Abstract
BACKGROUND AND PURPOSE:
Papillary craniopharyngiomas (PCPs) are particularly challenging lesions requiring accurate diagnosis to plan the best therapy. Our aim was to define a narrow duct-like recess identified on MR imaging at the base of papillary craniopharyngiomas with a strict third ventricle location.
MATERIALS AND METHODS:
A duct-like recess at the infundibular portion of craniopharyngiomas was observed on conventional T1WI and T2WI in 3 strict third ventricle papillary craniopharyngiomas in our craniopharyngioma series (n = 125). We systematically investigated this finding on the MR imaging of 2582 craniopharyngiomas and 10 other categories of third ventricle tumors (n = 690) published in the modern era (1986–2020). The diagnostic value and significance of this finding are addressed.
RESULTS:
The duct-like recess was recognized in 52 papillary craniopharyngiomas, including 3 of our own cases, as a narrow canal-shaped cavity invaginated at the tumor undersurface, just behind the optic chiasm. This structure largely involves papillary craniopharyngiomas with a strict third ventricle topography (96%), follows the same diagonal trajectory as the pituitary stalk, and finishes at a closed end. The duct-like recess sign identifies the papillary craniopharyngioma type with a specificity of 100% and a sensitivity of 38% in the overall craniopharyngioma population. This finding can also establish the strictly intra-third ventricle location of the lesion with a 90% specificity and 33% sensitivity. These recesses appear as hypointense circular spots on axial/coronal T1WI and T2WI. Their content apparently corresponds to CSF freely flowing within the suprasellar cistern.
CONCLUSIONS:
The presence of a duct-like recess at the infundibular portion of a third ventricle tumor represents a distinctive hallmark of papillary craniopharyngiomas that can be used as a simple MR imaging sign to reliably diagnose these lesions.
Papillary craniopharyngiomas (PCPs) comprise >10%–20% of all craniopharyngioma (CP) cases, affect almost exclusively adult patients, and typically grow within the third ventricle (3V) as purely solid masses or unilocular cysts lined with papillomatous excrescences of stratified squamous epithelium.1 From a surgical viewpoint, PCPs are a particularly challenging group of lesions owing to their intra-3V location and tenacious attachment to the infundibulo-tuberal region of the hypothalamus.2 The discovery of BRAF V600E oncogene mutations specific to this CP type has prompted the use of targeted chemotherapies with preliminary, successful results.3 Therefore, an accurate preoperative diagnosis of PCPs is essential for planning a proper surgical approach, or, alternatively, choosing nonsurgical therapies such as radiosurgery or chemotherapy.
During the past decade, our group has systematically analyzed the most pertinent information from conventional MR imaging to make reliable preoperative diagnoses of the PCP variant and to accurately define the tumor topography and adherence.4,5 After thoroughly examining MR images of >3000 CP records, we have identified some specific morphologic features predictive of the papillary type, such as tumor consistency, anatomic appearance of the pituitary stalk (PS), and the position of the hypothalamus relative to the tumor.5 In the present work, we describe and characterize an additional specific MR imaging sign identified in a subgroup of PCPs primarily developed within the 3V: a duct-like recess (DR) extending from the undersurface of the lesion toward its center and connected to the suprasellar cistern. This MR imaging finding, clearly distinguishable on midsagittal and coronal transinfundibular T1WI and T2WI, has never been observed in adamantinomatous CPs (ACPs) or in other pathologic categories of 3V tumors. Consequently, we consider that it constitutes a distinctive sign of the PCP type. The present work morphologically and neuroradiologically characterizes this CP finding and analyzes its potential diagnostic significance.
MATERIALS AND METHODS
DR Sign in Craniopharyngiomas: MR Imaging Definition
The DR is defined as a narrow and hollow duct-like cavity invaginated at the infundibular portion of a 3V tumor that follows a diagonal trajectory toward the center of the lesion through its midsagittal plane. DRs have a variable length, and they are closed at the top end and open at the bottom and show the same homogeneous hypointense signal on T1WI and hyperintensity on T2WI sequences as CSF, which can apparently flow freely between the suprasellar cistern and these tubular structures.
Case Series Generation and Inclusion Criteria
We conducted a systematic review of the well-described CP cases reported in the MR imaging era, from 1984 to June 2020 (n = 3352), according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (Online Supplemental Data). This study involved CPs reported in journals from the PubMed and MEDLINE databases (n = 2036) and CPs discussed either in specialized monographs (n = 796) or in professional Web pages (n = 520) focused on the treatment of pituitary/3V tumors. Likewise, an exhaustive search was made for primarily 3V tumors different from CPs published between 1984–2020 (n = 690), including 10 major pathologic categories detailed in the Online Supplemental Data. Finally, the medical charts and MR imaging studies of CPs treated from 1994 to 2020 in the authors’ departments of neurosurgery (n = 125; 106 ACPs/19 PCPs) were retrospectively examined.
From these databases, 2582 well-depicted CPs were deemed eligible for neuroradiologic assessment, among them 2426 ACPs and 156 PCPs, according to the following criteria: 1) Craniopharyngioma diagnosis was confirmed pathologically (CP histology verified in 81% of ACPs and 77% of PCPs, the remaining 23% showing MR imaging macroscopic features typical of the papillary type); 2) Preoperative midsagittal and/or coronal transinfundibular MR images of the lesion obtained with T1WI and/or T2WI sequences were given in the scientific report/Web page/clinical chart showing the presence of a DR at the tumor base; 3) A brief description of epidemiologic/clinical/pathologic and/or surgical findings of the case was provided. On the basis of these criteria, a collection of 52 CPs with a basal duct-like recess reported from 1994 to 2020 was finally included in this cohort, named the CPs with basal duct-like recess cohort (CP-DR).
