Abstract
Background:
During kidney procurement, after ice removal, kidneys located in the retroperitoneum are at risk for rewarming owing to the time taken to retrieve other abdominal and thoracic organs, which may lead to poorer outcomes. The purpose of this study was to evaluate the impact of prolonged kidney procurement time (PKP) on outcomes of kidney transplantation performed at the Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, Canada.
Methods:
We retrospectively reviewed the cases of all adult (age ≥ 18 yr) patients who underwent kidney transplantation at the Queen Elizabeth II Health Sciences Centre between Jan. 1, 2010, and Dec. 31, 2015. We included all patients who received kidney transplants from deceased donors with a minimum follow-up period of 3 years. We defined PKP as more than 65 minutes from aortic cross-clamp to final organ extraction, and standard procurement time (SP) as 65 minutes or less.
Results:
Among the 455 transplantation procedures performed during the study period, we reviewed the cases of 145 patients who received kidneys from Nova Scotian donors and were followed in Nova Scotia. No statistically significant differences were seen in outcomes between kidney-only (n = 46) and multiorgan (n = 99) procurement, although more organs from kidney-only donors than multiorgan donors had a Kidney Donor Profile Index score greater than 50% (32 [69.6%] v. 48 [48.5%], p < 0.01). Compared to the SP group (n = 115), the PKP group (n = 30) had a higher rate of 30-day graft loss (6.7% v. 0.0%, p < 0.01), a higher incidence of de novo formation of donor-specific antibodies (3 [10.0%] v. 1 [0.9%], p < 0.01) and a lower 5-year graft survival rate (90.0% v. 97.4%, p = 0.03). Left kidneys remained 11 minutes longer on the donor than right kidneys when multiorgan procurement was performed (p < 0.01), and their 5-year survival rate was significantly lower than that of right kidneys (p = 0.03).
Conclusion:
Procurement times longer than 65 minutes may be associated with poorer outcomes after kidney transplantation. Measures to reduce kidney exposure to rewarming during procurement may improve long-term outcomes.
Abstract
Contexte:
Au cours du prélèvement de greffons rénaux, après l’extraction de la glace, les reins situés dans le rétropéritoine risquent de se réchauffer en raison du temps nécessaire au prélèvement d’autres organes abdominaux et thoraciques, ce qui peut entraîner des conséquences moins favorables. L’objectif de cette étude était d’évaluer l’effet d’un temps de prélèvement rénal prolongé (PRP) sur les résultats des transplantations rénales réalisées au Queen Elizabeth II Health Sciences Centre, à Halifax (N.-É., Canada).
Méthodes:
Nous avons procédé à une analyse rétrospective des dossiers de tous les patients adultes (âge ≥ 18 ans) qui ont subi une transplantation rénale au Queen Elizabeth II Health Sciences Centre, entre le 1er janvier 2010 et le 31 décembre 2015. Nous avons inclus tous les patients qui ont reçu des reins provenant de donneurs décédés dont le suivi se déroulait depuis au moins 3 ans. Nous avons défini le temps de PRP à plus de 65 minutes, du clampage de l’aorte à l’extraction du dernier organe, et le temps de prélèvement standard (PS) à 65 minutes ou moins.
Résultats:
Parmi les 455 interventions réalisées pendant la période de l’étude, nous avons examiné les dossiers médicaux de 145 patients qui ont reçu des reins de donneurs provenant de la Nouvelle-Écosse et qui étaient suivis en Nouvelle-Écosse. Il n’y avait aucune différence statistiquement significative entre les prélèvements rénaux uniques (n = 46) et les prélèvements d’organes multiples (n = 99), bien qu’un nombre plus élevé de donneurs de reins uniques que de donneurs d’organes multiples présentaient un score au Kidney Donor Profile Index supérieur à 50 % (32 [69,6 %] contre 48 [48,5 %], p < 0,01). Comparativement au groupe de PS (n = 115), le groupe de PRP (n = 30) présentait un taux supérieur de perte du greffon à 30 jours (6,7 % contre 0,0 %, p < 0,01), une incidence accrue de formation récurrente d’anticorps spécifiques au donneur (3 [10,0 %] contre 1 [0,9 %], p < 0,01) et un plus faible taux de survie du greffon à 5 ans (90,0 % contre 97,4 %, p = 0,03). Lors des prélèvements d’organes multiples, les reins gauches sont demeurés 11 minutes de plus dans le donneur que les reins droits (p < 0,01) et leur survie à 5 ans était significativement inférieure à celle des reins droits (p = 0,03).
Conclusion:
Les temps de prélèvement supérieurs à 65 minutes peuvent être associés à des résultats moins favorables après la transplantation rénale. La prise de mesures pour diminuer l’exposition des reins au réchauffement pendant le prélèvement pourrait améliorer les résultats à long terme.
