Abstract
Forty two patients with high energy open fractures were involved into the study to investigate whether an indocyanine green (ICG)-based dynamic contrast-enhanced fluorescence imaging (DCE-FI) can be used to objectively assess bone perfusion and guide surgical debridement. For each patient, fluorescence images were recorded after 0.1 mg/kg of ICG was administered intravenously. By utilizing a bone-specific kinetic model to the video sequences, the perfusion-related metrics were calculated. The results of this study shown that the quantitative ICG-based DEC-FI can accurately assess the human bone perfusion during the orthopedic surgery.
Keywords: dynamic contrast-enhanced fluorescence imaging, indocyanine green, bone vascular perfusion, orthopaedic surgery, debridement
1. INTRODUCTION
Post-traumatic infection is one of the most prevalent and challenging complications facing orthopedic surgeons in both military and civilian populations, occurring after up to 60% of open bone fractures[1–7]. This converts a 6-month recovery into a several-year (or longer) recovery. Since high energy open fractures are associated with severe contamination and disruption in blood flow to the injured bone and soft tissue, disrupted blood flow (or perfusion) to bone and soft tissue is an important factor for subsequent complications and infections.[8] Because of this, management of open fractures and wound infection involves aggressive, thorough debridement (surgical removal) of bone and soft tissue lacking adequate blood flow.
Animal and human bone perfusion has been imaged using Dynamic Contrast Enhanced Magnetic Resonance Imaging (DCE-MRI)[9], Computer Tomography (CT) Angiography[10], combined Positron emission tomography (PET)/CT [8]. However, these imaging modalities are impractical in the orthopaedic surgical population because they cannot be translated to the operating room, have problems with image resolution particularly in the setting of metallic implants and lengthy time requirements for data acquisition. For providing a measurable or quantifiable method that can objectively assess the bone perfusion intraoperatively, and reduce the risk of complications and treatment failure due to the substantial practice variation, an indocyanine green (ICG)-based dynamic contrast-enhanced fluorescence imaging (DCE-FI) method has been developed to objectively assess bone perfusion and guide surgical debridement.
