Abstract
Objectives:
To assess associations between treatment and recurrence-free survival (RFS) among patients with isolated tumor cells (ITCs) in sentinel lymph nodes (SLN) and otherwise stage I/II endometrioid endometrial cancer (EC).
Methods:
A multi-institutional retrospective study of patients with SLN ITCs (<200 cells and <0.2 mm) was performed. Only patients with otherwise stage I/II EC, endometrioid histology, and no evidence of micro-or macrometastases were included. Univariate and multivariable Cox proportional hazard models were used to evaluate associations between treatment, tumor characteristics, and RFS.
Results:
175 patients were included. Median follow up time was 31 months. 39% stage IB and 12% stage II disease. 76 (43%) received no adjuvant therapy or vaginal brachytherapy only (NAT/VBT), 21 (12%) had external beam radiation (EBRT), and 78 (45%) received chemotherapy +/− radiation. Patients who received chemotherapy more often had tumors with deep myoinvasion, lymphovascular space invasion (LVSI), and higher grade. Nine (5.1%) patients recurred; 5 distant, 3 retroperitoneal, and 1 vaginal. Extra-vaginal recurrences were similar in patients with or without chemotherapy (5.2% vs 3.8%, p=0.68). After controlling for stage, LVSI and grade, chemotherapy and EBRT were not associated with RFS (HR=0.63, 95%CI 0.11–3.52, and HR=0.90, 95%CI 0.22–3.61, respectively). Type of lymph node dissection and ITC detection method were not associated with RFS.
Conclusions:
Risk of retroperitoneal and/or distant recurrence is low (4.6%) for patients with stage I/II endometrioid EC and ITCs in SLNs regardless of treatment. Our preliminary data suggests that adjuvant therapy may not be significantly associated with RFS. However, longer follow-up time and a larger sample size are needed before definitive recommendations regarding adjuvant therapy for patients with EC and only ITCs in SLN can be made.
Background
Sentinel lymph node (SLN) mapping has been widely accepted as part of surgical staging for endometrial cancer. Many studies have demonstrated that SLN mapping is feasible and safe[1–10]. Bilateral detection rates vary from 65–85% with a negative predictive value of 95–99%[1, 2, 7, 10, 11]. To achieve this high negative predictive value serial sectioning, or ultrastaging, of the sentinel lymph node is used to ensure all metastases are captured. Standard sectioning with a single cut through the lymph node and staining with hematoxylin and eosin (H&E) is able to detect most macrometastases. If the SLN is negative on standard sectioning, serial sectioning is done and immunohistochemistry (IHC) with cytokeratins (CK) is used to look for low volume metastases (LVM) such as micrometastases and isolated tumor cells. With increasing precision in lymph node identification and serial sectioning (ultrastaging) of sentinel lymph nodes, isolated tumor cells are identified in 3–10% of patients [1, 4, 5, 10, 12, 13].
In some studies of similar patient cohorts before and after the utilization of SLN mapping, lymph node positivity and thus stage IIIC, has significantly increased and doubled in some studies (from 15 to 30%)[3, 4, 6, 14]. As a result, the number of patients receiving adjuvant therapy including chemotherapy and/or radiation has also sharply increased in some studies and institutions. The goal of sentinel lymph node assessment is primarily to decrease the risk of complications of the procedure (such as lymphedema, lymphocele, infection, bleeding), and improved detection of low volume metastasis, especially micrometastasis, is a secondary goal. Nevertheless, we have to be mindful that we do not increase short- and long-term toxicity, as well as costs, from increased use of adjuvant therapies that may not be beneficial.
While it is clear that patients with macrometastases benefit from chemotherapy [15, 16], it remains unclear whether patients with low volume metastases, and in particular isolated tumor cells, would have a similar benefit from chemotherapy. A recent survey on SLN mapping among members of the Society of Gynecologic Oncology reported that 70% of respondents (198/1117 members surveyed) performed SLN mapping. Twenty-one percent reported that isolated tumor cells should be treated as node positive [17].
To avoid overtreatment, it is critically important to have a better understanding of the clinical significance of low volume metastases and to determine best practice for treatment of patients with endometrial cancer who are found to have ITC in the SLNs. The objective of this study was to estimate the recurrence free survival and to describe recurrence patterns of patients with isolated tumor cells in sentinel lymph nodes with and without adjuvant therapy and with and without completion lymphadenectomy.
