Abstract
Purpose
Coronavirus disease 2019 (COVID-19) has highlighted the need for new methods of pharmacovigilance. Here, we use adult community volunteers to obtain systematic information on vaccine effectiveness and the nature and severity of breakthrough infections.
Methods
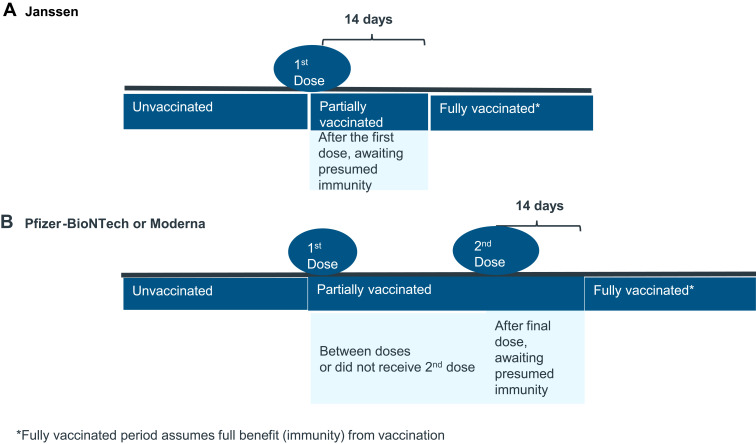
Between December 15, 2020 and September 16, 2021, 11,826 unpaid community-based volunteers reported the following information to an on-line registry: COVID-19 test results, vaccination (Pfizer, Moderna, or Johnson & Johnson) and COVID-19 symptoms. COVID-19 infections were described based on vaccination status at the time of infection: 1) fully vaccinated, 2) partially vaccinated (received first of two-dose vaccines or were <14 days post-final dose), or 3) unvaccinated.
Results
Among 8554 participants who received any COVID-19 vaccine, COVID-19 infections were reported by 74 (1.0%) of those who were fully vaccinated and 198 (2.3%) of those who were partially vaccinated at the time of infection. Among the 74 participants who reported a breakthrough infection after full vaccination, the median time from vaccination to reported positive test result was 104.5 days (interquartile range: 77–135 days), with no difference among vaccine manufacturers. One quarter (25.7%) of breakthrough infections in the fully vaccinated cases were asymptomatic and most (>97%) fully vaccinated participants reported no symptoms or only mild symptoms compared to 89.3% of the unvaccinated cases. Only 1.4% of fully vaccinated participants reported experiencing at least 3 moderate-to-severe symptoms compared to 7.8% in the unvaccinated.
Conclusion
Person-generated health data, also referred to as patient-reported outcomes, is a useful approach for quantifying breakthrough infections and their severity and for comparing vaccines.
Trial Registration
Clinicaltrials.gov NCT04368065, EU PAS Register EUPAS36240.
Keywords: COVID-19, vaccines, breakthrough infections, SARS-CoV-2, symptoms, patient-reported outcomes
Background
The three Coronavirus disease 2019 (COVID-19) vaccines authorized by the United States Food and Drug Administration (FDA) as of this writing have been found to be safe and highly efficacious in preventing symptomatic, laboratory-confirmed COVID-19 infections in randomized controlled trials.1–3 But, as is not uncommon with even highly effective vaccines, these vaccines confer infection-permissive or “non-sterilizing” immunity, which may permit infection while preventing symptoms, and especially severe symptoms.4
Infection in vaccinated persons is referred to as a “breakthrough” infection, defined by the US Centers for Disease Control and Prevention (CDC) as those who have detectable SARS-CoV-2 RNA or antigen on a respiratory specimen collected ≥14 days after completion of a vaccine regimen.1 While cases of COVID-19 do occur after vaccination, an excess of breakthrough infections could suggest a lack of vaccine effectiveness, consistent with the FDA definition of an adverse event as including “failure to produce the expected pharmacologic action”.5 For COVID, these concerns may be exacerbated by the emergence of viral mutations conferring possible vaccine escape, including some known variants of concern, making prompt detection of lack of effectiveness a public health imperative.6
While some serious safety concerns remain for COVID-19 vaccines, eg, anaphylaxis7,8 and Guillain-Barre Syndrome,9 lack of vaccine effectiveness is of particular importance given the potential risk of severe COVID-19 disease and mortality. Such information may also be useful for addressing the hesitancy occasioned by the accelerated development of these vaccines.10,11. While there are some reports quantifying the extent of breakthrough infections and severity of any associated symptoms based on surveillance or electronic medical record data,12–19 person-generated data, sometimes called patient-reported health data or patient reported outcomes provide additional insights, especially for symptoms that may not occasion medical care.
The objective of this work was to describe breakthrough infections in adults who received any of the three COVID-19 vaccines currently authorized for use in the US (BioNTech (Pfizer), Moderna, Inc. (Moderna), and Janssen Pharmaceuticals (Johnson & Johnson (J&J)) or who were not vaccinated. We describe the number of infections reported and participant-reported COVID-19 symptom information, stratified by vaccination status (fully vaccinated, partially vaccinated, and unvaccinated at the time of infection), illustrating how data generated by community reporters (here, people with confirmed COVID-19 infections) can be used for pharmacovigilance.
Materials and Methods
We leveraged data from a web-based registry, the COVID-19 Active Registry Experience (CARE, see www.helpstopcovid19.com). CARE was launched on April 2, 2020 to study COVID-19 symptoms and severity outside of the hospital setting to examine what factors, if any, mitigate the impact of infection with COVID-19.20–22 The protocol and web technology are updated periodically, with the COVID-19 vaccination questions reported here added on December 15, 2020. Participants are recruited via social media, targeting adult US residents, and are not compensated for participation. All provide informed consent online. Participants are asked to complete surveys once a week for the first month after enrollment, and then twice a month after the first month. They report information on their demographics, medical history, COVID-19-like symptoms and severity,20 COVID-19 tests, COVID-19 vaccination, and use of prescription and non-prescription medications and dietary supplements in the enrollment and follow-up surveys. Those who report having been vaccinated are also asked to provide the date of vaccination and side effects for first and second doses, as appropriate, manufacturer, and lot number. Participants are instructed to refer to their vaccination card to enhance data accuracy. This study was approved by an Institutional Review Board (Advarra) and registered with Clinicaltrials.gov NCT04368065, and the study complies with the Declaration of Helsinki.
