Abstract
Relapse to drug use is one of the major challenges in treating substance use disorders. Exposure to drug-related cues and contexts triggers drug craving, which drives cocaine seeking, and increases the probability of relapse. Clinical and animal studies have shown a progressive intensification of cocaine seeking and craving that develops over the course of abstinence, a phenomenon commonly referred to as incubation of cocaine craving. Although the neurobiology underlying incubation of cocaine craving has been examined – particularly within the context of glutamate plasticity– the extent to which increased cocaine craving engenders mesolimbic dopamine (DA) changes has received relatively little attention. To assess whether incubation of cocaine craving is associated with alterations in DA terminal neurotransmission in the nucleus accumbens core (NAc), we used ex vivo fast scan cyclic voltammetry in female and male rats to assess DA dynamics following short access, long access, or intermittent access to cocaine self-administration followed by 28 days of abstinence. Results indicated that both long access and intermittent access to cocaine produced robust incubation of cocaine craving, which was associated with increases in cocaine potency. In addition, intermittent access self-administration also produced a robust increase in DA uptake rate at baseline. In contrast, short access to cocaine did not engender incubation of cocaine craving, nor produce changes in DA neurotransmission. Together these observations indicate that incubation of cocaine craving coincides with changes in DA transmission, suggesting that underlying changes in mesolimbic DA signaling may contribute to the progressive intensification of drug craving that occurs across periods of abstinence.
Keywords: Dopamine, Incubation of drug craving, Cocaine self-administration, Dopamine transporter
1. Introduction
Cocaine users often experience intense desire for drug when encountering cues associated with cocaine [1,2]. Clinical and animal studies have demonstrated a time-dependent increase in cue-induced cocaine seeking that develops over the course of abstinence [3–5], and this phenomenon has been commonly referred to as incubation of cocaine craving [4]. Importantly, it is posited that incubation of cocaine craving contributes to the propensity for relapse, even after prolonged abstinence [6–9]. As such, identifying the neural processes that underlie incubation of cocaine craving may lead to the development of tolerable and effective treatments for cocaine use disorder.
Most research investigating the neural correlates of incubation of cocaine craving have focused on postsynaptic glutamatergic adaptations in the nucleus accumbens (NAc) [10–13]. However, emerging evidence suggests that dopamine (DA) adaptations after abstinence from cocaine may be associated with progressive increases in cocaine seeking and motivation to obtain cocaine. For example, a human study demonstrated increased DA transporter (DAT) availability in the striatum after a week of cocaine abstinence [14], and in rhesus monkeys, abstinence from cocaine self-administration increased DAT binding in the NAc [15]. Similarly, abstinence from cocaine increased DAT levels in the prefrontal cortex of rats and this persisted for up to 90 days [16]. Consistent with these findings, intermittent access (IntA) to cocaine self-administration increased DAT function and DAT sensitivity to cocaine after a week of abstinence and these changes were associated with increased motivation for cocaine [17]. Altogether, these studies suggest the possibility that the time-dependent increase in cocaine seeking and craving observed during abstinence may be associated with alterations in DA neurotransmission.
Previous studies have shown that consumption and temporal patterns of cocaine administration/access produce distinct DA terminal transmission changes. While long access (LgA) to cocaine has been associated with tolerance in DA responses to cocaine [18–21], IntA to cocaine has been shown to sensitize DA responses to cocaine [17,21,22]. Despite these observations, to what extent changes in DA neurotransmission exist after prolonged periods of cocaine abstinence, when incubation of drug craving is at its highest, has not been sufficiently studied.
In the current studies, we examined whether short access (ShA), LgA, and IntA to cocaine generate incubation of cocaine craving and DA neurotransmission using fast scan cyclic voltammetry (FSCV) after 28 days of abstinence. These schedules of reinforcement were selected because previous studies indicate that LgA to cocaine produces robust incubation of cocaine craving [10,12,16,23], while ShA typically does not have this effect [16,24]. IntA to cocaine was selected due to its potential translational relevance [17,25,26], and because there is emerging evidence that it may engender exaggerated drug-seeking behavior and considerable alterations in DA neurotransmission.
2. Methods
2.1. Animal housing and conditions
Female and male adult Sprague-Dawley rats (females 200–225g; males 325–350g; Envigo, Frederick, MD, USA) were maintained on a 12-h reverse light/dark cycle (lights on at 15:00; lights off at 03:00), and given ad libitum access to food, water, and enrichment material. After arrival, rats were given 7 days to acclimate before surgery. All protocols and animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals under the supervision of the Institutional Animal Care and Use Committee at Drexel University College of Medicine.
2.2. Intravenous catheter surgery
Rats were anesthetized using 2.5% isoflurane and implanted with a silastic catheter with an inner diameter (ID) of 0.012 in., and an outer diameter (OD) of 0.025 in. (Access Technologies, Skokie, IL) in the right jugular vein for intravenous delivery of cocaine. The catheter was connected to a cannula which exited through the skin on the dorsal surface in the region of the scapulae. Ketoprofen (Patterson Veterinary, Devens, MA; 5mg/kg s.c. of 5 mg/ml) and Enrofloxacin (Norbrook, Northern Ireland; 5 mg/kg s.c. of 5 mg/ml) were provided at the time of surgery and a second dose was given 12 h later. In addition, antibiotic/analgesic powder (Neopredef, Kalamazoo, MI) was applied around the chest and back incisions. Rats were subsequently singly housed and allowed to recover for 7 days prior to self-administration training. Intravenous catheters were manually flushed with saline every 2–3 days during recovery to maintain catheter patency. After recovery, rats were randomly assigned to three groups: ShA, LgA, or IntA.
2.3. Self-administration
All self-administration sessions took place in a standard test chamber (Med Associates, St Albans, VT) located inside sound-attenuating cabinets. A ventilating fan masked background noise. Within the test chamber, two levers were located 6 cm above the floor on the left- and right-hand side of the left wall, the right lever was designated active and the left inactive. A house light was located at the top center of the right wall. Pressing the active lever resulted in an intravenous infusion of cocaine hydrochloride (obtained from the National Institute on Drug Abuse) dissolved in 0.9% sterile saline. Pump times for each rat were adjusted based on body weight to deliver 2.5 mg/ml (ShA and LgA) or 5 mg/ml (IntA) of cocaine at a constant rate of 1.064 ml/min as previously described [21,27]. All measures were recorded using Med Associates software. Each rat underwent one cocaine self-administration session per day, with an average of 5 days per week in the middle of the dark phase. However, rats always self-administered the day prior to the cue-induced seeking test on abstinence day 1 (AD1). Controls were cocaine-naive rats housed in the same room as rats undergoing self-administration.
2.3.1. Short access
Rats were first trained to self-administer cocaine on a 6-h FR1 schedule whereby a single active lever press initiated an intravenous injection of cocaine (0.75 mg/kg, infused over 2.5 – 4 s) paired with a cue light. Acquisition occurred when a rat obtained ≥40 infusions in one session. During this acquisition period, sessions were terminated after a maximum of 40 infusions or after 6 h, whichever occurred first. Then, rats were allowed to self-administer cocaine on a 2-h FR1 schedule in which a single active lever press resulted in a single 0.75 mg/kg cocaine infusion over 2.5 – 4 s, paired with a cue light. At the start of each infusion, the stimulus light above the active lever was illuminated for the length of the infusion and the house light was illuminated for 20 s signaling no drug availability (i.e., timeout). Lever presses during this 20-s timeout were recorded but had no consequence. Inactive lever presses were recorded but had no consequence. Rats self-administered cocaine for 2 h per session for 5 sessions. Sessions were terminated after a maximum of 100 infusions or after 2 h, whichever occurred first. These experimental conditions are similar to those used previously to show incubation of cocaine craving [28].