2. MATERIALS AND METHODS
Under an approved Dartmouth-Hitchcock IRB protocol, we have acquired pilot ICG-based DCE-FI in 18 patients to date during orthopedic surgeries. Figure 1 shows the imaging set-up for a patient. The SPY Elite system was positioned 300mm from the surgical field and fluorescence images were recorded every 0.267 seconds for 4.5 minutes. Following 20 seconds of pre-injection imaging, 0.1 mg/kg of ICG was administered intravenously to the patient. After DCE-FI was acquired, a white-light image was taken.
Figure 1.
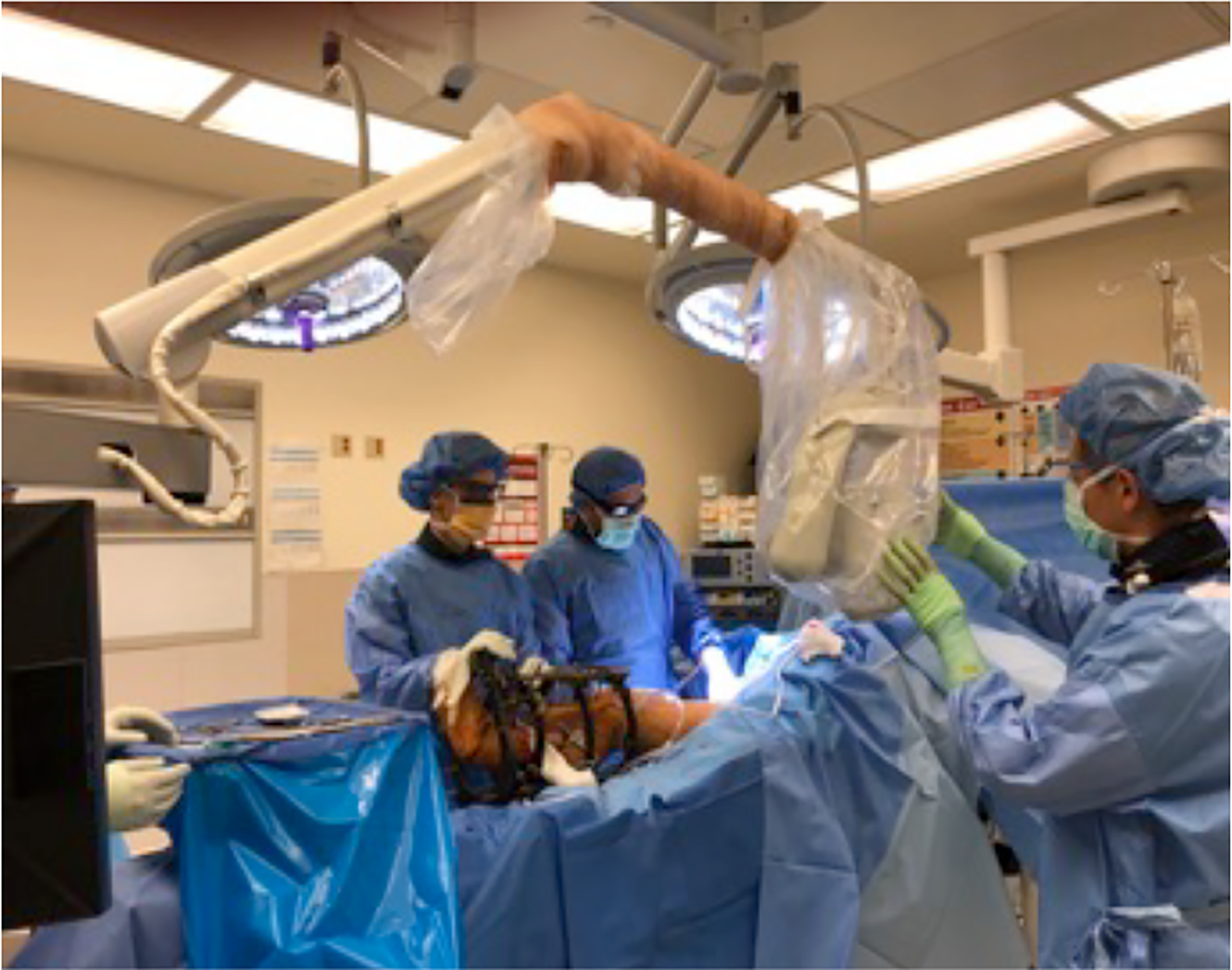
Intraoperative imaging set-up.
Since different arterial branches supply the periosteum and endosteal compartments, a bone-specific kinetic model [11, 12] to the fluorescence image-series has been developed. This model allows separating arrival and transit times in a hybrid plug-compartment (HyPC) and enables the recovery of a faster blood perfusion component which termed as ‘early bone perfusion’ (EBP) and a slower ‘late bone perfusion’ component (LBP). In addition to EBP and LBP, we further define the sum of EBP and LBP the total bone perfusion (TBP), and the fraction of LBP to TBP as late perfusion fraction (LPF), as well as peak intensity as the perfusion-related metrics. All these bone perfusion related parameters were calculated for assessing the damage level of the bone at different bone region of interest (ROI).
3. RESULTS
To improve the processing speed of dynamic video imaging process, a python code with Pydicom, Numpy, Scipy, and Imresize packages was developed. In this python code, 1024×768×1024 DICOM images were divided into many sub-images, and the image processing was performed on each sub-image parallelly. Finally, the processed sub-images were combined together to obtain the functional fluorescence images of the entire surgical view. The diagram of our new imaging processing is shown in Figure 2. By utilizing the python multiprocessing package, the processing time on a 1024×768×1024 DICOM image was improved to less 57 seconds, with 4 processes running parallel on a two-core computer. In the future, we could possibly run the code on a better computer with more parallel processes, or utilize GPU to further improve the time of processing.
