Purpose of review
Pulmonary hypertension (PH) is a common complication of chronic obstructive lung disease (COPD), but clinical presentation is variable and not always ’proportional’ to the severity of the obstructive disease. This review aims to analyze heterogeneity in clinical features of PH-COPD, providing a guide for diagnosis and management according to phenotypes.
Recent findings
Recent works have focused on severe PH in COPD, providing insights into the characteristics of patients with predominantly vascular disease. The recently recognized ’pulmonary vascular phenotype’, characterized by severe PH and mild airflow obstruction with severe hypoxemia, has markedly worse prognosis and may be a candidate for large trials with pulmonary vasodilators. In severe PH, which might be best described by a pulmonary vascular resistance threshold, there may also be a need to distinguish patients with mild COPD (pulmonary vascular phenotype) from those with severe COPD ('Severe COPD-Severe PH’ phenotype).
Summary
Correct phenotyping is key to appropriate management of PH associated with COPD. The lack of evidence regarding the use of pulmonary vasodilators in PH-COPD may be due to the existence of previously unrecognized phenotypes with different responses to therapy. This review offers the clinician caring for patients with COPD and PH a phenotype-focused approach to diagnosis and management, aimed at personalized care.
Keywords: chronic obstructive pulmonary disease, diagnosis, phenotypes, pulmonary hypertension, treatment
INTRODUCTION
Pulmonary hypertension (PH) is a complication of chronic obstructive pulmonary disease (COPD) [1] associated with increased morbidity [2,3] and mortality [4▪▪,5,6]. Prevalence, reported to be up to 90% [7–9], varies widely according to the different study populations (mild COPD to pretransplant stages) and different definitions of PH (including postcapillary PH and diverse thresholds for PH diagnosis) [9,10]. The threshold to define PH in lung disease has been lowered [1] to define mild-moderate PH as mean pulmonary artery pressure (mPAP) ≥21 mmHg in the presence of increased pulmonary vascular resistance (PVR ≥3 wood units, WU) or mPAP ≥25 mmHg, and severe PH as mPAP ≥35 mmHg or mPAP ≥25 mmHg with cardiac index (CI) <2 L/min/m2. PH is usually mild to moderate in COPD but is severe in 1–5% of cases [8,11], representing a distinct subgroup with markedly worse prognosis and predominant vascular impairment associated with relatively mild obstructive disease [6,12], which has been described as a ’pulmonary vascular phenotype’ (PVP) [13]. A paradigm shift is taking place in PH research, aimed at the analysis of phenotypic heterogeneity and ultimately precision medicine [14,15]. This review aims to update and orient the clinician on the changing landscape of COPD associated with PH, providing a guide for diagnosis and management according to phenotype.
Box 1.
no caption available
PATHOPHYSIOLOGY
The loss of pulmonary vessels due to emphysematous destruction and vascular remodeling of small muscular arteries and arterioles are the main mechanisms for PH in COPD [16]. Capillary density in COPD lungs appears to be dependent on PH severity [17]; vascular changes develop in different stages of COPD and in heavy smokers with preserved lung function [18–20]. The main histologic changes are: intimal hyperplasia through proliferation of smooth muscle cells [18,21,22], possibly by dedifferentiation and migration from the media to the intima [23]; medial thickening [24,25]; and the deposition of elastic and collagen fibers; all this produces thickening of the arterial wall and increased pulmonary vascular resistance [26]. The progressive luminal narrowing and loss of smaller distal vessels results in the ’pruning’ described in CT scan and angiography [27,28], and the loss of distal arterial vasculature is related to right ventricle (RV) enlargement [27].
PATHOGENESIS: ENVIRONMENTAL FACTORS AND GENETICS
The mechanisms leading to PH in COPD are related to endothelial dysfunction at early disease stages. Airborne particulates and tobacco smoke are triggers for pulmonary vascular alterations [29], even in patients with normal lung function [16] and prior to emphysema development [20,30,31]. Persistent hypoxia contributes to chronic vasoconstriction and vascular remodeling [1,18,32]. The relationship between pulmonary pressure and arterial oxygen (PaO2) is variable [9], and vascular alterations related to hypoxia might be partially reversible [33,34]. Finally, genetic heterogeneity might play a role in the development of a PVP in COPD patients. Some gene variants related to pulmonary arterial hypertension (PAH) are probably also implicated in PH and respiratory diseases [35,36]. Genes belonging to the retinol metabolism and extracellular matrix pathways [37] and to T-lymphocyte activation [38] are also implicated in vascular remodeling.
DIAGNOSTIC WORKUP
Symptoms of PH are nonspecific and common to COPD: exertional dyspnea, weakness and fatigue [39]. However, symptoms that seem excessive for the degree of obstructive disease should warn of vascular involvement. In advanced disease, more specific symptomatology of progressive right heart failure ensues: chest pain, syncope, peripheral edema, jugular venous dilation, and abdominal distension; however, no individual physical finding can accurately predict PH [40].
