86:13:1 CHCl3:MeOH:NH3) mp: 237–240°C (decomp.).
MBX-5066: Purchased from ChemDiv. white solid; 1H NMR (DMSO-d6) δ 13.36 (b s, 1H), 7.94 (d, 1H), 7.36 (d, 1H), 7.02 (m, 2H), 6.78 (m, 2H), 3.27 (br s, 4H), 2.88 (brs, 4H), 2.20 (s, 3H), 2.10 (s, 3H); MS: 415.1(M + 1).
MBX-3105: Prepared according to Method A using Compound I and 1-(pyridin-2-yl)piperazine. 239 mg (54%); light brown solid; 1H NMR (DMSO-d6) δ 13.30 (br s, 1H), 8.11-8.08 (m, 2H), 7.82 (d, 1H), 7.68 (d, 1H), 7.53 (m, 1H), 7.03 (d, 1H), 6.83 (d, 1H), 6.66 (dd, 1H), 3.63 (m, 4H), 3.06 (m, 4H); MS: 404.0 (M + 1); mp: > 300°C; Rf: 0.47 (86:13:1 CHCl3:MeOH:NH3).
MBX-2937: Prepared according to Method A using Compound I and 1-(pyridin-3-yl)piperazine. 60 mg (54%); white solid; 1H NMR (CDCl3:DMSO, 2:1, 1% trifluoroacetic acid -d) δ 8.52 (d, 1H), 8.18 (d, 1H), 7.87 (vr br d, 1H), 7.82 (d, 1H), 7.70 (br dd, 1H), 7.55 (s, 2H), 6.97 (d, 1H), 3.55 (m, 4H), 3.25 (m, 4H); MS: 404.3 (M + 1); mp: 287-289°C; Rf: 0.51 (86:13:1,CHCl3:MeOH:NH3).
MBX-3318: Prepared according to Method A using Compound I and 3-(piperazin-1-yl)pyridine-4-carbonitrile. 30 mg (39%); white solid; 1H NMR (DMSO-d6) δ 13.30 (s, 1H), 8.55 (s, 1H), 8.35 (dd, 1H), 8.13 (dd, 1H), 7.86 (dd, 1H), 7.74 (dd, 1H), 7.70 (d, 1H), 7.05 (d, 1H), 3.50–3.30 (m, 4H), 3.20–3.00 (m, 4H); MS: 429.1 (M + 1); mp: 296.298°C.
MBX-3477A: Prepared according to Method A using Compound I and 5-(piperazin-1-yl)pyridin-2-amine. 40 mg (19%); yellow solid; 1H NMR (DMSO-d6): 13.01 (br s, 1H), 8.12 (d, 1H), 7.88–7.84 (m, 2H), 7.71 (d, 1H), 7.63 (br s, 3H), 7.37 (d, 1H), 5.07 (d, 1H), 6.95 (d, 1H), 3.13 (m, 8H); 13MS: 419.2 (M + 1); mp: 147–149°C; Rf: 0.59 (86:13:1 CHCl3:MeOH:NH3).
MBX-3976: Prepared according to Method A using Compound I and 2-(piperazin-1-yl)pyrazine. 20 mg (27%); off white solid; 1H NMR DMSO: 13.28 (s, 1H), 8.31(s, 1H), 8.07 (d, 1H), 7.83 (dd, 2H), 7.68 (d, 2H), 7.02 (dd, 1H), 3.66 (m, 4H), 3.11 (m, 4H); MS: 405.1 (M + 1); mp: > 300°C (decomp.).
MBX-4055: Prepared according to Method A using Compound I and 2-methyl-3-(piperazin-1-yl)pyrazine. 49 mg (64%); yellow solid; 1H NMR (DMSO-d6) δ 13.30 (s, 1H), 8.10 (m, 3H), 7.85 (d, 1H), 7.70 (d, 1H), 7.04 (d, 1H), 3.27 (d, 4H), 3.18 (d, 4H), 2.39 (s, 3H); MS: 419.2 (M + 1); mp: > 278°C.
MBX-6513: Prepared according to Method A using Compound I and 2-(piperazin-1-yl)pyridin-1-ium-1-olate. 5 mg (61%); white solid; 1H NMR (DMSO-d6) δ 13.32 (s, 1H), 8.16 (m, 2H), 7.87 (s, 1H), 7.73 (d, 1H), 7.36 (m, 1H), 7.07 (m, 3H), 3.45 (br s, 4H), 3.14 (br s, 4H); MS: 420.1 (M + 1).
Parasite Culture
Laboratory-adapted P. falciparum lines were cultivated using O+ human erythrocytes obtained from commercial sources (University of Virginia Blood Bank, Charlottesville, VA) at 5% hematocrit in standard media or physiological growth inhibition medium (PGIM), a modified medium with more physiologic levels of critical nutrients (Pillai et al., 2012). PGIM was prepared with reduced concentrations of isoleucine and hypoxanthine (11.4 μM and 3.01 μM, respectively; Sigma-Aldrich, St. Louis, MO). Cultures were maintained at 37°C under 5% O2, 5% CO2, 90% N2 and synchronized using sorbitol according to standard methods. The clonal lines used were selected to represent the major malaria-endemic regions globally: Dd2 (derived from Indochina), 7G8 (Brazil), HB3 (Honduras), 3D7 (likely South Africa), Pf803 (Cambodia); these lines also cover the range of antimalarial drug resistance phenotypes.
Transport Studies Using Osmotic Lysis Measurements
Infected erythrocyte permeability to organic solutes and block by transport inhibitors was quantified using a kinetic assay that tracks light transmittance through a suspension of cells (Pillai et al., 2010). Osmotic lysis resulting from uptake of sorbitol, a sugar alcohol with primary uptake via PSAC and negligible permeability in uninfected erythrocytes, produces increased light transmittance. Synchronous trophozoite-stage cultures were enriched with the Percoll-sorbitol method, washed, and resuspended at 37°C in lysis buffer (280 mM sorbitol, 20 mM Na-HEPES, 0.1 mg/ml bovine serum albumin, pH 7.4) to initiate sorbitol uptake and osmotic lysis (GE Healthcare, Chicago, IL; Sigma-Aldrich, St. Louis, MO). Inhibitors were added from DMSO stock solutions without preincubation. Transmittance of 700 nm light through the cell suspension was then continuously tracked in a DU640 or DU800 spectrophotometer (Beckman Coulter, Brea, CA). Inhibitor K0.5 values were calculated from six-point dose responses using locally-developed scripts that interpolate the time required to reach a fractional lysis threshold, based on a two-compartment model of infected cell osmotic lysis in permeant solutes (Wagner et al., 2003). Iterative medical chemistry screens to define the inhibitor-ion channel structure-activity relationship (SAR) relied on single six-point dose response experiments; compounds selected for advancement were subjected to a minimum of three dose response experiments. Inhibitor affinities and solute permeability coefficients obtained from this method match estimates obtained using patch-clamp (Alkhalil et al., 2004).
