Abstract
Tumour markers have no established role in the monitoring of the course of metastatic breast cancer during antineoplastic therapy, yet cancer antigen 15.3 (CA15.3) and carcinoembryonic antigen (CEA) are commonly used in clinical practice to aid in the early detection of progression of disease (PD). In our multicentre, prospective, real-life study, we enrolled 142 consecutive patients with advanced breast cancer receiving endocrine therapy in combination with a CDK4/6 inhibitor from January 2017 to October 2020; 75 patients had PD at the time of database closure. We measured serum marker concentrations at regular 4-month intervals together with radiological tumour response assessments and in cases of clinical suspicion of PD. Appropriate descriptive and inferential statistical methods were used to analyse serum marker level trends amongst prespecified subgroups and at specific time points (baseline, best radiologically documented tumour response and first detection of PD) in the subpopulation of patients with PD at the time of database closure. Notably, the median time from treatment initiation to best tumour response was 4.4 months. We evaluated the presence of an association between baseline CA15.3 and CEA levels and prespecified clinical characteristics but found no clinically meaningful correlation. We assessed marker level variations at the time of best radiologically documented disease response and PD: in the subgroup of patients who responded to treatment before progressing, we detected a statistically significant correlation with tumour marker variation between the time of best response and progression; this finding was not confirmed in the subgroup of patients that did not benefit from treatment. In conclusion, serum tumour marker flares can be useful in the early diagnosis of PD but should not be used as the sole factor prompting a change in treatment strategy without radiological confirmation.
Keywords: breast cancer, CDK4/6 inhibitors, tumour markers
Introduction
In current clinical practice, the role of serum tumour markers in the diagnosis of breast cancer (BC) and during follow-up after adjuvant therapy is not completely established. According to the 5th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer,1 their use as an aid to evaluate response to treatment is considered acceptable, especially in patients with non-measurable metastatic disease. However, an isolated increase in tumour marker levels should not be the sole reason prompting a change in treatment. No serum marker has shown sufficient sensitivity and specificity to warrant its use as an indicator of progression during follow-up because they do not offer reliable prognostic information about the disease nor a comprehensive clinical and biological dynamic assessment of the disease course. Hence, according to these international guidelines, imaging is necessary to monitor for and diagnose progression of disease (PD).
Differently from randomized controlled trials (RCTs), which are conducted in ideal settings in the absence of confounding factors, patients enrolled in real-life studies match the actual population receiving the intervention of interest. Real-life studies are thus able to provide information about the effects of an intervention in routine clinical circumstances. Unlike RCTs, they are characterized by high generalizability but low internal validity.2
In this real-life study, we aimed to evaluate the prognostic role of variations in cancer antigen 15.3 (CA15.3) and carcinoembryonic antigen (CEA) levels in patients with metastatic hormone-receptor positive, human epidermal growth factor 2-negative (HR+/HER2−) BC (MBC) treated with cyclin-dependent kinase 4/6 (CDK4/6) inhibitors. The goal of our investigation is to consider whether it would be possible, in the near future, to use these markers as a monitoring tool to diagnose PD early, in a faster and more practical way compared to standard imaging procedures. This would allow more informed decisions regarding the continuation of treatment with a CDK4/6 inhibitor and changes in therapeutic strategy.
Patients and methods
In this prospective, observational study, we enrolled consecutive women treated at the Units of Medical Oncology at the ICS Maugeri IRCCS and at the Fondazione IRCCS Policlinico San Matteo. All patients had a diagnosis of HR+/HER2− MBC and had a clinical indication to receive antineoplastic treatment with endocrine therapy (ET) (consisting of either anastrozole, letrozole or fulvestrant) plus a CDK4/6 inhibitor (palbociclib or abemaciclib). We used an ‘all-comers’ design, where patients’ baseline levels of serum neoplastic markers could be within the normal range or increased.
Patient information was extrapolated from their medical records and semi-anonymized. No active patient participation was required for the research protocol.
The study was carried out according to the ethical regulations of ICS Maugeri IRCCS, following approval from the Institutional Ethics Committee (C.E. 2295, approved on 9 January 2017) and the signing of informed consent.
Data collection
Data collection started at the time of administration of the first dose of the CDK4/6 inhibitor. It included personal data in a semi-anonymous format, features of the disease, oestrogen receptor (ER) and HER2 status, type of adjuvant therapy, progression-free survival (PFS), site and number of metastases, tumour biology, number of previous lines of treatment, date of treatment initiation and termination, number of cycles received, and type of associated ET. Other recorded dates were those of best response (BR; defined as the best instrumentally documented response) and PD.