Methods
This multi-institutional retrospective cohort study was approved by the Institutional Review Board at each of the participating institutions. Data sharing agreements were in place. Patients were eligible if they had endometrioid adenocarcinoma (women with other histologies and mixed histologies were excluded), underwent surgical staging with sentinel lymph node mapping with or without completion lymphadenectomy between 2005–2017, and were found to have isolated tumor cells in sentinel lymph nodes. Sentinel lymph node mapping was performed following the NCCN SLN mapping guidelines and as published by Barlin et al[2]. Patients were excluded if they had evidence of micro-or macrometastases in sentinel or other lymph nodes, or if they had ITCs without serial sectioning of the lymph nodes. Serial sectioning (ultrastaging) was completed per institutional protocols and consisted of at least 5 levels per SLN. SLN were evaluated by hematoxylin and eosin (H&E) and if negative, immunohistochemistry with cytokeratin anti-AE1/AE-3 was performed to help identify ITCs. SLN isolated tumor cells (ITC) were defined as small clusters of cells not greater than 0.2 mm, present as either single tumor cells or clusters of <200 cells, in a single section (slide). ITCs could be detected by H&E or by IHC alone. The presence of ITCs was sub-classified as detected by H&E (ITC/H&E+) or as “ITC/CK+” if ITCs could only be detected by IHC. Patient characteristics, surgical procedures, pathology results, adjuvant treatment, recurrence and survival data were retrospectively collected from the medical records. Patients were considered to have high intermediate risk disease if they met GOG 249 criteria (age ≤ 50 with 3 risk factors, age 50–70 with 2 risk factors, age ≥70 with 1 risk factor; risk factors: myometrial invasion > 50%, grade 2 or 3, LVSI). Adjuvant treatment, which was prescribed as part of standard of care and physician preferences and was not part of the study protocol, was categorized as no adjuvant treatment (NAT) or vaginal brachytherapy (VBT), external beam radiation (EBRT), or chemotherapy with or without pelvic and/or vaginal cuff radiation.
Statistical analysis
The primary endpoint was recurrence-free survival (RFS) as measured from date of surgery to date of recurrence or death of disease (whichever comes first) or date of last follow up in the absence of recurrence or death. Secondary endpoints were time to recurrence as measured from date of surgery to date of recurrence, location of recurrence, overall survival as measured from date of surgery to death of disease, or date of last known to be alive (in the absence of death of disease). Recurrences were recorded by the dominant type of recurrence. For example, a patient with any component of a distant recurrence (lung, liver, brain, peritoneal, bone) was marked as a distant recurrence; as retroperitoneal if there was a retroperitoneal (LN) recurrence without evidence of distant disease; and vaginal if this was the only site of disease recurrence. There were no isolated pelvic recurrences.
We compared patient demographics and clinicopathologic characteristics according to adjuvant treatment using chi-square (or Fisher’s exact) tests, or Mann-Whitney U tests for nonparametric data as appropriate. For RFS and OS, Kaplan–Meier estimates and log-rank tests were used to compare survival distributions according to SLN metastasis size and treatment. We used Cox proportional hazards regression to estimate hazard ratios (HRs) and 95% CIs for associations between SLN, treatment and survival outcomes in models adjusted for stage, LVSI and grade.
Results
Our study population included 175 women with endometrioid adenocarcinoma and isolated tumor cells only. Those with macrometastases and/or micrometastases in any nodes were excluded. Ninety (51%) underwent SLN mapping, and 85 (49%) underwent SLN mapping followed by completion lymphadenectomy. ITCs were detected by in 85 (49%) patients by H&E, and by immunohistochemistry (cytokeratin) in 89 (51%). Eight-five (49%) had stage IA, 69 (39%) stage IB and 21 (12%) stage II disease; 100 (57%) had grade 1, 55 (31%) grade 2, 20 (11%) grade 3; and 118 (68%) had lymphovascular space invasion (Table 1). Combining patient and tumor characteristics for each patient, 77% of patients were considered high intermediate risk of recurrence according to GOG 249 criteria. Median follow up time for the entire cohort was 31 months (0.4–97.8). Seventy-six (43%) patients receive no adjuvant therapy (NAT) or vaginal brachytherapy only (VBT), while 21 (12%) received pelvic radiation (external beam radiation therapy/EBRT), and 78 (45%) chemotherapy with or without pelvic and/or vaginal cuff radiation. Pelvic radiation was more commonly used among patients with stage II endometrial cancer, and patients who received chemotherapy more often had tumors with deep myoinvasion, LVSI, and higher grade.
