Abstract
Hospital readmission rate is a ubiquitous measure of efficiency and quality. Individuals with life-limiting illnesses account heavily for admissions but evaluation is complicated by high mortality rates. We report a retrospective cohort study examining the association between palliative care (PC) and readmissions while controlling for post-discharge mortality with a competing risks approach. Eligible subjects were adult inpatients admitted to an academic, safety-net medical center (2009–2015) with at least one diagnosis of cancer, heart failure, chronic obstructive pulmonary disease, liver failure, kidney failure, AIDS/HIV and selected neurodegenerative conditions. PC was associated with reduced 30-, 60- and 90-day readmissions (subhazard ratios= 0.57, 0.53 and 0.52 respectively [all p<.001]). Hospital PC is associated with a reduction in readmissions, and this is not explained by higher mortality among PC patients. Performance measures only counting those alive at a given end point may underestimate systematically the effects of treatments with a high mortality rate.
Keywords: hospital readmissions, mortality, palliative care, retrospective studies
Introduction
Background
Avoidable hospital readmissions are an established marker of poor quality care and inadequate health system performance, and impose substantial costs: $17.8 billion annually for Medicare alone (Ness & Kramer, 2013). Eighty percent of American adults with serious and life-limiting conditions wish to avoid hospitalization in the terminal phase of illness but 90% of Medicare beneficiaries spend five or more nights in hospital in the last six months of life (Dartmouth Atlas, 2017).
For adults with serious life-limiting conditions, hospital-based palliative care (PC) may improve patient care and outcomes, including reduced readmissions, through expert symptom management, clarifying and communicating goals of subsequent care, and achieving effective transitions to home or other settings (Kavalieratos et al., 2016; Meier, 2011).
Studies to date have found a consistent pattern of palliative care reducing hospital costs during a given admission (May, Normand, et al., 2018; May, Normand, & Morrison, 2014), but have differed in their findings regarding the effects on readmissions. An early RCT found no association with 30-day readmissions (Gade et al., 2008), but a recent prospective cohort study found a significant reduction associated with the intervention (Adelson et al., 2017). Several retrospective studies have been reported (Chuang, Kim, Blank, Southern, & Fausto, 2017; O’Connor, Moyer, Behta, & Casarett, 2015; Tangeman, Rudra, Kerr, & Grant, 2014), but the direction, magnitude and significance of association between PC and readmissions have varied. None had fully ascertained survival (e.g., using data sources such as state vital statistics data for date of death, or later clinical encounters for evidence of continued survival); none had limited the 30-day readmission analyses to patients known to be alive at 30 days following admission.
Conceptual framework
Amounts and types of care received by patients near the end of life reflect not only clinical need but also other patient determinants, family preferences, physician attitudes and local practice patterns (Kelley, Morrison, Wenger, Ettner, & Sarkisian, 2010). Under this interpretation, palliative care is theorized to improve outcomes and reduce costs by improving decision-making by clinicians, patients and families; addressing symptoms and reducing distress; emphasizing communication, goals-of-care discussions and discharge planning; and reducing burdensome treatments and futile care (Kelley & Morrison, 2015).
Matching to control for observed confounding
Studies of end-of-life care are predominantly restricted to routinely collected data due to practical and ethical considerations in recruiting and retaining population-representative samples in prospective and randomized studies (Ewing et al., 2004; Higginson et al., 2013). Estimating treatment effect estimates in end-of-life care therefore typically requires controlling for confounding between those who received palliative care and those who did not using observational data (Earle & Ayanian, 2006). Propensity scoring is a well-established method for balancing differences between groups on observed confounders and is effectively an advanced matching method (Rubin, 2007). Potential confounders are those that are associated with outcome or with both treatment and outcome (Garrido, 2014). For example, in economic studies of palliative care it is important to balance treatment and comparison groups on comorbidity count since a greater number of comorbidities is associated with increased likelihood of receiving palliative care and with increased health care costs.
Differences in mortality between matched groups
A small number of randomized trials in palliative care show no evidence of inferior survival associated with treatment (Kavalieratos et al., 2016), and in some cases demonstrate extended survival (Temel et al., 2010). However, observational studies of palliative care typically do not analyze survival effects since people who receive palliative care are closer to end of life in ways that are not possible to control for with routinely collected data.
Differential mortality as a potential source of bias
The typical performance metric used by Centers for Medicare & Medicaid Services (CMS) is 30-day readmissions, which are calculated for patients who have continued enrollment for the 30 days (i.e. cannot be deceased) (Center for Medicare & Medicaid Services, 2018). When analyses include only those known to be alive at a given point post-discharge (treating decedents as censored), results may underestimate the effect of treatments whose subjects have a high mortality rate since reductions in post-discharge utilization among people who die within the timeframe are not captured. Conversely, where analysis does not account for differential mortality between treatment and comparison groups, results may overestimate the effect of interventions in samples with a high mortality rate since decedents’ utilization is analyzed as zero and not censored.
New contribution
Since care for the seriously ill is a policy priority, and research on this population remains reliant on observational study designs (Aldridge Carlson, 2013; Higginson et al., 2013; Langton et al., 2014), it is important that these biases be investigated and understood. Competing risks are events that may preclude the primary outcome of interest from occurring but which should not be treated as censored in analysis (Wolbers et al., 2014). Competing risks analyses offer a potentially useful way to understand post-discharge patterns of care for populations where mortality is not evenly distributed between treatment and comparison groups. They allow analyses of readmission rates to account for individuals’ mortality rather than counting the deceased as having zero utilization or excluding them altogether (Peter C. Austin & Fine, 2017). To our knowledge, no study has applied a competing risks analysis to these challenges in palliative care, although others have noted the limitations of treating death as a noninformative censoring event (Donoghoe & Gebski, 2017; Szychowski, Roth, Clay, & Mittelman, 2010).
