Abstract
The emergence of COVID-19 has drawn the attention of health researchers sharply back to the role that food systems can play in generating human disease burden. But emerging pandemic threats are just one dimension of the complex relationship between agriculture and infectious disease, which is evolving rapidly, particularly in low-income and middle-income countries (LMICs) that are undergoing rapid food system transformation. We examine this changing relationship through four current disease issues. The first is that greater investment in irrigation to improve national food security raises risks of vector-borne disease, which we illustrate with the case of malaria and rice in Africa. The second is that the intensification of livestock production in LMICs brings risks of zoonotic diseases like cysticercosis, which need to be managed as consumer demand grows. The third is that the nutritional benefits of increasing supply of fresh vegetables, fruit, and animal-sourced foods in markets in LMICs pose new food-borne disease risks, which might undermine supply. The fourth issue is that the potential human health risks of antimicrobial resistance from agriculture are intensified by changing livestock production. For each disease issue, we explore how food system transition is creating unintentional infectious disease risks, and what solutions might exist for these problems. We show that successfully addressing all of these challenges requires a coordinated approach between public health and agricultural sectors, recognising the costs and benefits of disease-reducing interventions to both, and seeking win–win solutions that are most likely to attract broad policy support and uptake by food systems.
Introduction
Food systems are broadly defined as the “production, marketing, transformation and purchase of food, and the consumer practices, resources and institutions involved in these processes”.1 In low-income and middle-income countries (LMICs), the focus of this Review, food systems are changing rapidly. These changes are largely demand driven, and linked to rising incomes, growing populations, and urbanisation. Although there are unique features of food system transition in each country, general patterns can be observed as countries move from low to middle and then to high incomes. The table characterises agricultural production and food supply changes as countries move from agrarian (usually low income), to transitioning (middle income), and modern (high income) stages, following broadly the approach used by the World Bank.2, 3, 4 Food system transitions have many positive benefits: increasing and diversifying food supply to growing cities and large towns, and offering opportunities for jobs and increasing incomes for many people. One general challenge is inclusion—ensuring that women, individuals from low-income households, and marginalised groups also benefit. From a public health perspective, there are also big challenges as food systems transform. Best known is the global pandemic of obesity and non-communicable diseases, associated with dietary transitions and the increased consumption of fats, sugar, salt, and calorie-dense foods.5 This Review will focus on another challenge: how the intensification of agricultural production and increasing complexity of food supply chains, particularly in transitioning African and Asian countries, change the risks and relative burdens of infectious diseases.
In transitioning food systems, intensification of irrigation for crop production and denser, more intensive livestock production are affecting vector-borne and zoonotic disease risk, while the increasing complexity of food supply systems, particularly for perishable foods—fish, meat, milk, eggs, vegetables, and fruits—is affecting food-borne disease risks and burdens.6, 7
In this Review, we propose that food systems in transition are likely to create unintentional infectious disease risks for rural and urban populations, associated with agricultural intensification and diversification aimed to meet changing consumer demand. We propose that many of these problems can be avoided or at least reduced, but this requires recognition and resolution of conflicts between agricultural and public health policy and practice. We explore this hypothesis using four case studies: vector-borne disease in irrigated agriculture, zoonotic diseases in livestock value chains, food safety, and antimicrobial resistance associated with food systems. For each study, we ask three questions. What aspects of food system transition are creating unintentional infectious disease risks? What solutions might exist for these problems? How would they require better coordination of agricultural and public health policy and practice?
Table.
Agricultural development, changes in production, and food systems
| Agrarian | Transitioning | Modern | |
|---|---|---|---|
| Priority outcomes | Food security | Diet diversification, food safety | Diet quality |
| Agricultural management | Minimal | Varied, coping | Systematically managed |
| Crop production | Minimal: land, labour, local water catchments | Increased: fertiliser, seeds, irrigation schemes | Packages of inputs, mechanisation |
| Livestock production | Small herds, dispersed, local breeds, and food | Growing densities, elite breeds, feed, and drugs | Systematically managed production units |
| Markets | Informal, short food chain | Mixed, informal and formal, urbanising | Formal, urbanised, long food chain |
| Regulations of production and inputs | Almost non-existent | Restricted capacity | Aligned and managed |
Case studies are drawn from a research collaboration under the Agriculture for Nutrition and Health programme, a collaboration between public health and agriculture research in LMICs, coordinated by the Consultative Group on International Agricultural Research.
Changing agricultural landscapes and vector-borne disease
Transitioning food systems also involve the intensification of production systems, which entails the conversion of natural habitats to agricultural landscapes, to maximise yield per input. This intensification can have both positive and negative effects on the distribution and abundance of disease vectors, but frequently, such agricultural development has led to increased risk of vector-borne diseases.7, 8, 9 Farming communities are exposed to disease from wildlife and their disease vectors, in adjacent natural habitats, with livestock creating a zoonotic pathway for transmission, whereas cultivation and irrigation expose communities to a range of soil-borne and vector-borne diseases. A meta-analysis in southeast Asia has shown that people who live or work in agriculture are 1·7 times more likely to be infected with a pathogen than those in non-farming professions.10 In Kenya, rural farming communities have been found to have high burdens of infectious disease, which are mainly zoonotic in origin.11 Agricultural intensification can lead to an increase in human and animal population densities and movement as migrant labour becomes more important, thus increasing spread of disease. Our case study focuses on the intensification of irrigation systems and unintended increase in malaria in Africa.
Over the past few decades, irrigation has played an important role in improving global crop yield in transitioning economies, especially in Africa, which has long been beset with food insecurity.12 The creation of dams and irrigation systems has frequently been linked to change in the risk of vector-borne diseases such as schistosomiasis,13 leishmaniasis,14 and malaria.15 The nature of this change depends on the specific ecology of the local vectors. For instance, in sub-Saharan Africa, the main vectors of malaria breed abundantly in irrigated rice fields, whereas in parts of southeast Asia they breed in small puddles within the forest and stream pools in the forest fringe.16 Expansion of rice production and reduction of forests in the Greater Mekong Subregion has contributed to reduced malaria prevalence,17 whereas in Africa, rice field expansion has increased the local population of malaria vectors. Our case study concerns current intensification of African rice production, a pan-African priority driven by concerns for food security and changing food demands of an urbanising population.18 This activity poses unintended disease risks, particularly as plans are made for elimination of malaria in this region.19, 20
The historical relationship between rice and malaria in Africa is complex. A series of studies in the 1990s and early 2000s compared malaria indicators in rice-growing and nearby non-rice-growing communities.21, 22, 23 Although rice-growing villages had much larger populations of the vector, Anopheles gambiae sensu lato, malaria prevalence was generally the same as, or slightly lower than, that in non-rice-growing villages. This observation, often called the paddies paradox, is thought to arise from interacting human and biological factors.21 In particular, new rice schemes often bring improvements to farmer income and community infrastructure, including better housing and access to mosquito nets and health services. These changes could enable farming communities to defend themselves more effectively against both mosquitoes and parasites. In this scenario, the social benefits of agricultural development tend to suppress transmission, counter-balancing the transmission-increasing effects of the additional mosquitoes.