Echocardiography is an accessible, noninvasive tool, crucial to corroborate the suspicion of PH. Besides estimating the systolic pulmonary arterial pressure, it allows the assessment of RV morphology and function, right atrium diameter, signs of fluid overload, valvular or other left heart abnormalities which might be the cause of PH, and which are highly prevalent [41] in patients with tobacco exposure [42,43]. Although echocardiography presents technical difficulties in COPD patients [44], it should be performed whenever PH is suspected. Common situations in clinical practice are disproportionate symptoms and/or low diffusing capacity for carbon monoxide (DLCO) with relatively preserved spirometry and lung parenchyma [45]. The size of the main pulmonary artery on computed tomography (CT), together with other signs suggesting RV overload, may suggest PH [3,46,47]. The role of biomarkers is controversial. Brain natriuretic peptide (BNP) or N-terminal pro-BNP are often increased in patients with COPD, reflecting pulmonary vascular and RV remodeling but also left heart dysfunction and other comorbidities [48–50]. A recent work [51▪▪] suggests the combination of echocardiographic, blood markers and CT parameters to reliably predict severe PH in COPD. Cardiopulmonary exercise tests are useful to distinguish ventilatory from circulatory limitation associated with PH in COPD patients [52,53].
After assessment with noninvasive tools, hemodynamic confirmation of PH by right heart catheterization (RHC) should be considered when severe PH is suspected [13] or in the work-up to lung transplant or lung reduction surgery evaluation.
PHENOTYPE PRESENTATION DURING DIAGNOSTIC WORK-UP
According to a classical paradigm, the physiopathology of PH is fundamentally driven by that of COPD [54] and caused by obstruction to airflow and hypoxic vasoconstriction. It would follow that PH is an inevitable, almost mechanical consequence of severe obstructive disease [55]. Although these mechanisms have indeed been confirmed experimentally as having a relevant role in the pathogenesis of PH [56], other patterns of pulmonary vascular disease in COPD have emerged in recent years, suggesting a more complex interplay.
RESPIRATORY FUNCTION AND HEMODYNAMIC PROFILES
PH is usually somewhat proportional to the degree of airflow obstruction [9,57], and its rate of progression relatively slow [58,59] compared to other lung diseases [60,61], increasing by approximately 0.5 mmHg of mPAP per year. It is difficult to establish prevalence and natural history of PH in COPD because patients are not systematically referred for RHC due to its limited indications. However, evidence [58] shows that approximately 25% of COPD patients without PH at a given time will develop it after 5 years, with increased risk for those with higher mPAP at rest and during exercise. The development of PH is correlated with worsening of hypoxemia, which might provide a clue in the absence of worsening of COPD [58]. Recently, the concept of a specific vascular phenotype [1] has been put forth. This phenotype is characterized by severe hemodynamic compromise, moderate airflow obstruction, marked reduction in DLCO[62▪] and severe hypoxemia, with normal arterial CO2 [11]. Thabut et al.[10] previously identified four clusters of COPD patients: patients without PH, with a moderate decrease in forced expiratory volume in one second (FEV1) and PaO2; patients with moderate PH, severe airflow obstruction and moderate hypoxemia; patients with severe airflow obstruction, severe hypoxemia and severe PH; and the PVP, with severe PH and hypoxemia but only moderate airflow obstruction. Thus, the level of hemodynamic compromise is not always proportional to that of the airflow obstruction, and patients with severe PH don’t always have mild airflow obstruction.
IMAGING PATTERNS
Due to the importance of early PH detection, research has focused on CT imaging, routinely performed on COPD patients. Although no correlation exists [9,57,63] between the extent of emphysema and PH severity, airway remodeling and pulmonary artery to aorta diameter ratio are proportional to the degree of PH [57,64] and in severe COPD patients a ratio >1 is predictive of PH [65]. Furthermore, patients with severe PH have increased remodeling of small pulmonary vessels on chest CT [66]. Finally, patients at earlier COPD stages and with milder emphysema present increased RV size and arterial pruning [27] than those with more advanced COPD, suggesting a similar profile to the PVP.
HEMODYNAMIC STATUS, EXACERBATIONS AND EXERCISE CAPACITY
PH (and even an mPAP >18 mmHg) is a risk factor for hospitalization from acute exacerbation of COPD [2]; furthermore, mortality in COPD patients with severe PH is increased with the severity and number of exacerbations requiring hospitalization in the previous year [62▪]. Interestingly, COPD patients admitted for an ’exacerbation’ episode may actually have decompensated heart failure from postcapillary PH [67].
PH decreases the exercise capacity of COPD patients, as evidenced by the six-minute walk test [68,69] and the cardio-pulmonary exercise test [70], independently from other concurring factors such as FEV1 or PaO2[69] and particularly in patients who are not candidates for lung-transplant [71]. Furthermore, patients with severe PH present a cardiovascular limitation to exercise [53] rather than exhausted ventilatory reserve seen in COPD patients without and with moderate PH.
OVERVIEW OF DIAGNOSIS AND MANAGEMENT ACCORDING TO PHENOTYPES
PH can take many forms in COPD (Fig. 1), partly explaining its variable prevalence [8–10,72–74]. All COPD patients, regardless of hemodynamic status, should be treated according to guidelines including oxygen (if in respiratory failure), physiotherapy and optimal medical therapy. Once RHC confirms PH, to avoid missing potentially treatable forms, possible causes of PAH (autoimmune disease, HIV, liver disease, congenital heart defects, pulmonary veno-occlusive disease/pulmonary capillary hemangiomatosis and schistosomiasis) should be considered, and chronic thromboembolic PH ruled out. If all these hypotheses are discarded, PH associated with COPD might be considered.
FIGURE 1.
