Abstract
BACKGROUND AND PURPOSE:
Carotid artery near-occlusion is a type of severe stenosis with complete or partial distal luminal collapse and intracranial collaterals. This study aimed to compare 30-day outcomes and 10-year survival in patients undergoing carotid artery stenting for near-occlusion with a control group of patients with severe stenosis.
MATERIALS AND METHODS:
We used data from a registry of 639 patients who underwent 789 carotid artery stenting procedures between 2005 and 2021. The primary end point was any stroke or death within 30 days after carotid artery stenting. Patients were matched using propensity scores based on 6 variables.
RESULTS:
Propensity score matching yielded 84 subjects in the near-occlusion group matched with 168 subjects in the control group. In the matched cohort, the primary end point occurred in 7 (8.3%) and 11 (6.6%) patients in the near-occlusion and control groups, respectively (P = .611). In the unmatched cohort, the primary end point occurred in 7 (8.3%) and 19 (4.1%) patients (P = .101). Survival in the near-occlusion group versus the control group in the matched cohort at 5 and 10 years was 69.8% (95% CI, 58.0%–78.8%) versus 77.3% (95% CI, 70.0%–83.1%) and 53.3% (95% CI, 39.9%–65.0%) versus 53.3% (95% CI, 44.5%–61.4%) (log-rank, P = .798).
CONCLUSIONS:
Carotid stent placement in patients with ICA near-occlusion was not associated with an increased 30-day risk of stroke or death compared with severe stenosis. Survival up to 10 years after carotid artery stenting was similar in both groups.
Carotid artery near-occlusion is a type of severe stenosis with complete or incomplete distal luminal collapse and intracranial collaterals.1 Various terms have been used to describe near-occlusion: subtotal stenosis or occlusion, functional occlusion, string sign, slim sign, critical stenosis, and others.2 Calculating the percentage stenosis for ICA near-occlusion according to the NASCET criteria3,4 is a fallacious approach. Near-occlusion can be confused with complete occlusion or severe stenosis when imaging with CTA or sonography is suboptimal.4 The multitude of terms, subtle variation in the definition, and diagnostic ambiguity lead to uncertainty about the true incidence, prognosis, and optimal treatment of ICA near-occlusion. Some early observational studies5,6 suggested that near-occlusion carries a high risk of stroke and should be promptly recognized and treated by endarterectomy. This suggestion was later negated by the re-analysis of the NASCET and the European Carotid Surgery Trial.3,7 Patients with near-occlusion were either excluded or not assessed or reported in further randomized clinical trials comparing surgery or conservative treatment of asymptomatic stenosis.2 Both current European and American guidelines state that there is no clear evidence that endarterectomy or carotid artery stenting (CAS) prevents stroke in patients with near-occlusion of the ICA.8,9 Another position paper admits that the prognoses of asymptomatic near-occlusion and periprocedural risks of CAS or endarterectomy are unknown.10 A recent meta-analysis showed that the 30-day risk of stroke or death after endarterectomy or CAS for symptomatic ICA near-occlusion was 2%.11
In this study, we report 30-day outcomes after CAS in patients with ICA near-occlusion and compare them with a control group of patients with severe stenosis after CAS using a propensity score matching analysis. Additionally, we report 10-year survival after CAS.