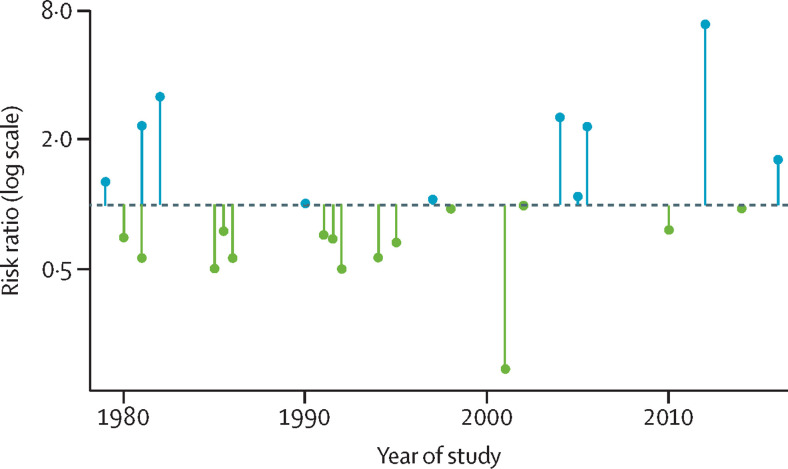
However, an updated analysis of comparisons between rice-growing and non-rice-growing villages suggests that rice-growing areas are now becoming malaria hotspots.24 What has changed? One possibility is that advances in malaria control have revealed the true effects of this agricultural driver on the disease. In the 1990s, malaria transmission was so intense in many parts of rural Africa that most people were infected most of the time, and for this reason, indices of prevalence were relatively insensitive to variations in transmission intensity.25 In the past two decades, with a massive scaling-up of anti-malaria interventions, malaria prevalence has declined dramatically.26 As a result, malaria indices are now more sensitive to variations in transmission. In addition, access to insecticide-treated bednets and effective drugs has now become more equitable and less dependent on the presence of a development project. Figure 1 illustrates how this relationship between rice growing and malaria prevalence has changed over recent decades. This trend looks set to continue—ie, as malaria continues to decline in Africa, its association with irrigated rice is likely to become a stronger and more conspicuous obstacle to elimination.
Figure 1.
The relationship between rice growing and malaria prevalence, by year of study
Data are from Chan and colleagues’ systematic review and meta-analysis.24 Each lollipop refers to a study comparing infection prevalence of malaria between rice-growing and non-rice-growing communities. Risk ratios less than 1 (green lollipops) indicate a lower prevalence of malaria in the rice-growing villages (ie, the paddies paradox); risk ratios higher than 1 (blue lollipops) indicate a higher malaria prevalence in the rice-growing villages. Studies conducted before 2003 in settings with relatively intense malaria transmission, generally observed less malaria in the irrigated rice villages; conversely, studies conducted since 2003 in settings with relatively low malaria transmission mostly found a higher prevalence of malaria in the rice field villages. This finding suggests that in the future, if malaria continues to decline in Africa, rice fields might become more prominent as hotspots of transmission.
There are potential technical solutions to address this problem, which involve reducing vector production in rice. Intermittent drying of paddies where mosquitoes breed can dramatically reduce vector populations.27, 28, 29 However, varying irrigation in this way has potential impacts on labour and yield. A study in Benin, for instance, indicates that intermittent irrigation can reduce mosquito production by 80%, but at the expense of a 10% loss in rice yield.30 By adding mosquito production as a factor (along with water use, labour, and yield) in designing and evaluating new rice intensification systems, this conflict between agricultural and public health policies might be resolved. Support for such an intervention could come from environmental considerations, as research has shown that a form of intermittent irrigation (called alternate wetting and drying) can also address national commitments to reduce water use and greenhouse gas production from irrigated rice.31, 32, 33
The importance of demonstrating cross-sectoral policy cobenefits has been shown in a successful programme to reduce malaria and water use in rice-producing areas of Peru.29 Similar success in Africa might remove an important obstacle to malaria elimination there, but this agriculture and public health trade-off might emerge in various forms elsewhere, as rapid global spread of a number of vector-borne diseases occurs,34, 35 and as climate change impacts rain-fed agriculture, which will drive a growing reliance on irrigation to meet food security needs.
Zoonotic diseases in changing food systems
Emergence of zoonotic disease has been occurring at greater frequency over the past century than ever observed before.36 The drivers causing these pathogens to spill over from animals into humans include features of countries in agricultural transition, including land use change, agricultural intensification, increasing trade, changes in human demography, and urbanisation.37 SARS-CoV-2, which has since spread globally, might have emerged from the food system, and complex, global food systems will no doubt continue to be routes of pathogen emergence with far reaching impacts.
Unlike the vector-borne diseases mentioned earlier, directly transmitted zoonoses have considerable potential to spread beyond rural farming environments. Intensification of livestock production in peri-urban areas to meet increased urban demand often results in more animals being kept within a limited space and therefore at a higher density. Such conditions lead to increased contact rates between animals, which can promote amplification of zoonotic pathogens in close proximity to humans. Where wildlife frequent peri-urban farms, livestock can also act as intermediate and amplifying hosts for wildlife-borne zoonoses, such as infections caused by Escherichia coli O157:H7,38 Leptospira spp,39, 40 Nipah virus,41 influenza A virus in southeast Asia,42 and potentially Ebola viruses in east Africa.43 As small-scale farmers adapt to more market-orientated production, while remaining relatively small scale in terms of production methods, the risk of transmission of zoonotic diseases, through the products produced on farms, extends to a much broader consumer group. Similar principles apply to the trade in wild meat, for which growing demand from urban centres could expose consumers living in major cities to zoonotic pathogen risks associated with wildlife that are harvested in rural and peri-urban areas.44, 45
The international public health community has for some years had its attention focused on emerging zoonotic diseases that have the potential to become pandemic, particularly in LMICs. The investment that has accompanied this attention predicted a COVID-19-type event, but was not sufficient to prevent its global spread when it came. Although this attention might now be expected to intensify in years to come, the principle and continuing burden of zoonotic disease in transitioning food systems in LMICs is nonetheless dominated by a group of quite different, endemic zoonotic diseases.46 These include echinococcosis, cysticercosis, brucellosis, Q-fever, leptospirosis, bovine tuberculosis, and several bacterial infections due to E coli, Staphylococcus aureus, Salmonella spp, and Campylobacter spp. In LMICs, an estimated 26% of the burden of infectious disease is contributed by these and other zoonoses.47
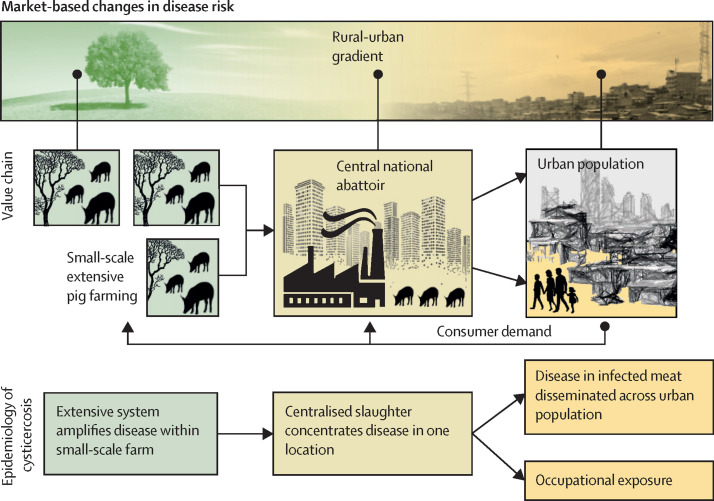
Focusing on cysticercosis infection with the pork tapeworm Taenia solium in Africa, as an illustration, highlights the complex interaction between food value chains and infectious disease. Figure 2 outlines its dynamics across pork food value chains in Africa, where small-scale pig farming in extensive rural or peri-urban settings can lead to infection rates of 30–40% in pigs at slaughter,48, 49 and growing urban demand50 means that many of these animals are slaughtered in central national abattoirs in peri-urban and urban areas,51, 52 exposing non-farming populations. Occupational exposure associated with operating in the food chain53 can lead to livestock-associated disease outbreaks far from farms.54 Disease surveillance approaches need to capture such market-based patterns in risks, including both farmers and consumers and rural and urban communities.