Rationale and objectives
The primary objective of this study was to estimate the effect of hospital PC for adult inpatients with life-limiting illness on hospital readmissions while controlling for post-discharge mortality. Specifically, we treated death as a competing risk in order to reduce the risk of a false positive due to higher mortality in the PC cohort.
Methods
Study design
This is a retrospective cohort study using routinely-collected data from Virginia Commonwealth University (VCU) Health System between November 2009 and October 2015. Our analytic sample included adults admitted to an acute hospital with one of seven life-limiting conditions and confirmed as dying within 365 days of admission (May, Garrido, et al., 2018).
In our primary analysis we estimated the association between receipt of palliative care and hospital readmission. We examined whether the subject was readmitted prior to death with evaluation of readmissions within 30, 60 and 90 days, since these are often used as performance metrics in reimbursement and evaluation (Center for Medicare & Medicaid Services, 2018; Kilgore, Patel, Kielhorn, Maya, & Sharma, 2017; McIlvennan, Eapen, & Allen, 2015). We used propensity score weights to control for observed baseline confounders associated with receipt of palliative care and outcomes (May, Garrido, et al., 2018). More details on hypothesized confounders are provided below.
Setting
VCU Health System is an academic, safety-net medical center and faculty practice. The main hospital has 774 beds and around 36,000 discharges annually including tertiary and quaternary services such as trauma, solid organ transplants, and stem cell transplants.
Intervention
At VCU, inpatient palliative care is provided using two modalities – via a palliative care consultation (PCC) team and in a palliative care unit (PCU) (Clinical Practice Guidelines for Quality Palliative Care, 2013; Morrison, 2013). The multidisciplinary team becomes involved in the care of hospitalized patients with life-limiting illness at the invitation of the primary attending physician. The most frequent causes for requesting consultation are for assistance with managing refractory or intractable symptoms and with reframing goals of care during this hospitalization and after discharge. Reframing goals of care occurs through elicitation of patient values and preferences and in-depth discussion of likely benefits and burdens of treatment options. Palliative care referral rates are low relative to need. For example, while the American Society of Clinical Oncology recommends palliative care be provided concurrently with active treatment for metastatic cancer from the time of diagnosis (Ferrell et al., 2017), a prospective observational study of advanced cancer patients at five large academic medical centers with well-established programs found a referral rate of 19% (Penrod et al., 2017). Reasons for low referral include lack of awareness of the potential value of palliative care among physicians, patients and families; and reluctance among physicians and families to engage palliative care (Hawley, 2017). Thus there are numerous patients hospitalized at this institution for whom specialist palliative care was likely appropriate but not engaged.
The 11-bed PCU first opened in 2000, and its approach shares the PCC goals but takes overall control of patient care. The PCC team includes a physician, an advance practice registered nurse, a social worker, a chaplain, and palliative care fellows. The PCU staff additionally includes a nurse manager, nurse clinician, specialty trained nurses, a volunteer coordinator, volunteers and a psychologist. The palliative care program serves about 1,330 unique adult patients per year, including approximately 400 unique patients in the PCU. The program has been accredited with Joint Commission (TJC) advanced certification since 2012, which indicates the program meets national consensus criteria, and also indicates consistency of care between the PCC and PCU. While palliative care units may not be feasible in all small hospitals, they are recommended for optimal care in larger hospitals (Weissman & Meier, 2008, 2009) and are common at academic cancer centers (Calton et al., 2016). Referral criteria for PCC and PCU are similar, both models of care are provided by the same overall program, and patients in both groups are receiving acute hospital care. Unlike hospice, PC is provided concurrently with “curative” (disease-focused) care, and is billed as specialist consultation encounters by the providers. For this study, our treatment group was recipients of either palliative care modality at any time during the hospitalization.
Participants and sample size
Adults admitted to VCU as inpatients during the study period were eligible if they were over 18 years of age, had a diagnosis of at least one of seven conditions (cancer (excluding benign malignancies), heart failure, chronic obstructive pulmonary disease (COPD), liver failure, kidney failure, AIDS/HIV and selected neurodegenerative conditions), and were recorded by the hospital database or the Social Security Death Index as dying within 365 days of their index admission. These seven diseases are identified as palliative care relevant for research purposes: progressive, life-limiting conditions often accompanied by high symptom burden, frequent hospitalizations, and decisions about further treatment (Kelley et al., 2016; Morrison et al., 2008). Patients were excluded if the index admission involved a transplant or trauma-related condition. More details about the construction of our analytic sample (N=6,761) are available elsewhere (May, Garrido, et al., 2018).
Variables
Independent variables
The primary independent variable was a binary treatment variable: did the subject receive palliative care during their index admission? Additional independent variables were recorded at the time of admission and were those hypothesized to be associated with likelihood of receiving palliative care and our designated outcomes of interest (Garrido, 2014): age (years); sex; race (black; white; neither black nor white); insurance status (Medicare [Medicare fee-for-service or Medicare Managed Care], Medicaid/none [Medicaid, Medicaid Managed Care, self-pay, or unable to pay] and other); primary diagnosis (noncancer, solid tumor, hematological tumor); first-day admission to the intensive care unit (ICU) or surgery; and number of comorbidities (Elixhauser index (Elixhauser, Steiner, Harris, & Coffey, 1998)) (Table 1). For race, insurance and primary diagnosis, categories were condensed from finer sub-categories; in each case, we aimed to minimize information loss while ensuring sufficient sample size to get good balance in propensity score weighting.
Table 1.