Table 1.
Characteristics of stage I/II endometrial cancer patients with isolated tumor cells, overall and by adjuvant treatment. NAT/VBT no adjuvant therapy/vaginal brachytherapy; LND lymph node dissection
| Overall N=175 | NAT/VBT N=76 | External beam radiation N=21 | Chemotherapy +/− radiation N=78 | p | |
|---|---|---|---|---|---|
| Age (median, range) | 63 (31 – 91) | 62 (34 – 91) | 65 (51 – 83) | 63 (31 – 78) | 0.64 |
|
| |||||
| # positive SLN | 1 (1 – 6) | 1 (1 – 4) | 1 (1 – 5) | 1 (1 – 6) | 0.009 |
|
| |||||
| # negative LN | 6 (0 – 50) | 7 (0 – 50) | 12 (1 – 37) | 5 (0 – 39) | 0.38 |
|
| |||||
| Grade | 0.05 | ||||
| 1 | 100 (57.1) | 52 (68.4) | 11 (52.4) | 37 (47.4) | |
| 2 | 55 (31.4) | 20 (26.3) | 8 (38.1) | 27 (34.6) | |
| 3 | 20 (11.4) | 4 (5.3) | 2 (9.5) | 14 (18.0) | |
|
| |||||
| LVSI | 0.003 | ||||
| No | 56 (32.0) | 36 (47.4) | 6 (28.6) | 14 (18.0) | |
| Yes | 118 (67.8) | 40 (52.6) | 15 (71.4) | 63 (81.8) | |
| Missing | 1 (0.6) | 0 (0.0) | 0 (0.0) | 1 (1.3) | |
|
| |||||
| Myoinvasion | 0.0006 | ||||
| <50% | 93 (53.1) | 50 (65.8) | 4 (19.1) | 39 (50.0) | |
| >50% | 82 (46.9) | 26 (34.2) | 17 (81.0) | 39 (50.0) | |
|
| |||||
| ITC detection method | <.0001 | ||||
| H&E | 85 (48.9) | 24 (31.6) | 6 (30.0) | 55 (70.5) | |
| Cytokeratin | 89 (51.2) | 52 (68.4) | 14 (70.0) | 23 (29.5) | |
| Missing | 0 (0.0) | 1 (4.8) | 0 (0.0) | ||
|
| |||||
| Stage | 0.001 | ||||
| 1A | 85 (48.6) | 47 (61.8) | 3 (14.3) | 35 (44.9) | |
| 1B | 69 (39.4) | 25 (32.9) | 12 (57.1) | 32 (41.0) | |
| 2 | 21 (12.0) | 4 (5.3) | 6 (28.6) | 11 (14.1) | |
|
| |||||
| Staging method | 0.0003 | ||||
| SLN only | 90 (51.4) | 38 (50.0) | 5 (23.8) | 47 (60.3) | |
| SLN+pelvic LND | 38 (21.7) | 20 (26.3) | 11 (52.4) | 7 (9.0) | |
| SLN+ pelvic+aortic LND | 47 (26.9) | 18 (23.7) | 5 (23.8) | 24 (30.8) | |
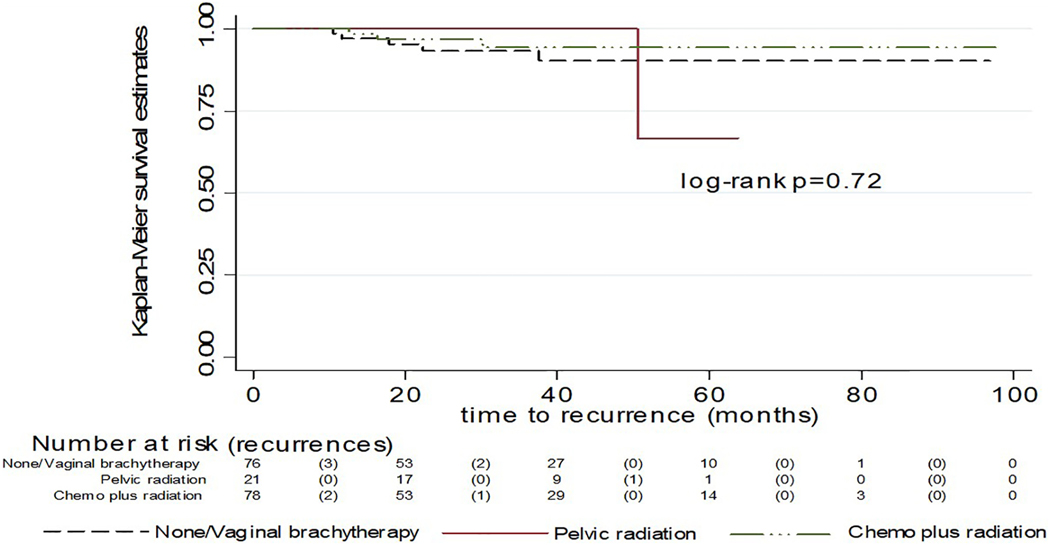
