Abstract
Introduction
Viral infections can induce autoimmune diseases in susceptible patients. SARS-CoV-2 has been associated with the development of rheumatic disease, especially small vessel vasculitis and arthritis. Typically, onset occurs days to weeks after the antigenic challenge and in patients with mild COVID-19. We report a case of large vessel vasculitis (LVV) temporally related to SARS-CoV-2 infection.
Case description
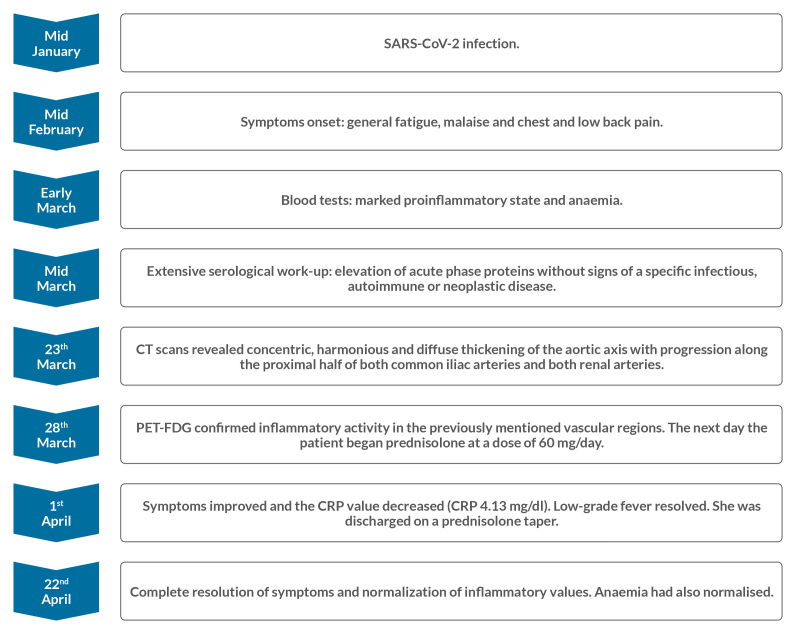
An otherwise healthy 19-year-old woman presented with fatigue, malaise, and chest and low back pain. The symptoms had begun 5 weeks earlier and 1 month after mild SARS-CoV-2 infection. Serological work-up revealed a marked proinflammatory state and anaemia without signs of infectious or autoimmune disease. Computerized tomography revealed thickening and blurring of the perivascular fat of the descending thoracic and abdominal aorta, progressing along the proximal iliac and renal arteries. Fluorodeoxyglucose positron emission tomography confirmed inflammatory activity. Symptoms and laboratory values normalized after prednisolone treatment.
Discussion
Recent SARS-CoV-2 infection may be a trigger for LVV, including Takayasu arteritis, as well as other rheumatic diseases. A prompt and thorough differential diagnosis is essential to exclude aortitis and LVV mimickers. Moreover, physicians should be aware of the potential spectrum of systemic and autoimmune diseases that could be precipitated by SARS-CoV-2 infection. This will allow timely diagnosis and treatment, with significant improvement in prognosis.
LEARNING POINTS
SARS-CoV-2 infection can trigger large vessel vasculitis and other rheumatic diseases.
Awareness of the association between COVID-19 and autoimmune phenomena allows for timely diagnosis and treatment with significant improvements in prognosis.
Vasculitis and other autoimmune diseases should be kept in mind in patients who develop proinflammatory states days to weeks after an initial antigenic challenge.
Keywords: Autoimmune diseases, case report, COVID-19, vasculitis
INTRODUCTION
Viruses are major environmental factors that can trigger autoimmune phenomena in genetically susceptible individuals[1]. Although the mechanisms underlying the association between viruses and autoimmunity are not entirely known, several models have been proposed and validated. It is thought that molecular mimicry is the predominant pathway in this association[1–3]. According to this theory, viruses carry epitopes which are structurally similar to self-epitopes, activating B and T cells and leading to a cross-reactive response against both self- and non-self-antigens, inducing tissue damage[1].