2.3.2. Long access
Rats were allowed to self-administer cocaine on a 6-h FR1 schedule in which a single active lever press resulted in a single 0.75 mg/kg cocaine infusion over 2.5 – 4 s, paired with a cue light. At the start of each infusion, the stimulus light above the active lever was illuminated for the length of the infusion and the house light was illuminated for 20 s signaling the timeout period. Lever presses during the 20-s timeout were recorded but had no consequence. Inactive lever presses were recorded but had no consequence. Rats self-administered cocaine for 6 h per session for 10 sessions. Sessions were terminated after a maximum of 100 infusions or after 6 h, whichever occurred first. Rats assigned to this schedule did not undergo an acquisition period; however, rats that did not receive on average >50 infusion during the last 5 days of LgA were excluded from the study. These experimental conditions are similar to those used previously to show incubation of cocaine craving [10,12].
2.3.3. Intermittent access
Rats were first trained to self-administer cocaine on a 6-h FR1 schedule whereby a single active lever press initiated an intravenous injection of cocaine (0.75 mg/kg, infused over 2.5 – 4 s) paired with a cue light. Acquisition occurred when an animal obtained ≥40 infusions in one session. During this acquisition period, sessions were terminated after a maximum of 40 infusions or after 6 h, whichever occurred first. Rats were then allowed to self-administer cocaine on a 6-h FR1 schedule in which active lever presses resulted in a single 0.375 mg/kg cocaine infusion delivered over 0.7 – 1.1 s, paired with a cue light. At the start of each infusion, the stimulus light above the active lever was illuminated for the length of the infusion. There was no timeout period following each infusion, to allow a binge-like pattern of consumption. During the 6-h session, rats had access to cocaine for 5-min trials followed by 25-min timeout periods, totaling 12 trials per session. During the 25-min timeout period, the levers were retracted. Rats self-administered cocaine for 6 h per session for 7 sessions. These experimental conditions are similar to those used previously to show increased motivation for cocaine after a period of abstinence [17,21].
2.4. Abstinence and cue-induced seeking tests
Following the last self-administration session, rats underwent a forced abstinence period of 28 days. During this phase, rats remained in their home cage (except for performing cue-induced drug seeking tests), in the absence of enrichment material. To assess incubation of cocaine craving, rats performed a cue-induced drug seeking test on abstinence day (AD) 1 and AD 28. During the 2-h seeking test, a single active lever press resulted in presentation of the cue light that was previously paired with cocaine, but no cocaine delivery. Under these conditions, the number of active lever presses was interpreted as representing the degree of cocaine seeking or craving.
2.5. Ex vivo fast scan cyclic voltammetry
Eighteen hours after the last seeking test, rats were anesthetized with 2.5% isoflurane for 5 min and subsequently decapitated. The brain was rapidly dissected and slices containing the NAc core were transferred to ice-cold, oxygenated artificial cerebrospinal fluid (aCSF) containing NaCl (126 mM), KCl (2.5 mM), NaH2PO4 (1.2 mM), CaCl2 (2.4 mM), MgCl2 (1.2 mM), NaHCO3 (25 mM), glucose (11 mM), and L-ascorbic acid (0.4 mM), with pH adjusted to 7.4. A vibrating microtome was used to produce 400 μm-thick coronal sections containing the NAc core. Slices were then transferred to room-temperature oxygenated aCSF and left to equilibrate for 45 min before being transferred into a recording chamber flushed with aCSF (32°C).
A bipolar stimulating electrode was placed on the surface of the tissue in the NAc core, and a carbon fiber microelectrode was implanted between the stimulating electrode leads. DA release was evoked every 3 min using a single electrical pulse (400μA; 4ms; monophasic) and measured using Demon Voltammetry and Analysis Software [29]. Once baseline DA release was stable (3 successive stimulations within <10% variation), the slice was exposed to a gradual increase in cocaine concentrations (0.3 – 30μM) as previously described [30–32].
2.6. Estrous cycle monitoring
Estrous cycle determination was based on a vaginal lavage obtained from all female rats immediately after seeking tests on AD1 and AD28, as well as immediately prior to decapitation for FSCV. Vaginal fluid samples were collected with a plastic pipette filled with 50 μL of sterile saline (NaCl 0.9%) by placing the end of the tip at the opening of the vaginal canal, taking care to not penetrate the orifice. Vaginal fluid was placed on glass slides. Unstained material was observed under a light microscope, with 10x and 40x objective lenses. Three types of cells could be recognized: 1) round and nucleated epithelial cells; 2) irregular shaped, anucleated cornified cells; and 3) small round leukocytes. The proportion among them was used for the determination of the estrous cycle phase [33]. A proestrus sample consisted of a predominance of nucleated epithelial cells an estrous sample primarily consisted of anucleated cornified cells. A metestrus sample consisted of the same proportion among leukocytes, cornified, and nucleated epithelial cells. A diestrus sample consisted of a predominance of leukocytes. As observed in Supplemental Fig. 1, there was a similar distribution of females in proestrus, estrous, metestrus, and diestrus after cue-induced seeking tests and for FSCV experiments.
2.7. Data analysis
All active and inactive lever presses were measured, including those made during the timeout period when no cocaine was delivered. The number of inactive lever presses served as a measure of nonspecific behavior. The rate of cocaine intake was calculated by dividing the intake (mg) by cocaine availability time. For example, 120 min for ShA and 360 min for LgA minus the 20 s time out period after every active lever press (active lever presses * 0.333 min), and 60 min for IntA.
Three ShA and 2 IntA rats were excluded during cocaine training because they did not meet acquisition criteria after 7 days. Two LgA rats were excluded because they did not meet the inclusion criteria (average >50 infusion during the last 5 sessions). Two IntA and 2 ShA rats were excluded because of faulty catheters.
Twelve rats were trained on ShA (6 females and 6 males), 16 on LgA (10 females and 6 males), and 11 on IntA (5 females and 6 males). As we were interested in the relationship between the progressive increase in cue-induced cocaine seeking and changes in DA neurotransmission after prolonged periods of abstinence, for FSCV experiments we excluded 2 ShA and 3 LgA that did not increase their active lever presses on AD 28 compared to AD 1. All IntA rats increased lever pressing on AD28 and thus no IntA rats were excluded from DA analyses.
DA concentrations were calculated by comparing currents at the peak oxidation potential for DA in consecutive voltammograms with electrode calibrations determined using an in situ calibration method as describe previously [31,32,34]. To determine whether different schedules of cocaine self-administration followed by prolonged abstinence influence DA terminal neurotransmission, we assessed stimulated DA release, DA uptake rate (Vmax), and cocaine-induced DA uptake inhibition (app Km) using a Michalis-Menten based model [35–37]. Baseline DA uptake was determined by setting Km values to 0.18 μM and all cocaine-induced alterations in uptake were attributed to changes in apparent Km. Inhibition constants (Ki) were determined to calculate the necessary cocaine concentration to produce 50% DA uptake inhibition and were then calculated using the equation Km/slope [17,31,38,39]. Demon Voltammetry and Analysis software [29] was used for all acquisition and analysis of FSCV data.
Sex differences were examined for all behavioral and neurochemical parameters evaluated here. A statistically significant interaction between sex and the main measure of interest suggests that sexes responded differently to the condition [40–42]. In all our studies, we observed no interactions between sex and measures of interest; therefore, female and male data were combined. Nevertheless, in Supplemental Data we show data separated by sex and provide further discussion comparing our findings to previous studies [26,27].