Nine (5.1%) patients were diagnosed with recurrence (Table 4); 5 (6.7%) of those who received NAT/VBT, 1 (4.8%) of patients with pelvic radiation only, and 3 (3.9%) of those with chemotherapy +/− radiation. The majority (n=5, 55%) of recurrences were distant (peritoneal, solid organ, and/or above the diaphragm), followed by retroperitoneal (pelvic and/or aortic; n=3, 33%), and 1 (11%) patient had a vaginal recurrence despite VBT. The 3- and 5-year RFS was 93% and 91% respectively, with a median time to recurrence of 17.8 months (range 10.5–50.5). Recurrence free survival for each treatment category is shown in Figure 1 and was not significantly different between treatment categories (log rank p=0.72).
Table 4.
Characteristics of patients who developed recurrent disease
| Age | Stage | Grade | LVSI | Positive SLN (#) | Type of LND | SLN detection | Adjuvant treatment | Location of recurrence | Time to recurrence (months) |
|---|---|---|---|---|---|---|---|---|---|
| 70 | 1A | 1 | - | 1 | SLN only | ITC/CK+ | None | Retroperitoneal | 11 |
| 70 | 1A | 1 | + | 1 | SLN + PV | ITC/H&E | None | Distant | 18 |
| 53 | 1B | 2 | - | 1 | SLN + PV/PA | ITC/CK+ | None | Retroperitoneal | 22 |
| 61 | 1B | 1 | + | 2 | SLN only | ITC/H&E | VBT | Vagina only | 12 |
| 43 | 1A | 1 | + | 1 | SLN only | ITC/H&E | VBT | Distant | 38 |
| 83 | 1B | 2 | + | 2 | SLN+PV | ITC/CK+ | EBRT | Distant | 51 |
| 61 | 1A | 1 | + | 1 | SLN only | ITC/H&E | Chemo + VBT | Retroperitoneal | 16 |
| 59 | 2 | 3 | + | 3 | SLN+ PV/PA | ITC/H&E | Chemo + EBRT | Distant | 13 |
| 58 | 1B | 3 | + | 1 | SLN only | ITC/H&E | Chemo + EBRT | Distant | 30 |
Figure 1.
Recurrence free survival
Univariate and multivariable adjusted associations between tumor characteristics and RFS showed no significant differences. There was no significant difference in RFS by surgical staging approach, adjuvant treatment category or by method of ITC detection (Table 3).
Table 3.
Unadjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between treatment and lymph node detection methods and RFS. NAT/VBT no adjuvant therapy/vaginal brachytherapy; LND lymph node dissection; RP retroperitoneal
| Recurrence (%) | Vaginal | RP | Distant | HR (95%CI) | |
|---|---|---|---|---|---|
| Treatment | |||||
| NAT/VBT (n=76) | 5 (6.6) | 1 | 2 | 2 | 1.00 |
| EBRT (n=21) | 1 (4.8) | 1 | 1 | 0.56 (0.13, 2.36) | |
| Chemo +/− RT (n=78) | 3 (3.9) | 2 | 0.68 (0.08, 5.87) | ||
|
| |||||
| SLN Dissection | |||||
| SLN only (n=90) | 5 (5.6) | 1 | 2 | 2 | 1.00 |
| SLN + pelvic LND (n=38) | 2 (5.3) | 0 | 0 | 2 | 0.72 (0.14, 3.72) |
| SLN+ pelvic +aortic LND (n=47) | 2 (4.3) | 0 | 1 | 1 | 0.84 (0.16, 4.34) |
|
| |||||
| ITC Detection | |||||
| H&E (n=85) | 6 (7.1) | 1 | 2 | 3 | 1.00 |
| IHC (n=89) | 3 (3.4) | 0 | 2 | 1 | 0.48 (0.12, 1.93) |
After controlling for stage, LVSI and grade, chemotherapy was not associated with risk of recurrence (HR=0.63, 95%CI 0.11–3.52, p=0.39). Extra-vaginal recurrences were similar in patients with or without chemotherapy (5.2% vs 3.8%, respectively, p=0.68); receiving chemotherapy was not associated with decreased RFS compared to NAT/VBT (HR=0.70, 95%CI 0.16,3.15). In the subset of patients with SLN detected by H&E, chemotherapy did not improve RFS or OS (for RFS HR=0.53, 95%CI 0.11–2.62; and for OS HR=1.09, 95% CI 0.10–12.07). EBRT (n=57) was not associated with RFS (HR 0.90, 95%CI 0.22–3.61). There was no difference in recurrence rates or patterns whether patients underwent SLN mapping only (5.6%) or SLN mapping with completion lymphadenectomy (4.7%) (HR 0.96; 95% CI 0.24, 3.87). We further evaluated if the number positive SLNs (positive for ITCs only) would increase the risk of recurrence. Six out of 124 (4.8%) patients with one positive SLN developed recurrence, compared to 2 of 31 (6.5%) of those with 2 positive SLNs (HR=1.38; 95%CI 0.28, 6.88) and 1 of 12 (8.3%) with 3 positive SLNs (HR=1.65; 95%CI 0.19, 13.71), while there were no recurrences among the 8 patients with 4 or more positive SLNs.
Discussion
SLN mapping is an important part of the standard of care for patients with endometrial cancer. Our multi-institutional cohort study of 175 patients with early stage endometrioid endometrial cancer and only isolated tumor cells in sentinel lymph nodes is the largest cohort to evaluate this rare and highly debated finding, and demonstrates that adjuvant therapy may not reduce the risk of recurrence or impact location of recurrence.
Although several studies to date have examined the significance of ITCs, all are limited by small numbers of patients with ITCs and most patients received adjuvant therapy. Two studies (cohorts of patients between 1986–1995[18] and 1997–2004[19]) retrospectively performed ultrastaging on all lymph nodes of patients with stage I or II disease. These studies reported that occult (IHC positive) lymph node metastases were associated with LVSI[18, 19] and deep myometrial invasion[19]. Recurrence was more common in patients with occult LN metastases, despite the fact that most had received chemotherapy and/or radiation based on uterine factors[18, 19]. Interestingly, recurrences occurred later in patients with occult lymph node metastases (at 42–59 months) compared to occult disease negative patients[19]. Although this has led many to conclude that adjuvant therapy should be prescribed, many of those patients still developed recurrent disease despite adjuvant therapy. Subsequent studies compared patients with ITCs, micrometastases, and macrometastases[5, 12, 20]. They found that patients with low volume metastases (LVM) have significantly better survival than patients with macrometastasis, however, LVM have not been shown to be a worse prognostic factor than negative nodes, and the small difference in PFS was not significant[5, 12, 20]. For example, St Clair et al retrospectively compared patient outcomes between those with ITCs, micrometastases and macrometastases. In the cohort of patients with endometrioid adenocarcinoma, they found 19/724 (2.6%) patients with ITCs; 96% received chemotherapy and/or radiation. Three-year RFS was 94% in patients with ITCs vs 93% for those with negative nodes, compared to 85% for those with macrometastases. Recurrence rates for those with negative nodes were 6.2% compared to 8.7% if ITCs were found (and 35% if macrometastases)[12]. Of note, follow up time was relatively short (median 26 months, range 0–108) and outcomes may be different with longer follow up[12]. Plante et al reported a large prospective SLN mapping study (n=519)[5]. None of the 28 patients with ITCs and endometrioid adenocarcinoma recurred, including 10 patients who received no adjuvant treatment or treatment with vaginal brachytherapy only. Others have also shown that isolated tumor cells in SLN may not need to change or increase adjuvant treatment recommendations [1].