Kidney transplantation is currently considered to be the most effective treatment for end-stage renal disease.1 The organ donation procedure has become longer and more complicated, as multiple teams must interact, and each organ has specific requirements and cannulation sequence. There has been a decline in harvest time priority for kidneys, and they are normally removed from the donor after all other vascularized organs (i.e., heart, lungs, pancreas, liver and small bowel) have been retrieved.2,3 This is probably because kidneys tend to tolerate ischemic injury better than other organs.
During organ procurement and after cross-clamp, cold preservation solution is flushed to remove blood and to allow organs to reach and maintain a temperature between 2°C and 4°C. Topical cooling with sterile ice is also typically performed in parallel to expedite hypothermia. This marks the beginning of the cold ischemia time. Organ cooling has been considered the first line of defence against hypoxic injury as it decreases the cellular metabolism and improves membrane integrity to minimize the well-known detrimental effects leading to tissue dysfunction and necrosis.4–6 Once the flush is complete, the ice is removed, and individual organ extraction begins.
The period between ice removal and kidney extraction is not typically measured, and it may vary depending on the number of organs to be retrieved and the complexity of retrieval, as well as the body habitus of the donor. Organ harvest procedures should last on average 30–60 minutes;7 however, kidney extraction times as high as 198 minutes have been reported.8 During this “waiting period,” kidneys located deep in the retroperitoneum gradually warm, and their temperature can reach up to 18°C before procurement.8
The deleterious effects of long cold ischemia time on kidney transplantation outcomes have been described.9,10 However, very little has been published on the impact of prolonged kidney procurement time (PKP) and its long-term consequences. The purpose of this study was to evaluate the impact of PKP during kidney-only and multiorgan procurement on kidney transplantation outcomes.
Methods
Setting and design
This was a retrospective cohort study including all patients who underwent kidney transplantation between Jan. 1, 2010, and Dec. 31, 2015, at the Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia, Canada. All kidney transplantation procedures for New Brunswick, Nova Scotia, Prince Edward Island, and Newfoundland and Labrador are performed at the centre. The study included all adult (age ≥ 18 yr) patients who received a kidney transplant from deceased donors with a minimum follow-up period of 3 years. Both donors after brain death (DBDs) and donors after circulatory death (DCDs) were included.
Kidney transplantation performed in pediatric (age < 18 yr) recipients or as part of a simultaneous kidney–pancreas transplantation procedure were excluded from the study, as were patients who did not achieve the minimum 3-year follow-up. Only organs harvested in Nova Scotia were included, as retrieval times were not consistently available for out-of-province procedures.
Transplantation procedure
Donation and transplantation procedures were conducted as per our standard of care, with kidneys retrieved locally, flushed and preserved with Static Preservation Solution (SPS-1, Organ Recovery Systems). Organs were nonrandomly preserved via hypothermic machine perfusion (LifePort Kidney Transporter, Organ Recovery Systems) or static cold storage, at the surgeon’s discretion. We measured the time from aortic cross-clamp to extraction of the last kidney. If an en block technique was used, the recorded time was equal for right and left kidneys.
Outcomes
Participants were classified into standard procurement time (SP) (≤ 65 min) and PKP (> 65 min) groups. We used a 65-minute cut-off time after cross-clamp as the PKP time based on the highest quartile of this data set. The 65-minute cut-off point was based on the technique described by Starzl and colleagues,11 which has a tolerance of 30–60 minutes from cross-clamp to cold storage. We also grouped and compared outcomes between organs from kidney-only and multiorgan procurements.
We matched donor information with recipient data while preserving confidentiality. We defined delayed graft function as a lack of decrease of more than 10% in creatinine level in the first 3 postoperative days,12 and early graft failure as graft nephrectomy or loss of kidney transplant function resulting in dialysis dependence within 30 days of transplantation. We defined extended-criteria donors as those aged more than 60 years, or more than 50 years with 2 or 3 of the following factors: history of hypertension, creatinine level of 133 μmol/L or higher, or death resulting from a stroke.13 We calculated the Kidney Donor Risk Index and Kidney Donor Profile Index (KDPI) according to the Organ Procurement and Transplantation Network KDPI calculator.14
Statistical analysis
We expressed categoric variables as proportions and compared them using the Pearson χ2 test. We expressed continuous variables as mean and standard deviation (SD), and analyzed them using the Student t test. Variables with an abnormal distribution were expressed as median and interquartile range (IQR), and were compared according to their distribution with the Mann–Whitney U test. We analyzed differences in graft survival using the Kaplan–Meier estimate and compared them using the log-rank test. A p value < 0.05 was considered significant at a 95% confidence interval. We analyzed the data using SPSS Statistics version 23 software (IBM Corp.) and Prism v9.0 (GraphPad).
Ethics approval
The study was approved by the Nova Scotia Health Authority Ethics Board (protocol 1024487). Consent from participants was waived, as all data were retrieved from our institutionally approved transplantation database.