Assessment of Histologic and Topographic Diagnostic Accuracy of the DR Sign
With the aim of ascertaining the diagnostic accuracy of the DR sign identified on MR imaging to establish the papillary histology and the strict 3V CP topography, we tested the specificity and sensitivity of this finding in the cohort of 200 CPs used by our group in 2017 to analyze the MR imaging reliability for the definition of the CP topography (CP MR imaging 200 control cohort).5 This series, which formed part of the data bank of 2582 CPs examined for the present study, included 14 CP-DR cases and had the advantage of having a balanced distribution of CP histologies (163 ACPs/37 PCPs), patient ages (148 adults/37 pediatric cases), and CP topographies (47% sellar/suprasellar; 32% infundibulotuberal; 21% strict 3V), all of these rates being similar to those observed in the overall CP population.
In addition, the diagnostic reliability of the DR MR imaging sign for establishing the papillary type was evaluated in a cohort of 106 strictly 3V CPs (CP strict 3V 106 control cohort) obtained from our recent review of the CP cases reported with a verified strictly 3V topography.6 This cohort, formed of 74 PCPs and 32 ACPs, included 49 CP-DR cases. Similarly, the DR MR imaging sign specificity and sensitivity for accurately classifying the strictly 3V tumor location were tested in a large cohort of 142 papillary CPs (PCP 142 control cohort) diagnosed with MR imaging and obtained from our recent comprehensive research on 350 well-described PCPs from the medical literature, which included 50 CP-DR cases.1
Comparison Cohort of CPs without Basal DR
To differentiate the clinicopathologic and MR imaging features of the tumors in the CP-DR cohort, formed of only adult patients, from the general population of adult CPs, a comparison cohort of adult CPs without a basal DR was created (CP-nDR). This comparison cohort formed part of the 2582 CP data bank eligible for this study and was gathered from our prior studies analyzing the MR imaging signs defining CP topography and adherence by arbitrarily selecting the consecutive 52 well-described CPs without a DR published in the most recent period (2004–2016).4,7 These cases met the same inclusion criteria as those defined for the CP-DR, except for the presence of a DR. Systematic bivariate analyses were then performed for clinicopathologic and MR imaging characteristics differentiating the CP-DR and the CP-nDR cohorts.
RESULTS
DR in Papillary CPs: MR Imaging Characteristics
The Online Supplemental Data present the main clinicopathologic and neuroradiologic features of the 52 CPs with a DR identified on preoperative MR images in the medical literature, including the 3 new cases from our own series. The DR can be observed on routine T1WI and T2WI midsagittal sections as a narrow hollow canal-shaped structure with a diagonal upward course along the midsagittal plane of the CP (Fig 1). All recesses follow the same diagonal trajectory as the PS and are open at the tumor undersurface, just at the area of the third ventricle floor (3VF), where the PS joins the infundibulum, connecting the recess cavity with the suprasellar or chiasmatic cistern. All DRs appear homogeneously hypointense on T1WI (Figs 1) and homogeneously hyperintense on T2WI (Fig 4B), with an intensity identical to that of the CSF, a sign indicating that CSF may flow freely into and out of the DR.
FIG 1.
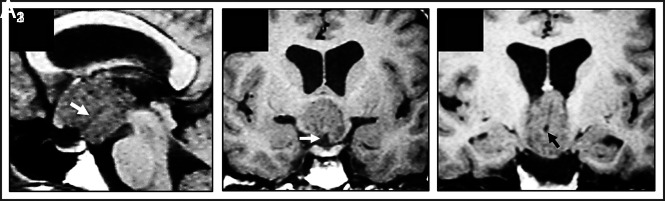
Basal recess in strictly 3V CPs of the papillary type: MR imaging evidence in 1 case identified from our series. Case 1 (Online Supplemental Data): This papillary CP was diagnosed in a 34-year-old woman with headaches, Fröhlich syndrome, poikilothermy, and emotional lability. Preoperative T1WI shows this strictly 3V homogeneously isointense lesion above the intact 3VF, with papillomatous excrescences at its surface. On the midsagittal (A1) and coronal (A2, A3) scans, a long basal recess can be identified as a tube-like hypointense structure extending from the suprasellar cistern to the center of the tumor (white arrows, A1 and A2; black arrow, A3).
FIG 4.