Our cohort is unique compared to other studies as it is larger than any other cohort reported to date. In addition, our cohort consists of 77% of patients with high intermediate risk (as defined by GOG 249 criteria) endometrial cancer. Although patients were not randomized and were treated according to institutional standards and physician preference, patient and tumor characteristics of patients who received adjuvant therapy and no adjuvant therapy were relatively balanced. Certainly, the wide variety in adjuvant therapy represents the broad range of options available as part of standard of care treatment for high intermediate risk endometrial cancer which include chemotherapy, radiation or observation. Our study suggests that the presence of ITCs should not be used as a predictive biomarker, independent of other disease factors, to decide whether to prescribe adjuvant therapy. The lack of benefit of adjuvant therapy may further be explained by the fact that the risk of positive non-SLN is much lower for those with low volume metastases and in particular for those with isolated tumor cells (0–10%)[1, 5, 10, 13, 21, 22] compared to when macrometastases are found (30–60%).
Our results are consistent with findings in other disease types. In cervical cancer (stage IA-IIB) ITCs did not have any prognostic significance, whereas SLN micrometastasis without macrometastasis was associated with decreased overall survival (compared to node negative or ITC positive patients), and overall survival of patients with macrometastasis and micrometastasis was similar[23]. Furthermore, the current National Comprehensive Cancer Network (NCCN) breast cancer guidelines state that routine IHC is not recommended and that treatment decisions should be based on H&E results. For patients with H&E negative nodes, further examination by cytokeratin IHC for patients with H&E negative nodes was not associated with improved overall survival [24]. Based on our study, detection of SLN ITCs with IHC may not be clinically relevant, should be staged as N0i, and consideration should be given to not alter treatment recommendations. In our cohort, we did note a non-significant trend toward decreased recurrence free survival with ITCs detected by H&E versus IHC, however, due to low numbers this could not be adjusted for other factors (patient, tumor, and treatment characteristics) and will require further exploration in larger studies. Regardless, performing ultrastaging with H&E only, and no IHC, may decrease costs to the health care system, as well as prevent patients from being exposed to adjuvant treatment with increased risk of toxicity and lack of clinical benefit.
As SLN mapping and ultrastaging has become widely accepted and utilized, it is important to note that isolated tumor cells may be detected in the initial (regular) sentinel lymph node section. Although serial sectioning does not need to be performed when macrometastases are found in SLNs, per definition isolated tumor cells can only be present in a single slide. Thus, it is critical that serial sectioning is performed to rule out that additional tumor cells are present in deeper layers before assigning “isolated tumor cells only (N0i)” and foregoing adjuvant therapy. If additional metastases are found on deeper levels of serial sectioning (ultrastaging), this node should not be classified as N0i, and instead should be classified as TxN1Mx (FIGO stage IIIC) at this time and treated as such.
Certainly, our study has several limitations. This is a retrospective study and treatment at most institutions was based on knowledge of isolated tumor cells and not blinded. Although this was not a randomized study, we were able to include a significant number of patients who received no adjuvant therapy and the treatment groups were relatively balanced. However, due to the retrospective nature of our observations with non-uniform assignment of therapies, it is difficult to determine the degree that adjuvant therapy impacted the observed recurrences of disease. Although we were able to include 175 patients with ITCs, recurrence rates were low and a larger study population with more events is needed to improve power. Follow up was short and longer follow up is needed to evaluate for later recurrences as no standard evaluation for recurrence was utilized during surveillance. Lastly, our study population consisted only of patients with endometrioid histology and our findings cannot be generalized or applied beyond this specific population.
In summary, the goal of SLN mapping is to provide prognostic information and to inform treatment decisions, while decreasing the morbidity of lymphadenectomy. Other studies have confirmed that adjuvant chemotherapy is beneficial for those with macrometastases [15, 16, 25]. For the first time, this study informs us that patients with only isolated tumor cells in sentinel lymph nodes may not receive a similar benefit from adjuvant therapy. Moreover, this study demonstrates that isolated tumor cells in sentinel lymph nodes that have undergone ultrastaging should be noted as N0i, and may be considered as stage I or II tumors depending on uterine factors. Adjuvant therapy should be based on uterine and/or molecular factors in order to avoid increased utilization and toxicity of adjuvant therapy.
Table 2.