Results
A total of 455 transplantation procedures were performed during the study period, of which 310 were excluded from the study (41 procedures were performed in pediatric recipients; in 261 cases, the kidney was donated or the recipient was followed outside of Nova Scotia; and in 8 cases, information was missing in the registry). The study thus included 145 participants, 115 in the SP group and 30 in the PKP group. The baseline characteristics of donors and recipients in the 2 groups were comparable, although we observed statistically significant differences in some variables. Donors in the SP group were relatively older (mean age 48.6 [SD 15.7] yr v. 41.3 [SD 17.3] yr, p = 0.03) and had a lower body mass index (mean 26.9 [SD 5.9] v. 29.6 [SD 6.4], p = 0.03) than those in the PKP group (Table 1). Compared to the PKP group, kidneys in the SP group were more likely to come from an extended-criteria donor (45 [39.1%] v. 5 [16.7%], p < 0.01), had a higher median Kidney Donor Risk Index score (1.1 [IQR 0.44] v. 0.88 [IQR 0.45], p < 0.01) and were more likely to come from donors with a KDPI score greater than 50% (71 [61.7%] v. 9 [30.0%], p < 0.01); however, there was no difference in the proportion of kidney-only versus multiorgan procurement in either group (p = 0.4). The mean procurement time was 55.7 (SD 12.6) minutes for the SP group and 89.0 (SD 14.9) minutes for the PKP group (p < 0.01).
Table 1.
Baseline characteristics of kidney donors and recipients according to kidney procurement time
| Characteristic | Procurement time; no. (%) of donors or recipients* | p value | |
|---|---|---|---|
| SP n = 115 |
PKP n = 30 |
||
| Donors | |||
| Body mass index, mean ± SD | 26.9 ± 5.9 | 29.6 ± 6.4 | 0.03 |
| Age, mean ± SD, yr | 48.6 ± 15.7 | 41.3 ± 17.3 | 0.03 |
| Height, mean ± SD, cm | 168.3 ± 11.5 | 170.0 ± 8.8 | 0.5 |
| Weight, mean ± SD, kg | 76.3 ± 17.4 | 85.8 ± 20.1 | < 0.01 |
| Creatinine level, mean ± SD, μmol/L | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.9 |
| Sex | |||
| Male | 62 (53.9) | 16 (53.3) | 1.0 |
| Female | 53 (46.1) | 14 (46.7) | 0.9 |
| African American ethnicity | 2 (1.7) | 0 (0.0) | 0.5 |
| History of hypertension | 37 (32.2) | 13 (43.3) | 0.2 |
| History of diabetes mellitus | 8 (7.0) | 2 (6.7) | 1.0 |
| Cause of death cerebrovascular | 9 (7.8) | 4 (13.3) | 0.4 |
| Extended-criteria donor | 45 (39.1) | 5 (16.7) | < 0.01 |
| Donor after circulatory death | 27 (23.5) | 3 (10.0) | 0.1 |
| KDRI score, median (IQR), % | 1.10 (0.4) | 0.88 (0.4) | 0.008 |
| KDPI score > 85% | 13 (11.3) | 4 (13.3) | 0.8 |
| KDPI score > 50% | 71 (61.7) | 9 (30.0) | < 0.01 |
| Kidney procurement time, mean ± SD, min | 55.7 ± 12.6 | 89.0 ± 14.9 | < 0.01 |
| Kidney-only procurement | 40 (34.8) | 11 (36.7) | 0.4 |
| Recipients | |||
| Sex | |||
| Male | 77 (67.0) | 22 (73.3) | 0.5 |
| Female | 38 (33.0) | 8 (26.7) | 0.5 |
| Age, mean ± SD, yr | 59.7 ± 11.3 | 57.1 ± 14.8 | 0.3 |
| Height, mean ± SD, cm | 169.9 ± 10.6 | 172.0 ± 8.7 | 0.3 |
| Weight, mean ± SD, kg | 80.6 ± 15.5 | 82.1 ± 20.3 | 0.7 |
| Body mass index, mean ± SD | 28.0 ± 4.9 | 27.5 ± 5.3 | 0.7 |
IQR = interquartile range; KDPI = Kidney Donor Profile Index; KDRI = Kidney Donor Risk Index; PKP = prolonged kidney procurement time (> 65 min); SD = standard deviation; SP = standard procurement time (≤ 65 min).
Except where noted otherwise.