MATERIALS AND METHODS
We retrospectively analyzed data from a single-center (Motol University Hospital) registry of 639 patients who underwent 789 CAS procedures between 2005 and 2021. Patients who had bilateral CAS or CAS for in-stent restenosis were excluded from the analysis. Some of the patients were included in previous studies.12-16
Patient Assessment, Procedure, and Follow-up
Patients were referred to carotid angiography and CAS by a neurologist, cardiologist, or vascular surgeon on the basis of Doppler sonography and/or CTA. Patient characteristics are listed in Table 1, and their medication before CAS is found in the Online Supplemental Data. Stenosis was quantified angiographically according to the NASCET criteria.3 Inclusion criteria were symptomatic (≥50%) or asymptomatic (≥70%) stenosis of the ICA in a patient who was considered eligible for CAS. Carotid stenosis was considered symptomatic if the patient had a stroke, TIA, or amaurosis fugax ipsilateral to the stenosis in the previous 6 months. All patients provided written informed consent for the procedure. Procedures were performed via the femoral artery using a 7F or 8F guiding catheter or a long 6F sheath. The antithrombotic regimen included administration of 500 mg of aspirin and 300 mg of clopidogrel before CAS in naïve patients; a bolus of heparin (70 IU/kg) was administrated at the beginning of CAS. Types and manufacturers of stents and embolic protection devices were at the operator’s discretion and current availability. Detailed angiographic and procedural characteristics are listed in the Online Supplemental Data. After CAS, patients were examined by a physician, and all symptomatic patients were examined by a neurologist. Postprocedural CTA or MR imaging was performed in all cases of clinically suspected stroke. Patients were discharged with dual antiplatelet therapy for 1 month and single antiplatelet therapy and high-dose statin therapy life-long. Follow-up consisted of a history and review of medical documentation if the patient had neurologic symptoms and Doppler sonography at 1, 6, and 12 months after CAS. Information about vital status was retrieved from the National Death Index. In the deceased patients, cause of death was adjudicated as cardiovascular or noncardiovascular. The study was conducted in accordance with the Declaration of Helsinki principles.
Table 1:
Patient characteristics
| Unmatched Cohort |
Matched Cohort |
|||||
|---|---|---|---|---|---|---|
| Near-Occlusion (n = 84 Patients) | Control Group (n = 460 Patients) | P Value | Near-Occlusion (n = 84 Patients) | Control Group (n = 168 Patients) | P Value | |
| Age (mean) (yr) | 70.3 (SD, 9.7) | 68.6 (SD, 8.3) | .091 | 70.3 (SD, 9.7) | 70.2 (SD, 7.7) | .943 |
| Men | 68% | 65% | .620 | 68% | 64% | .576 |
| Current smokers | 43% | 40% | .629 | 43% | 37% | .411 |
| Arterial hypertension | 88% | 88% | 1.000 | 88% | 88% | 1.000 |
| Total plasma cholesterol level (mean) (mmol/L) | 4.4 (SD, 1.1) | 4.2 (SD, 1.0) | .126 | 4.4 (SD, 1.1) | 4.4 (SD, 1.1) | .664 |
| LDL cholesterol level (mean) (mmol/L) | 2.6 (SD, 0.9) | 2.4 (SD, 0.8) | .171 | 2.6 (SD, 0.9) | 2.5 (SD, 0.9) | .629 |
| HDL cholesterol level (mean) (mmol/L) | 1.06 (SD, 0.31) | 1.09 (SD, 0.33) | .695 | 1.06 (SD, 0.31) | 1.09 (SD, 0.33) | .659 |
| Plasma triglyceride level (mean) (mmol/L) | 1.93 (SD, 1.2) | 1.8 (SD, 1.1) | .638 | 1.93 (SD, 1.2) | 1.78 (SD, 1.0) | .614 |
| CRP level (median) (IQR) (mg/L) | 3.6 (1.1–8.6) | 2.3 (0.8–5.5) | .008 | 3.6 (1.1–8.6) | 2.1 (0.9–4.9) | .010 |
| Body mass index (mean) | 28.6 (SD, 4.8) | 28.1 (SD, 4.4) | .483 | 28.6 (SD, 4.8) | 28.0 (SD, 4.1) | .540 |
| Diabetes | 33% | 42% | .184 | 33% | 37% | .676 |
| Chronic kidney disease | 27% | 22% | .324 | 27% | 24% | .644 |
| Chronic bronchopulmonary disease | 14% | 13% | .723 | 14% | 15% | 1.000 |
| Peripheral arterial disease | 32% | 39% | .271 | 32% | 33% | .888 |
| Heart failure with reduced ejection fraction | 15% | 11% | .320 | 15% | 11% | .374 |
| Previous coronary artery bypass | 20% | 18% | .649 | 20% | 17% | .490 |
| Need for heart surgery within 30 days | 5% | 9% | .281 | 5% | 10% | .224 |
| Previous myocardial infarction | 24% | 28% | .506 | 24% | 30% | .302 |
| Previous percutaneous coronary artery intervention | 23% | 32% | .094 | 23% | 24% | .876 |
| Known multivessel coronary artery disease | 39% | 40% | 1.000 | 39% | 35% | .490 |
| Previous stroke | 37% | 30% | .252 | 37% | 40% | .683 |
| Ipsilateral cerebral ischemic symptoms in the past month | 37% | 21% | .003 | 37% | 33% | .577 |
| Ipsilateral cerebral ischemic symptoms in the past 6 months (ie, symptomatic stenosis) | 45% | 29% | .005 | 45% | 46% | .894 |
| Patients with ≥1 risk factors for endarterectomya | 82% | 80% | .766 | 82% | 82% | 1.000 |
Note:—IQR indicates interquartile range; CRP, C-reactive protein.