Figure 2.
The relationship between cysticercosis caused by the tapeworm Taenia solium, and the farm and food chain environment
With increasing urbanisation, small-scale livestock production is connected to ever increasing numbers of consumers, creating disease risks distant from the farm environment.
These complex chains also point to potential interventions, for which efficacy needs to be assessed: tools for cysticercosis control, such as the pig-targeted vaccine designed to prevent infection and new dewormers that are effective at clearing existing infections,55 hold great promise for protecting consumers. Such interventions are agriculture sector focused with public health benefits potentially accruing to large populations. Work is urgently required to best understand how to deploy such interventions in a variety of epidemiological circumstances.
Much research to date on livestock-borne zoonoses has not addressed these local impacts of changing food systems. Rather, attention has been focused on low resolution, big data studies conducted at a global and regional scale, which enable epidemiologists to map the distribution of livestock-borne zoonoses and predict their response to immediate and long-term global change.56, 57, 58, 59 Although valuable in predicting large-scale changes in the risk envelope from zoonotic disease, such studies now need to be complemented by research that informs policy and practice at the level of local systems in which social, biological, and environmental factors will determine disease risk in food systems across farming and non-farming households.60, 61, 62 As food system change gathers pace, epidemiological research into livestock-borne zoonoses in LMICs must focus on establishing efficient local surveillance for zoonotic disease risks, to inform integrated agricultural and public health policy and interventions that are sensitive to capturing the dynamic nature of social and environmental factors. Examples of this approach include studies of zoonotic diseases in urban meat value chains in Nairobi,63, 64 and of zoonotic malaria in plantation farming in Malaysia.65 Integrating local-scale and broad-scale approaches described here into a systems approach58, 66 will inform the deployment of in-situ surveillance systems and epidemiological studies targeting the most susceptible food systems.
Food safety in informal markets
Infectious diseases originating in farming communities in LMICs encounter a broader, increasingly urban, population, through food products and through contact with waste generated by agricultural production.
Until recently, food-borne diseases were not seen as key health burdens in LMICs, relative to other infectious diseases. This perspective changed in 2016, when the first study on the global burden of food-borne diseases showed that the burden was too similar to those generated by malaria, HIV/AIDs, or tuberculosis.6 This landmark review also revealed that almost all the documented health burden (98%) fell on LMICs. Further, most of this burden (97%) was due to biological hazards, distinct from, for instance, chemical hazards in food such as toxins and pesticides. Unlike some other infectious diseases with a declining burden in LMICs, the burden of food-borne diseases appears to be increasing,67 consistent with the concept of a food safety life cycle that tracks economic development.3 As systems shift from traditional to transitioning, food risks increase, only to decrease again with transformation to modern systems.68
The major sources of food-borne diseases in LMICs are fresh livestock and fish products, and fruits and vegetables sold mainly in traditional or wet markets.68 Hence, foods associated with the greatest potential to improve nutrition in people living in low-income households in LMICs69 are also those associated with the greatest disease risks. Transitioning food systems combine increasing provision of these foods through traditional market systems, creating a particular challenge for food safety.
High-income countries have been relatively successful at managing food safety, using risk-based approaches that address food safety from the farm level to the consumer. Many LMICs successfully export safe food, but, to date, there are no examples of food safety approaches that work in mass domestic markets in LMICs that are both sustainable and scalable.70 This absence of workable food safety approaches is partly because there has been relatively little investment in food safety in domestic markets, and investments have not focused on hazards that have the highest burden on health,71 and partly because approaches tried to date might have limited potential to radically improve food safety.
Many initiatives have focused on modernisation of the food system, such as through promoting milk collection facilities, large abattoirs, or supermarkets. However, in many LMICs, these business models might not be competitive with the successful informal food sector. Indeed, modernised facilities do not always improve food safety indicators72, 73 and can paradoxically result in less safe food as they offer more opportunities for cross-contamination.74 Food safety interventions at farm level, in the form of good agricultural practices, have been promoted, but uptake has been low, and evidence for health outcomes is absent.75 A meta-analysis suggested some success in targeting households with food safety recommendations.76 However, the sustainability, scalability, and practicality of these remain uninvestigated.76 Moreover, mitigation at household level would require major behavioural and dietary change, which would be difficult to achieve across billions of households.
One area in which success has been achieved is in the targeting of informal sector actors. Research in Kenya to improve smallholder dairy production revealed that market access of farmers was under threat from the public health sector's belief that all milk should be pasteurised. Most milk was sold unpasteurised through informal sector traders and was cheaper and more accessible than milk provided by the formal dairy sector. Formal sector claims of the lack of a level playing field added to concerns about food safety.77 An innovative project focused on training and certifying informal market traders succeeded in showing that trained vendors produced acceptably safe milk, leading to a licensing and certification scheme that legitimised the traders.78 This secured livelihoods, provided markets for smallholder farmers, and ensured cheap milk was still available to consumers. An economic assessment found benefits of US$26 million a year.79
This landmark work on improving food safety and nutrition in LMICs has been followed by initiatives in several LMICs.80, 81, 82, 83 This emerging body of research suggests that three factors are crucial for success: an enabling regulatory environment with authorities on board; improvement in the capacity of value chain actors through training and simple technology; and implementation of incentives for behaviour change such as consumer demand, peer pressure, or changing power relations.