Disease status assessment
Assessment of tumour response was performed at regular 4-month intervals according to common clinical practices. Clinicians could decide to prescribe additional investigations in case of clinical suspicion of PD or for other specific clinical needs. The imaging methods used to re-evaluate disease status were CT scans (with or without contrast medium) or PET with 2-deoxy-2-[18F]-fluoro-D-glucose (18F-FDG-PET)/CT. Tumour response was reported according to the RECIST v.1.1 criteria: complete response (CR), partial response (PR), stable disease (SD) or PD.3
Determination of CEA and CA15.3 levels
Tumour marker levels were determined at each cycle of therapy for the first three cycles, concomitantly with imaging studies, and in case of clinical suspicion of PD. Measurements were performed with the chemiluminescent microparticle immunoassay (CMIA) technique, with the ‘Alinity’ kit.
Inclusion and exclusion criteria
Eligible patients were women with HR+ MBC and candidates to receive ET in association with a CDK4/6 inhibitor. Hormone-receptor positivity was defined as an expression of oestrogen and/or progesterone receptors on >10% of tumour cells detected by immunohistochemistry (IHC).4 Patients were either postmenopausal or had achieved a pharmacologically induced postmenopausal status with the use of luteinizing hormone-releasing hormone (LHRH) analogues or previous chemotherapy. The choice of CDK inhibitor was based on drug availability, clinician’s preference and specific drug toxicities. Patients needed to receive at least one complete cycle of therapy with a CDK4/6 inhibitor + ET before database closure for data analysis, which occurred in October 2020.
Additional inclusion criteria were HER2− disease (IHC 0–1 or IHC 2 confirmed by negative in situ hybridization), measurable lesions according to RECIST 1.1 criteria,3 and a life expectancy of at least 4 months. Enrolled patients were also required to have normal hepatic, renal and bone marrow function, consistently with recommendations from clinical practice guidelines for the administration of antineoplastic drugs.
Objectives and endpoints of the study
The objective of this study was to establish the existence of a correlation between marker level variation and disease course during treatment with a CDK4/6 inhibitor until the time of PD.
For this purpose, two fundamental points in the clinical course of the disease were identified: achievement of BR and subsequent PD. BR was defined as the lowest tumour burden to be radiologically documented before PD, as evaluated by a retrospective revision of imaging studies and in compliance with RECIST v1.1 criteria.3
The endpoints of the study, assessed in the subpopulation who had undergone PD at the time of data analysis, were as follows: (1) determination of baseline differences in tumour marker levels amongst subgroups of clinical interest (visceral versus non-visceral metastases, oligometastatic versus polymetastatic disease, primary versus secondary endocrine resistance as defined in the 5th edition of the Advanced Breast Cancer guidelines1); (2) determination of the percentage of patients that, at the time of BR, had a reduction in CEA and CA15.3 levels of at least 20% compared to baseline values, in the entire subpopulation and in the subgroups of patients with primary or secondary endocrine resistance; (3) determination of the percentage of patients that, at the time of BR, had an increase in CEA and CA15.3 levels of at least 20% compared to baseline values; (4) determination of the percentage of patients that, at the time of PD, had an increase in CEA and CA15.3 levels of at least 20% compared to the time of BR; (5) quantification of the time needed to achieve BR; and (6) establishment of the existence of a statistically significant relationship between an increase in tumour marker levels and radiologically documented PD.
Statistical analysis
Descriptive statistical methods were employed to report baseline characteristics of the entire study population (n=142). The distribution of numerical continuous variables was described by median value (25th–75th percentiles or interquartile range, IQR), whilst categorical variables were reported by their absolute and relative (%) frequency. The two-sided one-sample Wilcoxon test was used to test the null hypothesis of no change in terms of marker distribution (numeric, continuous) between two time points. The Friedman test was used to test the null hypothesis of no difference in terms of marker distribution (numeric, continuous) between three time points. The McNemar test was used to test the null hypothesis of the absence of change in terms of categorical variables between two time points. The Wilcoxon rank-sum test was applied to test the null hypothesis of no difference in terms of numeric continuous variable distribution between binary subgroups. The Kruskal–Wallis test was applied to test the null hypothesis of no difference in terms of numeric continuous variable distribution between subgroups characterized by three or more levels. The significance level α was set to 0.05. Statistical analyses were carried out with R statistical software tool v 4.0.5 ( www.r-project.org ).