One of the following: left ventricle ejection fraction of ≤40%, chronic bronchopulmonary disease, prior myocardial infarction, coronary artery bypass grafts, or age 75 years or older.
Definitions and End Points
Patients in the registry were divided into near-occlusion and control groups. Angiographic criteria described in the NASCET2,3 were used to distinguish near-occlusion from conventional stenosis: 1) partial or complete collapse of the distal lumen (diameter of the ipsilateral distal ICA less than that of the contralateral distal ICA), 2) diameter of ipsilateral distal ICA less than that of the ipsilateral external carotid artery (ECA), 3) delayed filling of the ipsilateral distal ICA, and 4) intracranial collaterals (Fig 1). Two of the 4 criteria were required for the diagnosis. In patients without acute neurologic symptomatology, intracranial collaterals must always be present in the near-occlusion. The primary end point consisted of any stroke or death within 30 days after CAS. Ischemic stroke was defined as an acute neurologic event with focal symptoms, lasting for ≥24 hours. Minor stroke was defined as a new neurologic deficit that resolved within 30 days without any limiting disability (≤1 on the mRS) or return to baseline status. Major stroke was defined as a new neurologic deficit with persisting disability (≥2 on mRS). TIA was defined as an episode of new neurologic dysfunction attributed to focal cerebral ischemia, with resolution within 24 hours.
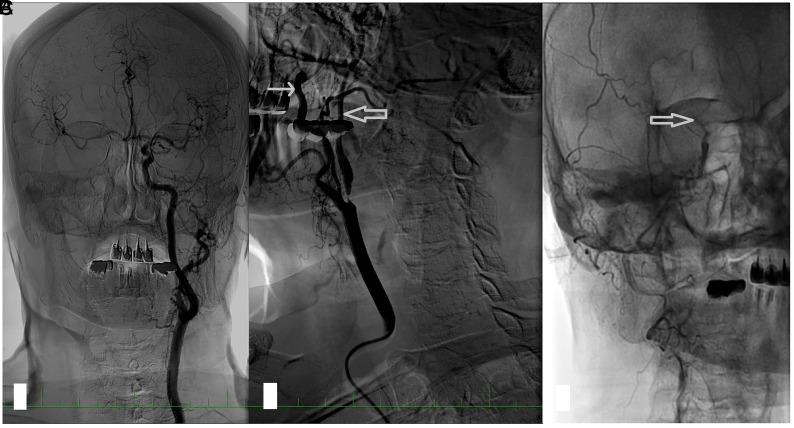
FIG 1.
An angiogram in a patient with right ICA near-occlusion. A, In a selective angiogram of the left carotid artery, no stenosis is present; the diameter of the ICA is larger than that of the ECA; and there is rapid filling of the intracranial circulation and intracranial collaterals to the right ACA and MCA. B, Near-occlusion of the right ICA. The diameter of the distal ICA (thick arrow) is smaller than that of the ECA (thin arrow) and much smaller than that of the contralateral ICA. Late filling of the distal ICA. C, In the late phase of the angiogram, the contrast filling stops at the level of carotid siphon (thick arrow). The intracranial circulation is not visualized because the contrast is diluted by collateral flow. ACA indicates anterior cerebral artery.