Antimicrobial resistance
Antimicrobial use in human health and agriculture is thought to be a key driver for the emergence of antimicrobial resistance, with the extensive use of such drugs leading to biological selection pressures favouring resistance.84 Agricultural and aquacultural use of antimicrobials prophylactically, for growth promotion, or disease prevention, and for treatment of disease, is widespread and increasing in transitioning food systems.85 As production inputs, antimicrobials are often unregulated or subject to poorly enforced regulations.85 This lack of regulation can lead to contamination of animal-source food chains with antimicrobial residues and antibiotic-resistant bacteria. Food crops are another agricultural source of these contaminants, particularly where manure and wastewater are used in crop production.86
Studies have suggested that widespread antimicrobial use in food animals might contribute to the development of resistance to antimicrobials commonly used in human medicine,87, 88 although evidence of the extent and frequency of this contribution remains scarce.89, 90, 91, 92, 93
However, antimicrobial susceptibility across both sectors needs to be regarded as a public good that needs safeguarding. A precautionary approach should therefore drive actions to reduce the use of antimicrobials in agricultural production. In high-income countries, where actions have been tied to regulation, levels of resistance have been shown to decrease in the absence of selective pressure,94 although this effect is not always found.95, 96, 97 Therefore, viewing antimicrobial resistance as an agricultural or medical problem alone is unlikely to tackle the issue.
LMICs could face a greater proportion of the emerging human antimicrobial resistance burden than high-income countries,98, 99 and levels of national economic development are negatively correlated with a number of antimicrobial resistance risk factors.100 Additional contributions from agriculture might arise because LMICs in food system transition will be where the most intensification of livestock production occurs in coming years.100 In some LMIC settings, veterinary services are often scarce, limiting resources for surveillance and empirical drug choices.
Patterns of use of antimicrobials in livestock in LMICs depend very much on the production system. For extensive smallholder livestock systems, much use is therapeutic and aimed at protecting health of small herds on which households depend.101 In more intensive systems, such as those associated with dairy, poultry, and pig keeping, larger quantities of drugs will often be used prophylactically, sometimes to compensate for poor hygiene and animal husbandry, to prevent disease and promote growth in order to protect profit margins, ensure reliability of supply, and meet export standards.102 Although inappropriate use of antibiotics occurs in all kinds of production, the rapid growth of intensive production in transitioning food systems deserves particular attention.
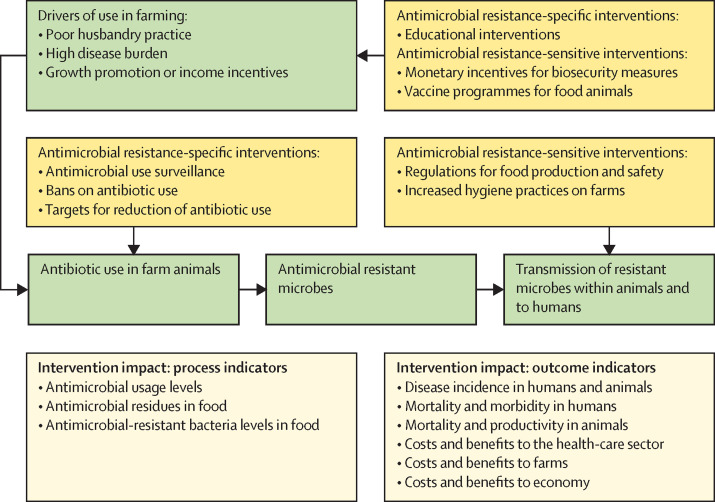
Targeting potential agricultural sources of antimicrobial resistance in LMICs can involve a range of interventions in livestock systems.103 Antimicrobial resistance interventions could be considered to be specific (ie, those directly targeting antimicrobial resistance) or sensitive (ie, those indirectly affecting antimicrobial resistance, such as biosecurity interventions). We illustrate in figure 3 how interventions can relate to antimicrobial use and antimicrobial resistance generation or transmission through farm antimicrobial use.
Figure 3.
Potential measures of the effect of antimicrobial resistance-related interventions within agriculture
Interventions can add costs to food production—eg, through the construction of biosecure production facilities. When demand and price can absorb these costs, such as in highly regulated export markets for meat and seafood, production in LMICs is capable of reducing antimicrobial resistance risks. But for local markets, higher costs will limit adoption of effective interventions. If the ultimate aim of interventions is to protect both agricultural production and human health, development of interventions should comprise measurement of process indicators for their effect on antimicrobial resistance reduction in livestock systems, as well as outcome indicators that relate to effects on both agricultural productivity and human health (figure 3). Health economic consequences of antimicrobial resistance risk from agriculture can be measured, and a cost–benefit approach, based on integrating agricultural and health economic models, might provide useful tools for bringing together stakeholders with different goals, and identifying the most promising interventions.104
Discussion
Food systems in transition are characterised by intensification and diversification of food production, as an increasingly urban and more wealthy population demands different diets. As our case studies have shown, these changes can generate unintentional infectious disease problems along the entire agricultural food chain. In principle, the disease risks in each case study can be reduced by public health interventions in agricultural systems to remove their causes: for instance, reducing vector populations by changing irrigation, de-intensifying livestock food production from urban communities, tightly regulating food safety, and removing antibiotics from animal production systems. But such unilateral action faces two challenges.
First, in transitioning agricultural systems, the regulatory capability necessary to reduce disease risk in food systems can be weak, as informal mechanisms often dominate the system organisation. In food safety, for instance, although well resourced private export sectors can meet international standards, requiring and enforcing safety regulations in local food systems with state resources is more challenging. Second, unilateral health-focused approaches can be counterproductive. Many countries in food system transition are historically agricultural economies. In these countries, agricultural growth has been shown to be the most effective at reducing poverty among the poorest communities compared with growth in other sectors.105
Agricultural development can bring low-income farming households into a better position to reduce disease risks and afford health care. The paddies paradox exemplifies these health benefits. An intensified and well functioning agriculture sector can also bring health benefits by reducing the costs and increasing the availability of highly nutritious foods, such as eggs, milk, meat, vegetables, and fruit. Increasing this capacity for nutrition-sensitive agriculture improves health.106, 107
Controlling disease risks by restricting agriculture in countries in food system transition can expose complex trade-offs. Public health interventions that undermine food security and nutrition might face low policy support or uptake. An integrated, cross-sectoral approach could have greater overall human welfare benefits than one based solely on addressing disease risks. This approach might begin, as we have indicated in some case studies earlier, with an assessment of the economic costs and benefits of food system interventions to the agricultural and public health sectors.
The COVID-19 pandemic has provided a dramatic example of the need for this intersectoral approach, magnifying the inter-relationships between health, food systems, and economics. Food systems and economies cannot function properly without control of SARS-CoV-2 transmission, but lockdowns and other public health measures can have dramatic impacts on food supply, nutrition, and livelihoods, particularly for people from low-income households involved in labour-intensive activities that cannot be done from home.108, 109
Finally, food system transformation exposes a range of poverty-related and gender-related issues that affect food security and nutritional outcomes.110, 111 We note that similar issues exist with respect to agriculturally related diseases. For instance, deeply rooted gender inequalities can exist within households with respect to livestock and animal-source food handling, creating very different risks for men and women.112 Zoonotic disease risks are already higher in marginalised farming populations than in the general population113, 114, 115 as a result of poor access to health services, poor or non-existent veterinary service provision, and inadequate estimates of disease impact that limit lobbying power for resources. While offering opportunities to address income and gender inequity through systems change, intensification of agriculture also has the potential to exacerbate differences in infectious disease risks from agriculture.