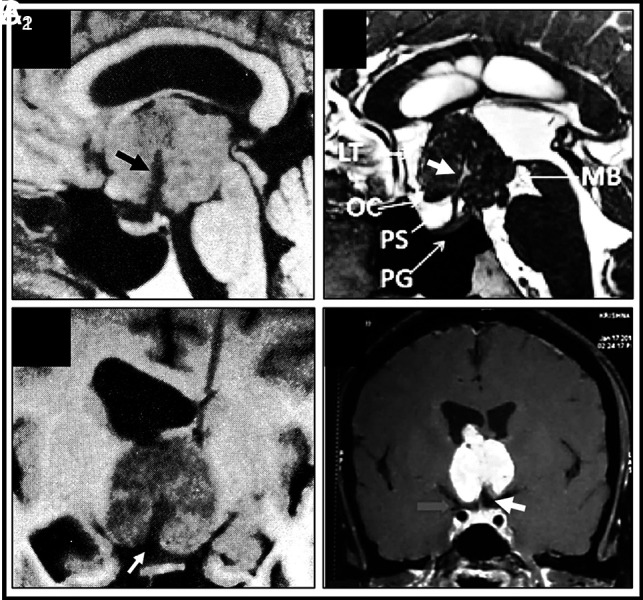
Basal recess at the infundibular portion of 3V papillary craniopharyngiomas. MR imaging characterization in seminal cases from the medical literature (A1–A2). Diverticulum-like recess at the base of a strict 3V papillary CP identified on preoperative MR imaging (Online Supplemental Data). On the midsagittal T1WI (A1), the hypointense recess (black arrowhead) is open at the suprasellar cistern and extends to the tumor center, following the same trajectory as the PS. The coronal-transinfundibular MR image (A2) shows the hypointense signal of the recess identical to CSF, indicating free passageway of CSF between the suprasellar cistern and the recess and the rounded dead end of the recess at the tumor center. Reproduced with permission from the Fukushima et al.8 B, Duct-like long-recess type in a strict papillary CP (Online Supplemental Data). This midsagittal T2WI shows the hyperintense signal of the duct, identical to that in the CSF (white arrow). Reproduced with permission Gu et al.21 C, Coronal-transinfundibular T1WI of a short-type basal DR in a strict 3V PCP (Online Supplemental Data). Note the isointense rim of the 3V floor covering the undersurface of this solid homogeneously gadolinium-enhancing tumor and how the 3VF invaginates at the midline of the basal pole of the CP to form the recess (white arrow). Reproduced with permission from Rambarki and Rajesh.22 PG indicates pituitary gland; MB, mammillary body; OC, optic chiasm.
According to their length, DRs can be classified into 2 major types: long DRs (Fig 1A1), with a length ranging between 10 and 15 mm (n = 33, 63.5%) and short DRs (Fig 4C), which invaginate into the tumor ≤5 mm (n = 19, 36.5%). No DR enhancement after gadolinium administration was observed in any of the cases, nor were any recognizable vascular structures identified, except in 1 case (with gadolinium). On coronal/axial MR imaging sections, all recesses have a circular outline and are engulfed by the central bulk of the tumor (Fig 1A3). From all these MR imaging data, the recesses seem to represent tubular invaginations of the 3VF infundibular region into the base of 3V CPs.
CP-DR Cohort: MR Imaging Characterization
The Online Supplemental Data show the categorization of the main clinicopathologic, neuroradiologic, and surgical variables analyzed in the CP-DR cohort. Contrary to the balanced sex division and age distribution in pediatric/adult cases in the general CP population, all CP-DR cases occurred in patients older than 20 years of age, affecting mostly men (73%) (Fig 2A). Clinically, headache was the most frequent symptom (75%), and it showed an almost perfect correlation with the presence of obstructive hydrocephalus (71%). Notably, the rates of psychiatric disturbances (58%) and other hypothalamic alterations (20.5%) were markedly high, in accordance with the expansion of these lesions within the 3V, causing mass effect on the hypothalamus. Conversely, visual defects and endocrine deficits due to hypopituitarism were described in a relatively small number of patients, given the preserved anatomic integrity of the optic chiasm and PS in many cases.
FIG 2.
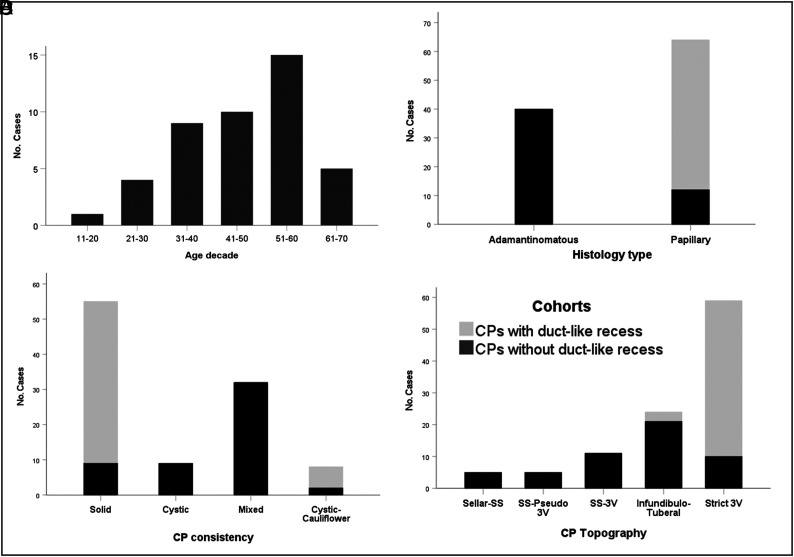
Epidemiologic, pathologic, and topographic characterization of the 52-craniopharyngioma cohort with a basal recess (CP-DR cohort). A, Bar chart shows the age distribution. Observe that no cases were diagnosed in the first decade. About 65% of cases occurred in adults between 30 and 60 years of age. B, Stacked bar chart comparing the distribution of histologic CP types between the CP-DR and the CP-nDR cohorts. All tumors with a DR belonged to the squamous-papillary type, only diagnosed in 23% of CPs without a DR in the adult CP population (P < .001). C, Stacked bar graph comparing tumor consistencies. Most CP-DR tumors had a homogeneous solid consistency (90%), whereas a mixed solid-cystic pattern was predominant among ACPs without a DR (61.5%) (P < .001). D, Topographic distribution of tumors. More than 80% of papillary CPs with a DR corresponded to strictly 3V tumors located above an anatomically intact 3VF. No CP-DR lesions originated at the sellar or suprasellar compartments below the 3VF (P < .001).