Parasite Growth Inhibition Assays
Parasite killing by PSAC inhibitors was quantified using a 72 hour SYBR Green I-based fluorescence assay that measures parasite nucleic acid in 96-well microplates (Pillai et al., 2012). Synchronized ring-stage cultures of indicated P. falciparum lines were washed and seeded at 1% parasitemia and 2% hematocrit in standard RPMI 1640 based medium or PGIM. Cultures were maintained at 37°C under 5% O2, 5% CO2, 90% N2 for 72 hours without medium changes. At harvest, the culture was lysed in 20 mM Tris, 10 mM EDTA, 0.016% saponin, 1.6% Triton X-100, pH 7.5, with SYBR Green I nucleic acid gel stain at a 5000-fold dilution (Thermo Fisher Scientific, Waltham, MA). The microplate was then incubated at RT for 30–45 minutes without light exposure before fluorescence measurements (excitation, 485 nm; emission, 528 nm). Inhibitor dose response experiments used triplicate well measurements at each concentration. IC50 values were estimated after subtraction of background fluorescence from in-plate controls killed with 20 µM chloroquine in the corresponding medium.
Mammalian Cytotoxicity
Inhibitor cytotoxicity was quantified by using human HeLa cells (CLL-2; American Type Culture Collection, Manassas, VA) seeded at 4000 cells/well in 96-well plates. Cultures were incubated with individual inhibitors for 72 hours at 37°C in minimal essential medium (Invitrogen, Waltham, MA) supplemented with 10% fetal calf serum (Gibco by Thermo Fisher Scientific, Waltham, MA). Cell viability was quantified by using the vital stain 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTT, Promega, Madison, WI), as described (Marshall et al., 1995). Mammalian cell toxicity (CC50) is defined as the concentration of inhibitor, which decreases cell viability by 50%.
Solubility
The solubility of compounds was determined by the nephelometric method of Bevan (Bevan and Lloyd, 2000); compounds were serially diluted in DMSO (Sigma-Aldrich, St. Louis, MO) and then added to water or alternate aqueous solutions. Results are reported as the highest concentration that does not cause a sharp increase in the slope of the relative absorption/concentration curve on a Nephelostar nephelometer (BMG Labtech, Cary, NC). Additionally, to confirm the nephelometry results, compounds were stirred with the test vehicle at 20°C for 4 hours. Additional compound was added if complete dissolution was obtained. The mixture was then filtered, and the filtrate assayed by High-performance liquid chromatography (Agilent, 6410 QQQ System, Santa Clara, CA) to quantify the amount of compound in solution.
Murine Liver Microsome and Serum Stability
These assays predict the stability of compounds when exposed to the liver and to serum in vivo. For microsomal stability measurements, compounds (concentration of 1 µM) were incubated with mouse microsomal proteins (Sekisui XenoTech, LLC, Kansas City, KS) for 30 minutes in buffer with NADPH (Sigma-Aldrich, St. Louis, MO) as described (Houston, 1994), and the resulting samples analyzed by LC/MS (Thermo Electron, LCQFL-10000, West Palm Beach, FL) to quantitate the remaining parent. The resulting data were reported as half-lives in minutes. For serum stability, compounds at 5 µM were exposed to 55% mouse serum (MP Biomedicals, LLC, Santa Ana, CA) at 37°C for 60 minutes and the resulting samples analyzed by LC/MS to quantitate the amount of parent consumed. Data were reported as percent of parent remaining.
Murine Serum Protein Binding
Protein binding for 99% mouse serum was determined after the equilibrium dialysis method (Banker et al., 2003). These assays were performed using a 96-well rapid equilibrium dialysis apparatus (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Propranolol and atenolol were included as high- and low-protein binding control compounds in every test run.
Cytochrome P450 Inhibition
Cytochrome P450 inhibition predicts compound interference with enzymes that metabolize drugs in vivo and was measured using fluorogenic substrates (Walsky and Obach, 2004; Cali et al., 2012). Test compound (3–50 µM) was added to an assay mix (50 µl) containing 200 mM potassium phosphate pH 7.4, 150 µg/ml mouse liver microsomes, 2.5 mM NADPH (Sigma-Aldrich, St. Louis, MO), and 12 µM Luciferin-IPA luminometric substrate (Promega, Madison, WI) specifically designed to detect only CYP450 3A4 activity. The reactions were incubated for 20 minutes at 37°C and Luciferin Detection Reagent (50 µl, Promega, Madison, WI) with esterase was added to stop the P450 reaction and initiate the light generating reaction with luciferase. Ketoconazole (Sigma-Aldrich, St. Louis, MO) was used as a positive control inhibitor that inhibits CYP450 3A4 activity by greater than 90% at 100 nM. Quinidine (Sigma-Aldrich, St. Louis, MO) was used as a negative control. After incubation at room temperature for 5 minutes, luminescence was read in an Envision plate reader (Perkin Elmer, Billerica, MA) and percent inhibition was calculated.
Caco-2 Permeability
The Caco-2 assay predicts the potential for oral bioavailability. Caco-2 cells were applied to wells of a collagen-coated BioCoat Cell Environment (BD Biosciences, Franklin Lakes, NJ) plate as described (Stewart et al., 1995). The test agent was added to the apical (A) side and the amount of permeation was determined on the basolateral (B) side by LC/MS/MS (Thermo Electron, LCQFL-10000, West Palm Beach, FL) analysis. The amount of material on the A and B sides was used to calculate a Papp value.
Safety Panel Studies
In vitro safety studies against a panel of 35 receptors, transporters, ion channels, and cytochrome P450 enzymes were performed using established protocols by Eurofins Discovery Services.