Recurrence outcomes in the overall study population and according to treatment. NAT/VBT no adjuvant therapy/vaginal brachytherapy; LND lymph node dissection
| Overall N=175 n (%) | NAT/VBT N=76 n (%) | EBRT N=21 n (%) | Chemotherapy+/− radiation N=78 n (%) | p | |
|---|---|---|---|---|---|
| Recurrence | 0.74 | ||||
| No | 166 (94.9) | 71 (93.4) | 20 (95.2) | 75 (96.2) | |
| Yes | 9 (5.1) | 5 (6.6) | 1 (4.8) | 3 (3.9) | |
|
| |||||
| Location of recurrence | 0.77 | ||||
| Vagina | 1 (11.1) | 1 (20.0) | 0 (0.0) | 0 (0.0) | |
| Retroperitoneal | 3 (33.3) | 2 (40.0) | 0 (0.0) | 1 (33.3) | |
| Distant | 5 (55.6) | 2 (40.0) | 1 (100.0) | 2 (66.7) | |
Highlights.
This is the largest cohort of stage I/II endometrial cancer and only ITCs in SLN
Adjuvant therapy may not reduce the risk of recurrence or location of recurrence
Adjuvant therapy should be based on uterine and/or molecular factors
ITCs in SLN does not warrant increased utilization and toxicity of adjuvant therapy
Acknowledgments
Dr. Leitao reports personal fees from JnJ/Ethicon, outside the submitted work; and Dr. Leitao is an ad hoc speaker for Intuitive Surgical, Inc. Dr. Abu-Rustum reports grants from GRAIL, grants from Stryker, outside the submitted work. Dr. Backes reports grants and personal fees from Eisai, grants and personal fees from Merck, grants from Immunogen, grants and personal fees from Clovis, personal fees from Agenus, personal fees from AstraZeneca, personal fees from Genentech, personal fees from GlaxoSmithKline, all outside the submitted work.
Footnotes
Conflict of interest statement
None of the other authors have a significant conflict of interest related to the current study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Backes FJ, Cohen D, Salani R, Cohn DE, O’Malley DM, Fanning E, et al. Prospective clinical trial of robotic sentinel lymph node assessment with isosulfane blue (ISB) and indocyanine green (ICG) in endometrial cancer and the impact of ultrastaging (NCT01818739). Gynecologic oncology. 2019;153:496–9. [DOI] [PubMed] [Google Scholar]
- [2].Barlin JN, Khoury-Collado F, Kim CH, Leitao MM Jr., Chi DS, Sonoda Y, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecologic oncology. 2012;125:531–5. [DOI] [PubMed] [Google Scholar]
- [3].Ducie JA, Eriksson AGZ, Ali N, McGree ME, Weaver AL, Bogani G, et al. Comparison of a sentinel lymph node mapping algorithm and comprehensive lymphadenectomy in the detection of stage IIIC endometrial carcinoma at higher risk for nodal disease. Gynecologic oncology. 2017;147:541–8. [DOI] [PubMed] [Google Scholar]
- [4].Holloway RW, Gupta S, Stavitzski NM, Zhu X, Takimoto EL, Gubbi A, et al. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecologic oncology. 2016;141:206–10. [DOI] [PubMed] [Google Scholar]
- [5].Plante M, Stanleigh J, Renaud MC, Sebastianelli A, Grondin K, Gregoire J. Isolated tumor cells identified by sentinel lymph node mapping in endometrial cancer: Does adjuvant treatment matter? Gynecologic oncology. 2017;146:240–6. [DOI] [PubMed] [Google Scholar]
- [6].Schlappe BA, Weaver AL, Ducie JA, Eriksson AGZ, Dowdy SC, Cliby WA, et al. Multicenter study comparing oncologic outcomes between two nodal assessment methods in patients with deeply invasive endometrioid endometrial carcinoma: A sentinel lymph node algorithm versus a comprehensive pelvic and paraaortic lymphadenectomy. Gynecologic oncology. 2018;151:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Soliman PT, Westin SN, Dioun S, Sun CC, Euscher E, Munsell MF, et al. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecologic oncology. 2017;146:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tanner E, Puechl A, Levinson K, Havrilesky LJ, Sinno A, Secord AA, et al. Use of a novel sentinel lymph node mapping algorithm reduces the need for pelvic lymphadenectomy in low-grade endometrial cancer. Gynecologic oncology. 2017;147:535–40. [DOI] [PubMed] [Google Scholar]