Baseline characteristics for all subjects at initial admission (N=6,761), before and after weighting
| Before weighting | After weighting | ||||||
|---|---|---|---|---|---|---|---|
| UC (n=5,264) | PC (n=1,497) | UC (n=5,264) | PC (n=1,497) | ||||
| Mean (SD) | Mean (SD) | ASD (VR) | Mean (SD) | Mean (SD) | ASD (VR) | ||
| Age | Years | 63.8 (14.4) | 64.1 (14.1) | 3% (1.1) | 64.2 (13.8) | 64.1 (14.1) | <0.5% (1.0) |
| Gender | Female | 44% | 45% | 2% | 45% | 45% | <0.5% |
| Race | Black | 45% | 41% | 7% | 41% | 41% | <0.5% |
| White | 51% | 54% | 5% | 54% | 54% | <0.5% | |
| Neither white nor black | 4% | 5% | 3% | 5% | 5% | <0.5% | |
| Insurance | Medicare | 57% | 55% | 2% | 55% | 55% | <0.5% |
| Low SES | 20% | 20% | 0% | 20% | 20% | <0.5% | |
| Other | 23% | 24% | 3% | 24% | 24% | <0.5% | |
| Primary dx | Noncancer | 74% | 71% | 8% | 71% | 71% | <0.5% |
| Cancer: solid tumor | 20% | 26% | 13% | 26% | 26% | <0.5% | |
| Cancer: heme | 5% | 4% | 8% | 4% | 4% | <0.5% | |
| ICU | Admitted via | 30% | 36% | 12% | 36% | 36% | <0.5% |
| Surgery | Admitted via | 16% | 12% | 12% | 12% | 12% | <0.5% |
| Comorbidities | Elixhauser index | 3.4 (1.6) | 3.9 (1.7) | 31% (0.9) | 3.9 (1.7) | 3.9 (1.7) | <0.5% (1.0) |
All data are baseline at admission for the participant’s first hospital admission during the study period. UC: Usual care; PC: Palliative care. Absolute standardized difference (ASD) measures the imbalance between groups on baseline characteristics, taking into account both means and variances; variance ratio (VR) measures the variance of continuous variables. ASD<10% and 0.9<VR<1.1 are rules of thumb for adequate balance in propensity score matching. SD: Standard deviation. SES: Socioeconomic status. ICU: Intensive Care Unit. Primary payer: ‘Medicare’ includes Medicare and Managed Medicare; ‘Low SES’ includes Medicaid, Managed Medicaid, Self-Pay, and uninsured, unable to pay, and not qualified for Medicaid; ‘Other’ incorporates all other insurers. ICU and Surgery: ‘On admission’. Patient visited ICU or underwent surgery on day of hospital admission. Comorbidities: Elixhauser index is an additive count of the presence/absence (1|0) of 31 specific conditions.
Dependent variables
In the primary analysis, we had three binary dependent variables: hospital readmission within 30, 60 and 90 days of discharge from the index hospitalization.
Data sources/measurement
All dependent and independent variable data were extracted from the hospital accounting and administrative databases, which included death data from the Social Security Death Index. Receipt of palliative care was identified using a free-standing database managed by the palliative care program documenting all patient encounters. The study is covered under VCU IRB protocol HM14959 and all data were de-identified prior to analyses.
Bias
Patient characteristics are likely associated with our intervention, receipt of palliative care, and our outcomes of interest. To control for selection bias, we balanced the treatment groups on all variables in Table 1 using the covariate balancing propensity score method (CBPS) (Imai & Ratkovic, 2014; R Core Team, 2016). We created inverse-probability-of-treatment-weights (IPTWs) from the estimated propensity score for use in analyses. Prior to estimating treatment effects on our outcomes of interest, we evaluated balance between treatment groups for the overall sample (Table 1) and across the distribution of the propensity score [data available from authors] (P. C. Austin, 2009; Garrido et al., 2014). Results are presented with and without weights for observed selection bias.
Statistical methods
In our primary analysis we modeled the association between palliative care and the risk of first readmission for 30-, 60- and 90-day readmissions, where a first readmission within the respective timeframe is treated as failure and death is treated as a competing risk. A first readmission within 30 days is therefore also counted as the failure event within 60 and 90 days.
Our treatment variable and other independent descriptors (Table 1) were employed as explanatory variables in our weighted sample. In secondary analyses, we examined summary post-discharge utilization data at 30-, 60- and 90-day points using only those subjects known to be alive (i.e. mirroring the CMS performance measurement perspective).
No patient in our final analytic sample had missing data in any dependent or independent variable, or in receipt of palliative care. Propensity scores were calculated in R (R Core Team, 2016); all other analyses were performed in Stata (version 12) (StataCorp, 2011).
Results
Descriptive data
Our analytic sample comprises 6,761 patients, 1,497 (22%) of whom received palliative care during the index hospitalization.
In the unweighted sample, treatment group patients had notably higher prevalence of solid cancer primary diagnosis and ICU admission, lower prevalence of surgery during admission, and higher comorbidity counts (Table 1). Following weighting, the two groups exhibited negligible difference on all observed confounders. Patients in this weighted sample had a mean age of 64 years and a mean of 3.9 comorbidities. A majority (60%) of patients had a primary diagnosis of something other than the seven life-limiting illnesses (i.e., they were eligible for inclusion because one of the seven life-limiting conditions was coded as a secondary diagnosis); the second most prevalent primary diagnosis was cancer (36%).
Outcome data
Readmission and mortality rates are presented in Table 2. Of our weighted sample, 49% died within 30 days of discharge, 64% died within 60 days and 71% died within 90 days. In each case, the proportion of subjects in the usual care who died within a given timeframe is smaller than the proportion in the palliative care group. Fourteen percent of our sample were readmitted within 30 days, 19% within 60 days, and 21% within 90 days. In each case, the proportion of subjects in the usual care group who were readmitted is larger than the proportion in the palliative care group. Therefore, in our sample palliative care patients had fewer readmissions and higher mortality than patients who received usual care, affirming the need to assess any association between these two trends.
Table 2.