Compound Formulations
MBX-2366: 10% dimethylacetamide (Sigma-Aldrich, St. Louis, MO), 10% Polysorbate 80 (Croda, Princeton, NJ), 80% PEG-400 (Fluka, Sigma-Aldrich, St. Louis, MO); MBX-3318: 10% DMSO, 1% Polysorbate 80, 80% PEG-400; MBX-3477A 5% DMSO, 5% Polysorbate 80, 25% (2-Hydroxpropyl)-β-cyclodextrin (Sigma-Aldrich, St. Louis, MO); MBX-4055: 6.25% DMSO, 12.5% polysorbate 80, 43.75% Labrasol (Gattefossé, Lyon, France), 1.25% hydroxypropyl methylcellulose (Sigma-Aldrich, St. louis, MO) in H2O.
Murine Pharmacokinetics
Compounds were formulated as described above, and male CD-1 mice (n = 3) were dosed with single dose levels of test compounds (3 mg/ml, 3.3–10 ml/kg, 10–30 mg/kg) by the intravenous and oral (PO) routes. Serial blood samples (approximately 20 µl each) were collected at 7 time points per route via tail vein, facial bleed, or cardiac puncture after inhalation anesthesia (for terminal bleeds). Samples were processed and compound content analyzed by LC/MS (Thermo Electron, LCQFL-10000, West Palm Beach, FL). Pharmacokinetic parameters were calculated using WinNonLin Phoenix 8 (Certara, Princeton, NJ).
Murine Tolerability
Compounds were dissolved at concentrations up to 20 mg/ml in various formulations. Male CD-1 mice were dosed with 4 dose levels (vehicle control plus 3 dose levels) of test compound by the intravenous or PO route. All mice were monitored at dosing and at 1-,3-, 8-, 24-, 48-, and 72-hours post dose for any adverse effects, and all abnormalities (such as lethargy, ataxia, breathing and digestive issues, etc.) were recorded and reported to the study director. Any animals observed to be moribund were euthanized by cardiac puncture with terminal blood collection after inhalation anesthesia.
In Vitro Selection with PSAC Inhibitors
The P. falciparum Dd2 clone was used for in vitro selections to examine the potential for acquired resistance to MBX-4055. Cultures were seeded at 1%–2% starting parasitemia and challenged with MBX-4055 at 600 nM (20x the growth inhibition IC50) in PGIM medium to limit nutrient availability. Cultures were observed by Giemsa staining of thin blood smears with medium replacement every 1–2 days. Drug pressure was maintained until consecutive smears were negative for viable parasites. The culture was then transferred to standard RPMI-based medium (Thermo Fisher Scientific, Waltham, MA) without drugs to allow outgrowth of potential mutants. When the parasites recovered to >1% parasitemia, an aliquot was taken for cryopreservation before repeating identical cycles of drug challenge and recovery for 6 months. The resulting parasite culture, termed Dd24055, was used for transport and growth inhibition studies.
Electrophysiology
Low-noise patch-clamp recordings were obtained from trophozoite-stage infected cells using the Dd2 strain. Seals were formed with freshly-pulled quartz capillaries with pipette resistances of 2–3 MΩ in symmetrical bath and pipette solutions of 1000 mM choline chloride, 115 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 20 mM Na-HEPES, pH 7.4 as described previously (Nguitragool et al., 2011). Seal resistances were typically >100 GΩ. Membrane potentials were applied from a holding potential of 0 mV using an Axopatch 200B amplifier and Digidata 1550 digitizer (Molecular Devices, San Jose, CA). Whole-cell patch-clamp recordings were obtained after application of brief, high-voltage electrical pulses to disrupt the membrane patch. Where indicated, inhibitor was added manually from preprepared stock solutions in the above bath solution. Inhibitor dose response experiments were tabulated after subtraction of small leak currents. All recordings were digitized at 100 kHz, filtered at 5 kHz (8-pole Bessel, Frequency Devices), and analyzed with Clampfit 10.0 (Molecular Devices, San Jose, CA) and SigmaPlot 10.0 (Systat, Chicago, IL).
Results
Iterative Medicinal Chemistry Yields a Potent Inhibitor Suitable for In Vivo Preclinical Studies
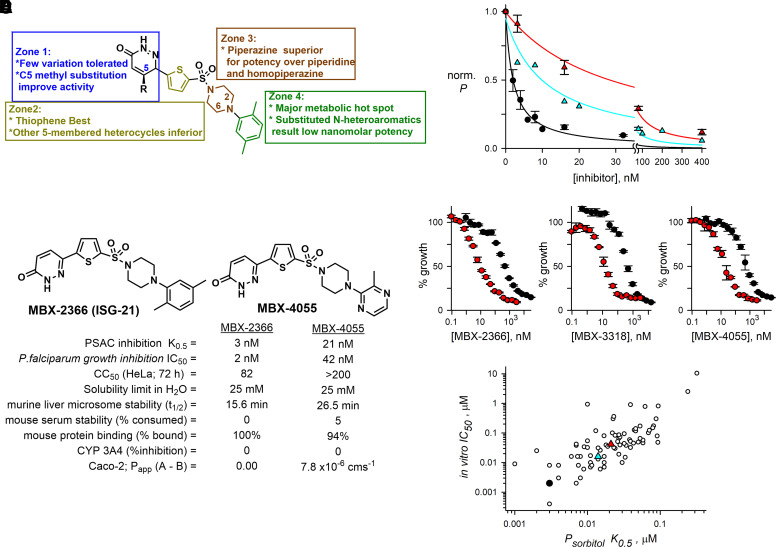
On the basis of the inhibition of infected cell permeability by the hit compound MBX-2366, targeted pyridazinones were synthesized in a convergent four-step sequence from readily available starting materials and literature protocols [Fig. 1; Supplemental Scheme 1 (Fang et al., 2010; Sengupta et al., 2015)]. We synthesized a total of 315 pyridazinones, modifying each zone of the molecule (Fig. 2A), and used a quantitative but straightforward cell-based permeability assay to drive iterative examination of structure-activity relationship (SAR). Although MBX-2366 displays low nanomolar potency, assessment of its in vitro pharmacokinetic properties revealed that the overall absorption, distribution, metabolism, excretion (ADME) profile renders it unsuitable for preclinical development (Fig. 1, entry 1; Fig. 2B). To enable murine efficacy in the humanized mouse model, newly synthesized derivatives were assessed for in vitro target potency (K0.5) in a transmittance-based erythrocyte osmotic lysis assay [Fig. 2C, (Wagner et al., 2003)], growth inhibition activity (IC50) [Fig. 2D, (Lisk et al., 2008)], and ADME properties (Fig. 1). Improved in vitro growth inhibition in assays using PGIM, a medium with more physiologic concentrations of key essential nutrients, independently confirmed action against parasite nutrient uptake (red symbols, Fig. 2D). These SAR studies demonstrated a high responsiveness to structural changes, consistent with binding specificity between the scaffold and the molecular target. Although target block in Plasmodium spp. is often not directly related to parasite killing, we found a statistically significant correlation between PSAC-blocking potency and in vitro parasite growth inhibition (Fig. 2E, Pearson’s correlation coefficient, r = 0.81, P < 10−4), suggesting that pyridazinones act specifically at the host membrane to inhibit uptake. The pyridazinone scaffold of MBX-2366 is composed of four partially modular units (denoted as “Zones”, Fig. 1; Fig. 2A). Our studies revealed that the pyridazinone moiety (Zone 1) plays a crucial role toward potency, with some replacements reducing the growth inhibition potential of the molecule (Fig. 1, entry 2) and substituents on the pyridazinone moiety maintaining potency without impact on overall ADME properties except solubility (entry 3). Similarly, the thiophene ring (Zone 2) plays a critical role in potency and cytotoxicity (entry 4), with very few modifications tolerated. We also observed that variations of the right terminal aromatic group (Zone 4) allow for broad tuning of both potency and ADME properties. For example, changing the xylene of MBX-2366 to a pyridine or substituted pyridine (entries 5–7) improves cytotoxicity and Caco-2 permeability, whereas addition of an amino group (entry 8) improves solubility and stability, albeit to the detriment of membrane permeability. Modification of Zone 4 to a substituted pyrazine improves cytotoxicity, permeability, and stability with a moderate improvement in solubility (entry 10) compared with entry 1 and the unsubstituted pyrazine (entry 9).