Algorithm for diagnosis and management of PH in COPD according to phenotype. Right heart catheterization is mandatory only if severe PH is suspected or in the work-up to lung transplant or lung reduction surgery evaluation. Assess COPD severity and manage according to current recommendations [98]. 6th World Symposium on PH classification is considered for grouped PH [86]. CI, cardiac index; COPD, chronic obstructive lung disease; IPAH, idiopathic pulmonary arterial hypertension; mPAP, mean pulmonary arterial pressure; PH, pulmonary hypertension.
In the following section we outline the different clinical scenarios where COPD can coexist with PH (Table 1).
Table 1.
Clinical phenotypes characteristics and treatment recommendations.
| Phenotype | Characteristics | Recommendations |
| PH during exercise | PAP abnormally rises during exercise Related to vascular remodeling or left heart dysfunction Risk to develop PH at rest | Treat COPD and cardiovascular risk factors Pulmonary vasodilator not recommended |
| Postcapillary PH | RHC: mPAP ≥21 mmHg and PAWP ≥ 15mmHg Frequently mild PH More cardiovascular risk factors Predominant LA enlargement LV diastolic or/and systolic dysfunction. Mitral valve disease, less frequent aortic valve disease. | Treat COPD and cardiovascular risk factors Treat heart failure and valve disease Consider diuretics Consider low-sodium diet Pulmonary vasodilator not recommended |
| Mild to moderate precapillary PH | Frequent type of PH at rest in COPD patients More frequent in severe and very severe COPD More endothelial dysfunction and arterial stiffness Ventilatory limitation during exercise: low breathing reserve, increased PaCO2 | Treat COPD Consider diuretics Pulmonary vasodilator not recommended |
| Pulmonary vascular phenotype | Mild to moderate airflow obstruction (FEV1 ≥ 60%) and severe PH (mPAP ≥ 35 mmHg or CI <2 L/min/m2) Severe hypoxemia with no hypercapnia Severe DLCO reduction Cardiovascular limitation during exercise with low peak VO2, lower PetCO2 | Treat COPD Consider diuretics Refer to a PH reference center and consider inclusion in large clinical trial with pulmonary vasodilators |
| Severe COPD – Severe PH phenotype | Severe airflow obstruction (FEV1 <50%) and severe PH (mPAP ≥ 35 mmHg or CI <2 L/min/m2) Severe gas impairment Increased mortality | Treat COPD Consider diuretics Refer to a PH reference center and consider inclusion in specific clinical trial |
CI, cardiac index; DLco, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in one second; LA, left atrium; LV, left ventricle; mPAP, mean pulmonary arterial pressure; PaCO2, partial arterial pressure of carbon dioxide; PAWP, pulmonary artery occlusion pressure; PetCO2, end-tidal carbon dioxide; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; UW, wood units; VO2, oxygen consumption.
PULMONARY HYPERTENSION DURING EXERCISE
Even in the absence of PH, COPD patients often suffer from systemic [75] and pulmonary [76] endothelial dysfunction. Abnormal hemodynamic response to exercise is common in COPD [58], and patients with mild COPD [22] and smokers without airflow obstruction [16] can develop pulmonary vascular remodeling, although it is unknown if and how these patients may progress to PH. Furthermore, elevation of mPAP during exercise might be a consequence of rising pulmonary capillary pressure from left heart dysfunction and only RHC can reliably distinguish the latter from pulmonary vascular remodeling [77]. Whatever the case, patients with PH during exercise are not candidates for treatment with pulmonary vasodilators; however, they should be monitored with echocardiography, especially during an otherwise unexplained worsening of symptoms or hypoxemia.
MILD-MODERATE PRE-CAPILLARY PULMONARY HYPERTENSION
One of the most common forms of PH in COPD, it is usually identified in later stages [8]; in these patients, endothelial dysfunction is even greater than in COPD without PH and accompanied by arterial stiffness [75], potentially explaining PH development. The clinical picture is thus characterized by severe or very severe airflow obstruction, moderate to severe hypoxemia, mild to moderate PH and ventilatory limitation to exercise. Pulmonary vasodilators are contra-indicated as they may have a detrimental effect on gas exchange [78]; in eligible patients, lung transplantation should be prioritized. Hemodynamic status should be monitored through echocardiography to detect the development of severe PH.
POST-CAPILLARY PULMONARY HYPERTENSION
Patients with COPD have increased cardiovascular risk [79] and COPD is a risk factor for cardiovascular events in patients with systemic hypertension [80]; even reduced lung function per se has the same effect [81]. It is therefore not surprising that COPD patients should present with PH due to left heart disease [82], frequent during exacerbations. A noteworthy cause of postcapillary PH in COPD patients is diastolic dysfunction, present in about half of moderate to severe COPD patients and correlated with exercise intolerance [83]. When probability of postcapillary PH is high [84] because of left heart disease or cardiovascular risk factors, RHC is not mandatory [85▪]. Since RHC is the only way to safely discriminate between pre and postcapillary PH, the prevalence of postcapillary PH in COPD is unknown, but pulmonary artery wedge pressure may be increased in >60% of COPD patients [9]. Postcapillary PH in COPD should be correctly categorized as group two of the PH Nice classification [86] and treated as such. There are no studies assessing the clinical characteristics and outcomes of combined pre and postcapillary PH in COPD.