2.8. Statistical analyses
Statistical analyses were conducted using IBM SPSS Statistics 24. Specific analyses are reported in the results section. Mauchly’s test of sphericity was used to confirm the assumption of sphericity in the two-way mixed ANOVA tests; when it was violated, a Greenhouse-Geisser correction was used to interpret the within-subjects effect and two-way interaction. Levene’s test for equality of variances was used to confirm the assumption of homogeneity in the one-way ANOVA tests; when it was violated, a Welch’s ANOVA was used to interpret differences between groups and Games-Howell post hoc tests were used for multiple comparisons.
3. Results
Rats underwent ShA, LgA or IntA to cocaine self-administration to generate incubation of cocaine craving and assess whether increases in cue-induced seeking were associated with changes in DA neurotransmission in the NAc. Similar to what has been shown previously, by allowing largely unfettered access to cocaine, the ShA and LgA schedules generated sustained cocaine intake [12,16]. In contrast, the IntA schedule restricts access to 5 min bouts every 25 min for 6 h, and thus generated a ‘binge-like’ pattern of cocaine intake, which is akin to what is typically observed in human cocaine use [25,43] (Fig. 1A). Cocaine self-administration schedules were replicated from prior studies that have shown incubation of cocaine craving [10,12,17,28]. For this reason, acquisition period, number of sessions, and cocaine dose differed among the three schedules of reinforcement.
Fig. 1.
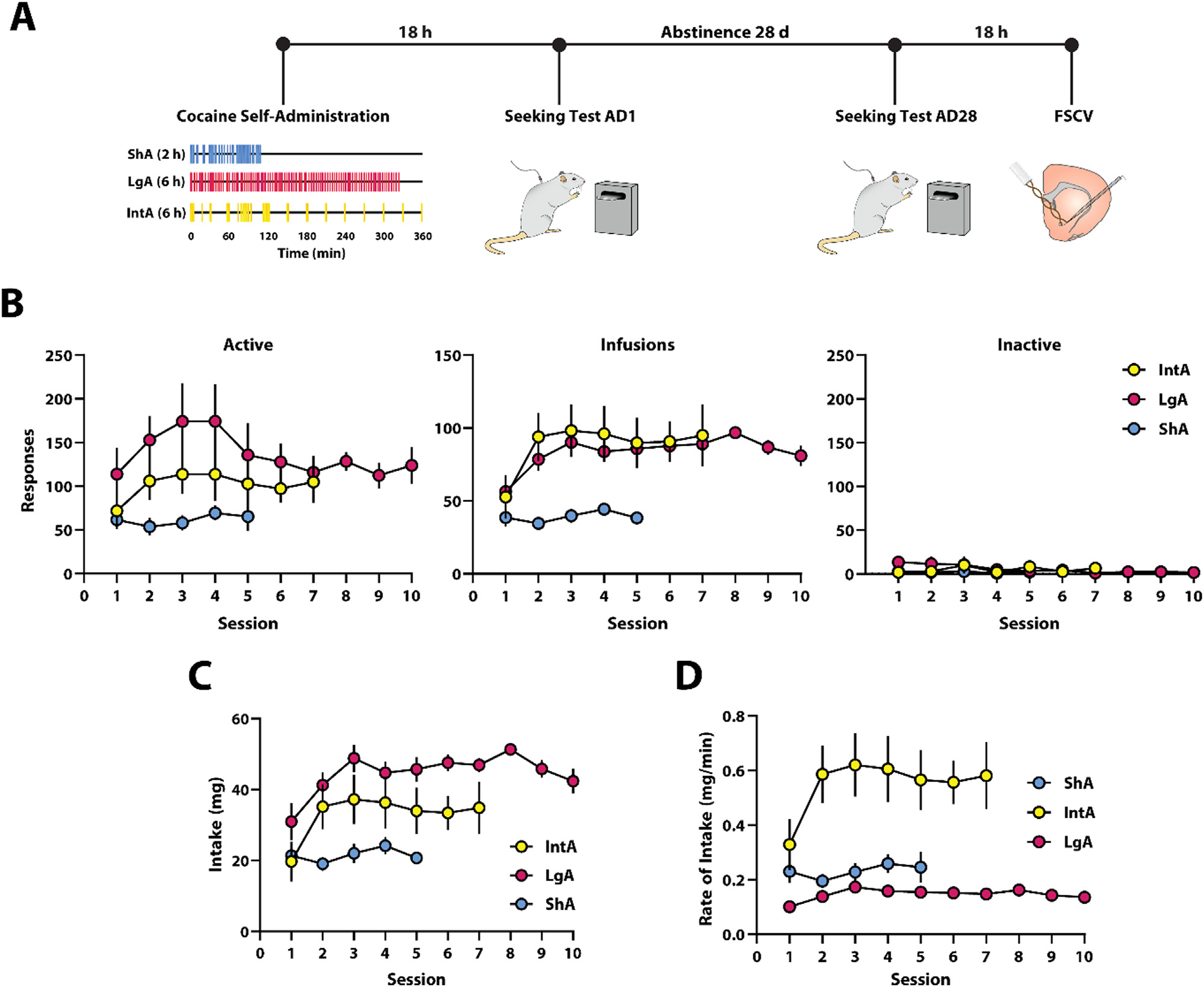
Relatively stable cocaine self-administration was observed during ShA, LgA and IntA to cocaine. (A) Experimental design. (B) Active and inactive lever presses, (C) cocaine intake, and (D) rate of cocaine intake during cocaine self-administration. ShA n=12 (6F / 6M). LgA n=16 (10F / 6M). IntA n=11 (5F / 6M). Data are shown as mean ± SEM.
3.1. Cocaine self-administration remained relatively stable for all schedules
To determine whether self-administration varied across test session, we examined active lever presses, cocaine intake, and rate of intake for each schedule. A one-way repeated measures ANOVA determined that there were no significant difference in active lever presses across test sessions for any of the schedules (ShA: F(4,44) = 0.503, p = 0.733; LgA: Greenhouse-Geisser correction; F(2.890,43.351) = 1.283, p = 0.292; IntA: Greenhouse-Geisser correction; F(3.400,33.997) = 1.701, p = 0.180, Fig. 1B). In addition, a one-way repeated measures ANOVA determined that there were no significant difference in infusions across test sessions for any of the schedules (ShA: Greenhouse-Geisser correction; F(2.251,24.77) = 0.7130, p = 0.5157). However, there were significant differences in cocaine intake across test sessions for LgA (Greenhouse-Geisser correction; F(3.832,57.48) = 1.283, p = 0.0035) and IntA (Greenhouse-Geisser correction; F(2.641,25.53) = 3.300, p = 0.0414, Fig. 1B). Moreover, a one-way repeated measures ANOVA determined that there were no significant differences in cocaine intake across test sessions for ShA (F(4,44) = 0.713, p = 0.588, Fig. 1C). However, there were significant differences in cocaine intake across test session for LgA (Greenhouse-Geisser correction; F(3.760,56.397) = 3.859, p = 0.009) and IntA (F(6,60) = 3.463, p = 0.005, Fig. 1C). Further, a one-way repeated measures ANOVA determined that there were no significant differences in rate of cocaine intake across test sessions for ShA (F(4,44) = 0.461, p = 0.746) or IntA (Greenhouse-Geisser correction; F(2.432,24.321) = 2.021, p = 0.147, Fig. 1D). However, there was a significant difference in rate of cocaine intake across test sessions for LgA (Greenhouse-Geisser correction; F(4.533,67.994) = 2.943, p = 0.022, Fig. 1D).