Morphologically, we differentiated 2 main tumor patterns: 1) the most common group of pure solid and round lesions, homogeneously isointense on T1WI, showing homogeneous gadolinium enhancement (90%); and 2) the less frequent subgroup of unilocular cystic lesions with a cauliflower-like solid nodule at the base of the cyst (Fig 2C). Both macroscopic architectures markedly differed from the multilocular, solid-cystic consistency usually found in ACPs and were certainly influenced by the papillary histology and the intra-3V development of all CP-DRs (Fig 2B, -D). Histologic confirmation of the papillary type was provided in all except 3 cases, which showed on MR imaging a gross berrylike morphology with papillary protrusions at the tumor surface, identical to all the other counterparts with a verified histology.
Diagnostic Accuracy of the DR Sign to Define the CP Histology and Topography
The DR occurred in 16% of PCPs in our own series (n = 125), a rate that increased to 27% when considering only the PCPs with a strict 3V topography. The DR was also present in 33% of the 156 PCPs with available MR imaging examined from the literature for this study. The specificity of the DR sign to define the papillary histology on MR imaging was 100% in both the CP MR imaging 200 and the CP strict 106 control cohorts. This finding validates the DR as a hallmark of PCPs. The sensitivity of this sign was 37.8% when tested in the CP MR imaging 200 control cohort and 66% in the CP strict 106 control cohort. This means that the finding of the DR alone permits the accurate diagnosis of the papillary histology in about 38% of PCPs spanning all kinds of topographies and in two-thirds of the PCPs with a strict 3V topography.
Concerning the definition of CP topography, the DR sign specificity for establishing the strictly 3V CP location was 100% in the CP MR imaging 200 control cohort and 90% in the CP papillary 142 control cohort, whereas the sensitivity rates for this strict 3V topography were 33.3% and 53%, respectively. This finding means that the DR sign by itself reliably allows the diagnosis of the strict 3V topography in one-third of CPs with such a topography and in more than half of all PCPs.
Pathologic and Topographic Differentiation of CPs with a Basal DR
The Table shows a comparative analysis of the clinical and anatomopathologic features that best differentiate the CP-DR cohort, formed exclusively of adult patients, and the CP-nDR comparison cohort, representative of the adult CP population. The essential pathologic characteristic differentiating both cohorts was the diagnosis of the papillary variant in 100% of the cases in the former (Fig 2B). This papillary architecture definitely influenced the predominant solid tumor consistency (Fig 2C) and lack of calcifications in CP-DR tumors. Second, most CP-DR cases primarily developed within the 3V, above an intact 3VF. This is an exceedingly rare topography among CPs, even among adult patients (Fig 2D). Such a pure intra-3V CP location determines the anatomic intactness of the pituitary-hypothalamic axis structures confirmed on preoperative MR imaging in many patients with CP-DR, in particular an anatomically intact PS in 84.5% of strictly 3V PCPs (Fig 3A). The position of the hypothalamus around the lower portion of the tumor on coronal transinfundibular MR images, observed in 74.5% of strict 3V cases, was also a defining feature of the CP-DR cohort (Fig 3C). However, even though these MR imaging signs strongly point to a strictly 3V CP location, the complete integrity of the 3VF beneath the tumor could only be confirmed in 61% of cases on preoperative MR imaging (Fig 3B). Notably, the identification of a DR yielded better results than all these aforementioned MR imaging signs to achieve an accurate topographic diagnosis of a strictly 3V CP in 96% of cases.
Differential characteristics between the CP-DR on MR imaging and a cohort of adults with CPs without such a structure
| CP Characteristics | Categories | CP-DR (n = 52) | Adult CP-nDR (n = 52) | P Value |
|---|---|---|---|---|
| Sex | Male | 73% | 50% | .012 |
| Female | 27% | 50% | ||
| Hydrocephalus | Present | 71% | 25.5% | <.001 |
| Headache | Present | 75% | 49% | .01 |
| Visual deficits | Present | 51% | 73.5% | .032 |
| Endocrine deficits | Present | 38.5% | 39% | NS |
| Hypothalamus disturbances | Present | 20.5% | 0% | .001 |
| Mental alterations | Present | 58% | 12% | <.001 |
| HTIC Sd | Present | 72% | 41% | .002 |
| Hypothalamic Sd | Present | 67.5% | 26.5% | <.001 |
| Tumor histology | Squamous-papillary | 100% | 23% | <.001 |
| Adamantinomatous | 0% | 77% | ||
| Tumor topography | Sellar/suprasellar | 0% | 40% | <.001 |
| Infundibulo-tuberal | 4% | 41% | ||
| Strictly 3V | 96% | 19% | ||
| Calcifications | Present nodular | 0% | 58% | <.001 |
| Tumor consistency | Pure solid | 90% | 17% | <.001 |
| Cystic-cauliflower | 10% | 4% | ||
| Pure cystic | 0% | 17.5% | ||
| Mixed solid-cystic | 0% | 61.5% | ||
| Sella turcica | Occupied by tumor | 0% | 31% | <.001 |
| PS status | Intact | 84.5% | 15.5% | <.001 |
| Lower portion visible | 15.5% | 27% | ||
| Infiltrated/invaded | 0% | 57.5% | ||
| Suprasellar cistern status | Tumor-free | 44% | 31% | .001 |
| Partially occupied | 54% | 32.5% | ||
| Wholly occupied | 2% | 36.5% | ||
| Hypothalamus position | Around CP lower third | 74.5% | 13.5% | <.001 |
| Around CP middle third | 26.5% | 60% | ||
| Around CP upper third | 0% | 26.5% | ||
| 3VF status | Visible intact | 61% | 6% | <.001 |
| Only MBs visible | 27.5% | 33% | ||
| Not visible | 11.5% | 61% |
Note:—HTIC indicates high intracranial pressure; MBs, mammillary bodies; NS, not significant; Sd, syndrome.