Mortality and readmission rates (N=6,761), before and after weighting
| Before weighting | After weighting | |||
|---|---|---|---|---|
| UC (n=5,264) | PC (n=1,497) | UC (n=5,264) | PC (n=1,497) | |
| Mortality | ||||
| 30-day | 30% | 64% | 34% | 64% |
| 60-day | 43% | 80% | 49% | 80% |
| 90-day | 53% | 85% | 58% | 85% |
| Readmissions | ||||
| 30-day | 20% | 9% | 20% | 9% |
| 60-day | 28% | 12% | 26% | 12% |
| 90-day | 32% | 13% | 30% | 13% |
Main results
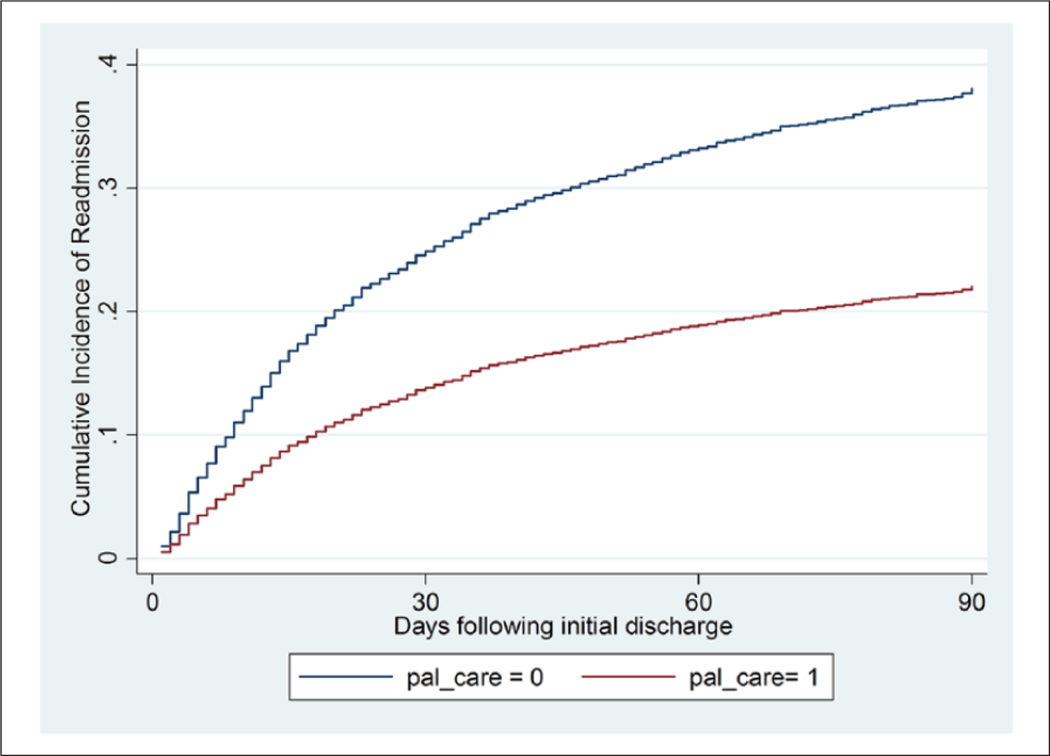
In all three time periods, patients who received hospital-based palliative care had a lower likelihood of readmission than patients who received usual care, even after accounting for the competing risk of mortality. Hospital-based palliative care was associated with reduced 30-, 60- and 90-day readmissions (subhazard ratios = 0.57, 0.53 and 0.52 respectively [all p<.001]). This reduced incidence is illustrated in Figure 1. Regression results for all predictors, with and without weights where 30-day readmissions were the outcome of interest, are presented in Table 3. In addition to palliative care, statistically significant associations are observable for hematological cancer diagnosis (positive association with 30-day readmissions), and age, Medicare coverage, admission via surgery and admission via ICU (negative associations).
Figure 1.
Cumulative incidence of readmissions for palliative care and usual care treating mortality as a competing risk after weighting (N=6,761).
Table 3.
Regression output where 30-day readmissions is outcome of interest and mortality is a competing risk (N=6,761), before and after weighting
| Before weighting | After weighting | |||||||
|---|---|---|---|---|---|---|---|---|
| SHR | P value | 95% | CI | SHR | P value | 95% | CI | |
| Palliative care | 0.58 | <0.01 | 0.48 | 0.69 | 0.57 | <0.01 | 0.47 | 0.68 |
| Age | 0.99 | <0.01 | 0.99 | 0.99 | 0.99 | <0.01 | 0.98 | 1.00 |
| Gender | 1.13 | 0.03 | 1.01 | 1.27 | 1.22 | 0.01 | 1.06 | 1.40 |
| Race: Black | 1.32 | 0.11 | 0.94 | 1.85 | 1.33 | 0.19 | 0.87 | 2.03 |
| Race: White | 1.36 | 0.07 | 0.97 | 1.90 | 1.40 | 0.12 | 0.92 | 2.13 |
| Insurance: Medicare | 0.67 | <0.01 | 0.57 | 0.78 | 0.58 | <0.01 | 0.47 | 0.71 |
| Insurance: Low SES | 0.90 | 0.18 | 0.77 | 1.05 | 0.81 | 0.03 | 0.67 | 0.98 |
| Primary dx: Solid tumor | 1.03 | 0.65 | 0.90 | 1.19 | 0.96 | 0.65 | 0.81 | 1.14 |
| Primary dx: Heme | 1.87 | <0.01 | 1.54 | 2.27 | 1.85 | <0.01 | 1.45 | 2.36 |
| ICU | 0.76 | <0.01 | 0.65 | 0.89 | 0.70 | <0.01 | 0.58 | 0.84 |
| Surgery | 0.77 | 0.01 | 0.64 | 0.93 | 0.71 | <0.01 | 0.56 | 0.89 |
| Comorbidities | 1.02 | 0.30 | 0.98 | 1.06 | 1.03 | 0.28 | 0.98 | 1.08 |
SHR: Sub-hazard ratio. CI: Confidence interval.