Figure 2.
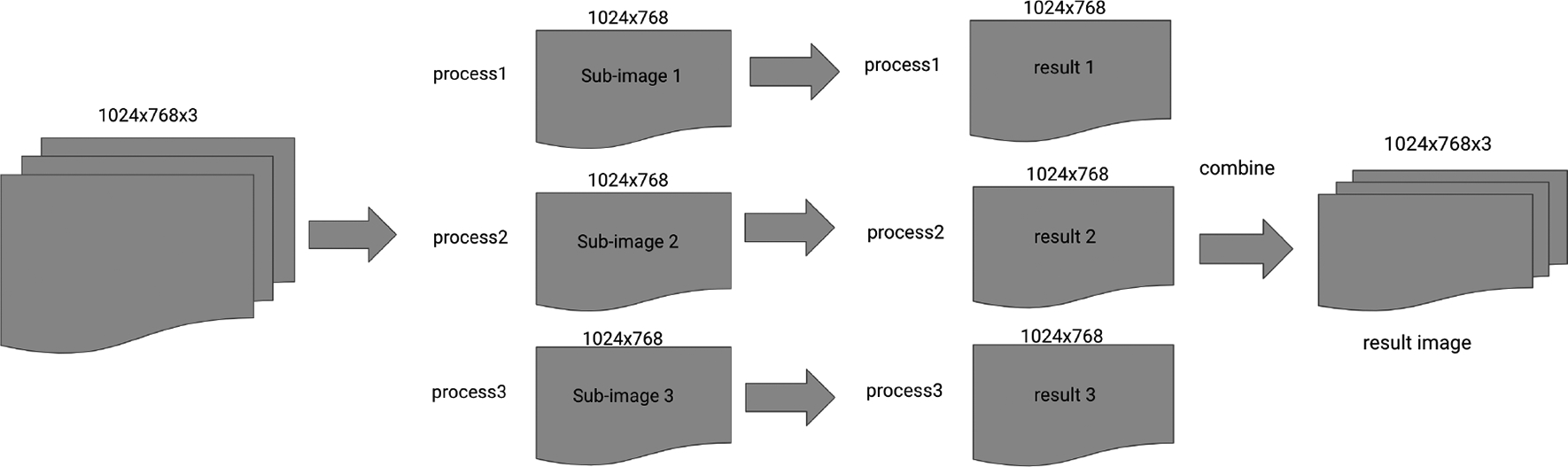
Diagram of our imaging processing.
Figure 3 summarizes the ICG based DCE-FI data we acquired from a patient with a Gustilo Type 3C open tibia fracture with segmental bone loss. In Fig.3, ROIs #1 was fracture, #2 was closest to the fracture/segmental bone defect and ROIs #3 and #4 are more proximal from the fracture/segmental bone defect. The more proximal ROIs (#3 and #4 in Fig.3(a)) were, as expected, better perfused than that of the ROIs in or close to the fracture site, which is demonstrated by the brighter regions in the fluorescence intensity images at 75 and 215 secs. (Fig.3(b)&(c)), and faster raises in the temporal dynamic curves (Fig.3(g)). This trend is further reflected in LPF (Fig.3(d)) and TBP (Fig.3(e)) where more proximal ROIs demonstrate brighter signal than more distal ROIs. Fig.3(f) demonstrates use of machine learning classification to establish a boundary between healthy and damaged bone based off the TBP and LPF (%) values, which were the variables that performed best in the porcine preclinical model.
Figure 3.