Extensive evidence has shown escalation of cocaine intake over the course of cocaine self-administration sessions for LgA and IntA [22,44–47]. Due to significant differences in cocaine intake across test sessions on LgA and IntA, post hoc tests were conducted between the first and last sessions of LgA and IntA to assess escalation. A Sidak’s post hoc test showed no significant difference between cocaine intake on the first session compared to the last session for either LgA (p = 0.986) or IntA (p = 0.310; Fig. 1C). Together these observations suggest no escalation of cocaine intake as a function of increasing self-administration experience.
3.2. LgA results in greater cocaine consumption than ShA and IntA, but IntA results in a faster rate of intake
To examine whether cocaine consumption varied across schedules, we analyzed total cocaine intake (i.e. cumulative intake across all sessions) for ShA, LgA, and IntA. A Welch’s ANOVA indicated that total cocaine intake was significantly different across schedules (F(2,18.391) = 138.727, p < 0.001). Games-Howell post hoc analyses revealed a significantly higher total intake in LgA compared to ShA (p < 0.001) and IntA (p < 0.001), as well as higher total intake in IntA compared to ShA (p = 0.030; Fig. 2A).
Fig. 2.
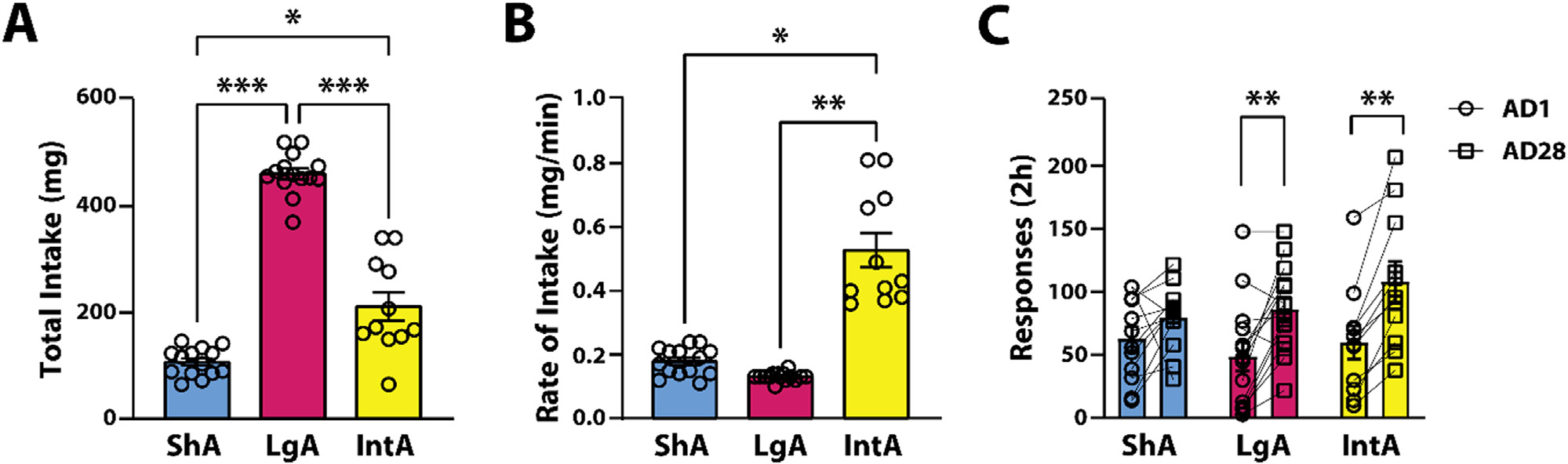
LgA and IntA to cocaine engender incubation of cocaine craving after prolonged abstinence. (A) Total cocaine intake and (B) average rate of cocaine intake across schedules of reinforcement. (C) Lever presses during cue-induced seeking tests on abstinence day 1 (AD1) and 28 (AD28). ShA n=12 (6F / 6M). LgA n=16 (10F / 6M). IntA n=11 (5F / 6M). Data are shown as mean ± SEM. * p < 0.05, **p < 0.01, ***p < 0.001
To examine whether rates of intake varied across schedules, we compared the average rate of intake for ShA, LgA, and IntA. A Welch’s ANOVA determined that there were significant differences in the rate of cocaine intake across schedules (F(2,15.510) = 13.374, p < 0.001). Games-Howell post hoc analysis revealed that IntA promoted a higher rate of cocaine intake (i.e., mg/minute) compared to ShA (p = 0.019) and LgA (p = 0.004; Fig. 2B). The faster rate of intake observed with IntA is likely associated with the temporal profile of cocaine access on this schedule and is consistent with a previous study [26].
3.3. LgA and IntA engender incubation of cocaine craving
To examine the impact of different patterns of cocaine consumption on incubation of cocaine craving, we compared active lever presses during the cue-induced seeking test on abstinence day 28 (AD28) to abstinence day 1 (AD1) across the three schedules of reinforcement. A two-way mixed ANOVA with schedule as the between-subjects variable and abstinence day as the within-subjects variable revealed no significant effect of schedule (F(2,36) = 0.638, p = 0.534), or schedule X abstinence day interaction (F(2,36) = 2.866, p = 0.070) on responses during seeking test. However, there was a significant effect of abstinence day (F(1,36) = 42.877, p < 0.001). Because of an a priori hypothesis that responses would vary between AD1 and AD28, post hoc analyses were conducted for each schedule. Sidak’s post hoc analysis revealed that responses on AD28 were significantly higher compared to AD1 for LgA (p < 0.001) and IntA (p < 0.001), but not for ShA (p = 0.2245).
An increase in responses on AD28 is the operational definition of incubation of cocaine craving [4]. Thefore, to further examine whether each schedule of reinforcement generated incubation of cocaine craving, responses on AD28 were compared to AD1. A paired samples t-test revealed a significant increase in active lever presses on AD28 compared to AD1 for LgA (t(12) = 3.706, p = 0.003; Fig. 2C). Further, because the data were not normally distributed, a Wilcoxon signed-rank test (nonparametric test) determined that there was a significant increase in active lever presses on AD28 compared to AD1 for IntA (z = 2.934, p = 0.002; Fig. 2C). However, a paired sample t-test revealed no significant difference in active lever presses on AD28 compared to AD1 for ShA (t(11) = 1.619, p = 0.134; Fig. 2C). Therefore, as shown previously, LgA and IntA, followed by prolonged periods of abstinence, engender incubation of cocaine craving [10,12,26]. However, animals trained on ShA did not show a significant increase in responses following abstinence [16] (Fig. 2C).
3.4. DA release did not differ between ShA, LgA or IntA compared to cocaine-naive rats
To assess whether ShA, LgA, or IntA to cocaine followed by abstinence alters DA release compared to cocaine naive rats, animals were sacrificed 18 h after the AD28 seeking test and DA release was examined in brain slices containing the NAc core using FSCV. Under baseline conditions, a one-way ANOVA demonstrated that DA release was not significantly different across schedules, (F(3,44) = 1.222, p = 0.313; Fig 3A), suggesting that cocaine self-administration followed by prolonged periods of abstinence did not alter electrically stimulated DA release in the NAc core.
Fig. 3.