There is growing evidence that infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is related to the development of autoimmunity[2,3]. Autoantibodies have been reported in patients with coronavirus disease 2019 (COVID-19): antinuclear antibodies in 35%, rheumatoid factor in 19% and lupus anticoagulant in 11%[3]. However, the presence of antibodies is not automatically diagnostic of new-onset rheumatic autoimmune diseases, which are actually quite rare, with vasculitis (in particular of small vessels) and arthritis being the most common. COVID-19 can induce vasculitis by a type 3 hypersensitivity reaction, without directly damaging the vessel wall. This inflammatory response appears days to weeks after the initial antigenic challenge and typically in patients with mild COVID-19[3]. Furthermore, it is thought that some cases of long COVID are associated with large vessel vasculitis (LVV), although evidence is limited[2].
Identification of the clinical and laboratory features of new-onset rheumatic autoimmune disease is essential for timely diagnosis and treatment. Therefore, we report a case of LVV which was temporally related to COVID-19.
CASE DESCRIPTION
A 19-year-old female Caucasian college student presented to the emergency department with general fatigue, malaise, and chest and low back pain. Her symptoms had begun about 5 weeks earlier and gradually increased. Her general practitioner initially prescribed symptomatic medication but later ordered blood tests when her symptoms failed to resolve. They revealed a marked proinflammatory state with a striking elevated erythrocyte sedimentation rate (ESR 109 mm/h), increased C-reactive protein (CRP 10.73 mg/dl) and normocytic anaemia (haemoglobin 10.4 g/dl).
At emergency department evaluation, the patient reported no cough, shortness of breath, myalgias, anorexia, significant weight loss, night sweats or arthralgia. She also denied a history of macroscopic loss of blood or menstrual cycle irregularities. She was not aware of any fever.
The patient’s medical history was unremarkable. She had had mild SARS-CoV-2 infection about 1 month before symptom onset. Her only regular medication was a combined oral contraception pill. She had no known contact with any ill people. She had a vaccinated pet and there was no history of travel outside of Portugal in the last 2 years. She had unprotected sexual intercourse only with a healthy male. There was family history (grandmother) of rheumatoid arthritis diagnosed in the third decade of life.
On examination, there were no relevant findings. Laboratory test results confirmed anaemia and a proinflammatory state. Electrocardiogram and computed tomography (CT) of the thorax were unremarkable. She was admitted for further work-up and management.
Further serological work-up confirmed elevation of acute phase proteins without signs of a specific infectious or autoimmune disease (Table 1). Protein electrophoresis was normal. On the seventh hospital day, CT scans of the chest, abdomen and pelvis after the administration of contrast were obtained. They revealed concentric and diffuse thickening of the descending thoracic and abdominal aorta with progression along the proximal half of both common iliac and renal arteries. Thickening was associated with a clear blurring of perivascular fat (Figs. 1 and 2). In order to obtain more diagnostic information, fluorodeoxyglucose positron emission tomography (FDG-PET) was performed 5 days later and confirmed the inflammatory activity (Figs. 3 and 4). The next day the patient started prednisolone at a dose of 60 mg/day. Three days later her symptoms had improved and the CRP value had decreased (CRP 4.13 mg/dl). Persistent low-grade fever was also documented during hospitalization but this also resolved. The patient was discharged on a prednisolone taper (5 mg decrease every 2 weeks).
Table 1.
Laboratory data.