Secondary analysis
In Table 4 the post-discharge utilization for the PC and UC groups are presented for those cases known to be alive at each timepoint. Among these subsets of the sample, PC patients had lower readmission rates at 30 and 60 days, but at 90 days both groups had the same rate (39%).
Table 4.
Summary readmissions data and associated utilization for those alive at relevant end points
| Alive at endpoint, All | Alive at endpoint, PC patients | Alive at endpoint, UC patients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Readmissions | N | Avg | SD | N | Avg | SD | N | Avg | SD | |
| <30 days | Proportion readmitted | 4,224 | 25% | 537 | 19% | 3,687 | 26% | |||
| Total readmissions | 4,224 | 0.29 | 0.55 | 537 | 0.22 | 0.49 | 3,687 | 0.31 | 0.56 | |
| LOS per readmit | 1,245 | 5 | 13.7 | 120 | 5 | 13.8 | 1,125 | 5 | 13.0 | |
| Cost per readmit ($) | 1,245 | 7116 | 39172 | 120 | 6842 | 63901 | 1,125 | 7206 | 35257 | |
| <60 days | Proportion readmitted | 3,283 | 34% | 302 | 31% | 2,981 | 34% | |||
| Total readmissions | 3,283 | 0.50 | 0.82 | 302 | 0.43 | 0.73 | 2,981 | 0.50 | 0.83 | |
| LOS per readmit | 1,629 | 5 | 11.8 | 131 | 5 | 11.9 | 1,498 | 5 | 11.6 | |
| Cost per readmit ($) | 1,629 | 7115 | 16896 | 131 | 6187 | 54954 | 1,498 | 7247 | 31419 | |
| <90 days | Proportion readmitted | 2,710 | 39% | 226 | 39% | 2,484 | 39% | |||
| Total readmissions | 2,710 | 0.65 | 1.03 | 226 | 0.64 | 1 | 2,484 | 0.66 | 1.03 | |
| LOS per readmit | 1,772 | 5 | 11.5 | 144 | 5 | 12.9 | 1,628 | 5 | 11.4 | |
| Cost per readmit ($) | 1,772 | 7181 | 35215 | 144 | 6159 | 50867 | 1,628 | 7298 | 33101 | |
Proportion readmitted: % of sample who were readmitted (0|1) during the timeframe. Total readmissions is all readmissions in the sample in the timeframe, i.e. it differs from ‘Proportion readmitted’ because subjects can have more than one readmission counted. LOS and costs reflect utilization associated with all readmissions.
Average: Mean for Rate and Total Readmissions; Median for LOS and Cost per readmit.
Discussion
Key results
We conducted a retrospective cohort study to evaluate the impact of palliative care on readmissions for hospitalized adults with serious illness, controlling for observed confounders using propensity score weights. In our weighted sample, the palliative care group had significantly lower readmissions but significantly higher mortality (Table 2), raising the possibility that observed reductions in post-discharge utilization are driven by selection bias (specifically, greater proximity to death at admission in the treatment group, which is inadequately controlled for by propensity scoring). We therefore treated readmission and death as competing risks, and found that palliative care was associated with reduced readmissions when accounting for differential mortality rates(Table 3, Figure 1). The observed lower readmissions and associated utilization for palliative care patients do not appear to be an artifact of higher mortality in the treatment group. Lower readmission rates for palliative care patients are consistent with the findings of a recent prospective cohort study (Adelson et al., 2017).
Examining utilization data using only those known to be alive at a given end point (Table 4) leads to differing conclusions. There is no difference in 90-day readmissions between groups from this perspective, compared to a substantial difference when mortality is controlled for as a competing risk (Figure 1). This suggests that performance measures only counting those alive at a given end point may fail to capture the full effects of treatments for populations with a high mortality rate. Treatment group patients who died prior to the end point but were not readmitted to hospital are excluded from the analysis yet their lower post-discharge utilization prior to death may be attributable to receipt of the treatment.
Although competing risks analyses are common in survival studies, to our knowledge this is the first study of palliative care and post-discharge utilization that uses a competing risks model. Readmission rate is a ubiquitous measure of efficiency or quality, used in both research and as a performance metric tied to reimbursement (Centers for Medicare & Medicaid Services, 2017). When used as a performance metric, readmission rates are only evaluated among patients known to be alive (Centers for Medicare and Medicaid Services, 2017). When examined in the context of palliative and end-of-life care, mortality must be ascertained, taken into account in analyses, and reported alongside the outcome of interest (Varadhan et al., 2010). In our study, despite propensity weighting using a variety of variables, the PC group had significantly more deaths at each time point than the control group. This is likely due to unobserved confounding (patients in the PC group have greater proximity to death than the UC group in ways for which we are unable to control).
Therefore, future studies with similar designs should consider employing a competing risk analysis to confirm that observed reductions in utilization following palliative care interventions are not driven by higher mortality in the treatment group. There are several data sources for ascertaining non-hospital mortality for quality improvement or research, which in the US include the National Death Index (NDI) (Centers for Disease Control and Prevention, 2017), the Social Security Death Master File (SSDMF) (National Technical Information Service, 2018), and each state’s vital records department.
Limitations
As a retrospective cohort study of routinely collected data, our results may be subject to the influence of unobserved confounding. We sought to minimize observed confounding with propensity score weights, but we are unable to account for unobserved potential confounders, such as differences in preferences for care across treatment groups. In analyses without propensity score weights (Brooks & Ohsfeldt, 2013), our main findings did not substantively change. The constraints we faced in this observational data analysis are typically faced by those engaged in research and performance measurement on this critical policy question.