Fig. 2.
Optimized pyridazinones inhibit PSAC and P. falciparum growth. (A) Screening hit MBX-2366, zones used to guide synthetic strategy, and key findings. (B) Structures and properties of MBX-2366 and optimized derivative MBX-4055. (C) Mean ± S.D. residual PSAC-mediated permeabilities (P) at indicated concentrations of MBX-2366, MBX-3318, and MBX-4055 (black, turquoise, and red symbols, respectively; n = 3–5 independent dose response trials for each inhibitor). Lines indicated best fits to the Langmuir isotherm: P = a/(1 + (x/K0.5)). (D) Normalized in vitro parasite growth with indicated inhibitor concentrations in PGIM and RPMI (red and black symbols, respectively). Symbols reflect mean ± S.D. of triplicate wells in a dose response experiment and are representative of 2–5 trials for each compound. Improved killing in PGIM indicates action on nutrient acquisition and establishes primary action on nutrient acquisition. (E) Correlation between PSAC block and in vitro growth inhibition for 79 pyridazinone derivatives. Black, turquoise, and red symbols represent MBX-2366, MBX-3318, and MBX-4055, respectively. Pearson correlation coefficient for K0.5 and IC50 values, r = 0.81; p < 10−4.
SAR studies of MBX-2366 identified four candidate leads: MBX-3318, MBX-3477A, MBX-3976, and MBX-4055 (Fig. 1; Fig. 2B), with favorable ADME properties predicting efficacy in animal studies. Each of these derivatives exhibits excellent potency against solute uptake in infected cells, in vitro parasite killing, murine serum stability, and plasma protein binding. Except for MBX-3477A, each also has acceptable membrane permeability in Caco-2 assays, an indicator of oral bioavailability that justifies oral dosing in animal studies. Despite improved murine liver microsomal stability of the lead MBX-4055 over the parent MBX-2366, we identified a metabolic liability within the Zone 4 nitrogen heterocycle, presumably through N-oxidation. Accordingly, introduction of a pyridine N-oxide in Zone 4 of the scaffold (Fig. 1, entry 11) improved the metabolic stability, but at the expense of a >10-fold reduction in target potency. In vitro analyses also suggested that MBX-4055 may have low toxicity in mammals. Assays for competitive binding with 45 mammalian receptors, inhibition of 7 cardiac ion channels and the 5 major liver CYP450 enzymes (Supplemental Table 1), and an Ames assay for genotoxicity revealed only minor inhibition of one mammalian receptor, indicating a desirable profile with negligible off-target activity and no genotoxicity (data not shown). Finally, determination of human liver microsome stability for MBX-4055 demonstrated very little difference between human and mouse metabolic stability (half-lives of 24.2 and 26.5 minutes, respectively), suggesting that mouse metabolism would be predictive of human metabolism.
Proof-of-Concept Murine Tolerability and Pharmacokinetics
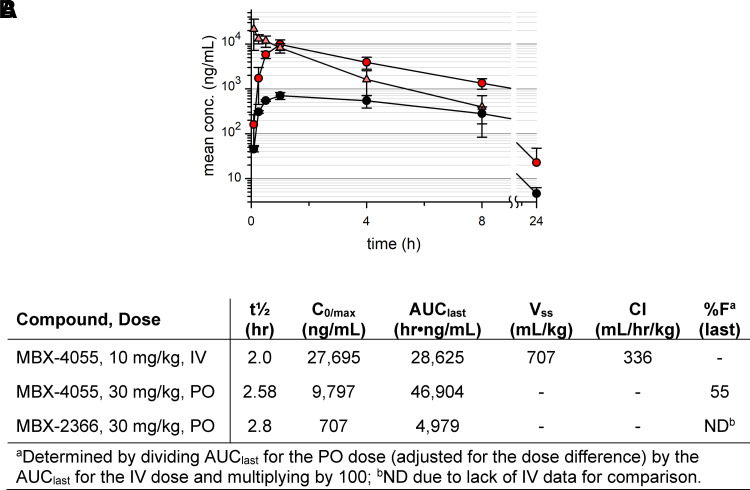
Based on preserved in vitro potency with improved ADME results of selected derivatives, we advanced compounds MBX-3318, MBX-3477A, MBX-3976, and MBX-4055 to murine studies. As shown in Fig. 3, A and B and Supplemental Table 2, the hit compound, MBX-2366, demonstrated low oral exposure that was improved upon with MBX-3318. MBX-3318 demonstrated improved oral exposure after a single dose in mice (Supplemental Fig. 1A; Supplemental Table 2), as well as moderate oral bioavailability. However, poor observed in vitro stability resulted in low intravenous plasma exposure (area under the curve), a short half-life, a greater volume of distribution, and a high clearance rate, making it less than optimal for future efficacy studies. MBX-3477A demonstrated improved intravenous exposure and half-life (Supplemental Fig. 1B; Supplemental Table 2) over that for MBX-3318, but demonstrated poor oral bioavailability as anticipated by a low Caco-2 permeability (Fig. 1). MBX-3976 exhibited excellent intravenous exposure, moderate oral bioavailability, lower clearance rate and volume of distribution (Supplemental Fig. 1C; Supplemental Table 2); however, it had a short oral half-life. In comparison, MBX-4055 revealed greater exposure over 24 hours after a single oral dose (Fig. 3, A and B), with nearly 3-fold improved oral bioavailability and preserved/improved liver microsome stability (Fig. 1), 20-fold greater exposure, a 2-fold longer half-life, and 6-fold slower clearance than MBX-3318. Additionally, we administered compounds MBX-2366, MBX-3318, MBX-3477A, MBX-3976, and MBX-4055 by the intravenous or PO routes to mice to determine tolerability. As shown in Supplemental Table 3, mice dosed with MBX-2366 by the intravenous route died immediately; however, dosing by the intravenous routes of the remaining compounds caused either no adverse effects (MBX-3318, MBX-3477A) or moderate effects that did not require euthanization (MBX-3976, MBX-4055). When dosed by the PO route, only MBX-3318 caused moderate effects of decreased activity, irregular breathing, and rough hair coat for up to 8 hours post dose; the remaining compounds produced only mild, short-lived effects. The maximum tolerated doses for MBX-3976 and MBX-4055 were estimated to be >65 and >100 mg/kg, respectively.