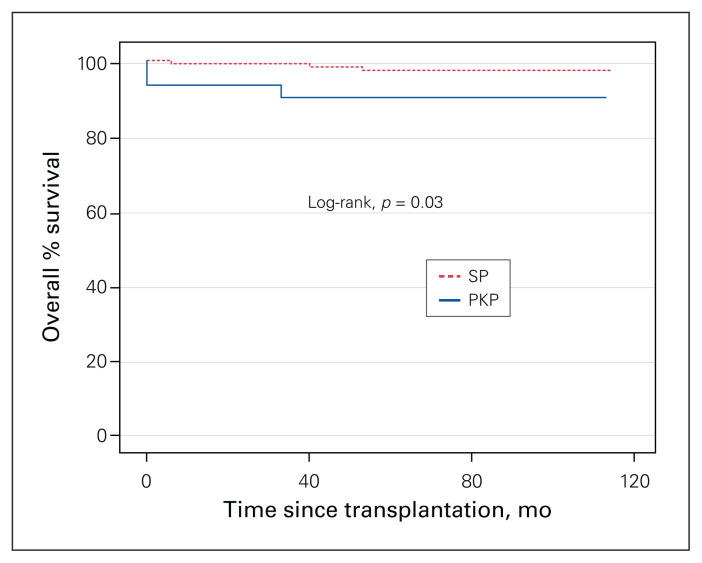
The incidence of delayed graft function was similar for the 2 groups, but 2 kidneys (6.7%) were lost early in the PKP group, versus none in the SP group (p < 0.01) (Table 2). Interestingly, 3 patients (10.0%) in the PKP group developed de novo donor-specific antibodies, compared to 1 patient (0.9%) in the SP group (p < 0.01), although this did not result in higher rates of acute rejection events (p = 0.3) or a higher rate of long-term graft loss (p = 0.7). A linear regression analysis with development of donor-specific antibodies as the outcome variable did not yield other statistically significant results. Five-year graft survival rates were lower in the PKP group than in the SP group (90.0% v. 97.4%, p = 0.03) (Figure 1).
Table 2.
Clinical outcomes according to kidney procurement time
| Outcome | Procurement time; no. (%) of recipients* | p value | |
|---|---|---|---|
| SP | PKP | ||
| Short-term | |||
| Cold ischemia time, mean ± SD, h | 9.6 ± 5.0 | 8.0 ± 2.5 | 0.4 |
| Delayed graft function | 7 (6.1) | 6 (20.0) | 0.2 |
| Early graft failure† | 0 (0.0) | 2 (6.7) | < 0.01 |
| Long-term | |||
| Donor-specific antibodies | 1 (0.9) | 3 (10.0) | < 0.01 |
| Acute rejection episode | 6 (5.2) | 3 (10.0) | 0.3 |
| Deceased with functioning graft | 8 (7.0) | 2 (6.7) | 1.0 |
| Graft failure | 3 (2.6) | 3 (10.0) | 0.7 |
PKP = prolonged kidney procurement time; SD = standard deviation; SP = standard procurement time.
Except where noted otherwise.
Graft nephrectomy or loss of kidney transplant function resulting in dialysis dependence within 30 days of transplantation.
Fig. 1.
Kaplan–Meier estimate of graft survival after kidney transplantation, prolonged kidney procurement time (PKP) (> 65 min) versus standard procurement time (SP) (≤ 65 min). Curve comparison using log-rank at 95% confidence interval.
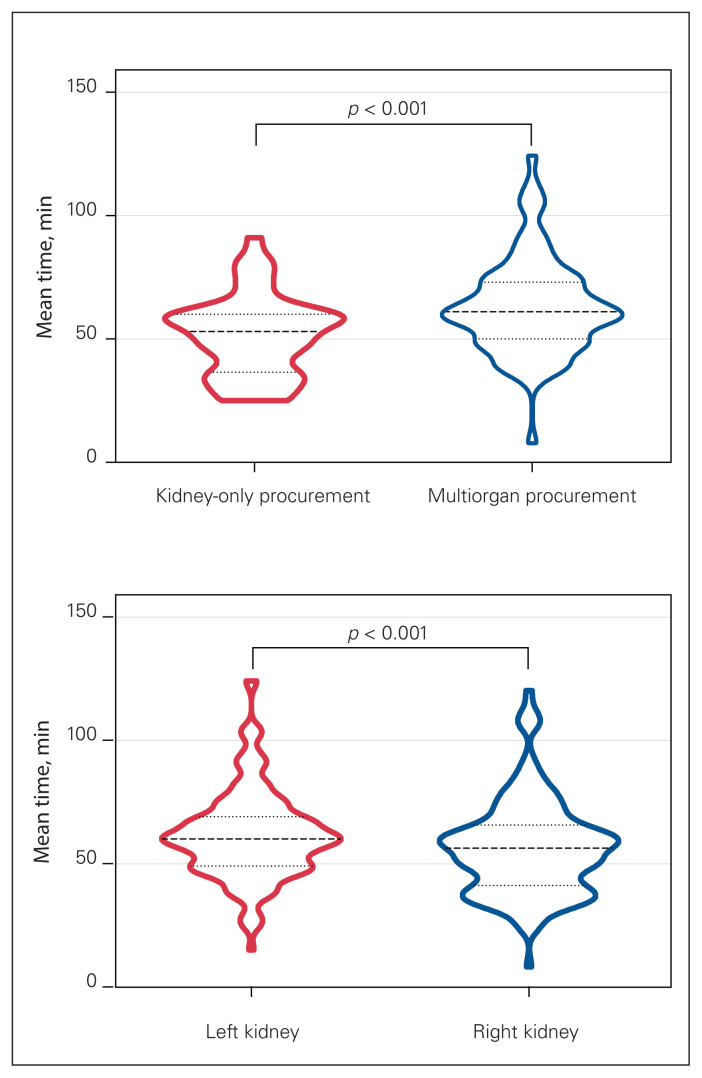
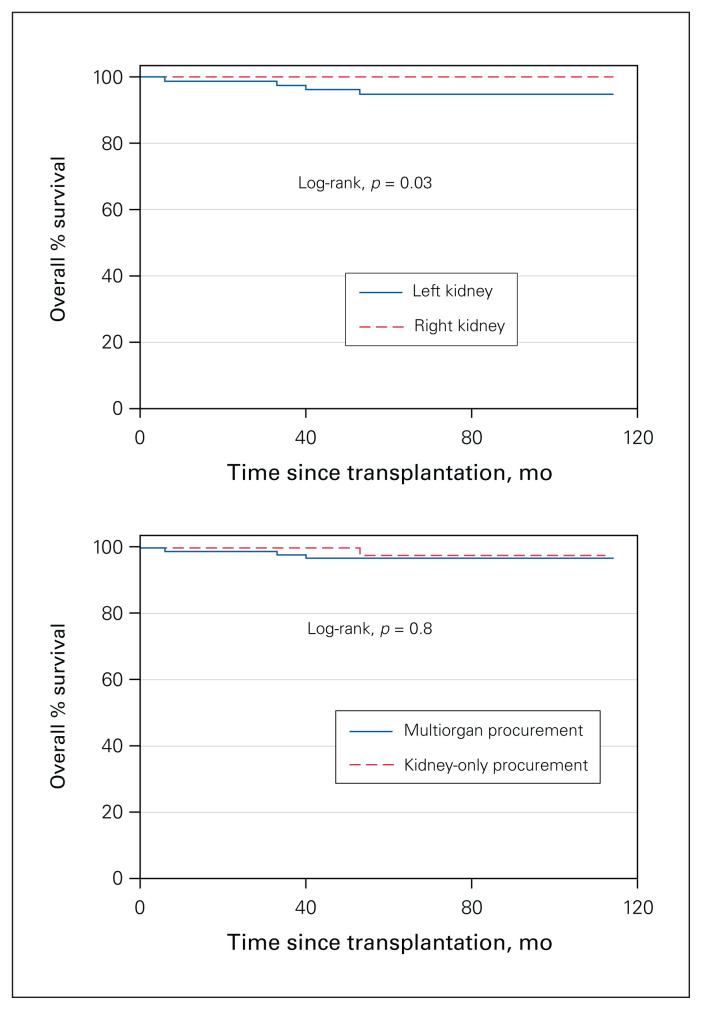