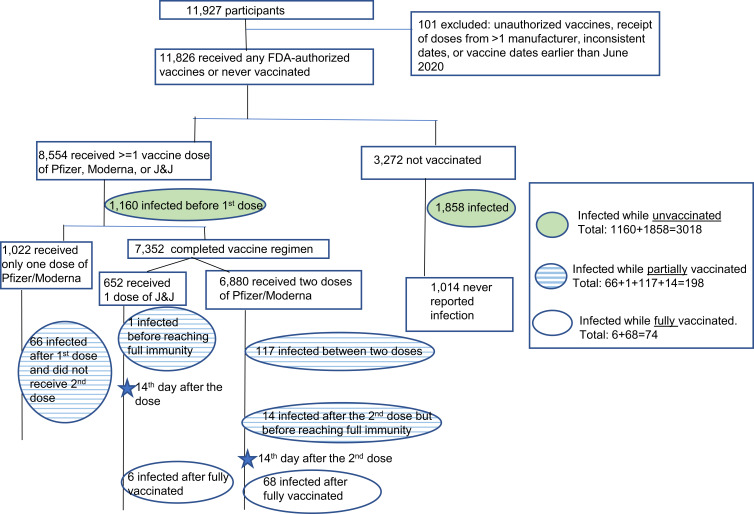
Surveys were submitted by 11,927 participants between December 15, 2020 and September 16, 2021. We excluded 101 reports of unauthorized vaccines, receipt of doses from more than one manufacturer, inconsistent dates, or vaccine dates earlier than June 2020. The analytic population consisted of 11,826 participants, including 8554 who had ever received a Pfizer, Moderna, or J&J vaccine, and 3272 who were not vaccinated during the study period. Among the 8554 participants, 7532 completed vaccination (ie, two doses of Pfizer or Moderna, or one dose of J&J), and 1022 had an incomplete vaccination regimen (ie, one dose of Pfizer or Moderna). Vaccination status was determined at the time of their positive COVID-19 test (Figure 1). For those who reported multiple positive COVID-19 test results, we considered tests within 90 days of each other as the same infection23 and used the first positive test to mark the start of the infection. Only a few participants (21 [0.2%] out of 11,826) have reported positive tests more than 90 days apart; for these participants we used the first positive test of the most recent course of infection. For participants who never received any COVID-19 vaccines during follow-up, we examined all positive tests occurring after December 15, 2020. Thus, participants only had one test result selected for this analysis and one corresponding vaccination status at the time of that test result, with no minimum requirement of follow-up time. For the analytic sample (n = 11,826), the average follow-up time is 73 days (median: 32 days, range: 0–502 days).
Figure 1.
COVID-19 breakthrough infection journey by manufacturer of COVID vaccine (A Janssen; B Pfizer-BioNTech or Moderna).
To assess potential confounding of other characteristics on COVID-19 infection symptoms, we performed two multivariate logistic regression models on these two outcomes respectively: (1) reporting at least one symptom (vs no symptom), and (2) reporting ≥1 moderate/severe symptom (vs no moderate/severe symptom). Covariates included in the models included age, gender, education, race, ethnicity, BMI category, smoking status, influenza vaccination status, cancer treatment status (yes/no), and any history of other medical condition (yes/no).
Results
Characteristics of Study Participants
Of 11,826 participants in our study population, 8554 reported receiving at least one dose of an FDA-authorized vaccine and 3272 did not report any vaccination. The average age among the study participants was 46.5 years (range: 18–91 years). The majority were female (82.9%), with some college or higher education (87.6%), White (86.5%), non-smokers (88.7%), and also had received prior vaccination for influenza (67.5%) (Table 1). Compared with non-vaccinated participants, those who received any vaccination were older (47.1% of ≥50 years vs 31.4%), had higher proportion of pregnant women (14.3% vs 0.0%), completed 4-year college or higher degree (60% vs 38.1%), were White (89.9% vs 77.5%) and non-Hispanic or Latino (93.5% vs 83.23%), non-smoker (90.4% vs 84.31%) and reported history of medical conditions such as depression and cardiovascular diseases.
Table 1.
Characteristics at Enrollment of Study Participants*
| All Study Participants N=11,826 | By Vaccination Status | ||
|---|---|---|---|
| Participants who Received at Least 1 COVID-19 Vaccine N=8554 | Participants who Did Not Receive Any COVID-19 Vaccine N=3272 | ||
| Age in years (N) | 11,823 | 8553 | 3270 |
| Mean (SD) | 46.5 (15.2) | 47.9 (15.42) | 42.8 (13.8) |
| Age Group (years, n[%]) | 11,823 | 8553 | 3270 |
| 18–29 | 1601 (13.5) | 994 (11.6) | 607 (18.6) |
| 30–39 | 2932 (24.8) | 2094 (24.5) | 838 (25.6) |
| 40–49 | 2238 (18.9) | 1439 (16.8) | 799 (24.4) |
| 50–59 | 2161 (18.3) | 1590 (18.6) | 571 (17.5) |
| ≥60 | 2891 (24.5) | 2436 (28.5) | 455 (13.9) |
| Gender, n (%) | 11,825 | 8554 | 3271 |
| Female | 9800 (82.9) | 7137 (83.4) | 2663 (81.4) |
| Male | 1772 (15.0) | 1200 (14.0) | 572 (17.5) |
| Transgender/Other/ Not Disclosed | 253 (2.1) | 217 (2.5) | 36 (1.1) |
| Pregnant, n (%) | 9800 | 7137 | 2663 |
| Yes | 1019 (10.4) | 1019 (14.3) | 0 (0.0) |
| Education, n (%) | 11,803 | 8538 | 3265 |
| High school or less | 1466 (12.4) | 782 (9.2) | 684 (21.0) |
| Some college | 3966 (33.6) | 2629 (30.8) | 1337 (41.0) |
| 4-yr college degree | 2865 (24.3) | 2212 (25.9) | 653 (20) |
| >4yr college degree | 3506 (29.7) | 2915 (34.1) | 591 (18.1) |
| Race, n (%) | 11,805 | 8514 | 3264 |
| White | 10,208 (86.5) | 7680 (89.9) | 2528 (77.5) |
| Black or African American | 384 (3.3) | 149 (1.7) | 235 (7.2) |
| Other** | 1213 (10.3) | 712 (8.3) | 501 (15.4) |
| Ethnicity, n (%) | 11,705 | 8491 | 3214 |