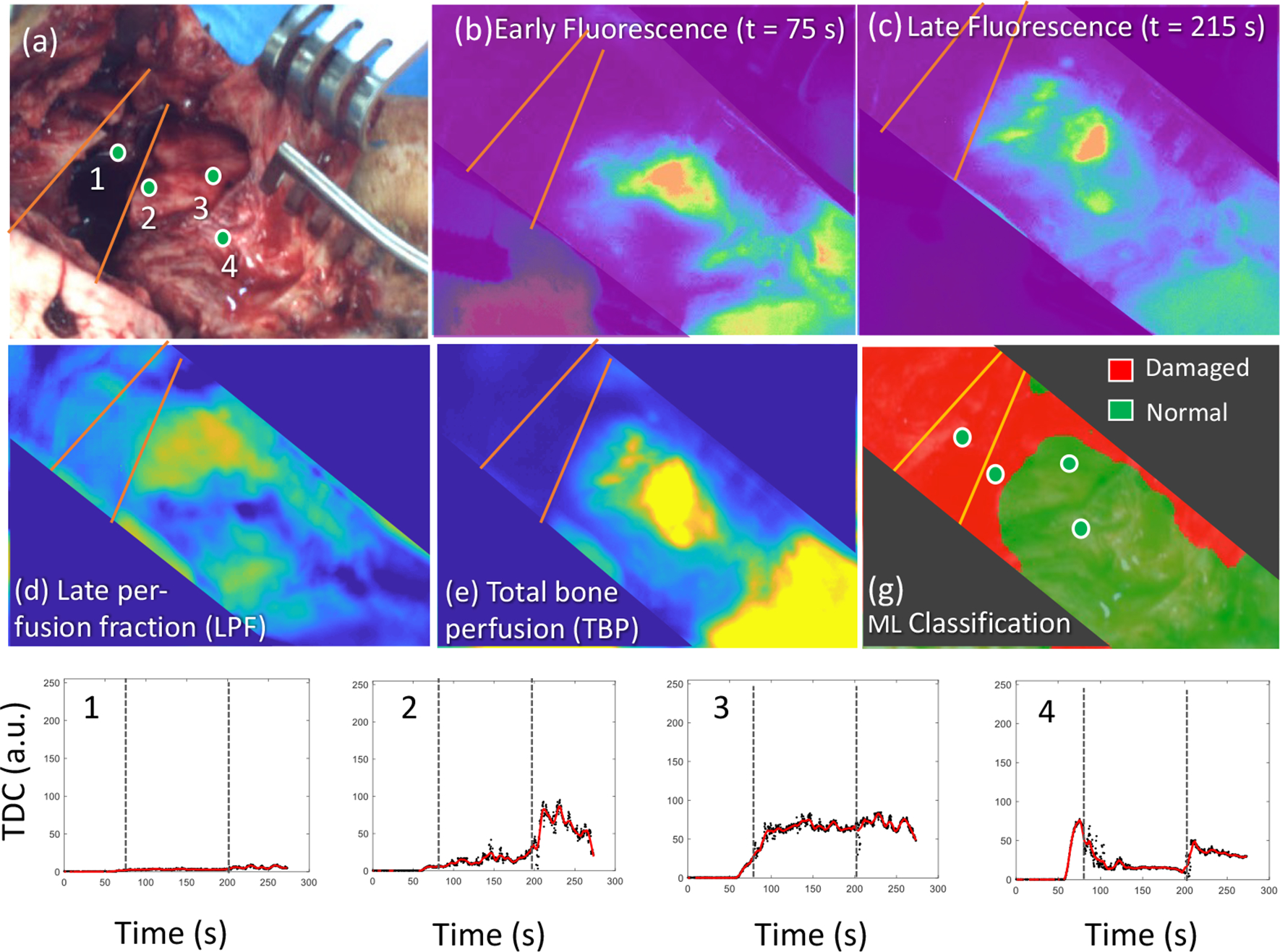
Images acquired intraoperatively during surgical treatment of a Gustilo Type 3C open tibia fracture with segmental bone loss. (a)White-light image, the circles #1-#4, were ROIs for imaging data analysis shown in (g); (b) and (c), fluorescence images were acquired at 75 sec. (b) and 215 sec. (c), after ICG injection; (d) and (e), LPF (d) and TBP(e) images; and (g), Temporal dynamic curves on ROIs shown in (a).