Results
The study population included 142 consecutive patients treated with a CDK4/6 inhibitor in combination with ET from January 2017 to October 2020. At the time of data analysis, 75 patients (population ‘A’, 52.8%) had suspended treatment because of PD, whilst 67 patients (population ‘B’, 47.1%) were still receiving study treatment.
The majority of patients were postmenopausal at the time of BC diagnosis (81.7%); 58.5% of patients had ≤65 years at the time of treatment initiation and 78.2% of primary tumours had ductal histology. Overall, 116 (81.7%) patients had received previous adjuvant therapy and disease-free survival (DFS) was >24 months in 92 patients (64.8%). Consistently with data reported in the literature, 26 (18.3%) patients presented with metastases at diagnosis. At the time of treatment initiation, 62 (43.7%) patients had visceral metastases and 67 (47.2%) had multiple sites of metastatic disease. Patients with primary endocrine resistance were less represented (14.8%) compared to those with secondary endocrine resistance (66.9%).
Regarding the choice of CDK 4/6 inhibitor, all but two patients received palbociclib; the remaining two patients received abemaciclib. In total, 78 (55%) patients received a CDK4/6 inhibitor as first-line therapy, 33 (23.2%) as second-line therapy and 31 (21.8%) as a subsequent line. During the enrolment phase, we observed a change in the use of CDK4/6 inhibitors, which were prescribed as earlier lines of treatment in the therapeutic strategy in accordance with evolving indications from regulatory agencies. Almost two-thirds of patients (63.4%) received a selective oestrogen receptor modulator (fulvestrant) in combination with a CDK4/6 inhibitor, in line with the proportion of patients pretreated with ET.
The pattern of tumour response was the following: 10 (7%) patients achieved a CR, 53 (37.3%) a PR and 51 (35.9%) an SD, with a clinical benefit rate of 80.2%; 28 (19.7%) patients had PD as BR. Table 1 summarizes baseline population characteristics.
Table 1.
Demographics and clinical characteristics of patients at baseline.
| n | % | |
|---|---|---|
| Total number of patients | 142 | 100 |
| Age at the beginning of treatment | ||
| ≤65 years | 83 | 58.5 |
| >65 years | 59 | 41.5 |
| Menopausal status at diagnosis | ||
| Postmenopausal | 116 | 81.7 |
| Premenopausal | 26 | 18.3 |
| Tumour histology | ||
| DCI | 111 | 78.2 |
| LCI | 31 | 21.8 |
| Adjuvant therapy | ||
| Yes | 116 | 81.7 |
| No | 26 | 18.3 |
| Mean DFS | ||
| ≤24 months | 50 | 35.2 |
| >24 months | 92 | 64.8 |
| Site of metastases | ||
| Visceral | 62 | 43.7 |
| Non-visceral | 80 | 56.3 |
| Metastatic pattern | ||
| Oligometastatic | 75 | 52.8 |
| Polymetastatic | 67 | 47.2 |
| Endocrine resistance pattern | ||
| Primary | 21 | 14.8 |
| Secondary | 95 | 66.9 |
| Metastatic at diagnosis | 26 | 18.3 |
| Line of therapy | ||
| First line | 78 | 55 |
| Second line | 33 | 23.2 |
| Beyond second line | 31 | 21.8 |
| Concomitant ET | ||
| Anastrozole | 9 | 6.3 |
| Letrozole | 43 | 30.3 |
| Fulvestrant | 90 | 63.4 |
| Best response | ||
| Complete response | 10 | 7.1 |
| Partial response | 53 | 37.3 |
| Stable disease | 51 | 35.9 |
| Disease progression | 28 | 19.7 |
| Therapy status | ||
| Ongoing | 67 | 47.1 |
| Stopped | 75 | 52.8 |
DFS, disease-free survival.
At the time of the treatment initiation, patients with marker levels above the upper limit of normal, defined as CEA >5 ng/mL and CA15.3 >25 UI/mL, were respectively 71 (50%) and 115 (81%). None of the prespecified subgroups showed a statistically and clinically relevant correlation with baseline marker elevation (Table 2).
Table 2.