PULMONARY VASCULAR PHENOTYPE VERSUS CHRONIC OBSTRUCTIVE PULMONARY DISEASE WITH IDIOPATHIC PULMONARY ARTERIAL HYPERTENSION
The PVP, with its characteristic profile of moderate airflow obstruction, severe hypoxemia and DLCO reduction, cardiovascular limitation to exercise, and poor prognosis [11,63], constitutes a real diagnostic challenge. This clinical picture may indeed represent either the PVP or just the coincidence of rare idiopathic PAH (iPAH) with the highly prevalent COPD in mild form[87]. This distinction, which might seem philosophical, has bearings on overall management since iPAH is treated with vasodilator drugs, for which there is no conclusive evidence in COPD patients with PH [1,88]; furthermore, it is unknown whether risk assessment scores and follow-up guidelines for iPAH apply to the PVP. Some clues from clinical studies [53,89], case series [90,91], and small randomized-controlled trials [92] point to this phenotype as a possible candidate for pulmonary vasodilators and patients should always be referred to an expert PH center for diagnosis and inclusion in a clinical trial and/or lung transplant workup.
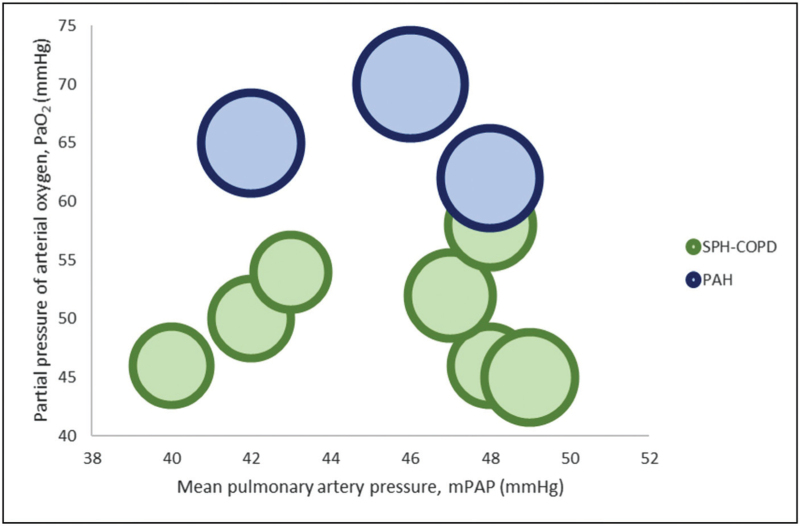
In the debate on how to best distinguish between the PVP and the coincidence of iPAH and COPD, we propose that the severity of hypoxemia, and not that of airflow obstruction, might provide a clue to favor the diagnosis of PVP (Fig. 2; Table 1 supplementary material). Patients with iPAH rarely show more than mild hypoxemia, in contrast with the severe hypoxemia existing in the PVP. Although the proportion of patients with iPAH and COPD in published series is unknown [93,94,95▪], obstructive disease is certainly not uncommon among iPAH patients [94] and despite being associated with a certain reduction in PaO2, the latter is rarely as severe as seen in case series of severe PH in COPD, in which PVP patients are included [10,11,62▪,63,76,88,95▪,96]. This proposal needs to be validated by future clinical and physiological studies.
FIGURE 2.
Relationship between PaO2, mPAP and FEV1 in COPD patients with severe PH (SPH-COPD) and in pulmonary arterial hypertension (PAH). Each bubble represents the relationship of mean values of mPAP and PaO2 reported in case series of SPH-COPD [10,11,62▪,63,88,95▪,96] and PAH [93,94,95▪]. The diameter of bubbles is proportional to the FEV1 reported in the same studies. Patients with COPD and severe PH are consistently more hypoxemic than PAH regardless of hemodynamic severity. Thus, when mild-moderate COPD is observed, PaO2 may be more helpful than FEV1 in differentiating patients with PVP over concurrent mild-moderate COPD and PAH. FEV1, forced expiratory volume in one second; mPAP, mean pulmonary artery pressure; PAH, idiopathic pulmonary arterial hypertension; PaO2, arterial oxygen partial pressure; PVP, pulmonary vascular phenotype; SPH-COPD, COPD and severe PH.
BEYOND THE PULMONARY VASCULAR PHENOTYPE: IS THERE A 'SEVERE CHRONIC OBSTRUCTIVE PULMONARY DISEASE -SEVERE PULMONARY HYPERTENSION’ PHENOTYPE?
Severe hemodynamic compromise is rare in clinically stable patients [8,39,97] and doesn’t always present with mild airflow obstruction, as in the PVP. In the study by Thabut et al., patients with severe COPD and severe PH formed a separate cluster from the PVP [10]; furthermore, two large registry cohorts [51▪▪,95▪] described patients with severe PH and similar FEV1 to those with milder PH, indicating that patients with PVP (who normally have significantly higher FEV1 than mild PH patients) were lumped together with patients with severe hemodynamic compromise and severe airflow obstruction. Interestingly, in the last of those studies [51▪▪], patients with advanced COPD stages and severe PH presented the worst survival, suggesting the importance of singling out this phenotype.