- [9].Casarin J, Multinu F, Tortorella L, Cappuccio S, Weaver AL, Ghezzi F, et al. Sentinel lymph node biopsy for robotic-assisted endometrial cancer staging: further improvement of perioperative outcomes. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2020;30:41–7. [DOI] [PubMed] [Google Scholar]
- [10].Rossi EC, Kowalski LD, Scalici J, Cantrell L, Schuler K, Hanna RK, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. The Lancet Oncology. 2017;18:384–92. [DOI] [PubMed] [Google Scholar]
- [11].Holloway RW, Gupta S, Stavitzski NM, Zhu X, Takimoto EL, Gubbi A, et al. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecologic oncology. 2016;141:206–10. [DOI] [PubMed] [Google Scholar]
- [12].St Clair CM, Eriksson AG, Ducie JA, Jewell EL, Alektiar KM, Hensley ML, et al. Low-Volume Lymph Node Metastasis Discovered During Sentinel Lymph Node Mapping for Endometrial Carcinoma. Annals of surgical oncology. 2016;23:1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Touhami O, Trinh XB, Gregoire J, Sebastianelli A, Renaud MC, Grondin K, et al. Predictors of non-sentinel lymph node (non-SLN) metastasis in patients with sentinel lymph node (SLN) metastasis in endometrial cancer. Gynecologic oncology. 2015;138:41–5. [DOI] [PubMed] [Google Scholar]
- [14].Raimond E, Ballester M, Hudry D, Bendifallah S, Darai E, Graesslin O, et al. Impact of sentinel lymph node biopsy on the therapeutic management of early-stage endometrial cancer: Results of a retrospective multicenter study. Gynecologic oncology. 2014;133:506–11. [DOI] [PubMed] [Google Scholar]
- [15].de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. The Lancet Oncology. 2018;19:295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Matei D, Filiaci V, Randall ME, Mutch D, Steinhoff MM, DiSilvestro PA, et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. The New England journal of medicine. 2019;380:2317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chambers LM, Vargas R, Michener CM. Sentinel lymph node mapping in endometrial and cervical cancer: a survey of practices and attitudes in gynecologic oncologists. Journal of gynecologic oncology. 2019;30:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yabushita H, Shimazu M, Yamada H, Sawaguchi K, Noguchi M, Nakanishi M, et al. Occult lymph node metastases detected by cytokeratin immunohistochemistry predict recurrence in node-negative endometrial cancer. Gynecologic oncology. 2001;80:139–44. [DOI] [PubMed] [Google Scholar]
- [19].Todo Y, Suzuki Y, Azuma M, Hatanaka Y, Konno Y, Watari H, et al. Ultrastaging of para-aortic lymph nodes in stage IIIC1 endometrial cancer: a preliminary report. Gynecologic oncology. 2012;127:532–7. [DOI] [PubMed] [Google Scholar]
- [20].Garcia Pineda V, Hernandez Gutierrez A, Gracia Segovia M, Siegrist Ridruejo J, Diestro Tejeda MD, Zapardiel I. Low-Volume Nodal Metastasis in Endometrial Cancer: Risk Factors and Prognostic Significance. Journal of clinical medicine. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Salvo G, Ramirez PT, Levenback CF, Munsell MF, Euscher ED, Soliman PT, et al. Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecologic oncology. 2017;145:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kennard JA, Stephens AJ, Ahmad S, Zhu X, Singh C, McKenzie ND, et al. Sentinel lymph nodes (SLN) in endometrial cancer: The relationship between primary tumor histology, SLN metastasis size, and non-sentinel node metastasis. Gynecologic oncology. 2019;154:53–9. [DOI] [PubMed] [Google Scholar]
- [23].Cibula D, Abu-Rustum NR, Dusek L, Zikan M, Zaal A, Sevcik L, et al. Prognostic significance of low volume sentinel lymph node disease in early-stage cervical cancer. Gynecologic oncology. 2012;124:496–501. [DOI] [PubMed] [Google Scholar]
- [24].Giuliano AE, Hawes D, Ballman KV, Whitworth PW, Blumencranz PW, Reintgen DS, et al. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. Jama. 2011;306:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. The Lancet Oncology. 2019;20:1273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]