| Variable | Patient value | Reference range |
|---|---|---|
| Haemoglobin (g/dl) | 10.4 | 12–15 |
| White cells (/μl) | 8300 | 4500–11500 |
| Platelet count (/μl) | 369,000 | 150,000–450,000 |
| Sodium (mmol/l) | 139 | 135–145 |
| Potassium (mmol/l) | 4.0 | 3.5–5.0 |
| Lactate dehydrogenase (IU/l) | 133 | 120–246 |
| C-reactive protein (mg/dl) | 10.73 | <0.5 |
| Procalcitonin (ng/ml) | <0.01 | 0.00–0.50 |
| Sedimentation rate (mm/h) | 109 | 0–20 |
| Prothrombin time (INR) | 1.09 | 0.9–1.1 |
| D-dimer (ng/ml) | 137 | <250 |
| Creatinine (mg/dl) | 0.7 | 0.5–1.2 |
| Urea nitrogen (mg/dl) | 26 | 19–49 |
| Alanine aminotransferase (U/l) | 18 | 3–31 |
| Aspartate aminotransferase (U/l) | 13 | 3–31 |
| Total bilirubin (mg/dl) | 0.3 | 0.3–1.2 |
| Albumin (g/dl) | 4.0 | 3.5–5.0 |
| Serum iron (μg/dl) | 33 | 50–150 |
| Serum ferritin (ng/ml) | 64 | 30–340 |
| Transferrin saturation (%) | 10.7 | 20–45 |
| Folic acid (ng/ml) | 4.5 | 1.0–20.0 |
| Vitamin B12 (pg/ml) | 363 | 179–1130 |
| Haptoglobin (mg/dl) | 383 | 36–195 |
| C3 (mg/dl) | 200.7 | 83.0–177.0 |
| C4 (mg/dl) | 32.2 | 12.0–36.0 |
| CH50 (U/ml) | >99.9 | 41.7–95.1 |
| IgG (mg/dl) | 887 | 650–1600 |
| IgA (mg/dl) | 116 | 40–350 |
| IgM (mg/dl) | 159 | 50–300 |
| Free kappa light chain (mg/l) | 13.19 | 3.30–19.40 |
| Free lambda light chain (mg/l) | 11.72 | 5.71–26.30 |
| Free kappa:lambda ratio | 1.13 | 0.3–1.7 |
| IgG subclass 1 (mg/l) | 5188 | 3400–10000 |
| IgG subclass 2 (mg/l) | 2141 | 1000–5400 |
| IgG subclass 3 (mg/l) | 357 | 200–1700 |
| IgG subclass 4 (mg/l) | 555 | 60–1300 |
| Human leukocyte antigen B27 | Negative | Negative |
| Antinuclear antibody | Negative | Negative |
| Anti-DNA antibody | Negative | Negative |
| Anti-double stranded DNA antibody | 0.7 | 0.0–15.0 |
| Extractable nuclear antigen screen | Negative | Negative |
| Antimitochondrial antibody | Negative | Negative |
| Antineutrophil cytoplasmic antibody | Negative | Negative |
| Rheumatoid factor (IU/ml) | 5.8 | <14.0 |
| Citrullinated peptide antibody (U/ml) | 1.2 | 0–10 |
| Cryoglobulins | Negative | Negative |
| Antiparietal cell antibody (U/ml) | 0.1 | 0.0–10.0 |
| Intrinsic factor antibody (U/ml) | 0.3 | 0.0–10.0 |
| Anti-basement membrane (U/ml) | 0.2 | 0–10 |
| HIV antigen and antibodies | Non-reactive | Non-reactive |
| Syphilis IgG antibodies | Negative | Negative |
| Epstein–Barr IgM antibodies (U/ml) | <10 | <20 |
| Interferon gamma release assay | Negative | Negative |
Figure 1.
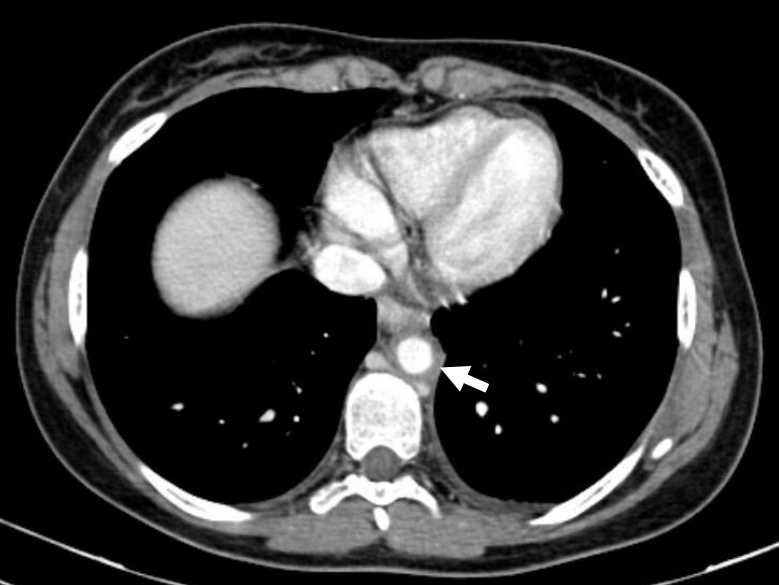
Contrast CT showing increased thickness of the descending thoracic aorta associated with a clear blurring of perivascular fat.
Figure 2.