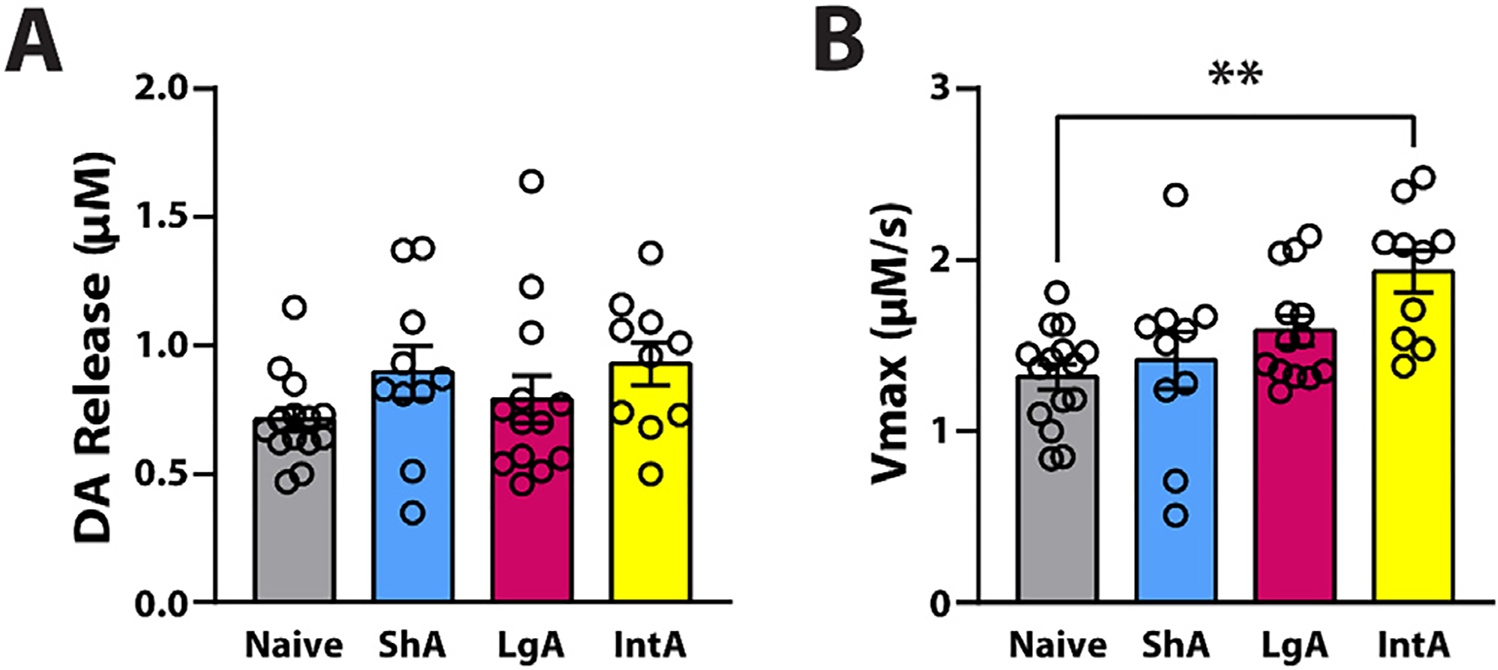
Abstinence from IntA to cocaine increases DA uptake in the NAc. (A) DA release and (B) maximal rate of DA uptake (Vmax) on AD29 of rats that incubated on ShA, LgA, and IntA. Naive; cocaine-naive rats n=15 (7F / 8M); ShA n=10 (5F / 5M); LgA n=13 (7F / 6M); IntA n=10 (5F / 5M). Data are shown as mean ± SEM. **p < 0.01.
3.5. IntA followed by prolonged abstinence generates a significant increase in maximal rate of DA uptake
To assess whether ShA, LgA, or IntA to cocaine followed by abstinence alters DA uptake (i.e., Vmax) compared to cocaine naive rats, animals were sacrificed 18 h after the AD28 seeking test and DA uptake was examined in brain slices containing the NAc core using FSCV. Under baseline conditions, a one-way ANOVA demonstrated that DA uptake was significantly different across schedules, (F(3,45) =5.658, p = 0.002). Further, Sidak’s post hoc analysis revealed that DA uptake was significantly faster in IntA (p = 0.001) compared to naïve rats indicating that the efficiency of DA uptake is higher in rats trained on IntA followed by abstinence (Fig. 3B).
3.6. LgA and IntA increase the effects of cocaine at inhibiting DA uptake after prolonged abstinence
After baseline recordings in the same rats, NAc core slices were exposed to five cumulative concentrations of cocaine. Two-way repeated measures ANOVA with schedule as the between-subjects variable and cocaine concentration as the within-subjects variable revealed a significant effect of schedule (F(3,43) = 8.250, p < 0.001), cocaine concentration (Greenhouse-Geisser correction; F(1.175,50.506) = 440.319, p < 0.001), and a significant interaction between schedule and cocaine concentration on cocaine potency (Greenhouse-Geisser correction; F(3.524,50.506) = 7.970, p < 0.001). Sidak’s post hoc analysis revealed that cocaine was significantly more effective at inhibiting DA uptake in the IntA schedule at 1 μM cocaine (p = 0.003), 3 μM cocaine (p < 0.001), 10 μM cocaine (p < 0.001), and 30 μM cocaine (p = 0.002) compared to the naive group (Fig. 4A–B). In addition, a significantly higher cocaine potency in LgA at 30 μM cocaine (p = 0.016) was observed compared to the naive group. These data suggest that rats trained on LgA and IntA show a greater effect of cocaine on the DAT compared to ShA and cocaine naive rats. Consistent with this, a one-way ANOVA revealed that the cocaine concentration required to inhibit 50% of uptake (Ki) was significantly different across groups, (F(3,42)= 5.742, p = 0.002). Sidak’s post hoc analysis revealed a significant decrease in Ki in LgA (p = 0.017) and IntA (p = 0.003) compared to naive rats (Fig. 4C).
Fig. 4.
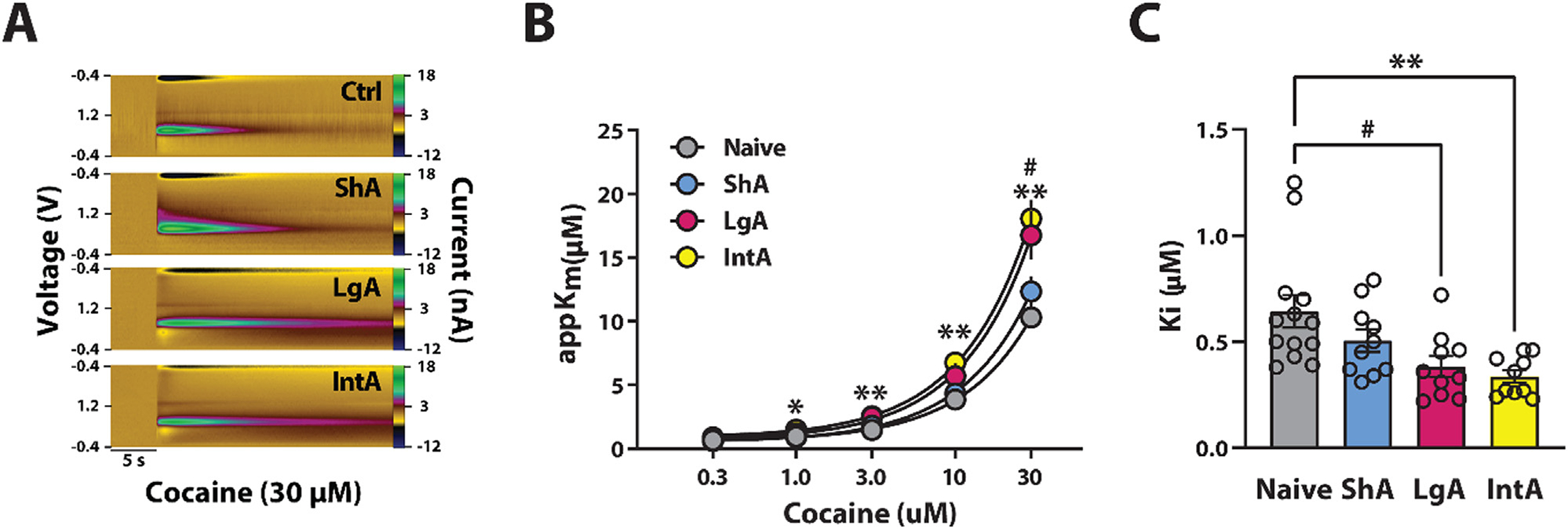
Abstinence from LgA and IntA to cocaine increases cocaine potency. (A) Example color plots after exposure to 30 μM of cocaine. (B) Inhibition of DA uptake across cocaine concentrations. (C) Inhibition constants (Ki) for naive rats and rats that incubated on ShA, LgA and IntA. Naive n=13 (6F / 7M); ShA n=10 (5F / 5M); LgA n=10 (4F / 6M); IntA n=10 (5M / 5F). Data are shown as mean ± SEM. # p < 0.05 LgA vs Naive. *p < 0.05, **p < 0.01 IntA vs naive.