4. CONCLUSIONS
In this work, a indocyanine green (ICG)-based dynamic contrast-enhanced fluorescence imaging (DCE-FI) was been utilized to the orthopedic surgeries to provide an objective measurement of bone perfusion. The results from the pilot patient exams demonstrated that this quantitative ICG-based DEC-FI can provide the reproducible, predictable and robust assess of the bone perfusion during the orthopedic surgery and differentiate ‘injured’ from ‘normal/healthy’ bone.
ACKNOWLEGMENTS
This work has been supported by a NIH grant R01 AR077157, a DOD award W81XWH-20-1-0319 and a Gillian Reny Stepping Strong Center for Trauma Innovation award. Authors have submitted US Provisional Patent Application No. 62/755,067 on the subject presented in this paper.
REFERENCES
- 1.Bosse MJ, MacKenzie EJ, Kellam JF, Burgess AR, Webb LX, Swiontkowski MF, Sanders RW, Jones AL, McAndrew MP, Patterson BM, McCarthy ML, Travison TG, and Castillo RC, “ An analysis of outcomes of reconstruction or amputation after leg-threatening injuries,” N Engl J Med 347, 1924–1931 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Harris AM, Althausen PL, Kellam J, Bosse MJ, Castillo R, and Lower G Extremity Assessment Project Study, “Complications following limb-threatening lower extremity trauma,” J Orthop Trauma 23, 1–6 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Keeling JJ, Gwinn DE, Tintle SM, Andersen RC, and McGuigan FX, “Short-term outcomes of severe open wartime tibial fractures treated with ring external fixation,” J Bone Joint Surg Am 90, 2643–2651 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Lerner A, Fodor L, and Soudry M, “Is staged external fixation a valuable strategy for war injuries to the limbs?,” Clin Orthop Relat Res 448, 217–224 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Owens BD, Kragh JF Jr., Wenke JC, Macaitis J, Wade CE, and Holcomb JB, “Combat wounds in operation Iraqi Freedom and operation Enduring Freedom,” J Trauma 64, 295–299 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Merritt K, “Factors increasing the risk of infection in patients with open fractures,” J Trauma 28, 823–827 (1988). [DOI] [PubMed] [Google Scholar]
- 7.Dellinger EP, Miller SD, Wertz MJ, Grypma M, Droppert B, and Anderson PA, “Risk of infection after open fracture of the arm or leg,” Arch Surg 123, 1320–1327 (1988). [DOI] [PubMed] [Google Scholar]
- 8.Jodal L, Nielsen OL, Afzelius P, Alstrup AKO, and Hansen SB, “Blood perfusion in osteomyelitis studied with [(15)O]water PET in a juvenile porcine model,” EJNMMI Res 7, 4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poot DHJ, van der Heijden RA, van Middelkoop M, Oei EHG, and Klein S, “Dynamic contrast-enhanced MRI of the patellar bone: How to quantify perfusion,” J Magn Reson Imaging 47, 848–858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Udagawa A, Sato S, Hasuike A, Kishida M, Arai Y, and Ito K, “Micro-CT observation of angiogenesis in bone regeneration,” Clin Oral Implants Res 24, 787–792 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Elliott JT, Jiang S, Pogue BW, and Gitajn IL, “Bone-specific kinetic model to quantify periosteal and endosteal blood flow using indocyanine green in fluorescence guided orthopedic surgery,” J Biophotonics 12, e201800427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott JT, Addante RR, Slobegean GP, Jiang S, Henderson ER, Pogue BW, and Gitajn IL, “Intraoperative fluorescence perfusion assessment should be corrected by a measured subject-specific arterial input function,” J Biomed Opt 25, 1–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