Statistical Analysis
Statistical analyses were performed using SAS software, Version 9.4 (SAS Institute). Data are presented as means (SD) or median and interquartile range or counts and proportions. The Student t test or Mann-Whitney U test was used to evaluate the difference among continuous variables, and the Fisher exact test was used for the evaluation among categoric variables. Kaplan-Meier survival analysis was used to estimate long-term survival in patients after CAS with 95% confidence intervals. Given the inherent differences between patients with near-occlusion and the control group, using the logit model, we calculated propensity scores for the following variables as covariates: age, sex, total plasma cholesterol, diabetes, previous percutaneous coronary intervention, and ipsilateral cerebral ischemic symptoms in the past 6 months. Other potential covariates were confirmed as nonsignificant or highly correlated to other variables. Matching on the propensity scores was performed using the 1:2 nearest neighbor method without replacement.17,18 A 2-sided P value ≤ .05 was considered to indicate statistical significance.
RESULTS
A total of 544 patients were analyzed, 84 (15%) in the near-occlusion group and 460 (85%) in the control group. Propensity score matching yielded 84 subjects in the near-occlusion group matched with 168 subjects in the control group (Table 1 and Online Supplemental Data). Patients in the unmatched cohort with near-occlusion had symptomatic stenosis significantly more often, higher median C-reactive protein levels, used less statin therapy, had longer fluoroscopic times, and more often required predilation (P < .05 for all) (Table 1 and Online Supplemental Data). After matching, patients with near-occlusion had significantly higher median C-reactive protein levels and longer fluoroscopy time and more often required predilation (P < .05 for all) (Table 1 and Online Supplemental Data).
Data on major adverse in-hospital and 30-day events were available in 544 (100%) and 527 (97%) patients, respectively. Vital status at the end of the follow-up was available in all patients from the National Death Index. In the unmatched cohort, the primary end point occurred in 7 (8.3%) patients in the near-occlusion group and 19 (4.1%) patients in the control group (P = .101) and in the matched cohort in 7 (8.3%) and 11 (6.6%) patients (P = .611). Individual components of the end point are summarized in Table 2. In the matched subgroup of symptomatic patients, the primary end point occurred in 4 (10.5%) patients in the near-occlusion group and 7 (9%) patients in the control group (P = .748). In the asymptomatic patients, the primary end point occurred in 3 (6.5%) patients in the near-occlusion group and 4 (4.4%) patients in the control group (P = .688). The results for the symptomatic and asymptomatic patients are summarized in the Online Supplemental Data.
Table 2:
Thirty-day major adverse events and long-term follow-up
| Unmatched Cohort |
Matched Cohort |
|||||
|---|---|---|---|---|---|---|
| Near-Occlusion (n = 84 Patients) | Control Group (n = 460 Patients) | P Value | Near-Occlusion (n = 84 Patients) | Control Group (n = 168 Patients) | P Value | |
| TIA during hospitalization (No.) (%) | 6 (7.1) | 12 (2.6) | .045 | 6 (7.1) | 7 (4.2) | .368 |
| A, Minor stroke during hospitalization (No.) (%) | 3 (3.6) | 5 (1.1) | .111 | 3 (3.6) | 3 (1.8) | .404 |
| B, Major stroke during hospitalization (No.) (%) | 1 (1.2) | 6 (1.3) | 1.000 | 1 (1.2) | 4 (2.4) | .668 |
| Hyperperfusion syndrome during hospitalization (No.) (%) | 0 | 2 (0.4) | 1.000 | 0 | 0 | |
| Myocardial infarction during hospitalization (No.) (%) | 1 (1.2) | 0 | .154 | 1 (1.2) | 0 | .333 |
| Death during hospitalization (No.) (%) | 2 (2.4) | 2 (0.4) | .115 | 2 (2.4) | 0 | .110 |
| C, Minor stroke in 30-day hospitalization (No.) (%) | 0 | 4 (0.9) | 1.000 | 0 | 2 (1.2) | .554 |
| D, Major stroke in 30-day hospitalization (No.) (%) | 1 (1.2) | 2 (0.4) | .396 | 1 (1.2) | 1 (0.6) | 1.000 |
| E, Death in 30 days (including during hospitalization) (No.) (%) | 3 (3.6) | 3 (0.7) | .050 | 3 (3.6) | 1 (0.6) | .109 |
| Primary end point: A + B + C + D + E (No.) (%) | 7 (8.3) | 19 (4.1) | .101 | 7 (8.3) | 11 (6.6) | .611 |
| Re-intervention for restenosis during follow-up (No.) (%) | 9 (10.7) | 19 (4.1) | .026 | 9 (10.7) | 6 (3.6) | .044 |
| All-cause mortality during follow-up (No.) (%) | 33 (39.3) | 202 (43.9) | .473 | 33 (39.3) | 76 (45.2) | .419 |
| Cardiovascular death (No.) (%) | 25 (29.8) | 129 (28.0) | .792 | 25 (29.8) | 53 (31.6) | .885 |
| Noncardiovascular death (No.) (%) | 6 (7.1) | 64 (13.9) | .110 | 6 (7.1) | 19 (11.3) | .374 |
| Unknown death (No.) (%) | 2 (2.4) | 9 (2.0) | .682 | 2 (2.4) | 4 (2.4) | 1.000 |
| Mortality per 100 patient-years | 6.6 | 6.8 | 6.6 | 6.5 | ||
Note:—TIA indicates transient ischemic attack.