To conclude, resolving unanticipated infectious disease consequences of food system transition requires constructive dialogue between agricultural and health sectors, and indeed within the health sector between the different groups responsible for reducing infectious disease risk and improving nutrition. As we have seen in our case studies, taking a cross-sectoral approach can even identify win–win solutions for health and food systems, such as new water management technologies that can lead to more sustainable rice intensification with less malaria risk, or programmes to inform food chain actors about food safety without undermining the important function of informal food markets by implementing stringent regulation. For areas, such as antimicrobial resistance, for which health risks from agriculture are still poorly understood, it is important to identify the potential agricultural and health outcomes of agricultural interventions to reduce health risks.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This Review captures work done by the Agriculture for Nutrition and Health programme of the Consultative Group for International Agricultural Research, and was partly funded by that programme. The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. It has its origins in a series of papers on agriculture and health designed with Alan Dangour at the London School of Hygiene & Tropical Medicine, and we are grateful to him and his team on the Sustainable and Healthy Food Systems project, funded by the Wellcome Trust, for supporting its development.
Contributors
JW and JM conceived the Review and drafted the introduction and discussion. Case studies were drafted as follows: vector borne disease by KC and JL, zoonotic diseases by EMF and JMH, food safety by DG, and antimicrobial resistance by NRN and BW. All authors contributed to revision of the entire paper in successive drafts. JW supervised the overall work and is the guarantor of the Review.
References
- 1.Global Panel on Agriculture and Food Systems for Nutrition Foresight Report 2.0. Future food systems: for people, our planet, and prosperity. 2020. https://www.glopan.org/wp-content/uploads/2020/09/Foresight-2.0_Future-Food-Systems_For-people-our-planet-and-prosperity.pdf
- 2.World Bank . World development report 2008: agriculture for development. World Bank; Washington, DC: 2007. [Google Scholar]
- 3.Jaffee S, Henson S, Unnevehr L, Grace D, Cassou E. The safe food imperative: accelerating progress in low- and middle-income countries. World Bank; Agriculture and food series. Washington, DC: 2019. [Google Scholar]
- 4.Berthe FCJ, Wadsworth J, Thiebaud A, Marquez PV, Baris E. Pulling together to beat superbugs: knowledge and implementation gaps in addressing antimicrobial resistance. World Bank; Washington, DC: 2019. [Google Scholar]
- 5.Swinburn BA, Kraak VI, Allender S, et al. The global syndemic of obesity, undernutrition, and climate change: The Lancet Commission report. Lancet. 2019;393:791–846. doi: 10.1016/S0140-6736(18)32822-8. [DOI] [PubMed] [Google Scholar]
- 6.Havelaar AH, Kirk MD, Torgerson PR, et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12:e1001923. doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottdenker NL, Streicker DG, Faust CL, Carroll CR. Anthropogenic land use change and infectious diseases: a review of the evidence. Ecohealth. 2014;11:619–632. doi: 10.1007/s10393-014-0941-z. [DOI] [PubMed] [Google Scholar]
- 8.Faust CL, McCallum HI, Bloomfield LSP, et al. Pathogen spillover during land conversion. Ecol Lett. 2018;21:471–483. doi: 10.1111/ele.12904. [DOI] [PubMed] [Google Scholar]
- 9.Jones BA, Grace D, Kock R, et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Scis. 2013;110:8399–8404. doi: 10.1073/pnas.1208059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah HA, Huxley P, Elmes J, Murray KA. Agricultural land-uses consistently exacerbate infectious disease risks in southeast Asia. Nature Comm. 2019;10:4299. doi: 10.1038/s41467-019-12333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fèvre EM, de Glanville WA, Thomas LF, Cook EAJ, Kariuki S, Wamae CN. An integrated study of human and animal infectious disease in the Lake Victoria crescent small-holder crop-livestock production system, Kenya. BMC Infect Dis. 2017;17:457. doi: 10.1186/s12879-017-2559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie H, Perez N, Anderson W, Ringler C, You L. Can sub-Saharan Africa feed itself? The role of irrigation development in the region's drylands for food security. Water Int. 2018;43:796–814. [Google Scholar]
- 13.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 14.Ghawar W, Toumi A, Snoussi M-A, et al. Leishmania major infection among Psammomys obesus and Meriones shawi: reservoirs of zoonotic cutaneous leishmaniasis in Sidi Bouzid (central Tunisia) Vector Borne Zoonotic Dis. 2011;11:1561–1568. doi: 10.1089/vbz.2011.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kibret S, Lautze J, Boelee E, McCartney M. How does an Ethiopian dam increase malaria? Entomological determinants around the Koka reservoir. Tropl Med Int Health. 2012;17:1320–1328. doi: 10.1111/j.1365-3156.2012.03077.x. [DOI] [PubMed] [Google Scholar]
- 16.Sinka ME, Bangs MJ, Manguin S, et al. The dominant anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trung HD, Van Bortel W, Sochantha T, et al. Malaria transmission and major malaria vectors in different geographical areas of southeast Asia. Trop Med Int Health. 2004;9:230–237. doi: 10.1046/j.1365-3156.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 18.Van Oort PAJ, Saito K, Tanaka A, et al. Assessment of rice self-sufficiency in 2025 in eight African countries. Glob Food Sec. 2015;5:39–49. [Google Scholar]
- 19.Feachem RGA, Chen I, Akbari O, et al. Malaria eradication within a generation: ambitious, achievable, and necessary. Lancet. 2019;394:1056–1112. doi: 10.1016/S0140-6736(19)31139-0. [DOI] [PubMed] [Google Scholar]
- 20.Emmanuel OI, Peter AF, Odeh UP, Uche AJ. Challenges of malaria elimination in Nigeria; a review. Int J Infect Dis Ther. 2017;2:79–85. [Google Scholar]
- 21.Ijumba JN, Lindsay SW. Impact of irrigation on malaria in Africa: paddies paradox. Med Vet Entomol. 2001;15:1–11. doi: 10.1046/j.1365-2915.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- 22.Keiser J, De Castro MC, Maltese MF, et al. Effect of irrigation and large dams on the burden of malaria on a global and regional scale. Am J Trop Med Hyg. 2005;72:392–406. [PubMed] [Google Scholar]
- 23.Chan K, Saito K, Lines J. Rice and malaria in Africa: a growing problem. Annual Meeting of the Multisectoral Working Group from the RBM Partnership to End Malaria; Feb 6, 2020. https://endmalaria.org/sites/default/files/u224/11_Jo%20Lines.pdf
- 24.Chan K, Tusting LS, Bottomley C, Saito K, Djouaka R, Lines J. Malaria transmission and prevalence in rice-growing versus non-rice-growing villages in Africa: a systematic review and meta-analysis. Lancet Planet Health. 2022;6:e257–e269. doi: 10.1016/S2542-5196(21)00349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molyneux DH. Common themes in changing vector-borne disease scenarios. Trans R Soc Trop Med Hyg. 2003;97:129–132. doi: 10.1016/s0035-9203(03)90097-6. [DOI] [PubMed] [Google Scholar]
- 26.Bhatt S, Weiss DJ, Cameron E, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Hoek W, Sakthivadivel R, Renshaw M, Silver JB, Birley MH, Konradsen F. Alternate wet/dry irrigation in rice cultivation: a practical way to save water and control malaria and Japanese encephalitis? Research report 47. International Water Management Institute; Colombo, Sri Lanka: 2001. [Google Scholar]
- 28.Keiser J, Utzinger J, Singer BH. The potential of intermittent irrigation for increasing rice yields, lowering water consumption, reducing methane emissions, and controlling malaria in African rice fields. J Am Mosq Control Assoc. 2002;18:329–340. [PubMed] [Google Scholar]
- 29.Chang J. Intermittent rice irrigation (IRI) for malaria control in Peru: a win-win intervention based on a multi-sector and trans-disciplinary approach. 2007. http://www.linksglobal.org/AMI/extras/Manuscript3.IntermittentRiceIrrigation.FINAL.100511.pdf (accesses May 4, 2022).