Kidney-only procurement was done in 46 donors (31.7%) overall, and these donors were older (mean age 63.3 [SD 10.5] yr v. 57.1 [SD 13.0] yr), more likely to have a KDPI score greater than 50% (32 [69.6%] v. 48 [48.5%]) and more likely to be DCDs (30 [65.2%] v. 0 [0.0%]) than multiorgan donors (all p < 0.01) (Table 3). The mean procurement time for the kidney-only procurement group was 51.4 (SD 16.2) minutes, compared to 63.2 (SD 18.9) minutes for the multiorgan procurement group (p < 0.01) (Figure 2). Overall, right kidneys were usually recovered first, which increased procurement time for left kidneys significantly (56.2 [SD 19.1] min v. 61.5 [SD 17.9] min, p < 0.01) (Figure 2). There was no difference in the incidence of delayed graft function between right and left kidneys; however, left kidneys had a lower 5-year survival rate than right kidneys (92.5% v. 100.0%, p = 0.03) (Figure 3). On average, left kidneys remained 11 minutes longer on the donor than right kidneys when multiorgan procurement was performed (65.0 [SD 17.7] min v. 54.1 [SD 19.4] min, p < 0.01); nonetheless, there were no differences in graft survival between kidneys from multiorgan procurement versus kidney-only procurement (p = 0.8) (Figure 3).
Table 3.
Characteristics of donors and clinical outcomes in multiorgan procurement group versus kidney-only procurement group
| Characteristic/outcome | Group; no. (%) of donors or recipients* | p value | |
|---|---|---|---|
| Multiorgan procurement n = 99 |
Kidney-only procurement n = 46 |
||
| Donors | |||
| Body mass index, mean ± SD | 27.8 ± 5.8 | 28.4 ± 6.5 | 0.2 |
| Donation after circulatory death | 0 (0) | 30 (65) | < 0.01 |
| Age, mean ± SD, yr | 57.1 ± 13.0 | 63.3 ± 10.5 | < 0.01 |
| KDPI score > 85% | 14 (14) | 3 (65) | 0.8 |
| KDPI score > 50% | 48 (48) | 32 (70) | < 0.01 |
| Kidney procurement time, mean ± SD, min | 63.2 ± 18.9 | 51.4 ± 16.2 | < 0.01 |
| Short-term | |||
| Cold ischemia time, mean ± SD, h | 9.5 ± 4.5 | 9.3 ± 5.2 | 0.9 |
| Delayed graft function | 9 (9) | 4 (9) | 0.9 |
| Early graft failure | 1 (1) | 1 (2) | 0.2 |
| Long-term | |||
| Donor-specific antibodies | 4 (4) | 0 (0) | 0.2 |
| Rejection episode | 5 (5) | 4 (9) | 0.4 |
| Deceased with functioning graft | 5 (5) | 5 (11) | 0.2 |
| Graft failure | 4 (4) | 2 (4) | 0.9 |
KDPI = Kidney Donor Profile Index; SD = standard deviation.