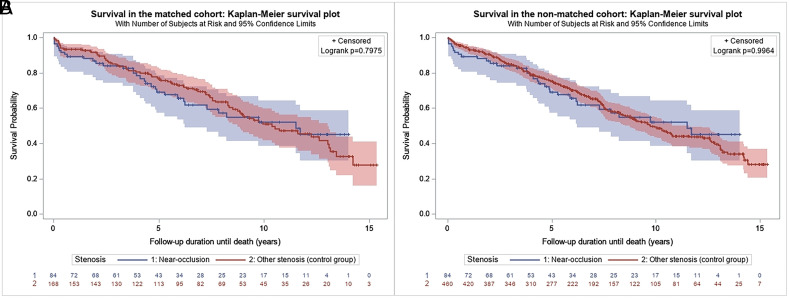
In the unmatched cohort, the mean follow-up was 5.9 (SD, 4.1) years in the near-occlusion group and 6.5 (SD, 4.1) years in the control group, which yielded 499 and 2980 patient-years of follow-up. Thirty-three (39.3%) patients in the near-occlusion group and 202 (43.9%) patients in the control group died (P = .473), which translated into 6.6 and 6.8 deaths per 100 patient-years, respectively. In the matched cohort, the mean follow-up was 5.9 (SD, 4.1) years in the near-occlusion group and 7.0 (SD, 4.2) years in the control group, which yielded 499 and 1176 patient-years of follow-up. In the matched cohort, 33 (39.3%) patients in the near-occlusion group and 76 (45.2%) patients in the control group died (P = .419), which translated into 6.6 and 6.5 deaths per 100 patient-years, respectively. In the matched cohort, survival in the near-occlusion group versus the control group at 5 and 10 years was 69.8% (95% CI, 58.0%–78.8%) versus 77.3% (95% CI, 70.0%–83.1%) and 53.3% (95% CI, 39.9%–65.0%) versus 53.3% (95% CI, 44.5%–61.4%) (log-rank, P = .798) (Fig 2A). In the unmatched cohort, survival at 5 and 10 years was similar (log-rank, P = .996) (Fig 2B).
FIG 2.
Kaplan-Meier estimate of 10-year survival after carotid stent placement in the near-occlusion-versus-control groups in the matched (A) and unmatched (B) cohorts.
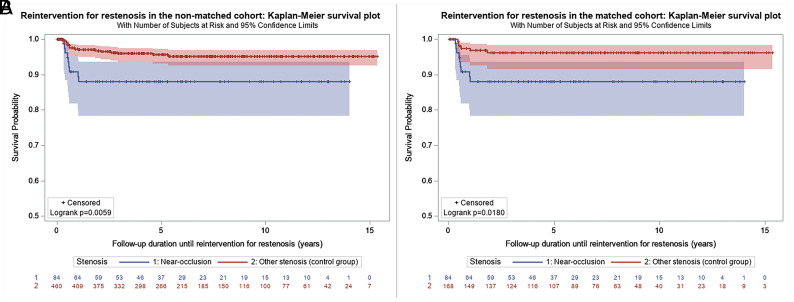
During the follow-up, 9 (10.7%) patients and 19 (4.1%) patients underwent re-intervention for in-stent restenosis in the near-occlusion and control groups, respectively (P = .026) (Fig 3A). After matching, the difference was still statistically significant, 10.7% versus 3.6% (P = .044) (Fig 3B).
FIG 3.