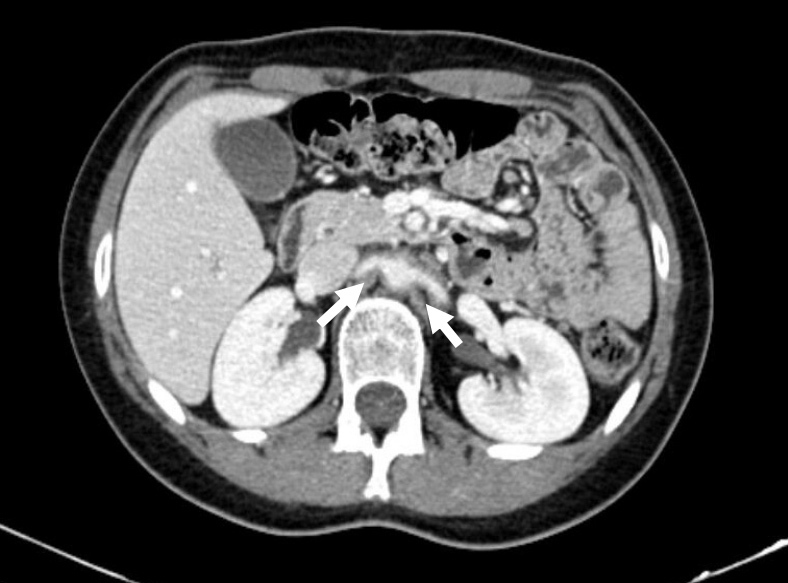
Contrast CT showing both renal arteries affected, especially in relation to their proximal third.
Figure 3.
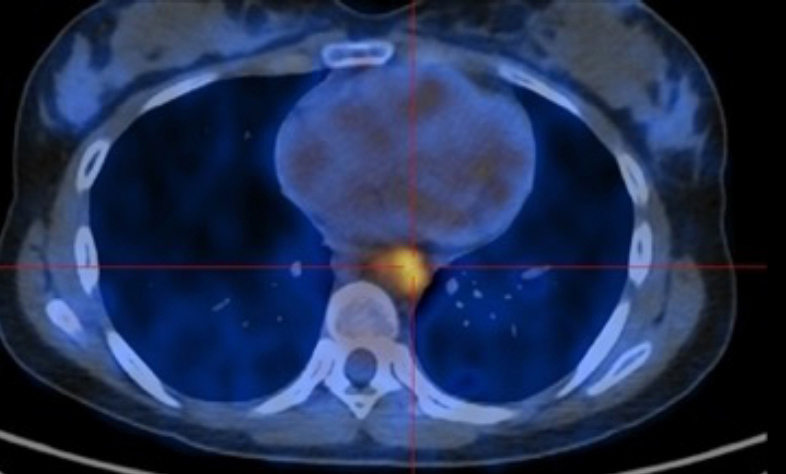
FDG-PET/CT showing increased uptake in the descending thoracic aorta.
Figure 4.
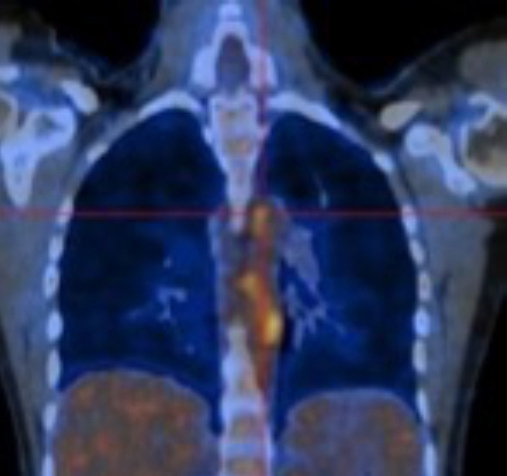
FDG-PET/CT showing increased uptake in the descending thoracic aorta.
Follow-up evaluations confirmed complete resolution of symptoms and normalization of inflammatory values and anaemia (Fig. 5).
Figure 5.
Disease work-up, course and treatment response.