Except where noted otherwise.
Fig. 2.
Violin plots of mean kidney procurement times (dashed lines). Dotted lines represent standard deviations. Top: Kidney-only versus multiorgan procurement. Bottom: Left versus right kidney.
Fig. 3.
Kaplan–Meier estimate of graft survival after kidney transplantation. Curve comparison using log-rank at 95% confidence interval. Top: Left versus right kidney. Bottom: Multiorgan versus kidney-only procurement.
Of the 145 donors, 115 (79.3%) were DBDs and 30 (20.7%) were DCDs. The 2 groups had similar demographic characteristics, and there were no significant between-group differences in mean procurement time or cold ischemia time (both p = 0.2) (Table 4). The rates of delayed graft function, early graft failure, rejection episodes and 3-year graft failure were comparable between the 2 groups. However, a higher proportion of patients died with a functioning graft in the DCD group than in the DBD group (p = 0.02).
Table 4.
Characteristics of donors and clinical outcomes in the donor after brain death group versus the donor after circulatory death group
| Characteristic/outcome | Group; no. (%) of donors or recipients* | p value | |
|---|---|---|---|
| DBD n = 115 |
DCD n = 30 |
||
| Donors | |||
| Body mass index, mean ± SD | 27.5 ± 6.3 | 27.1 ± 5.3 | 0.6 |
| Age, mean ± SD, yr | 46.4 ± 17.5 | 49.7 ± 10.5 | 0.0 |
| Extended-criteria donor | 39 (33.9) | 11 (36.7) | 0.8 |
| KDPI score > 85% | 14 (12.2) | 3 (10.0) | 0.7 |
| KDPI score > 50% | 60 (52.2) | 20 (66.7) | 0.2 |
| Kidney procurement time, mean ± SD, min | 65.5 ± 19.2 | 52.7 ± 14.3 | 0.2 |
| PKP | 27 (23.5) | 3 (10.0) | 0.1 |
| Short-term | |||
| Cold ischemia time, mean ± SD, h | 9.4 ± 4.2 | 9.4 ± 5.8 | 0.2 |
| Delayed graft function | 10 (8.7) | 3 (10.0) | 0.8 |
| Early graft failure | 2 (1.7) | 0 (0.0) | 0.5 |
| Long-term | |||
| Donor-specific antibodies | 4 (3.5) | 0 (0.0) | 0.3 |
| Rejection episode | 7 (6.1) | 2 (6.7) | 0.9 |
| Deceased with functioning graft | 5 (4.3) | 5 (16.7) | 0.02 |
| 3-yr graft failure | 5 (4.3) | 1 (3.3) | 0.8 |
DBD = donor after brain death; DCD = donor after circulatory death; KDPI = Kidney Donor Profile Index; PKP = prolonged kidney procurement time; SD = standard deviation.
Except where noted otherwise.
Discussion
Our study showed that kidney procurement times greater than 65 minutes may be associated with poorer outcomes after transplantation than procurement times of 65 minutes or less. Detrimental consequences have been reported with prolonged warm ischemia times during kidney transplantation,9 as this has been linked to a higher incidence of delayed graft function in both DBDs15 and DCDs.6
In our series, donor body mass index was associated with longer kidney procurement time. This was expected, as surgical dissection in patients with a larger body habitus may be more difficult, and the accumulation of adipose tissue around organ pedicles may add considerable time to the procurement of all abdominal organs. Prolonged kidney harvest time was also more likely to be observed in extended-criteria donors and DCDs. Surprisingly, kidney-only procedures were performed in almost half of our cases; however, this finding may be biased, as we excluded a substantial number of cases, and the study included a period when DCD donation was introduced in Atlantic Canada. The kidney transplantation program was the first to embrace this practice, and other organ transplantation programs lagged behind, increasing activity over the last 5 years. Therefore, some of the kidney-only procedures would be considered multiorgan today.
The mean kidney procurement time was 51.4 minutes for kidney-only procurement, compared to 63.2 minutes for multiorgan procurement; however, the incidence of procurement time longer than 65 minutes was similar with both types of donation. This may reflect the influence of trainees’ learning curves, as fellows usually retrieve organs, but it suggests the need for further refinement of retrieval techniques.16 Although we used hypothermic machine preservation in some of our cases, there was neither sufficient information available nor consistent use to enable analysis of the potential benefit of this technology, even when kidneys were procured after 65 minutes.