This study uses data from a single hospital system and may not generalize to other hospital systems. However, our sample was socio-demographically diverse and included patients with a variety of serious life-limiting diagnoses. The PC programs we studied are eligible for TJC;(The Joint Commission, 2014) certification, which is awarded to interdisciplinary teams containing a specialist physician, a nurse and a social worker, with chaplaincy and bereavement support. There is therefore a substantive level of standardization between the intervention we study and an estimated 135 other TJC-eligible programs nationally (Spetz et al., 2016). The program has participated in multi-site research such as the Palliative Care For Cancer study (May et al., 2015), which used palliative care provider trainings to ensure fidelity of palliative care across academic and community hospitals. Also, benchmark data for this program from the National Palliative Care Registry indicate it is similar to programs at other large hospitals in terms of staffing and volumes (Center to Advance Palliative care, 2018).
Our sample is defined by mortality within one-year of hospital admission in order to retrospectively identify a population with palliative care needs. This approach is sub-optimal since mortality is in principle an outcome that the intervention can impact, and as such all analyses are conducted in the context of multiple prior studies demonstrating that palliative care does not reduce survival but may in some cases extend it (Bakitas et al., 2014; Temel et al., 2010; Zimmermann et al., 2014).
Our data do not allow us to detect which readmissions are potentially avoidable, for example differentiating between those for an ambulatory care sensitive condition and those for a scheduled or needed procedure. Our results are therefore based on an implicit assumption that there are no major differences in proportion of potentially avoidable admissions between treatment and comparison groups. Further research could incorporate such distinctions into analysis (Fingar, Barrett, Elixhauser, Stocks, & Steiner, 2015).
For maximum usefulness, analyses of utilization should examine impact on overall resource use since this is the basis of all fundamental policy questions (May, Garrido, Cassel, Morrison, & Normand, 2016; Neumann, Sanders, Russell, Siegel, & Ganiats, 2017). In this analysis we do not model overall resource impact, only the risk of a first readmission, and as such we do not incorporate changing health states over time. Analyses of utilization in decedent cohort studies are only meaningful if there is an assumption of survival equivalence – if the treatment impacts survival in either direction, then comparing utilization for a defined time window prior to death does not compare overall resource utilization for both groups but rather underestimates the utilization of the group who live longer, potentially biasing results (Johnston, Normand, & May, 2017).
Finally, a recent meta-analysis of hospital utilization among people with life-limiting conditions has identified significant treatment effect heterogeneity (May, Normand, et al., 2018). It is therefore possible that the results we report vary by clinical factors (e.g. primary diagnosis, illness burden) and non-health determinants. Our study is not powered to detect such differences but measuring variance in treatment effect beyond hospital admission is an important subject for future research in this field.
Conclusion
Palliative care is associated with a reduction in time to first readmission compared to usual care, and this reduction is not explained by higher mortality in the treatment group. Performance measures only counting those alive at a given end point may fail to capture the full effects of treatments for populations with a high mortality rate. Given the policy importance of improving care for people with serious medical complexity and the strong reliance on observational data in studying this population, investigators should consider ascertaining mortality and incorporating competing risk analyses.
Financial support information
May was supported by the International Access, Rights and Empowerment (IARE) fellowship program, which is funded by The Atlantic Philanthropies (grant #24611). Garrido was supported by a Veterans Affairs HSR&D career development award (CDA 11-201/CDP 12-255); the views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of VA or the United States government. Garrido is now affiliated with the Boston VA Healthcare System and Boston University School of Public Health. Skoro was supported in part with funding from NIH-NCI Cancer Center Support Grant P30 CA016059. Lead author affirms there are no conflicts of interest to declare.
Footnotes
Permissions
There are no copyrighted materials or patient consent forms used in this study.
References
- Adelson K, Paris J, Horton JR, Hernandez-Tellez L, Ricks D, Morrison RS, & Smith CB (2017). Standardized Criteria for Palliative Care Consultation on a Solid Tumor Oncology Service Reduces Downstream Health Care Use. J Oncol Pract, 13(5), e431–e440. doi: 10.1200/jop.2016.016808 [DOI] [PubMed] [Google Scholar]
- Aldridge Carlson MD (2013). Research methods priorities in geriatric palliative medicine. J Palliat Med, 16(8), 838–842. doi: 10.1089/jpm.2013.9492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC (2009). Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med, 28(25), 3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC, & Fine JP (2017). Accounting for competing risks in randomized controlled trials: a review and recommendations for improvement. Statistics in Medicine, 36(8), 1203–1209. doi: 10.1002/sim.7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakitas M, Tosteson T, Li Z, Lyons K, Hull J, Li Z, . . . Dragnev KH (2014). The ENABLE III randomized controlled trial of concurrent palliative oncology care. Paper presented at the ASCO Annual Meeting, Chicago, IL. [Google Scholar]