FIG 3.
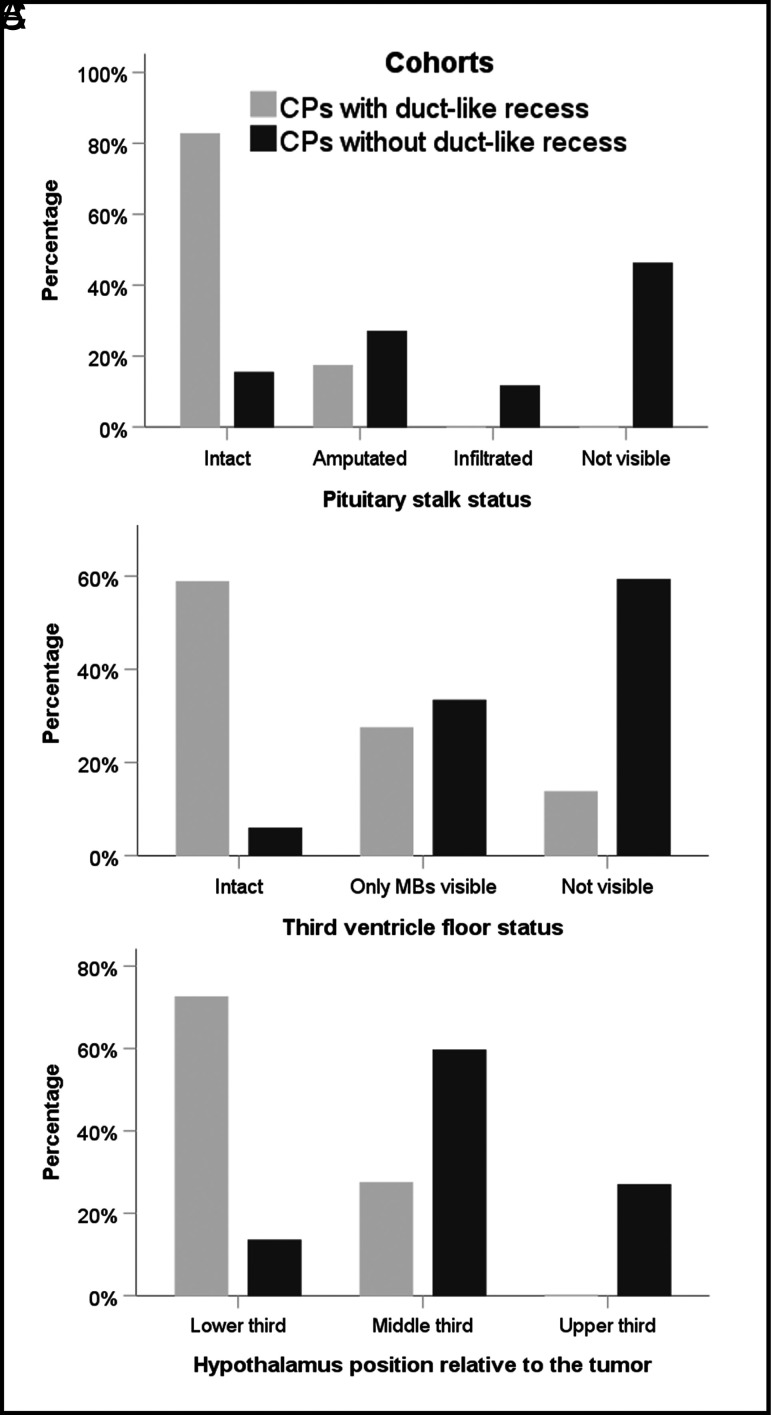
MR imaging characterization of the anatomic relationships between the tumor and the 3V in CPs with or without a DR. A, PS status. Among the lesions in the CP-DR cohort, the PS is observed intact under the basal recess (85%), whereas it is wholly or partially engulfed by the tumor in about 85% of adults with CPs without a DR (P < .001). B, Anatomic status of the 3VF. An intact 3VF was observed in 60% of CPs with a DR, whereas this structure cannot usually be distinguished from the tumor boundaries in CPs without a DR (P < .001). C, Bar graph shows the distribution of hypothalamus positions relative to the tumor. In almost 75% of papillary CPs with a DR, the hypothalamus is located around the lower third of the lesion. In contrast, it is observed encircling its central portion in 60% of adult CP cases without a DR (P < .001). MBs indicates mamillary bodies.