4. Discussion
In the present studies, we assessed whether incubation of cocaine craving engendered by different cocaine self-administration schedules was associated with changes in DA release, DA uptake, and cocaine potency in the NAc after a prolonged period of abstinence. Rats that self-administered cocaine on a LgA or IntA schedule showed significantly higher cue-induced seeking on AD28 compared to AD1, indicating incubation of cocaine craving. However, rats trained on ShA did not show incubation of cocaine craving. Regarding DA dynamics, we observed no difference in baseline DA release across schedules of reinforcement. However, IntA to cocaine followed by abstinence significantly increased DA uptake rate at baseline compared to cocaine naive rats. In addition, both LgA and IntA followed by abstinence engendered an increase in cocaine potency. In contrast, rats trained on ShA did not show an increase in cocaine potency. These results indicate that incubation of cocaine craving coincides with increases in cocaine potency suggesting underlying adaptations in DATs in the NAc.
4.1. IntA and LgA to cocaine engendered incubation of cocaine craving despite differences in cocaine consumption and patterns of intake
Rats that self-administered cocaine on LgA consumed significantly more cocaine than rats that self-administered cocaine on ShA or IntA schedules. However, similar to a prior observation, IntA to cocaine promoted a faster rate of consumption leading to rapid spikes in cocaine intake during the 5 min access to cocaine [25].
Despite differences in self-administration, both LgA and IntA produced incubation of cocaine craving, although ShA did not. Interestingly, neither LgA nor IntA produced escalation of cocaine intake, indicating that escalation of drug intake is not necessary to promote incubation of cocaine craving [44,47,48]. In addition, these data suggest that high cocaine intake and/or a binge-like pattern of consumption might be important factors for the development of incubation of cocaine craving.
Consistent with our observations, previous studies have shown that ShA to cocaine generates a relatively modest increase in drug-seeking after a month of abstinence, compared to LgA [16]. In our studies, rats were exposed to only 5 days of ShA; thus, in addition to low and continuous levels of cocaine intake, these rats experiences fewer and shorter cocaine self-administration sessions than rats that self-administered on the LgA and IntA schedules. Therefore, it is possible that the amount of cocaine or the number of days exposed to cocaine could differentiate the effects of ShA from the other schedules. However, previous studies have shown that even when rats were exposed to 10 or more ShA sessions, resulting in higher overall cocaine intake, ShA rats did not show a robust increase in drug seeking and motivation for cocaine after abstinence [16,21]. Additionally, the inability of ShA to engender incubation of cocaine craving might be associated with short sessions lengths compared to LgA and IntA, in which session length was three times longer. However, recent evidence suggests that a 2-h IntA schedule generates a similar increase in motivation for cocaine compared to rats exposed to a 6-h IntA [49]. Altogether, these results support previous findings suggesting that how fast and how frequent cocaine reaches the brain may play a crucial role in the development of neuronal adaptations associated with substance use disorders [25,47,48].
4.2. LgA and IntA to cocaine generated changes in DA dynamics
DA signaling has been implicated in the facilitation of cocaine cueevoked craving and susceptibility to relapse [22,50,51]. Thus, we hypothesized that the amount and patterns of cocaine intake that influenced incubation of cocaine craving after abstinence, might be associated with DA terminal adaptations. Although no change in DA release was observed under baseline conditions, there was an increase in DA uptake in rats exposed to IntA followed by 28 days of abstinence compared to cocaine naive rats. By comparison, ShA and LgA to cocaine did not generate significant changes in either DA release or uptake. These observations are similar to previous reports indicating that on the first day of abstinence from IntA to cocaine, DA release and uptake were both increased [17,21]. In contrast, LgA and ShA did not produce changes in DA neurotransmission after one day of abstinence [21]. Altogether, these observations suggest a time-dependent change in DA dynamics, where changes in DA release associated with IntA to cocaine might normalize over the course of abstinence, while DA uptake changes might remain the same or become potentiated as demonstrated previously after 7 days of abstinence [17]. Consistent with our ShA data, in vivo FSCV studies reported a decrease in DA release and uptake in the NAc core after prolonged abstinence compared to rats that self-administered saline [52], and no changes in DA release or uptake when comparing AD1 with AD30 [53]. Moreover, the increased DA uptake rate observed only in the IntA group suggests that the temporal patterns of cocaine intake might be driving this effect, perhaps by intermittent cocaine-DAT interactions.
Surprisingly, IntA followed by abstinence changed DA uptake but not DA release. We would predict that more efficient DA uptake biases DA intracellularly, which would then lead to greater release in response to stimulation [31,54]. However, there are mechanisms that may regulate DA release independently of DA uptake rate. For example, mobilization of vesicular DA reserve pools or changes in the expression of VMAT2 which transports DA into readily releasable vesicles intracellularly [55,56]. Further examination will be required to understand the mechanisms underlying the effects of IntA to cocaine on DA uptake but not DA release.
4.3. Changes in cocaine potency match the magnitude of incubation of cocaine craving
When NAc core slices were exposed to cocaine, we observed an increase in cocaine-induced DA uptake inhibition (i.e., cocaine potency) after abstinence from LgA and IntA to cocaine, but not following ShA. Studies during early abstinence have demonstrated that discontinuation of IntA to cocaine produced a sensitized cocaine response at the DAT. The opposite effect was observed after LgA (desensitization or tolerance), and no effect was observed after ShA compared to cocaine naive rats [19,21]. The increased cocaine potency after IntA has been shown to be further augmented following 7 days of abstinence [17] while the decreased cocaine potency after LgA has been shown to normalize following 15 days of abstinence [19]. Comparing these effects with the present studies, we suggest a time-dependent change in cocaine potency that matches the magnitude of incubation of cocaine craving. In the case of LgA, the initial decrease in cocaine potency observed early in abstinence [19,21], may transition into increased cocaine potency in late abstinence as observed herein. By comparison, IntA appears to increase cocaine potency both early in abstinence [17,21] and later in abstinence. In fact, only the schedules that engendered incubation of cocaine craving—LgA and IntA—were associated with increased cocaine potency, suggesting the possibility that DA terminal adaptations that contribute to increased cocaine potency may promote incubation of cocaine craving. Consistent with this, rats exposed to ShA followed by abstinence did not show changes in cocaine potency or incubation of cocaine craving. Similarly, a prior study showed that ShA followed by abstinence did not produce the glutamatergic changes in the NAc core necessary for the development of incubation of cocaine craving [57].