- 30.Djègbè I, Zinsou M, Dovonou EF, et al. Minimal tillage and intermittent flooding farming systems show a potential reduction in the proliferation of Anopheles mosquito larvae in a rice field in Malanville, Northern Benin. Mala J. 2020;19:333. doi: 10.1186/s12936-020-03406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrijo DR, Lundy ME, Linquist BA. Rice yields and water use under alternate wetting and drying irrigation: a meta-analysis. Field Crops Res. 2017;203:173–180. [Google Scholar]
- 32.Sander BO, Wassmann R, Palao LK, Nelson A. Climate-based suitability assessment for alternate wetting and drying water management in the Philippines: a novel approach for mapping methane mitigation potential in rice production. Carbon Manag. 2017;8:331–342. [Google Scholar]
- 33.Richards M, Sander BO. Alternate wetting and drying in irrigated rice. Implementation guidance for policymakers and investors. Consultative Group on International Agricultural Research Research Program on Climate Change, Agriculture and Food Security; Copenhagen, Denmark: 2014. [Google Scholar]
- 34.Kulkarni M. Global spread and impacts of emerging vector-borne diseases. Can Commun Dis Rep. 2016;42:198–199. doi: 10.14745/ccdr.v42i10a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weaver SC, Charlier C, Vasilakis N, Lecuit M. Zika, chikungunya, and other emerging vector-borne viral diseases. Annu Rev Med. 2018;69:395–408. doi: 10.1146/annurev-med-050715-105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones KE, Patel NG, Levy MA et al Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassell JM, Begon M, Ward MJ, Fèvre EM. Urbanization and disease emergence: dynamics at the wildlife–livestock–human interface. Trends Ecol Evol. 2017;32:55–67. doi: 10.1016/j.tree.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kauffman MD, LeJeune J. European starlings (Sturnus vulgaris) challenged with Escherichia coli O157 can carry and transmit the human pathogen to cattle. Lett Appl Microbiol. 2011;53:596–601. doi: 10.1111/j.1472-765X.2011.03163.x. [DOI] [PubMed] [Google Scholar]
- 39.Benavidez KM, Guerra T, Torres M, et al. The prevalence of Leptospira among invasive small mammals on Puerto Rican cattle farms. PLoS Negl Trop Dis. 2019;13:e0007236. doi: 10.1371/journal.pntd.0007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gamage CD, Koizumi N, Muto M, et al. Prevalence and carrier status of leptospirosis in smallholder dairy cattle and peridomestic rodents in Kandy, Sri Lanka. Vector Borne Zoonotic Dis. 2011;11:1041–1047. doi: 10.1089/vbz.2010.0153. [DOI] [PubMed] [Google Scholar]
- 41.Pulliam JRC, Epstein JH, Dushoff J, et al. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J R Soc Interface. 2012;9:89–101. doi: 10.1098/rsif.2011.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sims LD, Domenech J, Benigno C, et al. Origin and evolution of highly pathogenic H5N1 avian influenza in Asia. Vet Rec. 2005;157:159–164. doi: 10.1136/vr.157.6.159. [DOI] [PubMed] [Google Scholar]
- 43.Atherstone C, Smith E, Ochungo P, Roesel K, Grace D. Assessing the potential role of pigs in the epidemiology of Ebola virus in Uganda. Transbound Emerg Dis. 2017;64:333–343. doi: 10.1111/tbed.12394. [DOI] [PubMed] [Google Scholar]
- 44.Luiselli L, Hema EM, Segniagbeto GH, et al. Bushmeat consumption in large urban centres in West Africa. Oryx. 2020;54:731–734. [Google Scholar]
- 45.Kurpiers LA, Schulte-Herbrüggen B, Ejotre I, Reeder DAM. In: Problematic wildlife: a cross-disciplinary approach. Angelici FM, editor. Springer; Switzerland: 2016. Bushmeat and emerging infectious diseases: lessons from Africa; pp. 507–551. [Google Scholar]
- 46.Doble L, Fèvre EM. Focusing on neglected zoonoses. Vet Rec. 2010;166:546–547. doi: 10.1136/vr.c2373. [DOI] [PubMed] [Google Scholar]
- 47.Grace D, Gilbert J, Randolph T, Kang’ethe E. The multiple burdens of zoonotic disease and an ecohealth approach to their assessment. Trop Anim Health Prod. 2012;44(suppl 1):67–73. doi: 10.1007/s11250-012-0209-y. [DOI] [PubMed] [Google Scholar]
- 48.Thomas LF, de Glanville WA, Cook EAJ, et al. Modelling the risk of Taenia solium exposure from pork produced in western Kenya. PLoS Negl Trop Dis. 2017;11:e0005371. doi: 10.1371/journal.pntd.0005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas LF, Harrison LJS, Toye P, et al. Prevalence of Taenia solium cysticercosis in pigs entering the food chain in western Kenya. Trop Anim Health Prod. 2016;48:233–238. doi: 10.1007/s11250-015-0949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murungi MK, Muloi DM, Muinde P, et al. The Nairobi pork value chain: mapping and assessment of governance, challenges, and food safety issues. Front Vet Sci. 2021;8:1–18. doi: 10.3389/fvets.2021.581376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akoko JM, MacLeod E, Thomas LF, et al. Detection of circulating antigens for Taenia spp. in pigs slaughtered for consumption in Nairobi and surroundings, Kenya. Parasite Epidemiol Control. 2019;4:e00093. doi: 10.1016/j.parepi.2019.e00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shyaka A, Quinnell RJ, Rujeni N, Fèvre EM. Using a value chain approach to map the pig production system in Rwanda, its governance, and sanitary risks. Front Vet Sci. 2021;8:720553. doi: 10.3389/fvets.2021.720553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cook EAJ, De Glanville WA, Thomas LF, Kariuki S, Bronsvoort BM de C, Fèvre EM. Risk factors for leptospirosis seropositivity in slaughterhouse workers in western Kenya. Occup Environ Med. 2017;74:357–365. doi: 10.1136/oemed-2016-103895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cook EAJ, De Glanville WA, Thomas LF, Kariuki S, Bronsvoort BM de C, Fèvre EM. Working conditions and public health risks in slaughterhouses in western Kenya. BMC Public Health. 