DISCUSSION
This 19-year-old woman, who had a recent SARS-CoV-2 infection, presented with a proinflammatory state and aortitis extending to the iliac and renal arteries. Clinical and demographic features as well as laboratory data and imaging results were compatible with Takayasu arteritis, which is a rare non-specific inflammatory LVV, predominantly affecting the aorta and its main branches in young females[4]. Currently, there are no universally accepted diagnostic criteria for LVV[5] and imaging is essential for early diagnosis[6]. Timely diagnosis is crucial because the survival rate at 15 years ranges from 43% to 100% depending on disease stage[7].
In the differential diagnosis we considered diseases which mimic LVV, including common causes of infectious aortitis, such as syphilis, and mycobacterial and bacterial infection. We also ruled out other autoimmune pathologies, including ANCA-positive vasculitides, ankylosing spondylitis, systemic lupus erythematosus, rheumatoid arthritis and sarcoidosis as well as other entities, such as immunoglobulin G4-related disease and hereditary connective tissue disorders[5]. We also considered that a vasculitic process could have been triggered by self-limiting direct viral-induced vessel damage. However, the time between COVID-19 and disease onset as well as the lack of clinical or symptom improvement before immunosuppressive treatment challenge this hypothesis[3].
The possibility of an association between microorganisms and Takayasu arteritis has been extensively investigated. Viruses or bacteria, through a molecular mimicry mechanism, are likely to initiate or increase the auto-immune process in this disease[4].
This fact together with the autoimmune phenomena described in association with SARS-CoV-2, suggest SARS-CoV-2-induced Takayasu arteritis in our patient.
Some systematic reviews of case reports on COVID-19-associated vasculitis have been published[8,9]. Vasculitis secondary to COVID-19 is more frequent in females, in younger individuals and after a mild-to-moderate COVID-19 disease course[8]. Typically, the vasculitis appears approximately 1 month after SARS-CoV-2 infection and has an overall good prognosis with a positive response to treatment[9]. Our patient had several features similar to those of previous cases, although the scarcity and heterogeneity of available case reports makes it difficult to define typical patient characteristics. For that reason, case reports such as ours should continue to be published.
In our case, the clinical picture and temporal sequence suggest, but do not prove, that COVID-19 was a causal factor in LVV. Furthermore, we cannot completely rule out the possibility that there was a casual association or only isolated and idiopathic aortitis.
We believe that clinicians should be aware of the spectrum of rheumatic autoimmune diseases that may be precipitated by SARS-CoV-2 infection, as this will allow for the timely diagnosis and treatment of these conditions.
Footnotes
Conflicts of Interests: The authors declare there are no competing interests.
REFERENCES
- 1.Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11(8):762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zacharias H, Dubey S, Koduri G, D’Cruz D. Rheumatological complications of Covid 19. Autoimmun Rev. 2021;20(9):102883. doi: 10.1016/j.autrev.2021.102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gracia-Ramos AE, Martin-Nares E, Hernandez-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592. doi: 10.3390/cells10123592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espinoza JL, Ai S, Matsumura I. New insights on the pathogenesis of Takayasu arteritis: revisiting the microbial theory. Pathogens. 2018;7(3):73. doi: 10.3390/pathogens7030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keser G, Aksu K. Diagnosis and differential diagnosis of large-vessel vasculitides. Rheumatol Int. 2019;39(2):169–185. doi: 10.1007/s00296-018-4157-3. [DOI] [PubMed] [Google Scholar]
- 6.Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77(5):636–643. doi: 10.1136/annrheumdis-2017-212649. [DOI] [PubMed] [Google Scholar]
- 7.Águeda AF, Monti S, Luqmani RA, Buttgereit F, Cid M, Dasgupta B, et al. Management of Takayasu arteritis: a systematic literature review informing the 2018 update of the EULAR recommendation for the management of large vessel vasculitis. RMD Open. 2019;5(2):e001020. doi: 10.1136/rmdopen-2019-001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong K, Farooq Alam Shah MU, Khurshid M, Ullah I, Tahir MJ, Yousaf Z. COVID-19 associated vasculitis: a systematic review of case reports and case series. Ann Med Surg (Lond) 2022;74:103249. doi: 10.1016/j.amsu.2022.103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giryes S, Bragazzi NL, Bridgewood C, De Marco G, McGonagle D. COVID-19 vasculitis and vasculopathy-distinct immunopathology emerging from the close juxtaposition of type II pneumocytes and pulmonary endothelial cells. Semin Immunopathol. 2022;44(3):375–390. doi: 10.1007/s00281-022-00928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]