Fig. 3.
Murine pharmacokinetics for MBX-4055. (A) Mean ± S.D. MBX-4055 vs. MBX-2366 plasma concentrations in mice after single dose administration (n = 3 per timepoint). MBX-4055 at 30 mg/kg PO (red circles) or 10 mg/kg IV (orange triangles); MBX-2366 at 30 mg/kg PO (black circles). (B) Calculated pharmacokinetic parameters.
Direct Action on PSAC
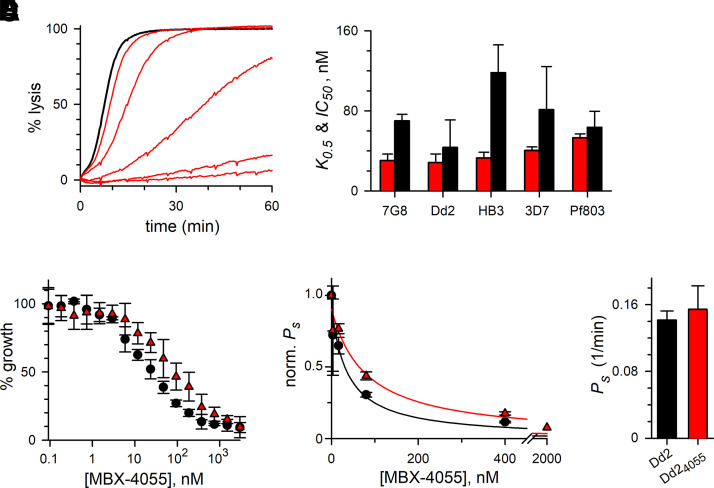
We examined mechanism of action for the MBX-4055 antimalarial lead and began by determining that this compound exhibits dose-dependent inhibition of uptake of sorbitol (Fig. 4A), a sugar alcohol that enters infected cells primarily via PSAC (Pillai et al., 2010). This permeability is conserved among divergent P. falciparum clones, including those resistant to various antimalarials. MBX-4055 potently blocked uptake in each clone, but exhibited modest variation in potency against the target and against in vitro parasite growth (Fig. 4B, K0.5 and IC50, red and black bars, respectively).
Fig. 4.
MBX-4055 exhibits broad-spectrum activity and is refractory to acquired resistance. (A) Continuous recording of sorbitol-induced osmotic lysis without (black trace) or with 3.2, 16, 80, 400, 2000 nM MBX-4055. PSAC-mediated permeability is inversely proportional to the time to hemolysis. (B) Mean ± S.D. PSAC block K0.5 and parasite growth inhibition IC50 values for MBX-4055 against indicated P. falciparum lines [red (left) and black (right) bars, respectively; n = 3–4 dose response experiments each]. The inhibitor retains broad activity against these lines, which represent all malaria-endemic continents and the full range of antimalarial susceptibilities. (C) MBX-4055 growth inhibition dose responses for Dd2 and Dd24055 (black circles and red triangles), showing unchanged potency against in vitro parasite growth after 6 months of in vitro selection (P = 0.1, n = 3). (D) Mean ± S.D. sorbitol permeability (Ps) at indicated [MBX-4055] for the parental Dd2 and Dd24055 lines (black circles and red triangles), normalized to 1.0 without inhibitor. There was a statistically insignificant change in MBX-4055 potency against the target (P = 0.1, n = 3). (E) Unchanged sorbitol permeability in Dd24055 (P = 0.49, n = 3), excluding upregulation of target activity by selective pressure.
To further examine mechanism of action and the potential for acquired resistance, we used in vitro selection through cyclical application of 600 nM MBX-4055 (∼20x IC50) in PGIM until cultures were microscopically sterilized. Between cycles, surviving parasites were allowed to recover in nutrient-rich standard RPMI-based media, permitting stringent selection of resistant mutants. After extensive cycling of high-dose MBX-4055 treatment, the resulting Dd24055 parasite line exhibited unchanged susceptibility to killing by MBX-4055 (Fig. 4C). Transport inhibition dose response studies also revealed unchanged MBX-4055 potency against the channel targets in the Dd24055 line (Fig. 4D). Finally, overexpression of the PSAC target was excluded by an unchanged sorbitol permeability (Fig. 4E). Although downregulation of channel-mediated transport after selection with toxins that require PSAC-mediated uptake has been observed (Mira-Martinez et al., 2013; Sharma et al., 2013, 2015), overexpression of this target risks premature osmotic lysis of infected cells (Cohn et al., 2003). These findings suggest a constrained inhibitor-binding pocket on the channel that is refractory to resistance mutations.