| Hispanic or Latino | 1092 (9.3) | 553 (6.5) | 539 (16.8) |
| BMI, n (%) | 11,607 | 8399 | 3208 |
| Underweight or Normal weight (15.0 ≤ BMI< 25.0) | 3156 (27.19) | 2240 (26.7) | 916 (28.6) |
| Overweight (25.0 ≤ BMI < 30.0) | 3146 (27.1) | 2328 (27.7) | 818 (25.5) |
| Obese (30.0 ≤ BMI ≤ 40.0) | 3871 (33.4) | 2820 (33.4) | 1051 (32.8) |
| Severe Obesity (BMI>40.0) | 1434 (12.4) | 1011 (12.0) | 423 (13.2) |
| Smoker, n (%) | 11,371 | 8217 | 3154 |
| Yes | 1284 (11.29) | 789 (9.6) | 495 (15.7) |
| Vaccinated for Influenza, n (%) | 10,954 | 8157 | 2797 |
| Yes | 7393 (67.5) | 6012 (73.7) | 1381 (49.4) |
| Medical Conditions, n (%) | 11,826 | 8554 | 3272 |
| Anxiety | 1963 (16.6) | 1459 (17.1) | 504 (15.4) |
| Autoimmune disease | 775 (6.55) | 593 (6.9) | 182 (5.6) |
| Blood disorder | 74 (0.63) | 69 (0.8) | 5 (0.15) |
| Cardiovascular disease | 320 (2.7) | 300 (3.5) | 20 (0.61) |
| Depression | 2251 (19.03) | 1742 (20.4) | 509 (15.6) |
| Diabetes | 973 (8.2) | 712 (8.3) | 261 (8.0) |
| Hypertension | 2416 (20.4) | 1794 (21.0) | 622 (19.0) |
| Insomnia or trouble sleeping | 678 (5.7) | 512 (6.0) | 166 (5.1) |
| Kidney disease | 34 (0.3) | 31 (0.4) | 3 (0.09) |
| Lung disease | 841 (7.1) | 625 (7.3) | 216 (6.6) |
| Organ transplant | 13 (0.1) | 13 (0.2) | 0 (0.0) |
| Whether on Treatment for Cancer, n (%) | 11,764 | 8529 | 3235 |
| Yes | 123 (1.1) | 99 (1.2) | 24 (0.7) |
Notes: *Percentages in this table are column percentages. **Including 226 Asians, 67 American Indian or Alaska Native, 12 Native Hawaiian or other Pacific Islander, and 908 Other.
Among vaccinated participants, comparison by vaccine manufacturer showed no difference in gender, race, and ethnicity, but slight differences in age and some comorbid conditions: age: 49.4 Moderna, 47.0 J&J, and 46.9 Pfizer (p < 0.01); cardiovascular disease: 4.3% Moderna vs 3.8% J&J and 2.8% Pfizer (p < 0.01); diabetes: 9.3% Moderna vs 8.0% J&J and 7.6% Pfizer (p = 0.02); hypertension: 23.9% Moderna vs 17.6% J&J and 19.1% Pfizer (p < 0.001); insomnia: 6.7% Moderna vs 7.1% J&J and 5.2% Pfizer (p < 0.01).
COVID-19 Infected Study Participants
The counts of participants who reported a positive COVID-19 test by vaccination status at the time of infection are shown in Figure 2 and Table 2. Among the 7532 fully vaccinated participants, 74 (1.0%) participants reported an infection, a proportion that was generally similar across manufacturers (n= 6 (0.9%) for J&J, n = 52 (1.4%) for Pfizer, and n = 16 (0.5%) for Moderna). The proportion of participants who reported an infection when they were partially vaccinated was 2.3% (n = 198), also comparable across manufacturers with 0.2% for J&J, 2.4% for Pfizer, and 2.6% for Moderna.
Figure 2.
Flowchart of study sample and participant counts with COVID-19 infection.
Table 2.
Number of Participants with COVID-19 Infections by Vaccination Status and Characteristics of Infection
| Total | Ever Vaccinated | Never Vaccinated | |||
|---|---|---|---|---|---|
| J & J | Pfizer | Moderna | |||
| Total number of study participants | 11,826 | 652 | 4322 | 3580 | 3272 |
|
8554 | 652 | 4322 | 3580 | |
| o Completed vaccine regimen | 7532 | 652 | 3788 | 3092 | |
| – Completed two doses of Pfizer/Moderna | 6880 | 3788 | 3092 | ||
| o Received only one dose of Pfizer/Moderna | 1022 | 534 | 488 | ||
|
3272 | 3272 | |||
| Total number of infected participants | 3290 | 108 | 743 | 581 | 1858 |
|
74 (1.0) | 6 (0.9) | 52 (1.4) | 16 (0.5) | |
|
198 (2.3) | 1 (0.2) | 103 (2.4) | 94 (2.6) | |
| o After final dose, awaiting presumed immunity (0–13 days after final dose) | 15 (0.2) | 1 (0.2) | 12 (0.3) | 2 (0.1) | |
| o Between two doses | 117 (1.7) | 64 (1.7) | 53 (1.7) | ||
| o After 1st dose and did not receive 2nd dose | 66 (6.5) | 27 (5.1) | 39 (8.0) | ||
|
3018 (25.5) | 101 (15.5) | 588 (13.6) | 471 (13.2) | 1858 (56.8) |
| o Infection prior to first dose | 1160 (13.6) | 101 (15.5) | 588 (13.6) | 471 (13.2) | |
| o Infection and never vaccinated | 1858 (56.8) | 1858 (56.8) | |||
| Time interval from COVID-19 vaccination to infection | |||||
|
74 | 6 | 52 | 16 | N/A |
| Mean (SD) | 101.4 (44.99) | 78.7 (61.17) | 100.1 (42.82) | 113.9 (44.64) | N/A |
| Median [IQR] | 104.5 [77.0–135.0] | 91.5 [10.0–127.0] | 104.0 [75.0–132.5] | 108.5 [88.5–148.0] | N/A |
| Min - Max | 2–196 | 2–150 | 12–196 | 14–190 | N/A |
|
198 | 1 | 103 | 94 | N/A |
| Mean (SD) | 11.3 (21.84) | 8.0 (-) | 10.4 (14.24) | 12.4 (28.03) | N/A |
| Median [IQR] | 8.0 [5.0–12.0] | 8.0 [8.0–8.0] | 8.0 [5.0–13.0] | 8.0 [5.0–12.0] | N/A |
| Min - Max | 0–223 | 8–8 | 0–128 | 0–223 | N/A |
Notes: *Percentages based on 7532 participants who had completed doses. €Percentages based on 8554 participants who received any vaccination. ΩPercentages based on all 11,826 participants.