Kaplan-Meier estimate of freedom from re-intervention for in-stent restenosis after carotid stent placement in the near-occlusion-versus-control groups in the unmatched (A) and matched (B) cohorts.
DISCUSSION
Results of our observational study suggest that patients with near-occlusion had a high incidence of stroke or death within 30 days after CAS (8.3%) and high annual mortality (6.7%). However, the risk was not significantly different from that of severe stenosis after the adjustment using the propensity score matching. Although we observed a trend toward higher periprocedural risk in the near-occlusion group, which was numerically 2-fold, it did not reach statistical significance. The difference in the primary end points was even smaller after the adjustment. We believe that this difference is due to the higher proportion of symptomatic patients in the near-occlusion group. However, it could be related to the number of patients in the registry and confounders. The NASCET and European Carotid Surgery Trial included 262 cases of near-occlusion.3,7 The risk of perioperative stroke and death was similar in near-occlusion (5.4%) and 70%–99% stenosis (6.2%),19 and lower periprocedural risks have been reported since then.11 Later, trials comparing endarterectomy and CAS either excluded patients with near-occlusion or did not assess near-occlusion. The real-world registry might provide valuable information about the early risk of CAS for near-occlusion in a population of patients with high cardiovascular comorbidity that is not typically represented in randomized trials. Given the differences in the population of patients with near-occlusion and conventional severe stenosis and a most notably higher incidence of symptomatic stenosis in the near-occlusion group, we decided to compare the groups with the propensity score matching analysis to balance the baseline characteristics in both groups and lower the risk of selection bias.
The high periprocedural risk of stroke in ICA near-occlusion might be explained by the slow flow of blood distal to the lesion, which promotes in situ thrombosis that is embolized during CAS or protrudes through the stent struts and embolizes in the early postprocedural period. Angiography might not be able to distinguish ruptured atherosclerotic plaque with mural thrombus or mural thrombus in the distal ICA. Some cases of near-occlusion are actually recanalized thrombotic occlusions. Indeed, Hirata et al20 reported that those old, organized thrombi were more frequently found in the endarterectomy specimens from near-occlusions than in high-grade stenoses. In our study, the 30-day stroke or death rate in the near-occlusion group (8.3%) was higher than that in a recent meta-analysis of some 703 patients with near-occlusion (226 underwent CAS; the 30-day stroke or death rate was 2.2%).11 Individual observational studies included in the meta-analysis reported a 30-day stroke or death rate after CAS between 0% and 7%.21-25 These studies had only 1 arm and did not compare the risk of CAS for near-occlusion with that in a control group. The high variability in the reported periprocedural risks might reflect different baseline characteristics and selection of patients rather than procedural techniques. It is also possible that the periprocedural stroke and death risk reported in the meta-analysis by Meershoek et al11 underestimated the true risk because of bias in the reporting of small observational studies with unfavorable results.
The high all-cause mortality rate in our study is in contrast to the much lower mortality in the long-term follow-up of a recent, large, randomized Second Asymptomatic Carotid Surgery Trial (ACST-2: 330 deaths in 1811 patients randomized to CAS with a mean follow-up of 5 years and an annual mortality of ≈3.6%).26 On the other hand, a study that analyzed data from Medicare beneficiaries treated with CAS reported a mortality rate of 8.0% per year after the periprocedural period.27 This study included older patients than in our registry, but other baseline characteristics were similar, with high cardiovascular comorbidity. These differences indicate that clinical trials enroll populations different from high-risk patients in whom the investigated technique is used in clinical practice.
We should carefully consider performing CAS in a patient with ICA near-occlusion, given the substantial periprocedural risk and life expectancy that might be shorter than 5 years reported by the European Society of Cardiology (ESC) and the European Society for Vascular Surgery (ESVS).28 Meershoek et al29 suggested that although the initial approach to symptomatic near-occlusion with full distal luminal collapse should be conservative, patients with recurrent events may be treated with endarterectomy. There is a need for further studies that could be based on large registries like the mandatory German Carotid National Registry.30 We believe that the treatment of patients with ICA near-occlusion is one of the important topics in the field of carotid interventions that will require continued research before more evidence-based recommendations can be made.