Incubation of cocaine craving is in part mediated by a progressive accumulation of calcium permeable AMPA receptors in the NAc. A previous study demonstrated that activation of CaMKII plays a key role as an interface between increases in DA and glutamate neurotransmission in the NAc that contribute to reinstatement of cocaine seeking [58]. In addition, it appears that AMPAR redistribution depends on cocaine-induced DAT inhibition [59]. Overall, these studies suggest possible ways in which cocaine potency and glutamate plasticity in the NAc could be connected.
Interestingly, IntA followed by prolonged abstinence engendered both increases in DA uptake rate and cocaine potency while LgA only generated increases in cocaine potency. Therefore, we and others speculate that the spiking pattern of high cocaine concentrations encountered with IntA contributes to DAT changes that promote faster uptake rates and greater cocaine potency, while the maintained high amounts of cocaine intake observed with LgA influence cocaine potency but leave DA uptake largely intact. Altogether, DA terminal adaptations after cocaine abstinence suggest that patterns of cocaine intake may be driving aberrant DAT function observed after prolonged periods of abstinence.
4.4. Increased cocaine potency may reflect alterations in DA neurotransmission that lead to enhanced drug craving
Exposure to drug cues during cocaine abstinence induces the activation of mesolimbic DA neurons, promoting an exaggerated incentive motivational state that primes individuals to seek drug and eventually relapse [60]. Consistent with this, prior evidence suggests that cocaine potency can influence behavior, with high cocaine potency associated with increases in cocaine self-administration and motivation to obtain cocaine [17,21,37], and reduced cocaine potency leading to reduced cocaine self-administration [21,61]. In the current studies, we observed an association between increased cocaine potency and incubation of cocaine craving. However, it is important to note that during cue-induced drug seeking tests there was no cocaine delivery, therefore a change in seeking behavior cannot be directly attributable to a change in the pharmacological effects of cocaine at the DAT. Consequently, our findings suggest that increased cocaine potency likely reflects a dysregulated DA state that promotes enhanced associations between the drug and drug-associated cues – even under conditions when no cocaine is present.
The mechanisms underlying changes in cocaine potency remain unclear. However, it has been posited that shifts in cocaine potency may reflect underlying adaptations in DAT function (e.g., post-translational modifications). Future experiments will need to examine DA neurotransmission in awake behaving rats to assess if re-exposure to cocaine or cocaine-associated cues promotes cue-induced DA signals that drive craving and motivation. In addition, to examine potential mechanisms underlying increased cocaine potency at DA terminals, studies may need to assess post-translational modifications and subcellular localization of the DAT after prolonged periods of cocaine abstinence.
5. Conclusions
Here we assessed whether incubation of cocaine craving engendered by different cocaine self-administration schedules is associated with changes in DA neurotransmission after prolonged periods of abstinence. Both LgA and IntA to cocaine followed by 28 days of abstinence engendered incubation of cocaine craving. However, rats trained on ShA did not show incubation of cocaine craving. In addition, IntA to cocaine followed by abstinence significantly increased DA uptake rate at baseline compared to cocaine naive rats and both LgA and IntA followed by abstinence engendered an increase in cocaine potency. In contrast, rats trained on ShA did not show an increase in cocaine potency. These results suggest that DA terminal adaptations may contribute to incubation of cocaine craving over the course of abstinence. Understanding the relationship between changes in DA neurotransmission and intensification of cocaine seeking observed after abstinence may provide critical information for the development of therapeutics to prevent cocaine relapse.
Supplementary Material
Funding resources
This work was supported by NIH grant DA031900 to R.A.E. and Drexel University Dean’s Fellowship for Excellence in Collaborative or Themed Research to I.P.A.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.addicn.2022.100029.
References
- [1].Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, P O’Brien C, Cue reactivity and cue reactivity interventions in drug dependence, NIDA Res. Monogr. 137 (1993) 73–95. [PubMed] [Google Scholar]
- [2].Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, P O’Brien C, Limbic activation during cue-induced cocaine craving, Am. J. Psychiatry 156 (1999) 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gawin FH, Kleber HD, Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations, Arch. Gen. Psychiatry 43 (1986) 107–113. [DOI] [PubMed] [Google Scholar]
- [4].Grimm JW, Hope BT, Wise RA, Shaham Y, Neuroadaptation. Incubation of cocaine craving after withdrawal, Nature 412 (2001) 141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Parvaz MA, Moeller SJ, Goldstein RZ, Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography, JAMA Psychiatry 73 (2016) 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wallace BC, Psychological and environmental determinants of relapse in crack cocaine smokers, J. Subst. Abuse Treat. 6 (1989) 95–106. [DOI] [PubMed] [Google Scholar]
- [7].McLellan AT, Lewis DC, O’Brien CP, Kleber HD, Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation, JAMA 284 (2000) 1689–1695. [DOI] [PubMed] [Google Scholar]
- [8].See RE, Neural substrates of conditioned-cued relapse to drug-seeking behavior, Pharmacol. Biochem. Behav. 71 (2002) 517–529. [DOI] [PubMed] [Google Scholar]
- [9].Sinha R, New findings on biological factors predicting addiction relapse vulnerability, Curr. Psychiatry Rep. 13 (2011) 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME, Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving, Nature 454 (2008) 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, Parlato R, Sprengel R, Luscher C, Schutz G, Spanagel R, Glutamate receptors on dopamine neurons control the persistence of cocaine seeking, Neuron 59 (2008) 497–508. [DOI] [PubMed] [Google Scholar]
- [12].Loweth JA, Tseng KY, Wolf ME, Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving, Neuropharmacology 76 (Pt B) (2014) 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wolf ME, Synaptic mechanisms underlying persistent cocaine craving, Nat. Rev. Neurosci. 17 (2016) 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Malison RT, McCance E, Carpenter LL, Baldwin RM, Seibyl JP, Price LH, Kosten TR, Innis RB, [123I]beta-CIT SPECT imaging of dopamine transporter availability after mazindol administration in human cocaine addicts, Psychopharmacology 137 (1998) 321–325. [DOI] [PubMed] [Google Scholar]
- [15].Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ, Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys, J Neurosci 21 (2001) 2799–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lu L, Grimm JW, Hope BT, Shaham Y, Incubation of cocaine craving after withdrawal: a review of preclinical data, Neuropharmacology 47 (1) (2004) 214–226 Suppl. [DOI] [PubMed] [Google Scholar]
- [17].Calipari ES, Siciliano CA, Zimmer BA, Jones SR, Brief intermittent cocaine self-administration and abstinence sensitizes cocaine effects on the dopamine transporter and increases drug seeking, Neuropsychopharmacology 40 (2015) 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR, Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation, Neuropsychopharmacology 30 (2005) 1455–1463. [DOI] [PubMed] [Google Scholar]
- [19].Ferris MJ, Mateo Y, Roberts DC, Jones SR, Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration, Biol. Psychiatry 69 (2011) 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ferris MJ, Calipari ES, Mateo Y, Melchior JR, Roberts DC, Jones SR, Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate, Neuropsychopharmacology 37 (2012) 1708–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR, Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter, Neuropsychopharmacology 38 (2013) 2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kawa AB, Valenta AC, Kennedy RT, Robinson TE, Incentive and dopamine sensitization produced by intermittent but not long access cocaine self-administration, Eur. J. Neurosci. 50 (2019) 2663–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Murray CH, Gaulden AD, Kawa AB, Milovanovic M, Caccamise AJ, Funke JR, Patel S, Wolf ME, CaMKII modulates diacylglycerol lipase-alpha activity in the rat nucleus accumbens after incubation of cocaine craving, eNeuro 8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE, Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use, Biol. Psychiatry 58 (2005) 751–759. [DOI] [PubMed] [Google Scholar]