This study could not identify differences in the incidence of delayed graft function between kidneys from multiorgan donors and those from kidney-only donors, or between the SP and PKP groups. Similar observations were reported by González Alfaro and colleagues.17 We observed a rate of early (within 30 d) graft loss of 6.7% in the PKP group, compared to no graft loss in the SP group (p < 0.01). This contrasts to previous reports indicating graft loss rates of up to 14.5% for kidneys procured after 60 minutes versus 8.1% for those procured within 60 minutes.18 Three-month graft loss occurred in 6 cases in the present series, with no difference between groups, as its cause was likely multifactorial.
We observed de novo donor-specific antibody formation in 3 patients (10.0%) in the PKP group versus 1 patient (0.9%) in the SP group (p < 0.01). The reason for this finding is not fully understood. Experimental studies have shown that hypoxic injury enhances both humoral and T cell responses,19,20 and may lead to the upregulation of factors that promote deposition of antibodies or enhance T cell attachment to the graft vasculature.21
An important finding is the difference in harvest time between right and left kidneys, with left kidneys receiving 11 more minutes of warm ischemia on average than right grafts. Traditionally, delayed nephrectomy time in the donor has been found to correlate directly with early graft failure.18 However, very little is published about the individual differences between left and right kidneys, and their impact on transplant outcomes. Even though we did not find a difference in immediate allograft function between left and right grafts, this seems to have some impact on 5-year graft survival. This may have been due to the anatomic differences between left and right kidneys, with easier access to the right kidney. Although some of these complexities may be attributed to the number of organs procured and the number of teams working, the experience of the procuring kidney surgeon is an important factor. Some en block surgical techniques minimize this added ischemia and may also offer added protection from vascular injuries.22
We did not observe any significant difference in procurement time or posttransplantation outcomes between DBDs and DCDs. This is surprising, as DCD retrievals are normally inherently faster procedures, with fewer organs harvested and with active efforts to minimize organ ischemia.23 Also noteworthy, there were no significant differences in immediate and longer-term outcomes between the 2 groups, which is counterintuitive, as DCDs have the added primary warm ischemia from the withdrawal of life support until the declaration of death and subsequent cold flushing. Consequently, using kidneys from DCDs may result in higher rates of delayed graft function compared to kidneys from standard-criteria donors.24 These findings are encouraging and support the notion that outcomes of transplantation using kidneys from DCDs may be equivalent to those with kidneys from standard-criteria donors.25
Finally, we observed a lower 5-year graft survival rate for kidneys with PKP than those with SP. Efforts to reduce exposure of kidneys to rewarming during procurement should be an area of focus of further research. Previous investigators have addressed this issue by implementing strategies to maintain a constant organ temperature during harvest using retroperitoneal cooling26 and total body cooling;27 however, neither of these are widely used. Current research is now incorporating the benefits of ex vivo normothermic organ perfusion to achieve zeroischemia procedures.28
Limitations
We excluded most of our initial study population because we could use information only from Nova Scotian recipients. Also, there were biases inherent to the retrospective design of this chart review study, as well as those related to the lack of a randomized sample, which was restricted by the characteristics of our transplantation program.
Conclusion
Kidney procurement times greater than 65 minutes may be associated with poorer outcomes after kidney transplantation. This is a potentially modifiable factor, and retrieving teams need to be acutely aware of time in order to avoid unnecessary delays during this phase of the procedure. Furthermore, clinical decisions based on procurement time may improve donor-to-recipient pairing and result in an overall improvement in transplantation outcomes. Given the negative impact of PKP, further research in this area is warranted.
Footnotes
Competing interests: None declared.
Contributors: F. Reyna-Sepulveda and B. Gala-Lopez designed the study. F. Reyna-Sepulveda acquired the data, which all authors analyzed. F. Reyna-Sepulveda and D. Badrudin wrote the manuscript, which B. Gala-Lopez critically revised. All authors gave final approval of the article to be published.