What factors favor the development of mild versus severe PH in patients with severe obstructive disease? As mentioned, the severity of emphysema is not correlated with hemodynamic compromise [9,57,63], and postcapillary was carefully excluded in works studying this group of patients [10,51▪▪,95▪]. Interestingly, studies using mPAP ≥40 mmHg as definition for severe PH [11,63] properly identified patients with the PVP, since their FEV1 was significantly higher than in the moderate PH group; this suggests that a higher mPAP threshold might be a better discriminant for the PVP. Recently it has been shown [4▪▪] that PVR >4 WU may be associated with poorer outcomes in COPD and thus a better descriptor of severe PH, but whether these patients conform to the PVP (statistically higher FEV1, lower PaO2 compared to patients with PVR ≤4 WU) is unknown. Finally, patients with severe PH and severe obstructive disease were included in a small randomized controlled trial showing positive results with pulmonary vasodilators in severe PH [92]. Overall, studies focusing on this specific phenotype of patients separately from the PVP may be warranted to evaluate its clinical presentation and outcomes.
FUTURE DIRECTIONS
Preliminary data focusing on severe PH patients have been encouraging and these patients may be a good target for future, dedicated large clinical trials with pulmonary vasodilators; it may also be useful to distinguish between the PVP and the ‘severe COPD-severe PH’ phenotype. Furthermore, the role of hypoxemia in the PVP versus iPAH should be investigated (Fig. 2).
Future studies should address the variety of presentations of PH in COPD (Table 1, Fig. 1) by focusing on deeply phenotyped cohorts, comparing clinical presentation and outcomes, harnessing registries to increase reproducibility with larger cohorts, and concentrating pharmacological trials on likely candidate phenotypes with carefully targeted compounds.
CONCLUSION
PH associated with COPD may present in a variety of phenotypes that require careful investigation, individualized management and, in the case of severe hemodynamic compromise, referral to an expert PH center. All patients require optimal management of their underlying condition; PH in COPD should also elicit referral to a lung transplant center in eligible patients. There is still not enough evidence favoring the use of pulmonary vasodilators in PH associated with COPD, likely due to insufficient discrimination between previously unrecognized phenotypes with different responses to therapy in historical randomized clinical trials.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2020; 53:1801914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler R, Faller M, Fourgaut G, et al. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159:158–164. [DOI] [PubMed] [Google Scholar]
- 3.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012; 367:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪▪.Zeder K, Avian A, Bachmaier G, et al. Elevated pulmonary vascular resistance predicts mortality in COPD patients. Eur Respir J 2021; 58:2100944. [DOI] [PubMed] [Google Scholar]; This study found PVR >5 WU to be the strongest independent hemodynamic predictor of mortality in COPD patients and may best identify COPD patients with severe pulmonary vascular disease.
- 5.Gall H, Felix JF, Schneck FK, et al. The Giessen Pulmonary Hypertension Registry: Survival in pulmonary hypertension subgroups. J Heart Lung Transplant 2017; 36:957–967. [DOI] [PubMed] [Google Scholar]
- 6.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: assessing the spectrum of pulmonary hypertension identified at a. Eur Respir J 2012; 39:945–955. [DOI] [PubMed] [Google Scholar]
- 7.Andersen KH, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant 2012; 31:373–380. [DOI] [PubMed] [Google Scholar]
- 8.Portillo K, Torralba Y, Blanco I, et al. Pulmonary hemodynamic profile in chronic obstructive pulmonary disease. Int J COPD 2015; 10:1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scharf SM, Iqbal M, Keller C, et al. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med 2002; 166:314–322. [DOI] [PubMed] [Google Scholar]
- 10.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 2005; 127:1531–1536. [DOI] [PubMed] [Google Scholar]
- 11.Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172:189–194. [DOI] [PubMed] [Google Scholar]
- 12.Chebib N, Mornex JF, Traclet J, et al. Pulmonary hypertension in chronic lung diseases: comparison to other pulmonary hypertension groups. Pulm Circ 2018; 8:2045894018775056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacs G, Agusti A, Barberá JA, et al. Pulmonary vascular involvement in chronic obstructive pulmonary disease is there a pulmonary vascular phenotype? Am J Respir Crit Care Med 2018; 198:1000–1011. [DOI] [PubMed] [Google Scholar]
- 14.Newman JH, Rich S, Abman SH, et al. Enhancing insights into pulmonary vascular disease through a precision medicine approach: a joint nhlbi-cardiovascular medical research and education fund workshop report. Am J Respir Crit Care Med 2017; 195:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes JW, Tonelli AR, Heresi GA, et al. Novel methods in pulmonary hypertension phenotyping in the age of precision medicine (2015 grover conference series). Pulm Circ 2016; 6:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos S, Peinado VI, Ramírez J, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J 2002; 19:632–638. [DOI] [PubMed] [Google Scholar]
- 17.Bunel V, Guyard A, Dauriat G, et al. Pulmonary arterial histologic lesions in patients with COPD with severe pulmonary hypertension. Chest 2019; 156:33–44. [DOI] [PubMed] [Google Scholar]
- 18.Ferrer E, Peinado VI, Castañeda J, et al. Effects of cigarette smoke and hypoxia on pulmonary circulation in the guinea pig. Eur Respir J 2011; 38:617–627. [DOI] [PubMed] [Google Scholar]
- 19.Sakao S, Voelkel NF, Tatsumi K. The vascular bed in COPD: pulmonary hypertension and pulmonary vascular alterations. Eur Respir Rev 2014; 23:350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seimetz M, Parajuli N, Pichl A, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell 2011; 147:293–305. [DOI] [PubMed] [Google Scholar]