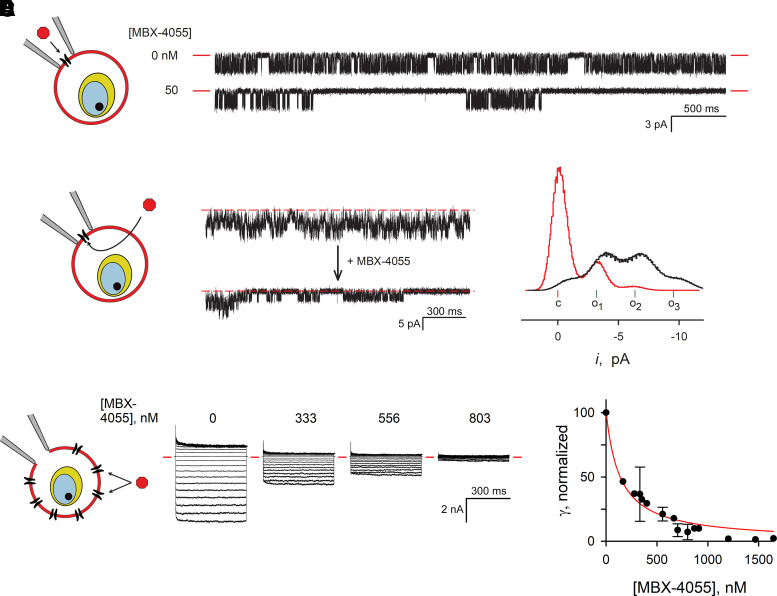
Cell-attached and whole-cell patch-clamp confirmed and extended these findings. We first used conventional cell-attached patch-clamp with MBX-4055 added symmetrically to bath and pipette compartments to ensure channels are exposed to the compound (Fig. 5A). In single channel recordings with this configuration, long blocked events (>1 second) were observed, as previously reported for unrelated potent inhibitors that do not meet our drug-likeness criteria (Pillai et al., 2010; Nguitragool et al., 2011).
Fig. 5.
Direct MBX-4055 action on PSAC in cell-attached and whole-cell patch-clamp. (A) Single channel recordings from trophozoite-infected erythrocytes at a -100 mV membrane voltage (Vm) with indicated [MBX-4055] added symmetrically to bath and pipette solutions. Red dashes indicate closed channel level. Note the long-blocked events when MBX-4055 is present. The schematic on the left shows the cell-attached patch-clamp configuration; inhibitor added to the pipette solution has direct access to channels in the membrane patch. (B) Cell-attached recording from a 3-channel patch before and after bath addition of MBX-4055 (top and bottom traces, respectively; final bath concentration, 210 nM). Vm, -100 mV. Dashed red lines represent zero current, observed when all three channels are closed. Schematic shows inhibitor must cross the membrane to access channels in the patch. (C) All-points histogram of currents from the recordings in panel B tallied from 18.7 seconds of recording before and after MBX-4055 addition (black and red traces, respectively). The current levels corresponding to all channels closed (c), and 1, 2, or 3 channels open (o1, o2, o3) are indicated. (D) MBX-4055 dose-response experiments, determined using the whole-cell patch clamp method. Ensemble currents in response to Vm between -100 and +100 mV, applied in 10 mV increments, are shown from a single cell before and after sequential addition of MBX-4055 to indicated concentrations. Red dashes indicate zero current levels. Schematic illustrates this configuration with inhibitor addition to bath. (E) Mean ± S.D. chord conductance (γ) calculated from currents between Vm of -100 and 0 mV, normalized to 100 without inhibitor; all measurements from 4 cells were pooled. Red line, best fit to Langmuir isotherm, γ = a/(1 + (x/K0.5)).
We then used a modified cell-attached configuration to circumvent concerns of molecule-to-molecule variation in channel gating (Fig. 5B, graphic). Here, a seal was formed on an infected cell without inhibitor; after obtaining baseline channel activity measurements, MBX-4055 was added to the bath compartment. For detectable inhibition under these conditions, the inhibitor must cross into the infected cell and diffuse to its binding site on the channel(s) in the patch; if the binding site is on the extracellular channel face, a second membrane crossing into the pipette is also required. Fig. 5B shows a recording from a patch with 3 identical channel molecules. MBX-4055 addition to the bath, at 10x its K0.5 value from sorbitol uptake studies, produced block almost immediately after addition (bottom trace). The complex transitions associated with activity from three independent channels was reduced to a primarily closed state with infrequent bursts of openings (Fig. 5C). Notably, this configuration provides independent evidence for MBX-4055 membrane permeability, as determined above using Caco-2 permeability studies (Fig. 1).
To obtain more quantitative estimates of PSAC block by MBX-4055, we next used the whole-cell configuration and performed dose response experiments on single cells (Fig. 5D). After obtaining inhibitor-free recordings at a range of membrane potentials, MBX-4055 was added in increasing doses with measurements of whole-cell currents after each addition. These dose response experiments revealed quantitative and complete inhibition of whole-cell currents, consistent with PSAC being the primary conductive pathway on the infected erythrocyte membrane (Nguitragool et al., 2011). By combining the dose response measurements from four infected cells, we estimated a K0.5 of 136 ± 16 nM for inhibition (Fig. 5E), a value somewhat greater than observed in our sorbitol permeability measurements. As these patch-clamp experiments were performed with molar [Cl-] at both channel faces and the sorbitol uptake studies used isotonic solutions, the higher K0.5 values with patch-clamp may reflect competition between permeating solutes and the inhibitor. Because PSAC exhibits low affinity binding for permeating solutes (26), competition between solutes and inhibitors has not been observed in previous studies of this channel. These findings are, nevertheless, consistent with PSAC inhibition as the primary mechanism of action against P. falciparum.
Discussion
Our studies define pyridazinones as a drug scaffold with a well characterized mechanism of action distinct from those of existing antimalarial chemotherapies. PSAC and the pyridazinone derivatives have several properties that are ideal for development as part of a combination therapy regimen for malaria cure. First, because PSAC is on the host erythrocyte membrane, it is more accessible to small molecule inhibitors in plasma. In contrast, all currently approved antimalarial drugs must cross at least three membranes to reach their intracellular parasite targets. Antimalarial drugs including chloroquine, mefloquine, and possibly artemisinin derivatives are also plagued by acquired resistance via extrusion by organic solute pumps (Krogstad et al., 1987; Veiga et al., 2016). Because such pumps are not present at the host membrane, PSAC inhibitors are not prone to this resistance mechanism. Second, the PSAC target is highly conserved in all examined Plasmodium spp. with conductance, gating, selectivity, and pharmacology largely unchanged between P. falciparum and the divergent P. knowlesi functional ortholog (Lisk and Desai, 2005). We submit that conservation not only of the responsible CLAG, RhopH2, and RhopH3 subunits but also of biophysical properties of channel-mediated permeation have resulted from marked constraints on the channel. Mutations that compromise subunit assembly into the RhopH complex (Counihan et al., 2017; Ito et al., 2017; Schureck et al., 2021), export and host membrane insertion (Nguitragool et al., 2011; Mira-Martinez et al., 2013; Ito et al., 2017), permeability of a broad range of essential nutrients (Kutner et al., 1982; Bokhari et al., 2008), or PSAC’s stringently low Na+ permeability (Overman, 1948; Cohn et al., 2003) would all compromise PSAC’s essential role and confer an unacceptable fitness cost. These constraints suggest that acquired resistance in the clinical setting may be limited or arise only after extended use. Third, the availability of robust, quantitative assays for PSAC has enabled the full spectrum of biochemical study, ranging from single-molecule studies of inhibitor action on the channel (Alkhalil et al., 2004), iterative medicinal chemistry with rapid inhibitor-target dose responses with a simple transmittance readout (the present study), and high-throughput screening technologies that have yielded potent and specific lead compounds (Pillai et al., 2010).