The study is not without limitations. First, data were collected from a single center for 16 years. Although operators and procedural techniques remained unchanged, protection devices and stents changed across time. Referral of patients for carotid angiography and the criteria for selection of patients who would benefit from CAS changed during that time, with updated ESC/ESVS guidelines and results of randomized controlled trials.26,28 The proportion of patients with near-occlusion and conventional stenosis who were selected for CAS compared with endarterectomy or medical therapy is unknown. Our results cannot be generalized to all patients with ICA near-occlusion. Second, propensity score matching is associated with inherent limitations. Although the matched cohort might seem well-balanced for baseline characteristics, there is always a risk of confounding bias. Third, we did not systematically collect data on major adverse cardiovascular events beyond the 30-day period after CAS; therefore, we reported only data on long-term survival.
CONCLUSIONS
Carotid stent placement in patients with ICA near-occlusion was not associated with an increased 30-day risk of stroke or death compared with severe stenosis. Survival up to 10 years after CAS was similar in both groups.
ABBREVIATIONS:
- CAS
carotid artery stenting
- ECA
external carotid artery
- ESC
European Society of Cardiology
- ESVS
European Society for Vascular Surgery
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Fox AJ, Eliasziw M, Rothwell PM, et al. Identification, prognosis, and management of patients with carotid artery near occlusion. AJNR Am J Neuroradiol 2005;26:2086–94 [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson E, Fox AJ. Carotid near-occlusion: a comprehensive review, Part 1: definition, terminology, and diagnosis. AJNR Am J Neuroradiol 2016;37:2–10 10.3174/ajnr.A4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgenstern LB, Fox AJ, Sharpe BL, et al. The risks and benefits of carotid endarterectomy in patients with near occlusion of the carotid artery: North American Symptomatic Carotid Endarterectomy Trial (NASCET) group. Neurology 1997;48:911–15 10.1212/WNL.48.4.911 [DOI] [PubMed] [Google Scholar]
- 4.Bartlett ES, Walters TD, Symons SP, et al. Quantification of carotid stenosis on CT angiography. AJNR Am J Neuroradiol 2006;27:13–19 [PMC free article] [PubMed] [Google Scholar]
- 5.Ringelstein EB, Berg-Dammer E, Zeumer H. The so-called atheromatous pseudo-occlusion of the internal carotid artery: a diagnostic and therapeutical challenge. Neuroradiology 1983;25:147–55 10.1007/BF00455734 [DOI] [PubMed] [Google Scholar]
- 6.O’Leary DH, Mattle H, Potter JE. Atheromatous pseudo-occlusion of the internal carotid artery. Stroke 1989;20:1168–73 10.1161/01.str.20.9.1168 [DOI] [PubMed] [Google Scholar]
- 7.Rothwell PM, Gutnikov SA, Warlow CP. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke 2003;34:514–23 10.1161/01.STR.0000054671.71777.C7 [DOI] [PubMed] [Google Scholar]
- 8.Bonati LH, Kakkos S, Berkefeld J, et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur Stroke J 2021;6:I–XLVII 10.1177/23969873211012121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: a Guideline From the American Heart Association/American Stroke Association. Stroke 2021;52:e364–467 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 10.Johansson E, Fox AJ. Carotid near-occlusion: a comprehensive review, Part 2: prognosis and treatment, pathophysiology, confusions, and areas for improvement. AJNR Am J Neuroradiol 2016;37:200–04 10.3174/ajnr.A4429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meershoek AJ, de Vries EE, Veen D, et al. ; NEON study group. Meta-analysis of the outcomes of treatment of internal carotid artery near occlusion. Br J Surg 2019;106:665–71 10.1002/bjs.11159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veselka J, Hajek P, Štěchovský C, et al. Long-term survival of carotid stenting patients with regard to single- or double-vessel carotid artery disease: a propensity score matching analysis. Arch Med Sci 2021;17:849–55 10.5114/aoms.2020.98167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Štěchovský C, Hájek P, Horváth M, et al. Near-infrared spectroscopy combined with intravascular ultrasound in carotid arteries. Int J Cardiovasc Imaging 2016;32:181–88 10.1007/s10554-015-0687-x [DOI] [PubMed] [Google Scholar]