DISCUSSION
Basal DR: A Hallmark of Strictly 3V CPs of the Papillary Type
In 1990, Fukushima et al8 first noticed a linear hypointense zone on the midsagittal T1WI of an intraventricular PCP (Fig 4A1 and A2). A more detailed description of a similar tube-shaped basal hypointensity in a 3V PCP was provided by Urbach et al,9 who pointed out that it could represent a diagnostic sign specific to the solid papillary CP variant. No additional insights about the nature or diagnostic significance of this MR imaging sign could be gained from the medical literature. The papillary variant constitutes a minority of CPs (10%–20% of cases), usually developing at the infundibulum or entirely within the 3V.1,6 Radical excision of 3V PCPs is commonly associated with a high surgical risk, owing to the mass effect on the hypothalamus and tight tumor attachments to the 3VF.2,7 In addition, many PCPs affect elderly patients in poor clinical condition, precluding any complex neurosurgical procedure. Consequently, achieving a correct preoperative diagnosis of 3V PCPs is essential to plan the most judicious treatment to diminish the risk of surgical hypothalamic injury.10
A papillary histology can be suspected on preoperative MR imaging mainly by assessing some CP features, such as a spherical shape, a homogeneous solid consistency, and a lack of calcifications within the tumor.4,5,10,11 The strictly 3V topography can be even more difficult to ascertain because CP boundaries are usually fused to the 3VF, which can become an unrecognizable structure on preoperative MR imaging.4,6,10 Moreover, many other strictly 3V tumors such as chordoid gliomas, astrocytomas, germinomas, and ependymomas are round with a solid consistency similar to features of PCPs.12 Therefore, verifying a duct-like recess at the base of a 3V tumor represents a novel, valuable diagnostic sign, permitting not only the pathologic diagnosis of a PCP but also a reliable definition of a strictly 3V topography in about 90% of cases.
A DR in a CP represents a morphologic feature 100% specific to the papillary type, with the sensitivity of this finding ranging between 33% in the global PCP population and 66% in the subpopulation of CPs with a strict 3V topography. The papillary variant represents between 10% and 20% of all CP cases, but because this type almost exclusively affects adult patients, the PCP rate can reach a peak of >30% in the age interval between 30 and 80 years, as observed in the recent study by Momin et al.13 Given the CP incidence of between 1.3 and 1.6 cases/million people observed in the United States, about 600 new cases/year, the identification of the DR sign alone on a preoperative MR imaging could confirm the certain diagnosis of a PCP in approximately 45 tumors from the pool of around 140 PCPs diagnosed every year in the United States.13 Moreover, the DR is an invaluable MR imaging sign to establish the strict 3V location of the lesion, with a 90% specificity and a sensitivity ranging between 33% in the overall CP population and 53% in the subpopulation of PCPs. This topographic information is of the utmost importance for neurosurgeons to plan the proper surgical approach for lesions known to be wholly confined within the 3V.
CP-DR Cohort: Differences from the General Adult CP Population
The unique pathologic characteristics of the CP-DR cohort, formed exclusively of PCPs with a 3V topography, contrasted markedly with those observed in the overall population of adult CP cases, which largely consist of ACPs developed either beneath the 3V (sellar/suprasellar, 60%) or within the 3VF itself (infundibulo-tuberal, 40%) (Table). These crucial differences definitely influenced the significant morphologic and clinical discrepancies between both cohorts. Symptoms of hypothalamic impairment, including psychiatric and cognitive disturbances, were prominent among CP-DR lesions, which caused severe compression and edema in the hypothalamic region.1,2 By contrast, visual disturbances due to optic chiasm stretching, usually caused CPs originating beneath the 3V, predominated in the CP-nDR cohort. The prevailing 3V topography among CP-DR lesions also determined the high rate of obstructive hydrocephalus and the round shape and solid consistency of these PCPs, tumor features that differed from the typical solid-cystic multilobulated morphology observed among ACPs. Finally, PCP-DR tumors affected male patients more frequently, a tendency not present among ACPs, plausibly attributable to genetic causes and deserving further investigation.
DR: A Sign Differentiating PCPs from Other 3V Tumors
The identification on conventional MR imaging of a hollow recess at the infundibular midline region of a solid 3V tumor distinguishes a particular subpopulation of 3V PCPs. We were able to confirm this finding after methodically reviewing the MR images displayed in a total of 2582 CPs. The DR was never observed in ACPs nor among PCPs growing beneath the 3VF. Very important, after systematically reviewing the MR images of >500 3V tumors of various pathologic diagnoses other than CP, we were unable to find any similar duct-like structures (Fig 1). Among originally developing 3V tumors, neither chordoid gliomas (n = 128) nor ependymomas (n = 116) showed, in any of the reports examined, a DR structure resembling those observed in the PCPs of the present cohort, nor in any of the secondary 3V tumor categories investigated, such as germinomas (n = 73), lymphomas (n = 58), epidermoids (n = 36), and teratomas (n = 66), could a similar tubular recess be recognized. This finding suggests the existence of a pathogenetic mechanism specific for the presence of a DR in 3V PCPs.