Pretreatment CA15.3 and CEA distribution by clinical characteristics of interest.
| Variable | CA15.3 | p value | CEA | p value |
|---|---|---|---|---|
| Endocrine-resistance patterns | ||||
| Primary | 55.2 (30–171) | 0.930 | 6.6 (2.5–15.9) | 0.653 |
| Secondary | 60.7 (29–138.7) | 4.4 (1.85–11.85) | ||
| Metastatic at diagnosis | 58.94 (28.78–146) | 4.6 (2.25–15.35) | ||
| Site of metastasis | ||||
| Visceral | 51 (29–119.47) | 0.596 | 5.9 (2.3–12.43) | 0.596 |
| Non-visceral | 73 (28.38–160.12) | 5 (1.8–13.85) | ||
| Line of treatment | ||||
| 1 | 76.3 (36.55–172) | 0.020* | 6.5 (2.1–19.2) | 0.122 |
| ≥2 | 53.9 (25.5–97.32) | 4.6 (1.96–9.45) | ||
Marker distribution is described in terms of median value (interquartile range). p values from biomarker analysis are derived from the two-sided Wilcoxon rank-sum test or the Kruskal test.
p<0.05.
According to data from the literature, tumours that show endocrine resistance are less likely to respond to combination therapy with ET and a CDK4/6 inhibitor compared to those with metastatic disease at presentation.5–7 In our study, only 21 patients showed primary endocrine resistance: at the time of BR, 10 (47.6%) and 7 (33.3%) patients had a decrease in CA15.3 and CEA levels, respectively. Out of 95 women with secondary endocrine resistance, 38 (40%) had a reduction in CA15.3 levels and 46 (48.4%) in CEA levels at the time of BR.
In the population with PD at the time of data analysis (n=75), the median time to reach BR was 4.4 months.
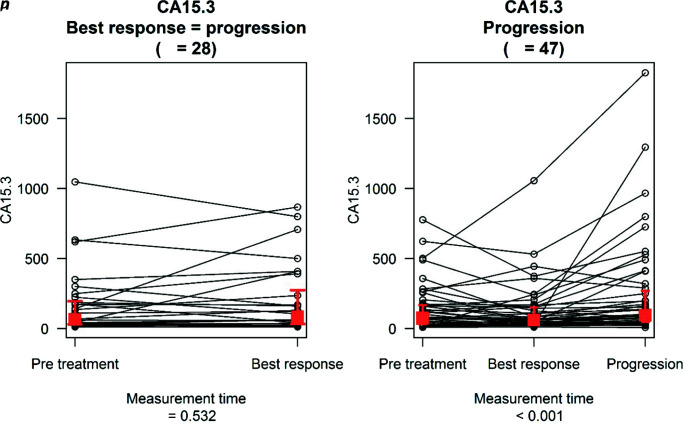
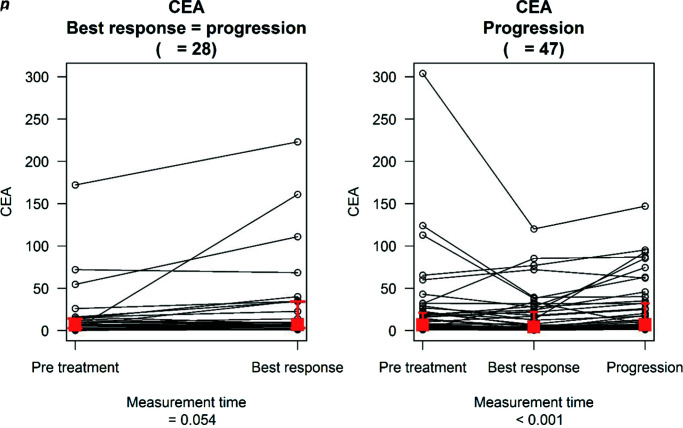
In the subgroup of patients that achieved PR, CR or SD (n=47), comparison between pretreatment and BR levels showed a median decrease of −3.5 and −0.3 for CA15.3 and CEA levels, respectively, a finding that was not statistically relevant (p=0.113 and p=0.110, respectively). Overall, 28 (37.3%) and 29 (38.7%) patients showed >20% decrease from baseline levels of CA15.3 and CEA, respectively. In 24.6% of cases, the reduction in tumour marker levels involved CEA and CA15.3 simultaneously; 21.3% and 29.8% of women showed a significant increase in either CEA or CA15.3 levels (i.e. >20% compared to baseline) at the time of BR and 10.6% of patients showed a simultaneous elevation of both tumour markers. Available data8,9 report a 24% discordance rate between serum tumour marker levels and imaging at the time of radiologically documented tumour response, a proportion coherent with the subpopulation from our study that showed an increase in tumour markers at the time of BR. The analysis of tumour marker level variation between BR and PD showed a statistically significant increase in both CA15.3 (median increase: 29; p<0.001) and CEA (1.9; p<0.001). We recorded an elevation in CEA and CA15.3 levels in 40 (85.1%) and 39 (83%) patients, respectively; whereas, 35 (74.5%) patients showed an increase in both tumour markers. CA15.3 levels at PD were also elevated compared to baseline values (median increase: 10.7; p=0.031) (Table 3).