Among 74 participants who reported a breakthrough infection after full vaccination, the median time from 14 days post-final vaccination dose to reported positive test result was 104.5 days, Interquartile range (IQR): 77–135 days, with no significant differences across vaccine manufactures: 91.5 days for J&J, 104.0 days for Pfizer, and 108.5 days for Moderna (p-value = 0.44) (Table 2). Among the 198 people infected after partial vaccination, the median time from the first dose to infection was 8.0 days (IQR: 5.0–12.0 days).
We also looked at the infection counts by age group (Table 3). Participants aged 60 years or more had slightly lower infection rates than younger participants at most time points of vaccine status.
Table 3.
Number of Participants with COVID-19 Infections by Age Group
| Total | By Age Group | |||||
|---|---|---|---|---|---|---|
| 18–29 Years | 30–39 Years | 40–49 Years | 50–59 Years | 60+ Years | ||
| Total number of study participants | 11,826 | 1601 | 2932 | 2238 | 2161 | 2891 |
|
8554 | 994 | 2094 | 1439 | 1590 | 2436 |
| o Completed vaccine regimen | 7532 | 827 | 1873 | 1224 | 1375 | 2233 |
| – Completed two doses of Pfizer/Moderna | 6880 | 734 | 1746 | 1104 | 1225 | 2071 |
| o Received only one dose of Pfizer/Moderna | 1022 | 167 | 221 | 215 | 215 | 203 |
|
3272 | 607 | 838 | 799 | 571 | 455 |
| Total number of infected participants | 3290 | 594 | 907 | 784 | 587 | 418 |
|
74(1.0%) | 12(1.5%) | 22(1.2%) | 14(1.1%) | 8(0.6%) | 18(0.8%) |
|
198(2.3%) | 25(2.5%) | 59(2.8%) | 43(3.0%) | 40(2.5%) | 31(1.3%) |
| o After final dose, awaiting presumed immunity (0–13 days after final dose) | 15(0.2%) | 2(0.2%) | 6(0.3%) | 3(0.2%) | 2(0.1%) | 2(0.1%) |
| o Between two doses | 117(1.7%) | 12(1.6%) | 33(1.9%) | 28(2.5%) | 28(2.3%) | 16(0.8%) |
| o After 1st dose and did not receive 2nd dose | 66(6.5%) | 11(6.6%) | 20(9.0%) | 12(5.6%) | 10(4.7%) | 13(6.4%) |
|
3018(25.5%) | 557(34.8%) | 826(28.2%) | 927(41.4%) | 539(24.9%) | 369(12.8%) |
| o Infection prior to first dose | 1160(13.6%) | 146(14.7%) | 266(12.7%) | 266(18.5%) | 265(16.7%) | 217(8.9%) |
| o Infection and never vaccinated | 1858(56.8%) | 411(67.7%) | 560(66.8%) | 461(57.7%) | 274(48.0%) | 152(33.4%) |
| Time interval from COVID-19 vaccination to infection | ||||||
|
74 | 12 | 22 | 12 | 8 | 18 |
| Mean (SD) | 101.4 (44.99) | 96.3 (46.90) | 109.3 (50.00) | 92.2 (45.14) | 99.4 (37.72) | 103.0 (43.19) |
| Median [IQR] | 104.5 [77.0–135.0] | 110.5 [46.5–138.0] | 105.5 [77.0–143.0] | 98.0 [77.0–120.0] | 105.0 [99.0–113.5] | 104.0 [79.0–127.0] |
| Min - Max | 2–196 | 29–150 | 2–196 | 10–150 | 14–146 | 13–178 |
|
198 | 25 | 59 | 43 | 40 | 31 |
| Mean (SD) | 11.3 (21.84) | 6.8 (3.77) | 9.0 (5.36) | 12.3 (18.74) | 17.9 (42.42) | 9.8 (12.05) |
| Median [IQR] | 8.0 [5.0–12.0] | 6.0 [4.0–9.0] | 8.0 [5.0–12.0] | 10.0 [5.0–14.0] | 7.0 [4.0–11.5] | 7.0 [3.0–13.0] |
| Min - Max | 0–223 | 0–15 | 0–28 | 1–128 | 0–223 | 0–66 |
Notes: *Percentages based on 7532 participants who had completed doses. € Percentages based on 8554 participants who received any vaccination. Ω Percentages based on all 11,826 participants.
Among 74 people infected after full vaccination, their average age was 44.5 years (Table 4). Most were female (79.7%), White (91.9%), and 40.5% had above a college degree. There were small differences in age across the three vaccination status groups of infected people, with 24.3% of people aged ≥60 years in the fully vaccinated group and 15.7% and 12.2% in partially vaccinated and unvaccinated, respectively. Among the partially vaccinated and unvaccinated, there were fewer male participants (13.1% for both partially vaccinated and unvaccinated versus 17.6% among fully vaccinated) and they had less education (31.8% and 20.3%, were college-educated versus 40.5% among fully vaccinated). Other demographics and medical characteristics including hypertension, diabetes, obesity, were similarly distributed.
Table 4.