- [25].Zimmer BA, Oleson EB, Roberts DC, The motivation to self-administer is increased after a history of spiking brain levels of cocaine, Neuropsychopharmacology 37 (2012) 1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You ZB, McCarthy MM, Shaham Y, Ikemoto S, Incubation of cocaine craving after intermittent-access self-administration: sex differences and estrous cycle, Biol. Psychiatry 85 (2019) 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Corbett CM, Dunn E, Loweth JA, Effects of sex and estrous cycle on the time course of incubation of cue-induced craving following extended-access cocaine self-administration, eNeuro 8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen B, Wang Y, Liu X, Liu Z, Dong Y, Huang YH, Sleep regulates incubation of cocaine craving, J. Neurosci. 35 (2015) 13300–13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yorgason JT, España RA, Jones SR, Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures, J. Neurosci. Methods. (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brodnik ZD, Black EM, Clark MJ, Kornsey KN, Snyder NW, España RA, Susceptibility to traumatic stress sensitizes the dopaminergic response to cocaine and increases motivation for cocaine, Neuropharmacology 125 (2017) 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Alonso IP, Pino JA, Kortagere S, Torres GE, España RA, Dopamine transporter function fluctuates across sleep/wake state: potential impact for addiction, Neuropsychopharmacology (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brodnik ZD, Black EM, España RA, Accelerated development of cocaine-associated dopamine transients and cocaine use vulnerability following traumatic stress, Neuropsychopharmacology 45 (2020) 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marcondes FK, Bianchi FJ, Tanno AP, Determination of the estrous cycle phases of rats: some helpful considerations, Braz. J. Biol 62 (2002) 609–614. [DOI] [PubMed] [Google Scholar]
- [34].Roberts JG, Toups JV, Eyualem E, McCarty GS, Sombers LA, In situ electrode calibration strategy for voltammetric measurements in vivo, Anal. Chem. 85 (2013) 11568–11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA, Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry, J. Neurosci. Methods 112 (2001) 119–133. [DOI] [PubMed] [Google Scholar]
- [36].Levy KA, Brodnik ZD, Shaw JK, Perrey DA, Zhang Y, España RA, Hypocretin receptor 1 blockade produces bimodal modulation of cocaine-associated mesolimbic dopamine signaling, Psychopharmacology 234 (2017) 2761–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Brodnik ZD, Alonso IP, Xu W, Zhang Y, Kortagere S, España RA, Hypocretin receptor 1 involvement in cocaine-associated behavior: therapeutic potential and novel mechanistic insights, Brain Res. 1731 (2020) 145894. [DOI] [PubMed] [Google Scholar]
- [38].John CE & Jones SR (2007) Fast Scan Cyclic Voltammetry of Dopamine and Serotonin in Mouse Brain Slices. In Michael AC, Borland LM (eds) Electrochemical Methods for Neuroscience, Boca Raton (FL). [PubMed] [Google Scholar]
- [39].Brodnik ZD, Xu W, Batra A, Lewandowski SI, Ruiz CM, Mortensen OV, Kortagere S, Mahler SV, España RA, Chemogenetic manipulation of dopamine neurons dictates cocaine potency at distal dopamine transporters, J. Neurosci. 40 (2020) 8767–8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Diester CM, Banks ML, Neigh GN, Negus SS, Experimental design and analysis for consideration of sex as a biological variable, Neuropsychopharmacology 44 (2019) 2159–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Garcia-Sifuentes Y, Maney DL, Reporting and misreporting of sex differences in the biological sciences, Elife (2021) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Radke AK, Sneddon EA, Monroe SC, Studying sex differences in rodent models of addictive behavior, Curr. Protoc 1 (2021) e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Beveridge TJR, Wray P, Brewer A, Shapiro B, Mahoney JJ, Newton TF, Analyzing human cocaine use patterns to inform animal addiction model development, Published Abstract for the College on Problems of Drug Dependence Annual Meeting, 2012. Palm Springs, CA. [Google Scholar]
- [44].Ahmed SH, Lin D, Koob GF, Parsons LH, Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels, J. Neurochem. 86 (2003) 102–113. [DOI] [PubMed] [Google Scholar]
- [45].Guillem K, Ahmed SH, Peoples LL, Escalation of cocaine intake and incubation of cocaine seeking are correlated with dissociable neuronal processes in different accumbens subregions, Biol. Psychiatry 76 (2014) 31–39. [DOI] [PubMed] [Google Scholar]
- [46].Li X, Caprioli D, Marchant NJ, Recent updates on incubation of drug craving: a mini-review, Addict. Biol. 20 (2015) 872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kawa AB, Bentzley BS, Robinson TE, Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior, Psychopharmacology 233 (2016) 3587–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Allain F, Minogianis EA, Roberts DC, Samaha AN, How fast and how often: The pharmacokinetics of drug use are decisive in addiction, Neurosci. Biobehav. J 56 (2015) 166–179. [DOI] [PubMed] [Google Scholar]
- [49].Allain F, Samaha AN, Roberts DC, Samaha AN, Revisiting long-access versus short-access cocaine self-administration in rats: intermittent intake promotes addiction symptoms independent of session length, Addict Biol 24 (2019) 641–651. [DOI] [PubMed] [Google Scholar]
- [50].Saunders BT, Yager LM, Robinson TE, Cue-evoked cocaine “craving”: role of dopamine in the accumbens core, J Neurosci 33 (2013) 13989–14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Berridge KC, Robinson TE, Liking, wanting, and the incentive-sensitization theory of addiction, Am Psychol 71 (2016) 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Saddoris MP, Terminal Dopamine Release Kinetics in the Accumbens Core and Shell Are Distinctly Altered after Withdrawal from Cocaine Self-Administration, eNeuro (2016) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cameron CM, Wightman RM, Carelli RM, One month of cocaine abstinence potentiates rapid dopamine signaling in the nucleus accumbens core, Neuropharmacology 111 (2016) 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ferris MJ, España RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R, Jones SR, Dopamine transporters govern diurnal variation in extracellular dopamine tone, Proc. Natl. Acad. Sci. USA 111 (2014) E2751–E2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pothos EN, Regulation of dopamine quantal size in midbrain and hippocampal neurons, Behav Brain Res 130 (2002) 203–207. [DOI] [PubMed] [Google Scholar]
- [56].Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM, Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool, J Neurosci 26 (2006) 3206–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Purgianto A, Scheyer AF, Loweth JA, Ford KA, Tseng KY, Wolf ME, Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self-administration regimens, Neuropsychopharmacology 38 (2013) 1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC, CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking, Nat. Neurosci. 11 (2008) 344–353. [DOI] [PubMed] [Google Scholar]
- [59].Brown MT, Bellone C, Mameli M, Labouebe G, Bocklisch C, Balland B, Dahan L, Lujan R, Deisseroth K, Luscher C, Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation, PLoS One 5 (2010) e15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Robinson TE, Berridge KC, The neural basis of drug craving: an incentive-sensitization theory of addiction, Brain Res Brain Res Rev 18 (1993) 247–291. [DOI] [PubMed] [Google Scholar]
- [61].Siciliano CA, Jones SR, Cocaine Potency at the Dopamine Transporter Tracks Discrete Motivational States During Cocaine Self-Administration, Neuropsychopharmacology 42 (2017) 1893–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