- Brooks JM, & Ohsfeldt RL (2013). Squeezing the balloon: propensity scores and unmeasured covariate balance. Health Serv Res, 48(4), 1487–1507. doi: 10.1111/1475-6773.12020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton BA, Alvarez-Perez A, Portman DG, Ramchandran KJ, Sugalski J, & Rabow MW (2016). The Current State of Palliative Care for Patients Cared for at Leading US Cancer Centers: The 2015 NCCN Palliative Care Survey. J Natl Compr Canc Netw, 14(7), 859–866. [DOI] [PubMed] [Google Scholar]
- Center to Advance Palliative Care. (2018). National Palliative Care Registry. Retrieved from https://registry.capc.org/
- Center for Medicare & Medicaid Services. (2018). QualityNet. Retrieved from https://www.qualitynet.org/
- Centers for Disease Control and Prevention. (2017, 2017 Mar 7). National Death Index. Retrieved from https://www.cdc.gov/nchs/ndi/index.htm
- Centers for Medicare & Medicaid Services. (2017). Condition-Specific Measures Updates and Specifications Report Hospital-Level 30-Day Risk-Standardized Readmission Measures. Retrieved from http://www.qualitynet.org/dcs/ContentServer?cid=1219069855841&pagename=QnetPublic%2FPage%2FQnetTier4&c=Page
- Centers for Medicare and Medicaid Services. (2017, 2017 Nov 30). Readmissions Reduction Program (HRRP). Retrieved from https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html
- Chuang E, Kim G, Blank AE, Southern W, & Fausto J. (2017). 30-Day Readmission Rates in Patients Admitted for Heart Failure Exacerbation with and without Palliative Care Consultation: A Retrospective Cohort Study. J Palliat Med, 20(2), 163–169. doi: 10.1089/jpm.2016.0305 [DOI] [PubMed] [Google Scholar]
- Clinical Practice Guidelines for Quality Palliative Care. (2013). Retrieved from Pittsburgh, PA: [Google Scholar]
- Dartmouth Atlas. (2017). Inpatient Days per Decedent During the Last Six Months of Life, by Gender and Level of Care Intensity for 2014. Retrieved from http://www.dartmouthatlas.org/data/topic/topic.aspx?cat=18
- Donoghoe MW, & Gebski V. (2017). The importance of censoring in competing risks analysis of the subdistribution hazard. BMC Med Res Methodol, 17(1), 52. doi: 10.1186/s12874-017-0327-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle CC, & Ayanian JZ (2006). Looking back from death: the value of retrospective studies of end-of-life care. J Clin Oncol, 24(6), 838–840. doi: 10.1200/JCO.2005.03.9388 [DOI] [PubMed] [Google Scholar]
- Elixhauser A, Steiner C, Harris DR, & Coffey RM (1998). Comorbidity measures for use with administrative data. Med Care, 36(1), 8–27. [DOI] [PubMed] [Google Scholar]
- Ewing G, Rogers M, Barclay S, McCabe J, Martin A, & Todd C. (2004). Recruiting patients into a primary care based study of palliative care: why is it so difficult? Palliat Med, 18(5), 452–459. [DOI] [PubMed] [Google Scholar]
- Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, . . . Smith TJ (2017). Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol, 35(1), 96–112. doi: 10.1200/jco.2016.70.1474 [DOI] [PubMed] [Google Scholar]
- Fingar KR, Barrett ML, Elixhauser A, Stocks C, & Steiner CA (2015). Trends in Potentially Preventable Inpatient Hospital Admissions and Emergency Department Visits. Retrieved from https://www.hcup-us.ahrq.gov/reports/statbriefs/sb195-Potentially-Preventable-Hospitalizations.pdf [PubMed]
- Gade G, Venohr I, Conner D, McGrady K, Beane J, Richardson RH, . . . Della Penna R. (2008). Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med, 11(2), 180–190. doi: 10.1089/jpm.2007.0055 [DOI] [PubMed] [Google Scholar]
- Garrido MM (2014). Propensity scores: a practical method for assessing treatment effects in pain and symptom management research. J Pain Symptom Manage, 48(4), 711–718. doi: 10.1016/j.jpainsymman.2014.05.014 [DOI] [PubMed] [Google Scholar]
- Garrido MM, Kelley AS, Paris J, Roza K, Meier DE, Morrison RS, & Aldridge MD (2014). Methods for constructing and assessing propensity scores. Health Serv Res, 49(5), 1701–1720. doi: 10.1111/1475-6773.12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley P. (2017). Barriers to Access to Palliative Care. Palliative Care, 10, 1178224216688887. doi: 10.1177/1178224216688887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginson IJ, Evans CJ, Grande G, Preston N, Morgan M, McCrone P, . . . Todd C. (2013). Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med, 11, 111. doi: 10.1186/1741-7015-11-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, & Ratkovic M. (2014). Covariate balancing propensity score. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 76(1), 243–263. doi:doi: 10.1111/rssb.12027 [DOI] [Google Scholar]
- Johnston BM, Normand C, & May P. (2017). Economics of Palliative Care: Measuring the Full Value of an Intervention. J Palliat Med, 20(3), 222–224. doi: 10.1089/jpm.2016.0446 [DOI] [PubMed] [Google Scholar]
- Kavalieratos D, Corbelli J, Zhang D, Dionne-Odom JN, Ernecoff NC, Hanmer J, . . . Schenker Y. (2016). Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA, 316(20), 2104–2114. doi: 10.1001/jama.2016.16840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AS, Covinsky KE, Gorges RJ, McKendrick K, Bollens-Lund E, Morrison RS, & Ritchie CS (2016). Identifying Older Adults with Serious Illness: A Critical Step toward Improving the Value of Health Care. Health Serv Res. doi: 10.1111/1475-6773.12479 [DOI] [PMC free article] [PubMed]
- Kelley AS, & Morrison RS (2015). Palliative Care for the Seriously Ill. N Engl J Med, 373(8), 747–755. doi: 10.1056/NEJMra1404684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AS, Morrison RS, Wenger NS, Ettner SL, & Sarkisian CA (2010). Determinants of treatment intensity for patients with serious illness: a new conceptual framework. J Palliat Med, 13(7), 807–813. doi: 10.1089/jpm.2010.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore ML, Patel HK, Kielhorn A, Maya JF, & Sharma P. (2017). Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Management and Healthcare Policy, 10, 63–70. doi: 10.2147/RMHP.S130341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton JM, Blanch B, Drew AK, Haas M, Ingham JM, & Pearson SA (2014). Retrospective studies of end-of-life resource utilization and costs in cancer care using health administrative data: a systematic review. Palliat Med, 28(10), 1167–1196. doi: 10.1177/0269216314533813 [DOI] [PubMed] [Google Scholar]