Pyridazinones have a distinct mechanism from those of existing antimalarial drugs, as evidenced by dramatically improved parasite killing in PGIM, a nutrient-limited medium that more closely approximates physiologic conditions (Pillai et al., 2012). A key limitation of compounds identified in phenotypic screens that have relied exclusively on in vitro growth inhibition using nutrient-rich RPMI-based media is that those assays may not reflect parasite growth and killing kinetics in human infections (Gamo et al., 2010; Guiguemde et al., 2010). Although nutrient deprivation is an experimentally validated outcome of PSAC block, the broad range of ions and organic solutes that permeate through this channel suggest that MBX-4055 and other potent PSAC inhibitors may also produce trapping of parasite metabolic waste within infected cells, which is also expected to be lethal to the intracellular parasite (Staines et al., 2007). PSAC’s permeability to inorganic cations may also produce changes in erythrocyte rigidity, leading to eryptosis and phagocytosis in the circulation, as has been observed for compounds that interfere with Na+ transport between host and parasite compartments (Jiménez-Díaz et al., 2014). These potential consequences of PSAC inhibition require more rigorous study. The potent and specific scaffold identified here provides a clear pharmacological avenue for these studies.
Lead compound MBX-4055 is highly specific for the PSAC target without activity against a battery of human channels, enzymes, and receptors. Further iterative medicinal chemistry and target studies, enabled by facile and robust assays for PSAC block, are ongoing and may provide curative treatment of malaria.
Acknowledgments
We thank Ryan Kissinger and Anita Mora for help with artwork.
Abbreviations
- ADME
absorption, distribution, metabolism, excretion
- PGIM
physiological growth inhibition medium
- PO
oral
- SAR
structure-activity relationship
- PSAC
plasmodial surface anion channel
Authorship Contributions
Participated in research design: Butler, Waidyarachchi, Nguyen, Jiménez-Díaz, Angulo-Barturen, Jacobs, Burrows, Aron, Bowlin, Desai.
Conducted experiments: Waidyarachchi, Shao, Nguyen, Ding, Cardinale, Morin, Kwasny, Ito, Gezelle.
Performed data analysis: Butler, Waidyarachchi, Shao, Nguyen, Cardinale, Morin, Kwasny, Ito, Gezelle, Desai.
Wrote or contributed to the writing of the manuscript: Butler, Waidyarachchi, Desai.
Footnotes
This work was supported by National Institutes of Health National Institute of Allergy and Infectious Diseases [Grant R43-AI100339 (Phase (M.M.B.) I)] and [Grant R44-AI100339 (Phases (M.M.B.) II and IIB)]; the Intramural Research Program of National Institutes of Health National Institute of Allergy and Infectious Diseases; (S.A.D.) and the Medicines for Malaria Venture (S.A.D.).
No author has an actual or perceived conflict of interest with the contents of this article.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Alkhalil A, Cohn JV, Wagner MA, Cabrera JS, Rajapandi T, Desai SA (2004) Plasmodium falciparum likely encodes the principal anion channel on infected human erythrocytes. Blood 104:4279–4286. [DOI] [PubMed] [Google Scholar]
- Anthony MP, Burrows JN, Duparc S, Moehrle JJ, Wells TN (2012) The global pipeline of new medicines for the control and elimination of malaria. Malar J 11:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker MJ, Clark TH, Williams JA (2003) Development and validation of a 96-well equilibrium dialysis apparatus for measuring plasma protein binding. J Pharm Sci 92:967–974. [DOI] [PubMed] [Google Scholar]
- Bevan CD, Lloyd RS (2000) A high-throughput screening method for the determination of aqueous drug solubility using laser nephelometry in microtiter plates. Anal Chem 72:1781–1787. [DOI] [PubMed] [Google Scholar]
- Bokhari AA, Solomon T, Desai SA (2008) Two distinct mechanisms of transport through the plasmodial surface anion channel. J Membr Biol 226:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer GBarbieri DDupuy FMarteau ASissoko AN’Dri MENeveu GBedault LKhodabux NRoman D, et al. (2020) Plasmodium falciparum sexual parasites regulate infected erythrocyte permeability. Commun Biol 3:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali JJ, Ma D, Wood MG, Meisenheimer PL, Klaubert DH (2012) Bioluminescent assays for ADME evaluation: dialing in CYP selectivity with luminogenic substrates. Expert Opin Drug Metab Toxicol 8:1115–1130. [DOI] [PubMed] [Google Scholar]
- Cohn JV, Alkhalil A, Wagner MA, Rajapandi T, Desai SA (2003) Extracellular lysines on the plasmodial surface anion channel involved in Na+ exclusion. Mol Biochem Parasitol 132:27–34. [DOI] [PubMed] [Google Scholar]
- Counihan NAChisholm SABullen HESrivastava ASanders PRJonsdottir TKWeiss GEGhosh SCrabb BSCreek DJ, et al. (2017) Plasmodium falciparum parasites deploy RhopH2 into the host erythrocyte to obtain nutrients, grow and replicate. eLife 6:e23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerman BK, Elsworth B, Cobbold SA, Nie CQ, McConville MJ, Crabb BS, Gilson PR (2016) Identification of inhibitors that dually target the new permeability pathway and dihydroorotate dehydrogenase in the blood stage of Plasmodium falciparum. Sci Rep 6:37502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AMNosten FYi PDas DPhyo APTarning JLwin KMAriey FHanpithakpong WLee SJ, et al. (2009) Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Li Y, Wang S, Meng Y, Peng J, Wang B (2010) Synthesis, characterization and electroluminescence properties of iridium complexes based on pyridazine and phthalazine derivatives with C^N N structure. Synth Met 160:2231–2238. [Google Scholar]
- Gamo FJSanz LMVidal Jde Cozar CAlvarez ELavandera JLVanderwall DEGreen DVKumar VHasan S, et al. (2010) Thousands of chemical starting points for antimalarial lead identification. Nature 465:305–310. [DOI] [PubMed] [Google Scholar]
- Guiguemde WAShelat AABouck DDuffy SCrowther GJDavis PHSmithson DCConnelly MClark JZhu F, et al. (2010) Chemical genetics of Plasmodium falciparum. Nature 465:311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston JB (1994) Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem Pharmacol 47:1469–1479. [DOI] [PubMed] [Google Scholar]