Characteristics of COVID-19 Positive Participants at Enrollment *
| All COVID-19 Infections N=3290 | Infected After Full Vaccination N=74 | Infected After Partial Vaccination N=198 | Infected While Not Vaccinated N=3018 | |
|---|---|---|---|---|
| Age in years (N) | 3290 | 74 | 198 | 3018 |
| Mean (SD) | 42.3 (13.40) | 44.5 (14.98) | 44.3 (13.03) | 42.2 (13.37) |
| Age Group (years, n[%]) | 3290 | 74 | 198 | 3018 |
| 18–29 | 594 (18.1) | 12 (16.2) | 25 (12.6) | 557 (18.5) |
| 30–39 | 907 (27.6) | 22 (29.7) | 59 (29.8) | 826 (27.4) |
| 40–49 | 784 (23.8) | 14 (18.9) | 43 (21.7) | 727 (24.1) |
| 50–59 | 587 (17.8) | 8 (10.8) | 40 (20.2) | 539 (17.9) |
| ≥60 | 418 (12.7) | 18 (24.3) | 31 (15.7) | 369 (12.2) |
| Gender, n(%) | 3290 | 74 | 198 | 3018 |
| Female | 2821 (85.7) | 59 (79.7) | 170 (85.9) | 2592 (85.9) |
| Male | 434 (13.2) | 13 (17.6) | 26 (13.1) | 395 (13.1) |
| Transgender/Other/ Not Disclosed | 35 (1.1) | 2 (2.7) | 2 (1) | 31 (1.0) |
| Pregnant, n(%) | 2821 | 59 | 170 | 2592 |
| Yes | 73 (2.6) | 12 (20.3) | 3 (1.8) | 58 (2.2) |
| Education, n(%) | 3289 | 74 | 198 | 3017 |
| High school or less | 564 (17.2) | 4 (5.4) | 21 (10.6) | 539 (17.9) |
| Some college | 1304 (39.7) | 21 (28.4) | 70 (35.4) | 1213 (40.2) |
| 4-yr college degree | 715 (21.7) | 19 (25.7) | 44 (22.2) | 652 (21.6) |
| >4yr college degree | 706 (21.5) | 30 (40.5) | 63 (31.8) | 613 (20.3) |
| Race, n(%) | 3286 | 74 | 198 | 3014 |
| Black or African American | 202 (6.2) | 3 (4.1) | 6 (3.0) | 193 (6.4) |
| White | 2640 (80.3) | 68 (91.9) | 179 (90.4) | 2393 (79.4) |
| Other** | 444 (13.5) | 3 (4.1) | 13 (6.6) | 428 (14.2) |
| Ethnicity | 3245 | 74 | 195 | 2976 |
| Hispanic or Latino | 538 (16.6) | 5 (6.8) | 20 (10.3) | 513 (17.2) |
| BMI, n(%) | 3242 | 73 | 198 | 2971 |
| Underweight or Normal weight (15.0 ≤ BMI< 25.0) | 795 (24.5) | 13 (17.8) | 36 (18.2) | 746 (25.1) |
| Overweight (25.0 ≤ BMI < 30.0) | 825 (25.5) | 22 (30.1) | 53 (26.8) | 750 (25.2) |
| Obese (30.0 ≤ BMI ≤ 40.0) | 1145 (35.2) | 28 (38.4) | 79 (39.9) | 1038 (34.9) |
| Severe Obesity (BMI>40.0) | 477 (14.7) | 10 (13.7) | 30 (15.2) | 437 (14.7) |
| Smoking status, n(%) | 3211 | 73 | 197 | 2941 |
| Smoker | 342 (10.7) | 8 (11.0) | 18 (9.1) | 316 (10.7) |
| Vaccinated previously for Influenza, n(%) | 3231 | 74 | 194 | 2963 |
| Vaccinated | 1867 (57.8) | 58 (78.4) | 162 (83.5) | 1647 (55.6) |
| Medical Conditions, n(%) | 3290 | 74 | 198 | 3018 |
| Anxiety | 608 (18.5) | 12 (16.2) | 41 (20.7) | 555 (18.4) |
| Autoimmune disease | 192 (5.8) | 6 (8.1) | 14 (7.1) | 172 (5.7) |
| Blood disorder | 10 (0.3) | 0 | 1 (0.5) | 9 (0.3) |
| Cardiovascular disease | 19 (0.6) | 3 (4.1) | 2 (1.0) | 14 (0.5) |
| Depression | 594 (18.1) | 12 (16.2) | 57 (28.8) | 525 (17.4) |
| Diabetes | 238 (7.2) | 5 (6.8) | 13 (6.6) | 220 (7.3) |
| Hypertension | 634 (19.3) | 13 (17.6) | 51 (25.8) | 570 (18.9) |
| Insomnia or trouble sleeping | 210 (6.4) | 3 (4.1) | 14 (7.1) | 193 (6.4) |
| Kidney disease | 4 (0.1) | 0 | 0 | 4 (0.1) |
| Lung disease | 221 (6.7) | 4 (5.4) | 14 (7.1) | 203 (6.7) |
| Organ transplant | 1 (0.03) | 0 | 0 | 1 (0.03) |
| Whether on Treatment for Cancer, n (%) | 3272 | 74 | 197 | 3001 |
| Yes | 29 (0.9) | 1 (1.4) | 3 (1.5) | 25 (0.8) |
Notes: *Percentages in this table are column percentages. **Including 69 Asians, 26 American Indian or Alaska Native, 4 Native Hawaiian or other Pacific Islander, and 345 Other.
COVID-19 Symptoms and Impact Among Vaccinated vs Unvaccinated
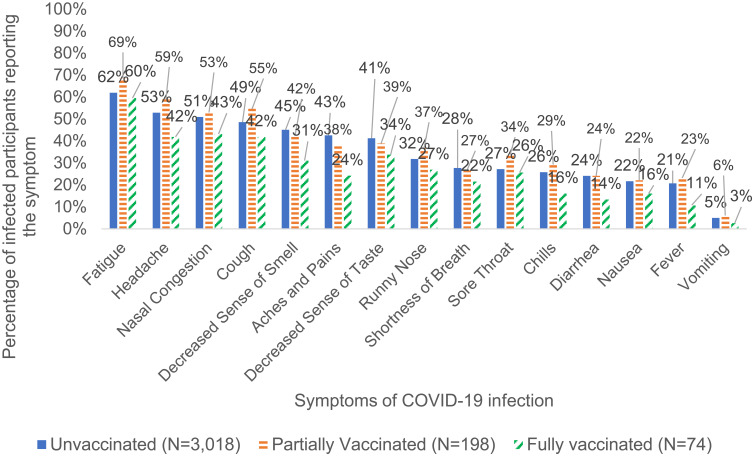
The fully vaccinated group with breakthrough infections had lower proportions of every COVID-19 symptom compared to the partially vaccinated and unvaccinated groups, including headache, aches and pains, decreased sense of taste and smell, chills, and diarrhea (Table 5 and Figure 3). Fully vaccinated breakthrough cases reported fewer mean number of COVID-19 symptoms (4.1) when compared to partially vaccinated (5.8) or unvaccinated cases (5.3).