Table 3.
CA15.3 and CEA distribution in patients with disease progression after achieving a complete/partial response or stable disease (n=47).
| Measurement time | Marker: CA15.3 | p value | Marker: CEA | p value |
|---|---|---|---|---|
| Pretreatment | 74 (31.56–168) | <0.001* (global difference) | 6.7 (2.1–21.05) | <0.001* (global difference) |
| Best response | 61 (28.4–150.05) | 4.4 (1.73–22.15) | ||
| Progression disease | 95 (47–270) | 6.8 (2.95–32.1) | ||
| Change | Marker: CA15.3 | p value | Marker: CEA | p value |
| Between PT and BR | −3.5 (−52.5 to 13.33) | 0.113 | −0.3 (−6.24 to 0.47) | 0.110 |
| Between BR and PD | 29 (5.5–106.3) | <0.001* | 1.9 (0.28–7.95) | <0.001* |
| Between PT and PD | 10.7 (−9.35 to 67.5) | 0.031* | 0.9 (−0.7 to 4.87) | 0.066 |
Tumour marker distribution and change between measurement times are described in terms of median value (interquartile range). p values from analysis of biomarker change are derived from the two-sided one-sample Wilcoxon signed rank test. p values for global differences between time are derived from the Friedman test.
p<0.05.
BR, best response; PD, progressive disease; PT, pretreatment.
In the subgroup of patients who had PD as BR (n=28), CEA and CA15.3 elevations by >20% were present in 57.1% and 42.9%, respectively. Comparison between pretreatment and PD levels showed a median increase of 3.55 for CA15.3 and of 1.07 for CEA; neither of these results reached statistical significance (Table 4).
Table 4.
CA15.3 and CEA distribution in patients with progressive disease as best response (n=28).
| Measurement time | Marker: CA15.3 | Marker: CEA | ||
|---|---|---|---|---|
| Pretreatment | 64.85 (29.75–195.20) | 6.60 (2.80–14.25) | ||
| Best response | 84.50 (33.05–274.95) | 6.90 (2.90–34.25) | ||
| Change | Marker: CA15.3 | p value | Marker: CEA | p value |
| Between PT and BR | 3.55 (−18.00 to 40.78) | 0.532 | 1.07 (−0.62 to 7.20) | 0.054 |
Tumour marker distribution and change between measurement times are described in terms of median value (interquartile range). p values from analysis of biomarkers change are derived from the two-sided one-sample Wilcoxon signed rank test. p values for global differences between time points are derived from the Friedman test.
p<0.05.
BR, best response; PD, progressive disease; PT, pretreatment.
Lastly, we evaluated the trend of tumour marker levels between BR and PD in the subpopulation that achieved CR, PR or SD, and between pretreatment and PD in the subgroup that did not. These data are illustrated in Figures 1 and 2, and analysis on the trend of tumour marker levels with respect to relevant thresholds is reported in Table 5. The only statistically relevant result regards the former group: 19 (40.43%) patients had CEA levels of ≤5 ng/mL both at the time of BR and of PD, whilst 8 (17.02%) patients had CEA levels of ≤5 ng/mL at BR but of >5 ng/mL at PD; amongst patients who already had elevated CEA levels at BR, 19 (40.43%) patients had CEA of >5 ng/mL both at BR and at PD, whilst 1 (2.13%) patient had CEA of >5 ng/mL at BR and of ≤5 ng/mL at PD.
Figure 1.
CA15.3 distribution at different measurement times.
The red squares describe the median value of the marker distribution at the different time points while the two whiskers indicate the 25th and 75th percentiles.
Figure 2.
CEA markers distribution at different measurement times.
The red squares describe the median value of the marker distribution at the different time points while the two whiskers indicate the 25th and 75th percentiles.
Table 5.