The category of 3V choroid plexus papillomas (n = 84), lesions with a macroscopic papillary architecture mimicking PCPs, may show multiple fissures and crevices somewhat resembling the DR.14 The DR in PCPs could then be interpreted as a large crack or fissure formed by the natural process of dehiscence of the squamous epithelium that gives these lesions a pseudopapillary appearance.1 However, small cracks and fissures are randomly present all around the tumor surface in both choroid plexus papillomas and PCPs, whereas the DR shows a consistent tubular shape and a constant position at the PCP midline undersurface, following the same course as the PS, evidence that goes against this view. Rather, the morphologic DR characteristics suggest a pathogenetic link between the intra-3V location of CP precursors and the embryogenesis of the pituitary-hypothalamic axis.
DR in 3V PCPs: Anatomic Clues Pointing toward an Embryologic Pathogenesis
The hollow, duct-like cavity identified in 3V PCPs in this study can also be defined as a tumor recess, because it presumably corresponds to the intratumor extension of the suprasellar (subarachnoid) space, filled with CSF. Anatomically, the 3VF is shaped like 2 recesses, the chiasmatic recess, a V-shaped 3V extension above the optic chiasm, and the infundibular recess, the funnel-shaped 3V downward extension into the PS.15 This anatomy raises the question as to whether the DR may represent a residual, patent portion of the infundibular recess invaginated within the lesion. Such an interpretation seems highly implausible because tumors expanding within the 3V, including CPs, usually compress the 3VF downward and obliterate the 3V recesses rather than filling in these spaces.6 Alternatively, the DR could be interpreted as a fibrovascular core providing the blood supply to the tumor. However, the basal stemlike fibrovascular attachment feeding 3V PCPs has a solid structure, not a hollow one.1 No blood vessels within the DR could be identified on the MR images available in this cohort, with the exception of the case by Sartoretti-Sheffer et al,1 in which a narrow vessel running along the anterior aspect of the PS penetrates the DR.16 Instead, the duct-like shape of the CP recess, its variable length, and, above all, its diagonal trajectory following the same course as the PS axis suggest that the DR formation could be linked to the embryologic process of the pituitary axis development from the Rathke pouch.
Most PCPs with a basal recess have a strict 3V location. How a tumor apparently originating from remnants of an extraventricular structure such as the Rathke pouch could develop exclusively within the 3V, a portion of the closed neural tube, has been a source of puzzlement since strictly 3V CPs were first identified at postmortem examination.17 In the 1980s, Ciric and Cozzens18 theorized that strictly 3V CPs could originate from the Rathke pouch cells if this embryonic structure merged with the 3VF before the pia mater was formed.15 Indeed, a close attachment between the ventral neural tube and the roof of an embryo’s primitive mouth occurs at the earliest stages of pituitary gland organogenesis, and this union could explain the inclusion of CP ectodermal cell precursors within the 3V.18 The presence of a DR at the base of 3V PCPs supports a dysembryogenetic origin of this histologic variant, linking PCPs with the developing course of stomodeal precursors moving with the Rathke pouch along the migratory pathway followed by the craniopharyngeal canal.19 The DR extending upward into the 3V can be judged to be an equivalent of the duct-like downward passageway between the 3V and the sella turcica, known as a persisting embryonal infundibular recess, likewise thought to be the result of a failed obliteration of the infundibular recess during pituitary organogenesis.20
CONCLUSIONS
The presence of a DR at the infundibular portion of a solid 3V tumor represents an MR imaging sign diagnostic of the papillary CP type with a specificity of 100% and a sensitivity of 33% in the global population of PCPs. This finding is also a reliable MR imaging sign for establishing the strictly 3V topography, with a specificity of 90% and a sensitivity of 33% in the overall CP population. The DR can be easily identified on midsagittal and coronal T1WI and T2WI as a hypointense canal-shaped signal of variable length. No similar duct-like structure has ever been observed in any other 3V tumor category. Therefore, the DR represents an invaluable sign for accurately diagnosing strictly 3V papillary CPs.
Acknowledgments
The authors wish to especially thank Crystal Smith and Liliya Gusakova, Reference Librarians of the National Library of Medicine, National Institutes of Health (Bethesda, Maryland) for their kind assistance during the process of searching and retrieving articles and monographs used in this study. We are also grateful to Melissa Grafe, Librarian for Medical History Library, Yale University (New Haven, Connecticut) and to Lucretia MacLure, Jack Eckert, and the staff at the Francis A. Countway Library of Medicine at Harvard Medical School (Boston, Massachusetts) for their invaluable help in obtaining some of the original research material used for this study. Finally, we are grateful to George Hamilton for his critical review of the language and style of the manuscript.