2017;17:1–12. doi: 10.1186/s12889-016-3923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kabululu ML, Ngowi HA, Mlangwa JED, et al. TSOL18 vaccine and oxfendazole for control of Taenia solium cysticercosis in pigs: a field trial in endemic areas of Tanzania. PLoS Negl Trop Dis. 2020;14:e0008785. doi: 10.1371/journal.pntd.0008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Intergovernmental Panel on Climate Change Climate change 2013: the physical science basis. Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel On Climate Change. 2013. https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_all_final.pdf
- 57.Sonwa DJ, Dieye A, El Mzouri EH, et al. Drivers of climate risk in African agriculture. Clim Dev. 2017;9:383–398. [Google Scholar]
- 58.Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet. 2015;386:1973–2028. doi: 10.1016/S0140-6736(15)60901-1. [DOI] [PubMed] [Google Scholar]
- 59.Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gill M. Zoonoses: designing a research programme to bridge multisectoral barriers. Outlook Agric. 2013;42:5–7. [Google Scholar]
- 61.Hassell JM, Begon M, Ward MJ, Fèvre EM. Urbanization and disease emergence: dynamics at the wildlife–livestock–human Interface. Trends Ecol Evol. 2017;32:55–67. doi: 10.1016/j.tree.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazet JAK, Clifford DL, Coppolillo PB, Deolalikar AB, Erickson JD, Kazwala RR. A “One health” approach to address emerging zoonoses: the HALI project in Tanzania. PLoS Med. 2009;6:e1000190. doi: 10.1371/journal.pmed.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alarcon P, Dominguez-Salas P, Häsler B, et al. Mapping of beef, sheep and goat food systems in Nairobi—a framework for policy making and the identification of structural vulnerabilities and deficiencies. Agric Syst. 2017;152:1–17. doi: 10.1016/j.agsy.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed S, Haklay M, Tacoli C, et al. Participatory mapping and food-centred justice in informal settlements in Nairobi, Kenya. Geo. 2019;6:e00077. [Google Scholar]
- 65.Grigg MJ, Cox J, William T, et al. Individual-level factors associated with the risk of acquiring human Plasmodium knowlesi malaria in Malaysia: a case-control study. Lancet Planet Health. 2017;1:e97–104. doi: 10.1016/S2542-5196(17)30031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pongsiri MJ, Gatzweiler FW, Bassi AM, Haines A, Demassieux F. The need for a systems approach to planetary health. Lancet Planet Health. 2017;1:e257–e259. doi: 10.1016/S2542-5196(17)30116-X. [DOI] [PubMed] [Google Scholar]
- 67.Kristkova ZS, Grace D, Kuiper M. The economics of food safety in India—a rapid assessment. Wageningen University and Research; Wageningen, Netherlands: 2017. [Google Scholar]
- 68.Grace D. Food safety in low and middle income countries. Int J Environ Res and Public Health. 2015;12:10490–10507. doi: 10.3390/ijerph120910490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grace D, Dominguez-Salas P, Alonso S, et al. The influence of livestock-derived foods on nutrition during the first 1,000 days of life. ILRI Research Reports 44. International Livestock Research Institute; Nairobi, Kenya: 2018. [Google Scholar]
- 70.Unnevehr L, Grace D. Aflatoxins: finding solutions for improved food safety. 2013. https://www.ifpri.org/publication/aflatoxins-finding-solutions-improved-food-safety
- 71.Global Food Safety Partnership . Food safety in Africa: past endeavors and future directions. World Bank; Washington, DC: 2019. [Google Scholar]
- 72.Nyokabi SN, de Boer IJM, Luning PA, et al. Milk quality along dairy farming systems and associated value chains in Kenya: an analysis of composition, contamination and adulteration. Food Control. 2021;119:107482. [Google Scholar]
- 73.Alonso S, Muunda E, Ahlberg S, Blackmore E, Grace D. Beyond food safety: socio-economic effects of training informal dairy vendors in Kenya. Glob Food Sec. 2018;18:86–92. [Google Scholar]
- 74.Fahrion A, Lapar M, Nguyen NT, Do NT, Grace D. Food-borne hazards in a transforming pork value chain in Hanoi: basis for future risk assessments. Viet J Prev Med. 2013;23:18–25. [Google Scholar]
- 75.Grace D. White paper: food safety in developing countries: research gaps and opportunities. International Livestock Research Institute; Nairobi, Kenya: 2017. [Google Scholar]
- 76.Woldt M, Moy GG. Literature review on effective food hygiene interventions for households in developing countries. 2015. https://www.fantaproject.org/sites/default/files/resources/Food%20Hygiene%20Literature%20Review.pdf
- 77.Leksmono C, Young J, Hooton N, Muriuki H, Romney D. Informal traders lock horns with the formal milk industry: the role of research in pro-poor dairy policy shift in Kenya ODI/ILRI. Working Paper 266. 2006. https://hdl.handle.net/10568/1692
- 78.Omore A, Baker D. Integrating informal actors into the formal dairy industry in Kenya through training and certification. Towards priority actions for market development for African farmers. Alliance for a Green Revolution in Africa Conference; May 13–15, 2009.