Frequency distribution of patients characterized by CA15.3 and CEA values above and below the relevant threshold before treatment at different time points.
| CA15.3 | CEA | ||||||
|---|---|---|---|---|---|---|---|
| Best response: PD (n=28) | Best response: PD (n=28) | ||||||
| PT | PD | n (%) | p value | PT | PD | n (%) | p value |
| <25 | <25 | 1 (3.57%) | 0.450 | ≤5 | ≤5 | 8 (28.57%) | 1 |
| <25 | ≥25 | 2 (7.14%) | ≤5 | >5 | 2 (7.14%) | ||
| ≥25 | <25 | 5 (17.86%) | >5 | ≤5 | 2 (7.14%) | ||
| ≥25 | ≥25 | 20 (71.43%) | >5 | >5 | 16 (57.14%) | ||
| Best response: CR/PR/SD ( n =47) | Best response: CR/PR/SD ( n =47) | ||||||
| BR | PD | n (%) | p value | BR | PD | n (%) | p value |
| <25 | <25 | 3 (6.25%) | 0.617 | ≤5 | ≤5 | 19 (40.43%) | 0.045* |
| <25 | ≥25 | 3 (6.25%) | ≤5 | >5 | 8 (17.02%) | ||
| ≥25 | <25 | 1 (2.08%) | >5 | ≤5 | 1 (2.13%) | ||
| ≥25 | ≥25 | 40 (85.42%) | >5 | >5 | 19 (40.43%) | ||
p values are derived from the McNemar test.
p<0.05.
BR, best response; PD, progressive disease; PT, pretreatment.
Discussion
Unlike in the adjuvant setting, where the intent of treatment is curative, the aim of therapy in patients with advanced BC is palliation. Therefore, quality of life and tolerability are important aspects impacting treatment strategy and should be balanced with potential gains in disease regression and survival.6 It follows that an accurate and rapid test to assess response to treatment after its initiation is essential to make therapeutic decisions: if a patient is responding and toxicity is acceptable, treatment should be continued; whereas, if the tumour shows intrinsic or acquired resistance, an alternative therapy should be considered. One of the most convenient and objective methods to determine the efficacy of antineoplastic treatment is by measuring serum markers such as CA15.3.10–12 Apart from the MUC1-related markers, several others have been associated with BC, but none have been shown to be superior to CA15.3.13
Even though several studies evaluated the relationship between changes in CA15.3 levels and response to chemotherapy in patients with MBC,10–12 this application of biomarkers in clinical practice has not been properly investigated in the last decade and especially after the introduction of CDK4/6 inhibitors.
In one of the largest studies conducted so far, Tampellini et al.5 measured CA15.3 levels at baseline and after 3 and 6 months of anthracycline-based first-line treatment in 526 patients enrolled in prospective phase II and III clinical trials. The results of this study showed that, at 6 months, the median time to progression was 15.3 months in women with low marker levels throughout the study, 11.7 months in those with a CA15.3 decrease of >25%, 9.6 months in those with elevated baseline levels but without further increase during therapy and 8.6 months in those with an increase in marker levels during treatment (p<0.001). Overall, there was a significant relationship between changes in CA15.3 levels and clinical response but several individual discrepancies were noted: indeed, CA15.3 variations paralleled disease response in only about half of patients. These data support the inadequacy of CA15.3 level monitoring as the sole method of treatment response assessment in patients with advanced BC.
In another study by Lee et al.,14 an increase in CA15.3 levels was detected in 55.6% of patients at the time of systemic recurrence, and elevated CEA levels were observed in 36% of patients. Similar results were obtained in other studies, with the proportion of patients showing a correlation between marker elevation and radiologically documented PD ranging between 54% and 80% for CA15.3 and between 30% and 50% for CEA.15–19 These studies suggest that the tumour markers CA15.3 and CEA may be prognostic for survival in MBC and that elevated levels at the time of recurrence may be associated with poor outcomes.
A study by Bartsch et al.20 evaluated the prognostic value of monitoring CA15.3 and CEA during treatment with fulvestrant. The results of this study showed that CA15.3 could increase in patients with PD but also in those experiencing clinical benefits. This is further proof that tumour marker elevation cannot be taken as a sign of PD without verification by imaging studies. Moreover, in this trial both CA15.3 and CEA were poor prognostic markers for determining progression in patients receiving fulvestrant, thus raising the issue that variations in tumour markers may also be affected by specific antineoplastic agents.