- 21.Peinado VI, Gómez FP, Barberà JA, et al. Pulmonary vascular abnormalities in chronic obstructive pulmonary disease undergoing lung transplant. J Heart Lung Transplant 2013; 32:1262–1269. [DOI] [PubMed] [Google Scholar]
- 22.Barberà JA, Riverola A, Roca J, et al. Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994; 149:423–429. [DOI] [PubMed] [Google Scholar]
- 23.Rabinovitch M. Elastase and the pathobiology of unexplained pulmonary hypertension. In: Chest Chest 1998; 114:213S–224S. [DOI] [PubMed] [Google Scholar]
- 24.Magee F, Wright JL, Wiggs BR, et al. Pulmonary vascular structure and function in chronic obstructive pulmonary disease. Thorax 1988; 43:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright JL, Petty T, Thurlbeck WM. Analysis of the structure of the muscular pulmonary arteries in patients with pulmonary hypertension and COPD: National Institutes of Health Nocturnal Oxygen Therapy Trial. Lung 1992; 170:109–124. [DOI] [PubMed] [Google Scholar]
- 26.Eddahibi S, Chaouat A, Morrell N, et al. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation 2003; 108:1839–1844. [DOI] [PubMed] [Google Scholar]
- 27.Washko GR, Nardelli P, Ash SY, et al. Arterial vascular pruning, right ventricular size, and clinical outcomes in chronic obstructive pulmonary disease a longitudinal observational study. Am J Respir Crit Care Med 2019; 200:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Synn AJ, Margerie-Mellon Cde, Jeong SY, et al. Vascular remodeling of the small pulmonary arteries and measures of vascular pruning on computed tomography. Pulm Circ 2021; 11:20458940211061284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco I, Piccari L, Barberà JA. Pulmonary vasculature in COPD: the silent component. Respirology 2016; 21:984–994. [DOI] [PubMed] [Google Scholar]
- 30.Weissmann N, Lobo B, Pichl A, et al. Stimulation of soluble guanylate cyclase prevents cigarette smoke-induced pulmonary hypertension and emphysema. Am J Respir Crit Care Med 2014; 189:1359–1373. [DOI] [PubMed] [Google Scholar]
- 31.Hoeper MM, Vonk-Noordegraaf A. Is there a vanishing pulmonary capillary syndrome? Lancet Respir Med 2017; 5:676–678. [DOI] [PubMed] [Google Scholar]
- 32.Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on-the cells of the pulmonary vasculature. Eur Respir J 2007; 30:364–372. [DOI] [PubMed] [Google Scholar]
- 33.Sakao S, Tatsumi K, Voelkel NF. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Bio 2010; 43:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heath D, Williams D, Rios-Dalenz J, et al. Small pulmonary arterial vessels of Aymara Indians from the Bolivian Andes. Histopathology 1990; 16:565–571. [DOI] [PubMed] [Google Scholar]
- 35.Morrell NW, Aldred MA, Chung WK, et al. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J 2019; 53:1801899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neubert L, Borchert P, Stark H, et al. Molecular profiling of vascular remodeling in chronic pulmonary disease. Am J Pathol 2020; 190:1382–1396. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann J, Wilhelm J, Marsh LM, et al. Distinct differences in gene expression patterns in pulmonary arteries of patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis with pulmonary hypertension. Am J Respir Crit Care Med 2014; 190:98–111. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann J, Wilhelm J, Olschewski A, Kwapiszewska G. Microarray analysis in pulmonary hypertension. Eur Respir J 2016; 48:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 2008; 32:1371–1385. [DOI] [PubMed] [Google Scholar]
- 40.Braganza M, Shaw J, Solverson K, et al. A prospective evaluation of the diagnostic accuracy of the physical examination for pulmonary hypertension. Chest 2019; 155:982–990. [DOI] [PubMed] [Google Scholar]
- 41.Agustí AGN. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2:367–370. [DOI] [PubMed] [Google Scholar]
- 42.Frost A, Badesch D, Gibbs JS, et al. Diagnosis of pulmonary hypertension. Eur Respir J 2019; 53:1801904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakayama S, Chubachi S, Sakurai K, et al. Characteristics of chronic obstructive pulmonary disease patients with pulmonary hypertension assessed by echocardiography in a three-year observational cohort study. Int J COPD 2020; 15:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greiner S, Jud A, Aurich M, et al. Reliability of noninvasive assessment of systolic pulmonary artery pressure by doppler echocardiography compared to right heart catheterization: Analysis in a large patient population. J Am Heart Assoc 2014; 3:e001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailey KL. The importance of the assessment of pulmonary function in COPD. Med Clin N Am 2012; 96:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yagi M, Taniguchi H, Kondoh Y, et al. CT-determined pulmonary artery to aorta ratio as a predictor of elevated pulmonary artery pressure and survival in idiopathic pulmonary fibrosis. Respirology 2017; 22:1393–1399. [DOI] [PubMed] [Google Scholar]
- 47.Coste F, Benlala I, Dournes G, et al. Assessing pulmonary hypertension in COPD. Is there a role for computed tomography? Int J Chron Obstruct Pulmon Dis 2019; 14:2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leuchte HH, Baumgartner RA, el Nounou M, et al. Brain natriuretic peptide is a prognostic parameter in chronic lung disease. Am J Respir Crit Care Med 2006; 173:744–750. [DOI] [PubMed] [Google Scholar]
- 49.Hawkins NM, Khosla A, Virani SA, et al. B-type natriuretic peptides in chronic obstructive pulmonary disease: a systematic review. BMC Pulm Med 2017; 17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian F, Song W, Wang L, et al. NT-pro BNP in AECOPD-PH: old biomarker, new insights-based on a large retrospective case-controlled study. Respir Res 2021; 22:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51▪▪.Kovacs G, Avian A, Bachmaier G, et al. Severe Pulmonary Hypertension in COPD. Chest 2022; S0012-3692(22)00192-1. [DOI] [PubMed] [Google Scholar]; This single-center retrospective work showed that the contribution of severe PH and severe airflow limitation to impaired survival is independent and comparable, with the combination of severe COPD and severe PH showing the poorest survival. It also proposes a noninvasive score to predict severe PH in COPD.