- 14.Štěchovský C, Hájek P, Horváth M, et al. Effect of stenting on the near-infrared spectroscopy-derived lipid core burden index of carotid artery plaque. Eurointervention 2019;15:e289–96 10.4244/EIJ-D-17-01054 [DOI] [PubMed] [Google Scholar]
- 15.Veselka J, Špaček M, Horváth M, et al. Impact of coexisting multivessel coronary artery disease on short-term outcomes and long-term survival of patients treated with carotid stenting. Arch Med Sci 2016;12:760–65 10.5114/aoms.2016.60964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veselka J, Cerná D, Zimolová P, et al. Feasibility, safety, and early outcomes of direct carotid artery stent implantation with use of the FilterWire EZ Embolic Protection System. Catheter Cardiovasc Interv 2009;73:733–38 10.1002/ccd.21936 [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. The American Statistican 1985;39:33–38 10.1080/00031305.1985.10479383 [DOI] [Google Scholar]
- 18.Fraeman KH. An introduction to implementing propensity score matching with SAS. NESUG 2010: Applications Development. https://www.lexjansen.com/nesug/nesug10/ad/ad05.pdf Accessed September 07, 2021
- 19.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107–16 10.1016/S0140-6736(03)12228-3 [DOI] [PubMed] [Google Scholar]
- 20.Hirata Y, Sakata N, Inoue T, et al. Histopathological features with angiographic correlates of internal carotid artery pseudo-occlusion: impact of plaque compositions—clinical article. J Neurosurg 2011;115:350–58 10.3171/2011.3.JNS101434 [DOI] [PubMed] [Google Scholar]
- 21.Oka F, Ishihara H, Kato S, et al. Cerebral hemodynamic benefits after carotid artery stenting in patients with near occlusion. J Vasc Surg 2013;58:1512–17 10.1016/j.jvs.2013.05.103 [DOI] [PubMed] [Google Scholar]
- 22.González A, Gil-Peralta A, Mayol A, et al. Internal carotid artery stenting in patients with near occlusion: 30-day and long-term outcome. AJNR Am J Neuroradiol 2011;32:252–58 10.3174/ajnr.A2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda Y, Terada T, Okada H, et al. Angiographic characteristics of pseudo-occlusion of the internal carotid artery before and after stenting. Neurosurgery 2016;79:832– 38 10.1227/NEU.0000000000001345 [DOI] [PubMed] [Google Scholar]
- 24.Son S, Choi DS, Kim SK, et al. Carotid artery stenting in patients with near occlusion: a single-center experience and comparison with recent studies. Clin Neurol Neurosurg 2013;115:1976–81 10.1016/j.clineuro.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto S, Kiura Y, Kajihara Y, et al. Carotid artery stenting using the proximal or dual protection method for near occlusion of the cervical internal carotid artery. Neurosurg Rev 2013;36:551–58 10.1007/s10143-013-0481-y [DOI] [PubMed] [Google Scholar]
- 26.Halliday A, Bulbulia R, Bonati LH, et al. ACST-2 Collaborative Group. Second asymptomatic carotid surgery trial (ACST-2): a randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet 2021;398:1065–73 10.1016/S0140-6736(21)01910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jalbert JJ, Nguyen LL, Gerhard-Herman MD, et al. Outcomes after carotid artery stenting in Medicare beneficiaries, 2005 to 2009. JAMA Neurol 2015;72:276–86 10.1001/jamaneurol.2014.3638 [DOI] [PubMed] [Google Scholar]
- 28.Aboyans V, Ricco JB, Bartelink MEL, ESC Scientific Document Group, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European Stroke Organization (ESO), the Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816 10.1093/eurheartj/ehx095 [DOI] [PubMed] [Google Scholar]
- 29.Meershoek AJ, Vonken EP, Nederkoorn PJ, et al. Carotid endarterectomy in patients with recurrent symptoms associated with an ipsilateral carotid artery near occlusion with full collapse. J Neurol 2018;265:1900–05 10.1007/s00415-018-8939-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kallmayer MA, Tsantilas P, Knappich C, et al. Patient characteristics and outcomes of carotid endarterectomy and carotid artery stenting: analysis of the German mandatory national quality assurance registry - 2003 to 2014. J Cardiovasc Surg (Torino) 2015;56:827–36 [PubMed] [Google Scholar]