- 79.Kaitibie S, Omore A, Rich K, Kristjanson P. Kenyan dairy policy change: influence pathways and economic impacts. World Dev. 2010;38:1494–1505. [Google Scholar]
- 80.Grace D, Dipeolu M, Olawoye J, et al. Evaluating a group-based intervention to improve the safety of meat in Bodija Market, Ibadan, Nigeria. Trop Anim Health Prod. 2012;44(suppl 1):61–66. doi: 10.1007/s11250-012-0208-z. [DOI] [PubMed] [Google Scholar]
- 81.Lindahl JF, Deka RP, Melin D, et al. An inclusive and participatory approach to changing policies and practices for improved milk safety in Assam, northeast India. Glob Food Sec. 2018;17:9–13. [Google Scholar]
- 82.Lindahl JF, Deka RP, Asse R, Lapar L, Grace D. Hygiene knowledge, attitudes and practices among dairy value chain actors in Assam, north-east India and the impact of a training intervention. Inf Ecoy Ep. 2018;8:1555444. [Google Scholar]
- 83.Alonso S, Muunda E, Ahlberg S, Blackmore E, Grace D. Beyond food safety: socio-economic effects of training informal dairy vendors in Kenya. Glob Food Sec. 2018;18:86–92. [Google Scholar]
- 84.Holmes AH, Moore LSP, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387:176–187. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 85.Schar D, Sommanustweechai A, Laxminarayan R, Tangcharoensathien V. Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: optimizing use and addressing antimicrobial resistance. PLoS Med. 2018;15:e1002521. doi: 10.1371/journal.pmed.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brunn A, Kadri-Alabi Z, Moodley A, et al. Characteristics and global occurrence of human pathogens harboring antimicrobial resistance in food crops: a scoping review. Front Sustain Food Syst. 2022;6:824714. [Google Scholar]
- 87.Chatterjee A, Modarai M, Naylor NR, et al. Quantifying drivers of antibiotic resistance in humans: a systematic review. Lancet Infect Dis. 2018;18:e368–e378. doi: 10.1016/S1473-3099(18)30296-2. [DOI] [PubMed] [Google Scholar]
- 88.Tang KL, Caffrey NP, Nóbrega DB, et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health. 2017;1:e316–e327. doi: 10.1016/S2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mather AE, Matthews L, Mellor DJ, et al. An ecological approach to assessing the epidemiology of antimicrobial resistance in animal and human populations. Proc Biol Scis. 2012;279:1630–1639. doi: 10.1098/rspb.2011.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muloi D, Ward MJ, Pedersen AB, Fèvre EM, Woolhouse MEJ, Van Bunnik BAD. Are food animals responsible for transfer of antimicrobial-resistant Escherichia coli or their resistance determinants to human populations? A systematic review. Foodborne Pathog Dis. 2018;15:467–474. doi: 10.1089/fpd.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Muloi D, Kiiru J, Ward MJ, et al. Epidemiology of antimicrobial-resistant Escherichia coli carriage in sympatric humans and livestock in a rapidly urbanizing city. Int J Antimicrob Agents. 2019;54:531–537. doi: 10.1016/j.ijantimicag.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Otalu OJ, Kwaga JKP, Okolocha EC, Islam MZ, Moodley A. High genetic similarity of MRSA ST88 isolated from pigs and humans in Kogi State, Nigeria. Front Microbiol. 2018;9:1–8. doi: 10.3389/fmicb.2018.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ludden C, Raven KE, Jamrozy D, Gouliouris T, Blane B, Coll F. One health genomic surveillance of Escherichia coli demonstrates distinct lineages and mobile genetic elements. mBio. 2019;10:1–12. doi: 10.1128/mBio.02693-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aarestrup FM, Seyfarth AM, Emborg HD, Pedersen K, Hendriksen RS, Bager F. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob Agents Chemother. 2001;45:2054–2059. doi: 10.1128/AAC.45.7.2054-2059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Der Steen M, Leenstra T, Kluytmans JAJW, Van Der Bij AK. Trends in expanded-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae among Dutch clinical isolates, from 2008 to 2012. PLoS One. 2015;10:e0138088. doi: 10.1371/journal.pone.0138088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Bunnik BAD, Woolhouse MEJ. Modelling the impact of curtailing antibiotic usage in food animals on antibiotic resistance in humans. R Soc Open Sci. 2017;4:161067. doi: 10.1098/rsos.161067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Emes D, Naylor N, Waage J, Knight G. Quantifying the relationship between antibiotic use in food-producing animals and antibiotic resistance in humans. Antibiotics (Basel) 2022;11:66. doi: 10.3390/antibiotics11010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okeke IN, Laxminarayan R, Bhutta ZA, et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5:481–493. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 99.Okeke IN, Klugman KP, Bhutta ZA, et al. Antimicrobial resistance in developing countries. Part II: strategies for containment. Lancet Infect Dis. 2005;5:568–580. doi: 10.1016/S1473-3099(05)70217-6. [DOI] [PubMed] [Google Scholar]
- 100.World Bank Group . Drug-resistant infections: a threat to our economic future. World Bank; Washington, DC: 2017. [Google Scholar]
- 101.Gemeda BA, Amenu K, Magnusson U, et al. Antimicrobial use in extensive smallholder livestock farming systems in Ethiopia: knowledge, attitudes, and practices of livestock keepers. Front Vet Sci. 2020;7:55. doi: 10.3389/fvets.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rushton J, Gilbert W, Coyne L, Thomas L. Interactions between intensifying livestock production for food and nutrition security, and increased vulnerability to AMR and zoonoses. Science Forum 2018 Background Papers. Consultative Group for International Agricultural Research Independent Science and Partnership Council; Rome, Italy: 2018. [Google Scholar]
- 103.Berthe FCJ, Wadsworth J, Thiebaud A, Marquez PV, Baris E. Pulling together to beat superbugs: knowledge and implementation gaps in addressing antimicrobial resistance. World Bank; Washington, DC: 2019. [Google Scholar]
- 104.Naylor NR, Lines J, Waage J, Wieland B, Knight GM. Quantitatively evaluating the cross-sectoral and one health impact of interventions: a scoping review and application to antibiotic resistance. One Health. 2020;11:100194. doi: 10.1016/j.onehlt.2020.100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Christiaensen L, Demery L, Kuhl J. The (evolving) role of agriculture in poverty reduction—an empirical perspective. J Dev Econ. 2011;96:239–254. [Google Scholar]
- 106.Kadiyala S, Harris J, Headey D, Yosef S, Gillespie S. Agriculture and nutrition in India: mapping evidence to pathways. Ann N Y Acady Sci. 2014;1331:43–56. doi: 10.1111/nyas.12477. [DOI] [PubMed] [Google Scholar]
- 107.Ruel MT, Quisumbing AR, Balagamwala M. Nutrition-sensitive agriculture: what have we learned so far? Glob Food Sec. 2018;17:128–153. [Google Scholar]
- 108.Béné C, Bakker D, Chavarro MJ. Impacts of COVID-19 on people's food security: foundations for a more resilient food system. February, 2021. https://ebrary.ifpri.org/digital/collection/p15738coll2/id/134298
- 109.McDermott J, Swinnen J. COVID-19 and global food security: 2 years later. 2022. https://ebrary.ifpri.org/digital/collection/p15738coll2/id/135017
- 110.Harris-Fry H, Nur H, Shankar B, Zanello G, Srinivasan C, Kadiyala S. The impact of gender equity in agriculture on nutritional status, diets, and household food security: a mixed-methods systematic review. BMJ Glob Health. 2020;5:e002173. doi: 10.1136/bmjgh-2019-002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Njuki J, Eissler S, Malapit H, Meinzen-Dick R, Bryan E, Quisumbing A. A review of evidence on gender equality, women's empowerment, and food systems. July, 2021. https://ebrary.ifpri.org/digital/collection/p15738coll2/id/134469 [PubMed]
- 112.Mulema AA, Wakjira KW, Lemma M, et al. Clapping with two hands: transforming gender relations and zoonotic disease risks through community conversations in rural Ethiopia. Hum Ec. 2020;48:651–663. [Google Scholar]
- 113.Floyd JR, Ruktanonchai NW, Wardrop N, Tatem AJ, Ogola J, Fèvre EM. Exploring fine-scale human and livestock movement in western Kenya. One Health. 2019;7:100081. doi: 10.1016/j.onehlt.2019.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.WHO . The control of neglected zoonotic diseases: a route to poverty alleviation. World Health Organization; Geneva: 2006. [Google Scholar]
- 115.WHO Integrated control of neglected zoonotic diseases in Africa. Wkly Epidemiol Rec. 2009;84:147–148. [PubMed] [Google Scholar]