In our study, we analysed data from a population of 142 consecutive patients receiving treatment for their MBC. Enrolled patients were representative of the real-life population with respect to prognostic factors such as the site of metastases (visceral versus non-visceral) and metastatic pattern (oligometastatic versus polymetastatic). The majority of patients (67%) showed secondary endocrine resistance; 78 (55%) women were given CDK4/6 inhibitors as first-line treatment. The vast majority of our patients received palbociclib as the CDK4/6 inhibitor of choice. This fact can be explained by the earlier approval and availability of palbociclib in this clinical setting compared to abemaciclib and ribociclib.
In order to analyse the tumour marker trends, the fundamental moments during the course of antineoplastic treatment were identified: baseline (before the initiation of treatment), radiologically documented BR, and PD. This study also evaluated the time required to achieve BR whilst receiving CDK4/6 inhibitors (4.4 months). This parameter carries great clinical significance and suggests that a rapid cytoreduction can be realistically achieved in patients being treated with these agents.
In our study, there was no statistically significant decrease of serum markers at the time of BR, but the increase in tumour marker levels between baseline and PD in the population who had a radiologically established tumour response reached statistical significance. We consider this finding to be clinically relevant, even though we acknowledge the presence of limitations in our analysis, including the lack of an adequate sample size and of centralized imaging review. Our small sample size precluded further analysis of the impact of clinical and primary tumour histological characteristics on prognosis and response to treatment.
We could not find strong evidence in the literature regarding the trend of tumour markers during antineoplastic treatments. However, the study by Tampellini et al.8 reported that between 50% and 60% of patients show a biochemical response during treatment with anthracyclines for advanced BC. This percentage was significantly lower in our study, probably because of the differences in pharmacodynamics with specific anticancer agents. Therefore, it is necessary to evaluate the response to treatment through radiological studies, nuclear medicine and quality of life assessments.
To further support this, a relevant proportion of patients in our study had an increase in marker levels at the time of BR. These results correspond with data found in the literature5 and underline the necessity to confirm biochemical progression with imaging before considering a change in treatment. Nevertheless, our data suggest the presence of a tumour marker flare that could be used as an aid to prompt radiological disease assessments with an earlier diagnosis of PD.
The analysis of the impact of an increase in tumour marker levels on PFS was omitted because of the impossibility to stratify patients for multiple, well-known factors with an impact on prognosis. Additionally, the analysis of marker level variations in specific subgroups was omitted because the study itself was not designed to answer such a question.
Conclusion
Despite the lack of evidence about the existence of a specific population of patients in whom an increase in tumour marker levels is always detected at PD and despite the individual heterogeneity of tumour marker trends, this study showed a correlation between CEA and CA15.3 elevations during treatment with CDK4/6 inhibitors and PD. Indeed, at the time of radiologically established PD, an increase in tumour markers (more relevantly with CA15.3) was observed both in patients who had PD as BR and in those who initially responded to treatment and later had PD, though statistical significance was reached only in the latter group.
Our data are significant enough to justify an anticipation of radiological disease assessments in patients showing an increase in serum tumour marker levels. Further confirmatory studies are necessary to validate this finding.
Acknowledgements
None.
Footnotes
Contributions: FS, EF, BT and RP conceived the content of the article. EF, BT, RP, EQ, CT and EB provided patient data and revised the final draft. LM, SP, SS and AP collected patient data and compiled the study database. AM performed the statistical analysis. FS and CL revised the final draft. All authors approved the final manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/08/dic.2022-1-3-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article nor for the associated research.
Correct attribution: Copyright © 2022 Sottotetti F, Ferraris E, Tagliaferri B, Palumbo R, Quaquarini E, Teragni C, Balletti E, Leli C, Premoli A, Mollica L, Puglisi S, Sardi S, Malovini A, Pedrazzoli P, Bernardo A. https://doi.org/10.7573/dic.2022-1-3. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
This article is part of the Tackling clinical complexity in breast cancer Special Issue: https://www.drugsincontext.com/special_issues/tackling-clinical-complexity-in-breast-cancer/
References
- 1.Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saturni S, Bellini F, Braido F, et al. Randomized controlled trials and real life studies. Approaches and methodologies: a clinical point of view. Pulm Pharmacol Ther. 2014;27:129–138. doi: 10.1016/j.pupt.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1 – update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AIOM. Linee guida: neoplasie della mammella. 2020. [Accessed July 29, 2022]. https://www.aiom.it/wp-content/uploads/2021/11/2021_LG_AIOM_Neoplasie_Mammella_11112021.pdf.pdf .