ABBREVIATIONS:
- ACP
adamantinomatous craniopharyngioma
- CP
craniopharyngioma
- CP-DR
CPs with a basal duct-like recess
- CP-nDR
CPs without a basal duct-like recess
- DR
duct-like recess
- PCP
papillary craniopharyngioma
- PS
pituitary stalk
- 3V
third ventricle
- 3VF
third ventricle floor
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Prieto R, Barrios L, Pascual JM. Papillary craniopharyngioma: a type of tumor primarily impairing the hypothalamus: a comprehensive anatomo-clinical characterization of 350 well-described cases. Neuroendocrinology 2021. Dec 28. [Epub ahead of print] 10.1159/000521652 [DOI] [PubMed] [Google Scholar]
- 2.Pascual JM, Prieto R, Rosdolsky M. Craniopharyngiomas primarily affecting the hypothalamus. Handb Clin Neurol 2021;181:75–115 10.1016/B978-0-12-820683-6.00007-5 [DOI] [PubMed] [Google Scholar]
- 3.Juratli TA, Jones PS, Wang N, et al. Targeted treatment of papillary craniopharyngiomas harboring BRAF 600 mutations. Cancer 2019;125:2910–14 10.1002/cncr.32197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pascual JM, Prieto R, Carrasco R, et al. Displacement of mammillary bodies by craniopharyngiomas involving the third ventricle: surgical-MRI correlation and use in topographical diagnosis. JNS 2013;119:381–405 10.3171/2013.1.JNS111722 [DOI] [PubMed] [Google Scholar]
- 5.Prieto R, Pascual JM, Barrios L. Topographic diagnosis of craniopharyngiomas: the accuracy of MRI findings observed on conventional T1 and T2 images. AJNR Am J Neuroradiol 2017;38:2073–80 10.3174/ajnr.A5361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prieto R, Barrios L, Pascual JM. Strictly third ventricle craniopharyngiomas: pathological verification, anatomo-clinical characterization and surgical results from a comprehensive overview of 245 cases. Neurosurg Rev 2022;45:375–94 10.1007/s10143-021-01615-0 [DOI] [PubMed] [Google Scholar]
- 7.Prieto R, Pascual JM, Rosdolsky M, et al. Craniopharyngioma adherence: a comprehensive topographical categorization and outcome-related risk stratification model based on the methodical examination of 500 tumors. Neurosurg Focus 2016;41:E13 10.3171/2016.9.FOCUS16304 [DOI] [PubMed] [Google Scholar]
- 8.Fukushima T, Hirakawa K, Kimura M, et al. Intraventricular craniopharyngioma: its characteristics in magnetic resonance imaging and successful total removal. Surg Neurol 1990;33:22–27 10.1016/0090-3019(90)90220-J [DOI] [PubMed] [Google Scholar]
- 9.Urbach H, Behrens E, von Deimling A, et al. Solides Kraniopharyngiom im III: Ventrikel-Differential-diagnostiche Aspekte [in German]. Aktuelle Radiol 1998;8:95–97 [PubMed] [Google Scholar]
- 10.Pascual JM, Prieto R, Castro-Dufourny I, et al. Topographic diagnosis of papillary craniopharyngiomas: the need for an accurate MRI-surgical correlation. AJNR Am J Neuroradiol 2015;36:E55–56 10.3174/ajnr.A4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue Q, Yu Y, Shi Z, et al. Prediction of BRAF mutation status of craniopharyngioma using magnetic resonance imaging features. J Neurosurg 2018;129:27–34 10.3171/2017.4.JNS163113 [DOI] [PubMed] [Google Scholar]
- 12.Glastonbury CM, Osborn AG, Salzman KL. Masses and malformations of the third ventricle: normal anatomic relationships and differential diagnoses. Radiographics 2011;31:1889–1905 10.1148/rg.317115083 [DOI] [PubMed] [Google Scholar]
- 13.Momin AA, Recinos MA, Cioffi G, et al. Descriptive epidemiology of craniopharyngiomas in the United States. Pituitary 2021;24:517–22 10.1007/s11102-021-01127-6 [DOI] [PubMed] [Google Scholar]
- 14.Buckle C, Smith JK. Choroid plexus papilloma of the third ventricle. Pediatr Radiol 2007;37:725 10.1007/s00247-007-0474-5 [DOI] [PubMed] [Google Scholar]
- 15.Tsutsumi S, Hori M, Ono H, et al. The infundibular recess passes through the entire pituitary stalk. Clin Neuroradiol 2016;26:465–69 10.1007/s00062-015-0391-1 [DOI] [PubMed] [Google Scholar]
- 16.Sartoretti-Schefer S, Wichmann W, Aguzzi A, et al. MR differentiation of adamantinomatous and squamous-papillary craniopharyngiomas. AJNR Am J Neuroradiol 1997;18:77–87 10.1007/s00062-015-0391-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pascual JM, Rosdolsky M, Prieto R, et al. Jakob Erdheim (1874–1937): father of hypophyseal-duct tumors (craniopharyngiomas). Virchows Arch 2015;467:459–69 10.1007/s00428-015-1798-4 [DOI] [PubMed] [Google Scholar]
- 18.Ciric IS, Cozzens JW. Craniopharyngiomas: transsphenoidal method of approach: for the virtuoso only? Clin Neurosurg 1980;27:169–87 10.1093/neurosurgery/27.CN_suppl_1.169 [DOI] [PubMed] [Google Scholar]
- 19.Kollias SS, Ball WS, Prenger EC. Review of the embryologic development of the pituitary gland and report of a case with hypophyseal duplication detected by MRI. Neuroradiology 1995;37:3–12 10.1007/BF00588511 [DOI] [PubMed] [Google Scholar]
- 20.Steno A, Popp AJ, Wolfsberger S, et al. Persisting embryonal infundibular recess. J Neurosurg 2009;110:359–62 10.3171/2008.7.JNS08287 [DOI] [PubMed] [Google Scholar]
- 21.Gu Y, Zhang X ,Hu F, et al. Suprachiasmal translamina terminalis corridor used in endoscopic endonasal approach for resecting third ventricular craniopharyngioma. J Neurosurg 2015;122:1166–72 10.3171/2015.1.JNS132842 [DOI] [PubMed] [Google Scholar]
- 22.Rambarki O, Rajesh A. Third ventricular craniopharyngioma. Neurol India 2016;64:834–35 10.4103/0028-3886.185370 [DOI] [PubMed] [Google Scholar]