Table 5.
COVID-19 Infection Symptoms Among COVID-19 Positive Participants*
| All COVID-19 Infections N=3290 | Infected After Full Vaccination N=74 | Infected After Partial Vaccination N=198 | Infected While Not Vaccinated N=3018 | |
|---|---|---|---|---|
| COVID-19 symptoms, n (%) | ||||
| Aches and Pains | 1381 (42.0) | 18 (24.3) | 76 (38.4) | 1287 (42.6) |
| Chills | 850 (25.8) | 12 (16.2) | 58 (29.3) | 780 (25.8) |
| Decreased Sense of Smell | 1469 (44.7) | 23 (31.1) | 84 (42.4) | 1362 (45.1) |
| Cough | 1606 (48.8) | 31 (41.9) | 108 (54.6) | 1467 (48.6) |
| Decreased Sense of Taste | 1349 (41.0) | 25 (33.8) | 77 (38.9) | 1247 (41.3) |
| Diarrhea | 784 (23.8) | 10 (13.5) | 48 (24.2) | 726 (24.1) |
| Fatigue | 2051 (62.3) | 44 (59.5) | 136 (68.7) | 1871 (62.0) |
| Fever | 680 (20.7) | 8 (10.8) | 46 (23.2) | 626 (20.7) |
| Nausea | 712 (21.6) | 12 (16.2) | 44 (22.2) | 656 (21.7) |
| Nasal Congestion | 1677 (51.0) | 32 (43.2) | 105 (53.0) | 1540 (51.0) |
| Runny Nose | 1054 (32.0) | 20 (27.0) | 73 (36.9) | 961 (31.8) |
| Shortness of Breath | 906 (27.5) | 16 (21.6) | 53 (26.8) | 837 (27.7) |
| Sore Throat | 906 (27.5) | 19 (25.7) | 67 (33.8) | 820 (27.2) |
| Vomiting | 164 (5.0) | 2 (2.7) | 12 (6.1) | 150 (5.0) |
| Headache | 1744 (53.0) | 31 (41.9) | 117 (59.1) | 1596 (52.9) |
| Number of symptoms | ||||
| Mean (SD) | 5.3 (3.87) | 4.1 (3.73) | 5.8 (3.90) | 5.3 (3.87) |
| Median [IQR] | 5.0 [2.0–8.0] | 4.0 [0.0–6.0] | 5.0 [2.0–9.0] | 5.0 [2.0–8.0] |
| Min-Max | 0–15 | 0–14 | 0–15 | 0–15 |
| Category of number of symptoms, n (%) | ||||
| 0 symptoms | 542 (16.5) | 19 (25.7) | 27 (13.6) | 496 (16.4) |
| 1–2 symptoms | 418 (12.7) | 10 (13.5) | 27 (13.6) | 381 (12.6) |
| 3+ symptoms | 2330 (70.8) | 45 (60.8) | 144 (72.7) | 2141 (70.9) |
| Category of number of moderate to severe symptoms, n(%) | ||||
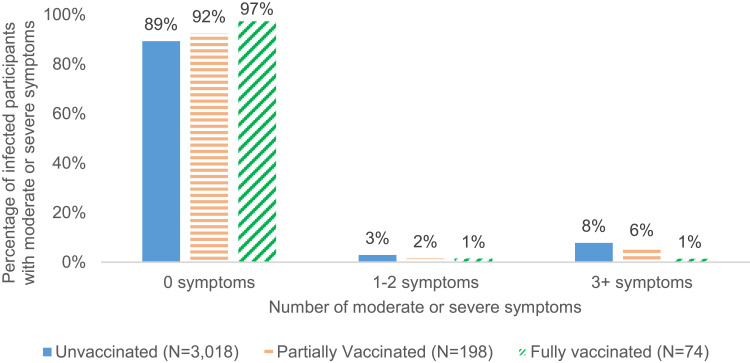
| 0 moderate or severe symptoms | 2945 (89.7) | 72 (97.3) | 182 (92.4) | 2691 (89.3) |
| 1–2 moderate or severe symptoms | 91 (2.8) | 1 (1.4) | 3 (1.5) | 87 (2.9) |
| 3+ moderate or severe symptoms | 247 (7.5) | 1 (1.4) | 12 (6.1) | 234 (7.8) |
| Hospitalization | ||||
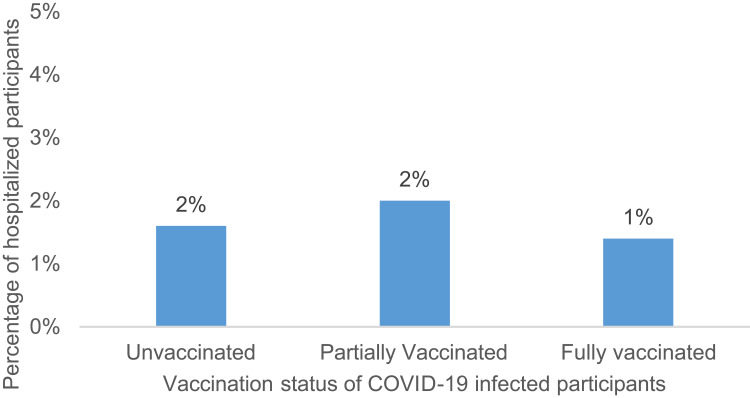
| Hospitalized | 54 (1.6) | 1 (1.4) | 4 (2) | 49 (1.6) |
Note: *Percentages in this table are column percentages.
Figure 3.
Percentage of COVID-19 infected participants with symptoms by vaccination status.