- 52.Weatherald J, Farina S, Bruno N, Laveneziana P. Seminar for Clinicians cardiopulmonary exercise testing in pulmonary hypertension. Ann Am Thorac Soc 2017; 14:84–92. [DOI] [PubMed] [Google Scholar]
- 53.Boerrigter BG, Bogaard HJ, Trip P, et al. Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest 2012; 142:1166–1174. [DOI] [PubMed] [Google Scholar]
- 54.Simonneau G, Galiè N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004; 43: (12 Suppl S): S5–S12. [DOI] [PubMed] [Google Scholar]
- 55.Mal H. Prevalence and diagnosis of severe pulmonary hypertension in patients with chronic obstructive pulmonary disease. Curr Opin Pulm Med 2007; 13:114–119. [DOI] [PubMed] [Google Scholar]
- 56.Agusti AGN, Barbera JA. Contribution of multiple inert gas elimination technique to pulmonary medicine 2. Chronic pulmonary diseases: chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Thorax 1994; 49:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dournes G, Laurent F, Coste F, et al. Computed tomographic measurement of airway remodeling and emphysema in advanced chronic obstructive pulmonary disease. Correlation with pulmonary hypertension. Am J Respir Crit Care Med 2015; 191:63–70. [DOI] [PubMed] [Google Scholar]
- 58.Kessler R, Faller M, Weitzenblum E, et al. ‘Natural history’ of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med 2001; 164:219–224. [DOI] [PubMed] [Google Scholar]
- 59.Weitzenblum E, Sautegeau A, Ehrhart M, et al. Long-term course of pulmonary arterial pressure in chronic obstructive pulmonary disease. Am Rev Respir Dis 1984; 130:993–998. [DOI] [PubMed] [Google Scholar]
- 60.Nathan SD, Shlobin OA, Ahmad S, et al. Serial development of pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Respiration 2008; 76:288–294. [DOI] [PubMed] [Google Scholar]
- 61.Teramachi R, Taniguchi H, Kondoh Y, et al. Progression of mean pulmonary arterial pressure in idiopathic pulmonary fibrosis with mild to moderate restriction. Respirology 2017; 22:986–990. [DOI] [PubMed] [Google Scholar]
- 62▪.Dauriat G, Reynaud-Gaubert M, Cottin V, et al. Severe pulmonary hypertension associated with chronic obstructive pulmonary disease: A prospective French multicenter cohort. J Heart Lung Transplant 2021; 40:1009–1018. [DOI] [PubMed] [Google Scholar]; This prospective analysis of a French cohort reports on the clinical picture of severe PH in COPD, characterized by highly impaired exercise capacity, frequent exacerbations, and poor prognosis.
- 63.Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: Results from the ASPIRE registry. Eur Respir J 2013; 41:1292–1301. [DOI] [PubMed] [Google Scholar]
- 64.Wu XG, Shi YJ, Wang XH, et al. Diagnostic value of computed tomography-based pulmonary artery to aorta ratio measurement in chronic obstructive pulmonary disease with pulmonary hypertension: A systematic review and meta-analysis. Clin Respir J 2022; 16:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iyer AS, Wells JM, Vishin S, et al. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest 2014; 145:824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coste F, Dournes G, Dromer C, et al. CT evaluation of small pulmonary vessels area in patients with COPD with severe pulmonary hypertension. Thorax 2016; 71:830–837. [DOI] [PubMed] [Google Scholar]
- 67.Krahnke JS, Abraham WT, Adamson PB, et al. Heart failure and respiratory hospitalizations are reduced in patients with heart failure and chronic obstructive pulmonary disease with the use of an implantable pulmonary artery pressure monitoring device. J Card Fail 2015; 21:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cuttica MJ, Kalhan R, Shlobin OA, et al. Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir Med 2010; 104:1877–1882. [DOI] [PubMed] [Google Scholar]
- 69.Blanco I, Valeiro B, Torres-Castro R, et al. Effects of pulmonary hypertension on exercise capacity in patients with chronic obstructive pulmonary disease. Arch Bronconeumola 2020; 56:499–505. [DOI] [PubMed] [Google Scholar]
- 70.Thirapatarapong W, Armstrong HF, Bartels MN. Comparing cardiopulmonary exercise testing in severe COPD patients with and without pulmonary hypertension. Heart Lung Circ 2014; 23:833–840. [DOI] [PubMed] [Google Scholar]
- 71.Torres-Castro R, Gimeno-Santos E, Vilaró J, et al. Effect of pulmonary hypertension on exercise tolerance in patients with COPD: a prognostic systematic review and meta-analysis. Eur Respir Rev 2021; 30:200321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weitzenblum E, Sautegeau A, Ehrhart M, et al. Long-term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1985; 131:493–498. [DOI] [PubMed] [Google Scholar]
- 73.Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest 1995; 107:1193–1198. [DOI] [PubMed] [Google Scholar]
- 74.Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006; 28:523–532. [DOI] [PubMed] [Google Scholar]