- Ito D, Schureck MA, Desai SA (2017) An essential dual-function complex mediates erythrocyte invasion and channel-mediated nutrient uptake in malaria parasites. eLife 6:e23485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Díaz MBEbert DSalinas YPradhan ALehane AMMyrand-Lapierre MEO’Loughlin KGShackleford DMJustino de Almeida MCarrillo AK, et al. (2014) (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc Natl Acad Sci USA 111:E5455–E5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad DJ, Gluzman IY, Kyle DE, Oduola AM, Martin SK, Milhous WK, Schlesinger PH (1987) Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science 238:1283–1285. [DOI] [PubMed] [Google Scholar]
- Kutner S, Baruch D, Ginsburg H, Cabantchik ZI (1982) Alterations in membrane permeability of malaria-infected human erythrocytes are related to the growth stage of the parasite. Biochim Biophys Acta 687:113–117. [DOI] [PubMed] [Google Scholar]
- Lisk G, Desai SA (2005) The plasmodial surface anion channel is functionally conserved in divergent malaria parasites. Eukaryot Cell 4:2153–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisk G, Pain M, Gluzman IY, Kambhampati S, Furuya T, Su XZ, Fay MP, Goldberg DE, Desai SA (2008) Changes in the plasmodial surface anion channel reduce leupeptin uptake and can confer drug resistance in Plasmodium falciparum-infected erythrocytes. Antimicrob Agents Chemother 52:2346–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luth MR, Gupta P, Ottilie S, Winzeler EA (2018) Using in vitro evolution and whole genome analysis to discover next generation targets for antimalarial drug discovery. ACS Infect Dis 4:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NJ, Goodwin CJ, Holt SJ (1995) A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul 5:69–84. [PubMed] [Google Scholar]
- Mira-Martínez S, Rovira-Graells N, Crowley VM, Altenhofen LM, Llinás M, Cortés A (2013) Epigenetic switches in clag3 genes mediate blasticidin S resistance in malaria parasites. Cell Microbiol 15:1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguitragool W, Bokhari AA, Pillai AD, Rayavara K, Sharma P, Turpin B, Aravind L, Desai SA (2011) Malaria parasite clag3 genes determine channel-mediated nutrient uptake by infected red blood cells. Cell 145:665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM; Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium (2008) Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359:2619–2620. [DOI] [PubMed] [Google Scholar]
- Overman RR (1948) Reversible cellular permeability alterations in disease; in vivo studies on sodium, potassium and chloride concentrations in erythrocytes of the malarious monkey. Am J Physiol 152:113–121. [DOI] [PubMed] [Google Scholar]
- PATH (2011) Staying the Course? Malaria Research and Development in a Time of Economic Uncertainty, Program for Appropriate Technology in Health, Seattle. [Google Scholar]
- Phyo APNkhoma SStepniewska KAshley EANair SMcGready Rler Moo CAl-Saai SDondorp AMLwin KM, et al. (2012) Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AD, Addo R, Sharma P, Nguitragool W, Srinivasan P, Desai SA (2013) Malaria parasites tolerate a broad range of ionic environments and do not require host cation remodelling. Mol Microbiol 88:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AD, Nguitragool W, Lyko B, Dolinta K, Butler MM, Nguyen ST, Peet NP, Bowlin TL, Desai SA (2012) Solute restriction reveals an essential role for clag3-associated channels in malaria parasite nutrient acquisition. Mol Pharmacol 82:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AD, Pain M, Solomon T, Bokhari AA, Desai SA (2010) A cell-based high-throughput screen validates the plasmodial surface anion channel as an antimalarial target. Mol Pharmacol 77:724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schureck MADarling JEMerk AShao JDaggupati GSrinivasan POlinares PDBRout MPChait BTWollenberg K, et al. (2021) Malaria parasites use a soluble RhopH complex for erythrocyte invasion and an integral form for nutrient uptake. eLife 10:e65282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta D, Gharbaoui T, Krishnan A, Buzard DJ, Jones RM, Ma YA, Burda R, Montalban AG, Semple G (2015) An efficient scale-up process for the preparation of the APD334 precursor 4-Chloromethyl-1-cyclopentyl-2-(trifluoromethyl)benzene. Org Process Res Dev 19:618–623. [Google Scholar]
- Sharma P, Rayavara K, Ito D, Basore K, Desai SA (2015) A CLAG3 mutation in an amphipathic transmembrane domain alters malaria parasite nutrient channels and confers leupeptin resistance. Infect Immun 83:2566–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Wollenberg K, Sellers M, Zainabadi K, Galinsky K, Moss E, Nguitragool W, Neafsey D, Desai SA (2013) An epigenetic antimalarial resistance mechanism involving parasite genes linked to nutrient uptake. J Biol Chem 288:19429–19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines HMAlkhalil AAllen RJDe Jonge HRDerbyshire EEgée SGinsburg HHill DAHuber SMKirk K, et al. (2007) Electrophysiological studies of malaria parasite-infected erythrocytes: current status. Int J Parasitol 37:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BH, Chan OH, Lu RH, Reyner EL, Schmid HL, Hamilton HW, Steinbaugh BA, Taylor MD (1995) Comparison of intestinal permeabilities determined in multiple in vitro and in situ models: relationship to absorption in humans. Pharm Res 12:693–699. [DOI] [PubMed] [Google Scholar]
- Veiga MI, Dhingra SK, Henrich PP, Straimer J, Gnädig N, Uhlemann AC, Martin RE, Lehane AM, Fidock DA (2016) Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nat Commun 7:11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MA, Andemariam B, Desai SA (2003) A two-compartment model of osmotic lysis in Plasmodium falciparum-infected erythrocytes. Biophys J 84:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsky RL, Obach RS (2004) Validated assays for human cytochrome P450 activities. Drug Metab Dispos 32:647–660. [DOI] [PubMed] [Google Scholar]