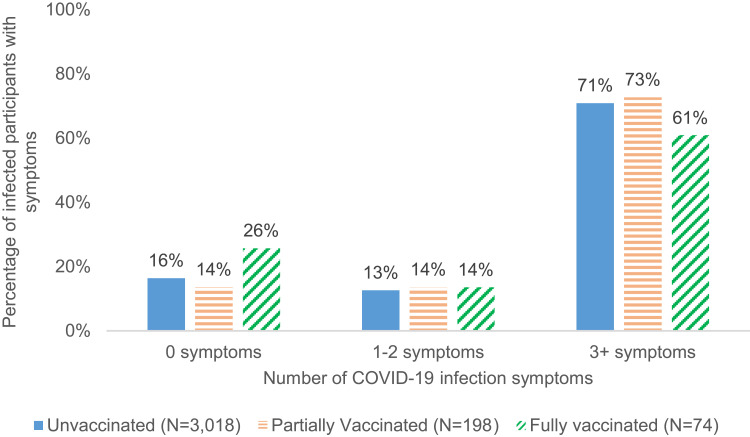
Approximately one quarter (25.7%) of fully vaccinated breakthrough cases reported no symptoms compared with 13.6% and 16.4% among partially vaccinated and unvaccinated cases, respectively (Figure 4). Fully vaccinated breakthrough cases were also more likely to report only mild symptoms and not report moderate or severe symptoms (97.3%) compared with partially vaccinated or unvaccinated cases (92.4% and 89.3%, respectively) (Table 5 and Figure 5). Hospitalization due to COVID infection was rare, with 1.4% reporting hospitalization among the 74 infected after full vaccination, 1.6% among the 198 infected after partial vaccination, and 2.0% among the 3018 infected while unvaccinated, respectively (Table 5 and Figure 6).
Figure 4.
Number of symptoms among COVID-19 infected participants by vaccination status.
Figure 5.
Number of moderate or severe symptoms among COVID-19 infected participants by vaccination status.
Figure 6.
Percentage of hospitalized participants among COVID-19 infected by vaccination status.
When accounting for the effects of potential confounders, results of both models suggested a protective effect of full vaccination on symptoms although the associations were not statistically significant (Odds Ratios < 1, p-values = 0.13 and 0.24 for association between vaccination status and reporting any symptom or reporting any moderate/severe symptom, respectively, Supplemental Tables 1 and 2).
Discussion
Systematic collection of person-generated health data as illustrated by the CARE registry demonstrates the value of coupling patient symptom information with COVID-19 test data to provide meaningful and timely information about vaccine effectiveness. This study corroborates other work showing that vaccination affords significant protection against severe symptoms of COVID-19 disease, and that it also confers protection against infection overall.12–18 Here 1.0% of all participants reported infections after full vaccination, consistent with the low rates reported elsewhere.24 Fully vaccinated individuals who experienced breakthrough infections reported fewer symptoms than partially vaccinated or unvaccinated at the time of study, with only a small fraction of fully vaccinated cases reporting moderate or severe symptoms and about one quarter experienced no symptoms at all. Although the multivariate logistic regression models suggested protection against symptom occurrence, the lack of statistical significance could be due to the relatively small study size and the large number of covariates.
In this study, there were a few differences between those who reported COVID-19 infection after full vaccination and those who reported infection while partially vaccinated. Those fully vaccinated had a slightly higher proportion of infection among males, individuals aged 60 years or above and those with at least a college education. These older and more educated participants may have been vaccinated earlier, and therefore had a longer “at risk” time for infection and/or potentially waning immunity after being fully vaccinated. Infection counts by age group in Table 3 showed that participants aged 60 years or more had slightly lower infection rates than younger participants at most time points of vaccine status. However, the results should be cautiously interpreted given the small sample sizes.
Also of interest, looking at all fully vaccinated participants, the median time to breakthrough infection was 105 days, ranging from 2 to 196 days. Other studies have found similarly long time to breakthrough infections, including a study in Massachusetts that reported a median time of 86 days after final vaccine dose.13,25 Interestingly, in this study the average time to breakthrough infection by manufacturer was not significantly different between manufacturers, with 91.5, 104.0, and 108.5 days for J&J, Pfizer, and Moderna, respectively. The proportions of participants reporting infections after completing the vaccination regimen were low, but they were notably higher (1.4%) for participants vaccinated with Pfizer as compared to J&J (0.9%) and Moderna (0.5%).
Among the 198 people infected after partial vaccination, the median time to infection was 8.0 days. The proportion of participants reporting infection within 14 days of completing at least one vaccination were similar for those vaccinated with Pfizer (2.4%) and Moderna (2.6%), and lower for those vaccinated for J&J (0.2%). Although this average time is much shorter than those who were fully vaccinated, it should be noted that the window for having infection while partially vaccinated was, by definition, shorter for J&J with a window of only 14 days until full immunity, compared to about 28 days between doses plus 14 days post-2nd dose (42 days) for those receiving Pfizer or Moderna.
Of note, several factors that might be expected to be associated with an increased risk of infection, such as hypertension, diabetes, and obesity, were not associated with COVID-19 infection in the fully vaccinated. This observation might offer encouraging mechanistic support for vaccine effectiveness.
This study has a number of strengths that enhance its information value. The rich symptom data permits more contextualization of the effectiveness of the vaccine than simply reporting a positive or negative COVID-19 test result. Vaccine reporting is likely to be more complete than the vaccination status recorded in most real-world data sources such as insurance claims data and/or electronic health record datasets, considering how vaccines were distributed in the United States and the lack of any single accessible central source of vaccination status information. Similarly, COVID-19 test results are also likely to be more complete than in many real-world databases, since participants were systematically queried to see if they were tested at every bi-weekly follow-up.
Nonetheless it is important to keep in mind that, like other online registries that survey volunteers without intervention or collaboration from medical care providers, the CARE registry relies on participants to contribute information completely, faithfully, and accurately. Missing data, especially outcomes of interest, are possible. Also, there was no independent validation of COVID-19 test result reporting, nor were there any clinical assessments of these person-generated symptoms. However, CARE emphasized the importance of data accuracy for example, by instructing participants to refer to their vaccination card when entering vaccination history. Furthermore, the validity of reports from lay people on medication use has been demonstrated,26 suggesting that reporting quality is likely to apply at least as well to vaccine reporting.
There also may be underreporting of breakthrough cases and misclassification of their timing and severity. Participation in follow-up surveys depends on sustained personal interest, and participants may have tested positive after reporting their vaccine information but not completed any follow-up surveys. It is also possible that individuals with asymptomatic breakthrough infections were not aware of their infection in the absence of widespread systematic surveillance testing in most parts of the US at the time these data were collected. Very serious cases of COVID-19 that required hospitalization or resulted in death may be underreported. Analytically, if only one positive test was reported, we assumed the positive test date reflected the start of the infection, whereas the infection may have started earlier and gone undetected prior to vaccination initiation.