- 5.Schettini F, Giuliano M, Giudici F, et al. Endocrine-based treatments in clinically-relevant subgroups of hormone receptor-positive/HER2-negative metastatic breast cancer: systematic review and meta-analysis. Cancers. 2021;13(6):1458. doi: 10.3390/cancers13061458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Souza A, Spicer D, Lu J. Overcoming endocrine resistance in metastatic hormone receptor-positive breast cancer. J Hematol Oncol. 2018;11(1):80. doi: 10.1186/s13045-018-0620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartkopf AD, Grischke EM, Brucker SY. Endocrine-resistant breast cancer: mechanisms and treatment. Breast Care. 2020;15(4):347–354. doi: 10.1159/000508675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tampellini M, Berruti A, Bitossi R, et al. Prognostic significance of changes in CA 15-3 serum levels during chemotherapy in metastatic breast cancer patients. Breast Cancer Res Treat. 2006;98:241–248. doi: 10.1007/s10549-005-9155-y. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso F, Bedard PL, Winer EP, et al. International guidelines for management of metastatic breast cancer: combination vs sequential single-agent chemotherapy. J Natl Cancer Inst. 2009;101:1174–1181. doi: 10.1093/jnci/djp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson JFR, Jaeger W, Syzmendera JJ, et al. The objective measurement of remission and progression in metastatic breast cancer by use of serum tumor markers. Eur J Cancer. 1999;35:47–53. doi: 10.1016/s0959-8049(98)00297-4. [DOI] [PubMed] [Google Scholar]
- 11.Van Dalen A, Heering KJ, Barak V, et al. Treatment response in metastatic breast cancer: a multicenter study comparing UICC criteria and tumor marker changes. Breast. 1996;5:82–88. doi: 10.1016/S0960-9776(96)90126-5. [DOI] [Google Scholar]
- 12.Kurebayashi J, Nishimura R, Tanaka K, et al. Significance of serum tumor markers in monitoring advanced breast cancer patients treated with systemic therapy: a prospective study. Breast Cancer. 2004;11:389–395. doi: 10.1007/BF02968047. [DOI] [PubMed] [Google Scholar]
- 13.Duffy MJ, Evoy D, McDermott EW. CA 15-3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta. 2010;411(23–24):1869–1874. doi: 10.1016/j.cca.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Park S, Park JM, et al. Elevated levels of serum tumor markers CA 15-3 and CEA are prognostic factors for diagnosis of metastatic breast cancers. Breast Cancer Res Treat. 2013;141(3):477–484. doi: 10.1007/s10549-013-2695-7. [DOI] [PubMed] [Google Scholar]
- 15.Molina R, Zanon G, Filella X, et al. Use of serial carcinoembryonic antigen and CA 15.3 assays in detecting relapses in breast cancer patients. Breast Cancer Res Treat. 1995;36:41–48. doi: 10.1007/BF00690183. [DOI] [PubMed] [Google Scholar]
- 16.Hayes DF, Zurawski VR, Jr, Kufe DW. Comparison of circulating CA15-3 and carcinoembryonic antigen levels in patients with breast cancer. J Clin Oncol. 1986;4:1542–1550. doi: 10.1200/JCO.1986.4.10.1542. [DOI] [PubMed] [Google Scholar]
- 17.Robertson JF, Pearson D, Price MR, et al. Assessment of four monoclonal antibodies as serum markers in breast cancer. Eur J Cancer. 1990;26:1127–1132. doi: 10.1016/0277-5379(90)90268-x. [DOI] [PubMed] [Google Scholar]
- 18.Robertson JF, Pearson D, Price MR, et al. Prospective assessment of the role of five tumour markers in breast cancer. Cancer Immunol Immunother. 1991;33:403–410. doi: 10.1007/BF01741602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams MR, Turkes A, Pearson D, et al. The use of serum carcinoembryonic antigen to assess therapeutic response in locally advanced and metastatic breast cancer: a prospective study with external review. Eur J Surg Oncol. 1988;14:417–422. [PubMed] [Google Scholar]
- 20.Bartsch R, Wenzel C, Pluschnig U, et al. Prognostic value of monitoring tumour markers CA 15-3 and CEA during fulvestrant treatment. BMC Cancer. 2006;6:81. doi: 10.1186/1471-2407-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]