References
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341: 1725–30. [DOI] [PubMed] [Google Scholar]
- 2.Jang HJ, Kim SC, Kim SK, et al. Comparison of cadaveric renal allograft survival between multiorgan donors and kidney donors. Transplant Proc 1998;30:3664–5. [DOI] [PubMed] [Google Scholar]
- 3.Castelo D, Campos L, Moreira P, et al. Does multiorgan versus kidney-only cadaveric organ procurement affect graft outcomes? Transplant Proc 2013;45:1248–50. [DOI] [PubMed] [Google Scholar]
- 4.Carden DL, Granger DN. Pathophysiology of ischaemia–reperfusion injury. J Pathol 2000;190:255–66. [DOI] [PubMed] [Google Scholar]
- 5.Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation 1997;63:968–74. [DOI] [PubMed] [Google Scholar]
- 6.Pieringer H, Biesenbach G. Risk factors for delayed kidney function and impact of delayed function on patient and graft survival in adult graft recipients. Clin Transplant 2005;19:391–8. [DOI] [PubMed] [Google Scholar]
- 7.Starzl TE, Miller C, Broznick B, et al. An improved technique for multiple organ harvesting. Surg Gynecol Obstet 1987;165:343–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Rademaker E, Rebers PM, Hagenaars HJAM, et al. Impact of extraction time during organ procurement on kidney function after transplantation [abstract]. Transplantation 2018;102:S767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tennankore KK, Kim SJ, Alwayn IPJ, et al. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int 2016;89:648–58. [DOI] [PubMed] [Google Scholar]
- 10.Kukla U, Cholewa H, Chronowska J, et al. Effect of the second warm ischemia time and its components on early and long-term kidney graft function. Transplant Proc 2016;48:1365–9. [DOI] [PubMed] [Google Scholar]
- 11.Starzl TE, Miller C, Broznick B, et al. An improved technique for multiple organ harvesting. Surg Gynecol Obstet 1987;165:343–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Mallon DH, Summers DM, Bradley JA, et al. Defining delayed graft function after renal transplantation: simplest is best. Transplantation 2013;96:885–9. [DOI] [PubMed] [Google Scholar]
- 13.Young A, Dixon SN, Knoll GA, et al. The Canadian experience using the expanded criteria donor classification for allocating deceased donor kidneys for transplantation. Can J Kidney Health Dis 2016; 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the Kidney Donor Risk Index. Transplantation 2009;88:231–6. [DOI] [PubMed] [Google Scholar]
- 15.Nishikido M, Noguchi M, Koga S, et al. Kidney transplantation from non-heart-beating donors: analysis of organ procurement and outcome. Transplant Proc 2004;36:1888–90. [DOI] [PubMed] [Google Scholar]
- 16.Baranski AG, Lam HD. A modified, rapid and safe technique of kidney only procurement from donors after circulatory and brain death (DCD, DBD). Open J Organ Transplant Surg 2016;6:23–8. [Google Scholar]
- 17.González Alfaro A, Campos Hernández P, Gómez Gómez E, et al. Variations in initial renal transplant function by type of organ retrieval. Transplant Proc 2013;45:3603–5. [DOI] [PubMed] [Google Scholar]
- 18.Osband AJ, Zaki RF. Extraction time of kidneys during organ procurement impacts function. Clin Transplant 2011;25:235–8. [DOI] [PubMed] [Google Scholar]
- 19.Goto R, Issa F, Heidt S, et al. Ischemia–reperfusion injury accelerates human antibody-mediated transplant vasculopathy. Transplantation 2013;96:139–45. [PubMed] [Google Scholar]
- 20.Yi T, Fogal B, Hao Z, et al. Reperfusion injury intensifies the adaptive human T cell alloresponse in a human–mouse chimeric artery model. Arterioscler Thromb Vasc Biol 2012;32:353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori DN, Kreisel D, Fullerton JN, et al. Inflammatory triggers of acute rejection of organ allografts. Immunol Rev 2014;258:132–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakazato PZ, Concepcion W, Bry W, et al. Total abdominal evisceration: an en bloc technique for abdominal organ harvesting. Surgery 1992;111:37–47. [PubMed] [Google Scholar]
- 23.Shemie SD, Baker AJ, Knoll G, et al. National recommendations for donation after cardiocirculatory death in Canada: donation after cardiocirculatory death in Canada. CMAJ 2006;175:S1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maggiore U, Oberbauer R, Pascual J, et al. ; ERA-EDTA-DESCARTES Working Group. Strategies to increase the donor pool and access to kidney transplantation: an international perspective. Nephrol Dial Transplant 2015;30:217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaapherder A, Wijermars LGM, de Vries DK, et al. Equivalent long-term transplantation outcomes for kidneys donated after brain death and cardiac death: conclusions from a nationwide evaluation. EClinicalMedicine 2018;4–5:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salazar-Bañuelos A, Monroy-Cuadros M, Henriquez-Cooper H. Retro-peritoneal cooling for kidney preservation from multi-organ cadaver donors. Am J Surg 2018;215:802–3. [DOI] [PubMed] [Google Scholar]
- 27.Valero R, Manyalich M, Cabrer C, et al. Total body cooling for organ procurement. In: Touraine JL, Traeger J, Bétuel H, et al., editors. Organ shortage: the solutions. Proceedings of the 26th Conference on Transplantation and Clinical Immunology, 13–15 June 1994. Transplantation and Clinical Immunolog y series vol. 26. Dordrecht (The Netherlands): Springer, Dordrecht; 1995. [Google Scholar]
- 28.He X, Chen G, Zhu Z, et al. The first case of ischemia-free kidney transplantation in humans. Front Med (Lausanne) 2019;6:276. [DOI] [PMC free article] [PubMed] [Google Scholar]