The representativeness and generalizability of observational, volunteer-driven studies like CARE might be questioned. CARE has broad geographic representation, with participants from all 50 states but CARE participants are not a systematic sample of the US adult population. CARE participants are older, more likely to be female, and have less racial and ethnic diversity than adults living in the US. Respondents include those with internet access and sufficient availability and interest to participate in surveys. Projects like this tend to attract more females as well as those “worried well”, with higher participation among those with autoimmune disorders as well as anxiety, depression and insomnia. Nonetheless, it is unlikely that comparisons made here between vaccine manufacturers were biased as differential participation by vaccine manufacturers is unlikely, especially since there no isolated widespread new events drawing attention to one of these vaccines or another.
Conclusion
The systematic collection of person-generated health data shows that only a small proportion of vaccinated people became infected after vaccination, a majority of whom were asymptomatic or had fewer and milder symptoms than the unvaccinated, and that while cases of COVID-19 may occur in vaccinated individuals, these are uncommon and generally mild in nature. This efficient approach of direct-to-patient research can be used obtain information not readily available in most real-world data sources, and can be used effectively to study adverse events as well as medical product effectiveness.
Acknowledgments
We thank Lisa Albert for her work on preliminary analyses, Savitha Pallipuram for leading the technical team, and Alex Secora for his thoughtful editing contributions.
Funding Statement
This work was supported in part by a contract with the US Food and Drug Administration. The bulk of the funding was provided by IQVIA.
Data Sharing Statement
Due to data privacy and security regulations the researchers are not able to share participant level data.
Consent for Publication
Consent for publication of research finding was provided online.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Baden LR, El Sahly HM, Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384(5):403–416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi A, Ibanez LI, Strohmeier S, Krammer F, Garcia-Sastre A, Schotsaert M. Non-sterilizing, infection-permissive vaccination with inactivated influenza virus vaccine reshapes subsequent virus infection-induced protective heterosubtypic immunity from cellular to humoral cross-reactive immune responses. Front Immunol. 2020;11:1166. doi: 10.3389/fimmu.2020.01166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration. Guideline for Postmarketing Reporting of Adverse Drug Experiences. Rockville, MD: US Food and Drug Administration; 1992. [Google Scholar]
- 6.Thompson RN, Hill EM, Gog JR. SARS-CoV-2 incidence and vaccine escape. Lancet Infect Dis. 2021;21(7):913–914. doi: 10.1016/S1473-3099(21)00202-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(2):46–51. doi: 10.15585/mmwr.mm7002e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(2):125–129. doi: 10.15585/mmwr.mm7004e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao SC, Wang CH, Chang KC, Hung MJ, Chen HY, Liao SC. Guillain-barre syndrome associated with COVID-19 vaccination. Emerg Infect Dis. 2021;27(12):3175–3178. doi: 10.3201/eid2712.211634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misu T, Kortepeter CM, Munoz MA, Wu E, Dal Pan GJ. An evaluation of “Drug Ineffective” postmarketing reports in drug safety surveillance. Drugs Real World Outcomes. 2018;5(2):91–99. doi: 10.1007/s40801-018-0131-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geneva: Council for International Organizations of Medical Sciences (CIOMS). Development and Rational Use of Standardised MedDRA Queries (Smqs): Retrieving Adverse Drug Reactions with MedDRA. 2nd ed. 2016. [Google Scholar]
- 12.Ontario Agency for Health Protection and Promotion (Public Health Ontario). SARS-Cov-2 Infections After Vaccination – What We Know so Far. Toronto, ON: Queen’s Printer for Ontario; 2021. [Google Scholar]
- 13.Teran RA, Walblay KA, Shane EL, et al. Postvaccination SARS-CoV-2 infections among skilled nursing facility residents and staff members - Chicago, Illinois, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):632–638. doi: 10.15585/mmwr.mm7017e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang L, Hijano DR, Gaur AH, et al. Asymptomatic and symptomatic SARS-CoV-2 infections after BNT162b2 vaccination in a routinely screened workforce. JAMA. 2021;325(24):2500–2502. doi: 10.1001/jama.2021.6564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pera M. Marin records 21 “breakthrough” infections after COVID-19 vaccines. The Mercury News; 2021.
- 16.Haas R. Oregon reports small number of COVID cases in vaccinated people. OPB; 2021.
- 17.Washington State Department of Health. SARS-CoV-2 vaccine breakthrough surveillance and case information resource; 2022. Available from: https://doh.wa.gov/sites/default/files/2022-02/420-339-VaccineBreakthroughReport.pdf. Accessed August 9, 2022.
- 18.Scobie HM, Johnson AG, Suthar AB, et al. Monitoring Incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination Status - 13 U.S. Jurisdictions, April 4-July 17, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(37):1284–1290. doi: 10.15585/mmwr.mm7037e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Kaelber DC, Xu R, Berger NA. Breakthrough SARS-CoV-2 infections, hospitalizations, and mortality in vaccinated patients with cancer in the US between December 2020 and November 2021. JAMA Oncol. 2022;4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreyer NA, Reynolds M, DeFilippo Mack C, et al. Self-reported symptoms from exposure to Covid-19 provide support to clinical diagnosis, triage and prognosis: an exploratory analysis. Travel Med Infect Dis. 2020;38:101909. doi: 10.1016/j.tmaid.2020.101909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreyer N, Petruski-Ivleva N, Albert L, et al. Identification of a vulnerable group for post-acute sequelae of SARS-CoV-2 (PASC): people with autoimmune diseases recover more slowly from COVID-19. Int J Gen Med. 2021;14:3941–3949. doi: 10.2147/IJGM.S313486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreyer NA, Reynolds MW, Albert L, et al. How frequent are acute reactions to COVID-19 vaccination and who is at risk? Available from: https://medrxiv.org/cgi/content/short/2021.10.14.21265010v1. Accessed August 9, 2022. [DOI] [PMC free article] [PubMed]
- 23.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) 2021 case definition; 2021.
- 24.CDC COVID-19 Vaccine Breakthrough Case Investigations Team. COVID-19 vaccine breakthrough infections reported to CDC — United States, January 1–April 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:792–793. doi: 10.15585/mmwr.mm7021e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1059–1062. doi: 10.15585/mmwr.mm7031e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreyer NA, Blackburn SC, Mt-Isa S, et al. Direct-to-patient research: piloting a new approach to understanding drug safety during pregnancy. JMIR Public Health Surveill. 2015;1(2):e22. doi: 10.2196/publichealth.4939 [DOI] [PMC free article] [PubMed] [Google Scholar]