- 75.Piccari L, Pozo Rdel, Blanco I, et al. Association between systemic and pulmonary vascular dysfunction in COPD. Int J COPD 2020; 15:2037–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Domingo E, Grignola JC, Aguilar R, et al. Pulmonary arterial wall disease in COPD and interstitial lung diseases candidates for lung transplantation. Respir Res 2017; 18:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herve P, Lau EM, Sitbon O, et al. Criteria for diagnosis of exercise pulmonary hypertension. Eur Respir J 2015; 46:728–737. [DOI] [PubMed] [Google Scholar]
- 78.Blanco I, Gimeno E, Munoz PA, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med 2010; 181:270–278. [DOI] [PubMed] [Google Scholar]
- 79.Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Lancet Respir Med 2015; 3:631–639. [DOI] [PubMed] [Google Scholar]
- 80.Perticone M, Maio R, Caroleo B, et al. COPD significantly increases cerebral and cardiovascular events in hypertensives. Sci Rep 2021; 11:7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramalho SHR, Shah AM. Lung function and cardiovascular disease: a link. Trends Cardiovasc Med 2021; 31:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lepida D, Papathanasiou A, Galiatsou E, et al. The contribution of left heart disease in COPD patients with pulmonary hypertension. Hellenic J Cardiol 2018; 59:160–165. [DOI] [PubMed] [Google Scholar]
- 83.Faludi R, Hajdu M, Vértes V, et al. Diastolic Dysfunction Is a Contributing Factor to Exercise Intolerance in COPD. COPD 2016; 13:345–351. [DOI] [PubMed] [Google Scholar]
- 84.Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019; 53:1801897.30545974 [Google Scholar]
- 85▪.Kanwar MK, Tedford RJ, Thenappan T, et al. Elevated pulmonary pressure noted on echocardiogram: a simplified approach to next steps. J Am Heart Assoc 2021; 10:e017684. [DOI] [PMC free article] [PubMed] [Google Scholar]; This mini-review gives simplified, case-based directions for the diagnostic approach of elevated pulmonary artery pressure on echocardiogram to general practitioners and clinicians.
- 86.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blanco I, Tura-Ceide O, Peinado VI, Barberà JA. Updated perspectives on pulmonary hypertension in COPD. Int J COPD 2020; 15:1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Girard A, Jouneau S, Chabanne C, et al. Severe pulmonary hypertension associated with COPD: Hemodynamic improvement with specific therapy. Respiration 2015; 90:220–228. [DOI] [PubMed] [Google Scholar]
- 89.Brewis MJ, Church AC, Johnson MK, Peacock AJ. Severe pulmonary hypertension in lung disease: phenotypes and response to treatment. Eur Respir J 2015; 46:1378–1389. [DOI] [PubMed] [Google Scholar]
- 90.Abuserewa ST, Selim A, Youssef A, Zolty R. Role of selexipag in chronic obstructive pulmonary disease (COPD) patients with out-of-proportion pulmonary hypertension. Cureus 2021; 13:e16520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Calcaianu G, Canuet M, Schuller A, et al. Pulmonary arterial hypertension-specific drug therapy in copd patients with severe pulmonary hypertension and mild-to-moderate airflow limitation. Respiration 2016; 91:9–17. [DOI] [PubMed] [Google Scholar]
- 92.Vitulo P, Stanziola A, Confalonieri M, et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: A randomized controlled multicenter clinical trial. J Heart Lung Transplant 2017; 36:166–174. [DOI] [PubMed] [Google Scholar]
- 93.Stubbe B, Seyfarth HJ, Kleymann J, et al. Monotherapy in patients with pulmonary arterial hypertension at four German PH centres. BMC Pulm Med 2021; 21:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoeper MM, Pausch C, Grünig E, et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant 2020; 39:1435–1444. [DOI] [PubMed] [Google Scholar]
- 95▪.Vizza CD, Hoeper MM, Huscher D, et al. Pulmonary hypertension in patients with COPD: results from the comparative, prospective registry of newly initiated therapies for pulmonary hypertension (COMPERA). Chest 2021; 160:678–689. [DOI] [PubMed] [Google Scholar]; This analysis of the COMPERA registry reports that male sex, low exercise tolerance and high PVR are predictors of death in COPD-PH, and that PH-targeted therapy may improve exercise capacity and functional class in a subgroup of patients with severe COPD-PH.
- 96.Tanabe N, Taniguchi H, Tsujino I, et al. Multiinstitutional retrospective cohort study of patients with severe pulmonary hypertension associated with respiratory diseases. Respirology 2015; 20:805–812. [DOI] [PubMed] [Google Scholar]
- 97.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016; 4:306–322. [DOI] [PubMed] [Google Scholar]
- 98.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med 2017; 195:557–582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.