- May P, Garrido MM, Cassel JB, Kelley AS, Meier DE, Normand C, . . . Morrison RS (2015). Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol, 33(25), 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P, Garrido MM, Cassel JB, Morrison RS, & Normand C. (2016). Using Length of Stay to Control for Unobserved Heterogeneity When Estimating Treatment Effect on Hospital Costs with Observational Data: Issues of Reliability, Robustness, and Usefulness. Health Serv Res, 51(5), 2020–2043. doi: 10.1111/1475-6773.12460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P, Garrido MM, Del Fabbro E, Noreika D, Normand C, Skoro N, & Cassel JB (2018). Does modality matter? Palliative care units associated with more cost-avoidance than consultations. J Pain Symptom Manage, Mar;55(3), 766–774. doi: 10.1016/j.jpainsymman.2017.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P, Normand C, Cassel JB, Del Fabbro E, Fine RL, Menz R, . . . Morrison RS (2018). Economics of Palliative Care for Hospitalized Adults With Serious Illness: A Meta-analysis. JAMA Intern Med, 178(6), 820–829. doi: 10.1001/jamainternmed.2018.0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P, Normand C, & Morrison RS (2014). Economic impact of hospital inpatient palliative care consultation: review of current evidence and directions for future research. J Palliat Med, 17(9), 1054–1063. doi: 10.1089/jpm.2013.0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvennan CK, Eapen ZJ, & Allen LA (2015). Hospital Readmissions Reduction Program. Circulation, 131(20), 1796–1803. doi: 10.1161/CIRCULATIONAHA.114.010270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier DE (2011). Increased access to palliative care and hospice services: opportunities to improve value in health care. Milbank Q, 89(3), 343–380. doi: 10.1111/j.1468-0009.2011.00632.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RS (2013). Models of palliative care delivery in the United States. Curr Opin Support Palliat Care, 7(2), 201–206. doi: 10.1097/SPC.0b013e32836103e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RS, Penrod JD, Cassel JB, Caust-Ellenbogen M, Litke A, Spragens L, & Meier DE (2008). Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med, 168(16), 1783–1790. doi: 10.1001/archinte.168.16.1783 [DOI] [PubMed] [Google Scholar]
- National Technical Information Service. (2018, 2018 Mar 2). Social Security Death Master File. Retrieved from https://www.ssdmf.com/
- Ness D, & Kramer W. (2013). Reducing Hospital Readmissions: It’s About Improving Patient Care. Health Affairs Blog. Retrieved from http://healthaffairs.org/blog/2013/08/16/reducing-hospital-readmissions-its-about-improving-patient-care/
- Neumann PJ, Sanders GD, Russell LB, Siegel JE, & Ganiats TG (2017). Cost effectiveness in health and medicine (Second edition. ed.). Oxford ; New York: Oxford University Press. [Google Scholar]
- O’Connor NR, Moyer ME, Behta M, & Casarett DJ (2015). The Impact of Inpatient Palliative Care Consultations on 30-Day Hospital Readmissions. J Palliat Med, 18(11), 956–961. doi: 10.1089/jpm.2015.0138 [DOI] [PubMed] [Google Scholar]
- Penrod JD, Garrido MM, McKendrick K, May P, Aldridge MD, Meier DE, . . . Morrison RS (2017). Characteristics of Hospitalized Cancer Patients Referred for Inpatient Palliative Care Consultation. J Palliat Med. doi: 10.1089/jpm.2017.0111 [DOI] [PMC free article] [PubMed]
- R Core Team. (2016). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from https://www.R-project.org [Google Scholar]
- Rubin DB (2007). The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med, 26(1), 20–36. doi: 10.1002/sim.2739 [DOI] [PubMed] [Google Scholar]
- Spetz J, Dudley N, Trupin L, Rogers M, Meier DE, & Dumanovsky T. (2016). Few Hospital Palliative Care Programs Meet National Staffing Recommendations. Health Aff (Millwood), 35(9), 1690–1697. doi: 10.1377/hlthaff.2016.0113 [DOI] [PubMed] [Google Scholar]
- StataCorp. (2011). Stata Statistical Software: Release 12. College Station, TX: StataCorp LP. [Google Scholar]
- Szychowski JM, Roth DL, Clay OJ, & Mittelman MS (2010). Patient Death as a Censoring Event or Competing Risk Event in Models of Nursing Home Placement. Stat Med, 29(3), 371–381. doi: 10.1002/sim.3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangeman JC, Rudra CB, Kerr CW, & Grant PC (2014). A hospice-hospital partnership: reducing hospitalization costs and 30-day readmissions among seriously ill adults. J Palliat Med, 17(9), 1005–1010. doi: 10.1089/jpm.2013.0612 [DOI] [PubMed] [Google Scholar]
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, . . . Lynch TJ (2010). Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med, 363(8), 733–742. doi: 10.1056/NEJMoa1000678 [DOI] [PubMed] [Google Scholar]
- The Joint Commission. (2014). Advanced Certification for Palliative Care Programs. Retrieved from http://www.jointcommission.org/certification/palliative_care.aspx
- Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, & Boyd C. (2010). Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care, 48(6 Suppl), S96–105. doi: 10.1097/MLR.0b013e3181d99107 [DOI] [PubMed] [Google Scholar]
- Weissman DE, & Meier DE (2008). Operational features for hospital palliative care programs: consensus recommendations. J Palliat Med, 11(9), 1189–1194. doi: 10.1089/jpm.2008.0149 [DOI] [PubMed] [Google Scholar]
- Weissman DE, & Meier DE (2009). Center to advance palliative care inpatient unit operational metrics: consensus recommendations. J Palliat Med, 12(1), 21–25. doi: 10.1089/jpm.2008.0210 [DOI] [PubMed] [Google Scholar]
- Wolbers M, Koller MT, Stel VS, Schaer B, Jager KJ, Leffondré K, & Heinze G. (2014). Competing risks analyses: objectives and approaches. Eur Heart J, 35(42), 2936–2941. doi: 10.1093/eurheartj/ehu131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, . . . Lo C. (2014). Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet, 383(9930), 1721–1730. doi: 10.1016/S0140-6736(13)62416-2 [DOI] [PubMed] [Google Scholar]