Abstract
Naturally occurring noncytotoxic vacA type s2 strains of Helicobacter pylori have a 12-residue extension to the vacuolating cytotoxin (VacA) compared with cytotoxic type s1 strains. We show that adding the region encoding this extension to type s1 vacA completely abolishes vacuolating cytotoxin activity but has no effect on VacA production.
Essentially all strains of the gastric pathogen Helicobacter pylori possess vacA, the gene encoding its vacuolating cytotoxin (4). However, vacA alleles vary between strains, and one important difference is that two versions of the region encoding the signal peptide commonly occur, termed s1 and s2 (1). This is medically important, because in the United States, H. pylori strains with type s1 vacA are usually cytotoxic in vitro and are associated with high levels of gastric inflammation in vivo and a high prevalence of peptic ulceration (2). In contrast, strains with type s2 vacA are not cytotoxic in vitro, are associated with less inflammation in vivo, and are rarely isolated from patients with peptic ulcers (2). Why differences in the region encoding the VacA signal peptide have these associations is unclear, but it has been speculated that there may be differences in signal peptide cleavage efficiency or that the signal region differences may be a marker for more subtle sequence differences in vacA, either upstream (affecting level of transcription) or downstream (affecting VacA structure). In previous investigations, we have shown that the signal sequence cleavage site differs between s1 and s2 VacA: mature type s1 VacA has a hydrophobic N terminus, but type s2 VacA has a short, predominantly hydrophilic, 12-amino-acid, N-terminal extension (1). This led us to hypothesize that the naturally occurring N-terminal extension of s2 forms of VacA may “block” toxin activity. We have been encouraged in this work by recent reports from de Bernard et al. and Ye et al., who have worked on the toxigenic s1 form of VacA and who have shown that deleting the hydrophobic N terminus (9 and 17 amino acid residues, respectively) from s1 VacA abolishes vacuolating activity when the modified toxin is expressed intracellularly (5, 7).
To investigate the importance of the s2-specific hydrophilic extension, we inserted the DNA encoding this region from the nontoxigenic s2 strain Tx30a (ATCC 51932) into the corresponding position in vacA in the toxigenic s1 strain 60190 (ATCC 49503). To do this, we first cloned the 5′ 274 bp of vacA from 60190 into pBluescript SK together with the 3′-terminal region of the upstream gene cysS. Next, we inserted a chloramphenicol resistance marker 74 bp upstream of the vacA promoter, and constructed a 60190 control strain by transforming 60190 with this construct. We then introduced the 36-bp insertion encoding the s2-specific 12-amino-acid extension (nucleotides 436 to 471 in the Tx30a vacA sequence, GenBank accession number U29401) into the cloned vacA sequence immediately downstream of the region encoding the signal peptide (between codons 33 and 34 in the 60190 vacA sequence, GenBank number U05676). We did this by inverse PCR using primers which annealed to 60190 vacA adjacent to the site of insertion (896 to 923 nucleotides and 868 to 895 nucleotides respectively). Each primer contained half of the sequence to be inserted at its 5′ end. Following inverse PCR, we recircularized the linear product by blunt-end ligation and confirmed the presence of the correct 36 bp insertion by nucleotide sequence analysis. We introduced the resulting construct into the chromosomal vacA gene of 60190 by allelic exchange, using natural transformation and marker rescue. We confirmed the presence of the insertion in 60190 vacA by PCR analysis using allelic type-specific primers (1). To confirm that the signal peptide cleavage site was not altered by the presence of the hydrophilic extension, we determined the N-terminal amino acid sequence of the mature, secreted VacA protein of the 60190 N-terminal insertion mutant strain (Applied Biosystems 473A, Warrington, United Kingdom). The N-terminal sequence, NTPNDP, confirmed the presence of the s2-specific hydrophilic extension.
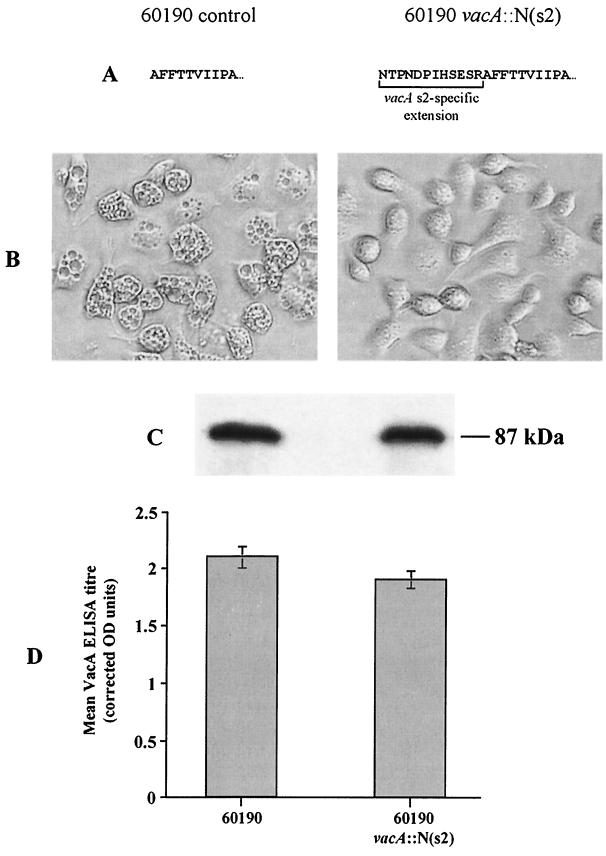
Next, we assessed the vacuolating activity of the 60190 control and its isogenic N-terminal insertion mutant derivative, 60190 vacA::N(s2), by incubating HeLa, AGS (a human gastric adenocarcinoma cell line), and RK-13 (a rabbit kidney epithelial cell line) cells overnight with unconcentrated 48-h broth culture supernatants (grown in Oxoid Iso-Sensitest broth supplemented with 5% fetal calf serum). For all six control 60190 broth supernatants assayed, essentially all cells were vacuolated for each of the three cell lines, whereas incubation with the six supernatants from 60190 vacA::N(s2) produced no vacuolation in any of the cell lines tested (Fig. 1). To confirm that loss of vacuolating activity was not due to a loss of VacA expression in our mutant strain, we performed immunoblots and enzyme-linked immunosorbent assays (ELISAs) for mature VacA protein in the same 48-h broth culture supernatants by using an antiserum raised against the recombinant midregion of VacA from strain 60190 (kindly donated by T. L. Cover, Vanderbilt University, Nashville, Tenn.). The immunoblot showed that a protein of similar size (∼87 kDa) and amount was detected in all six 60190 controls and all six 60190 vacA::N(s2) mutant broth supernatants tested, confirming that full-sized, mature VacA protein was consistently secreted by the mutant strain. ELISAs showed that similar levels of VacA were produced by 60190 vacA::N(s2) and by the 60190 control (means ± standard deviations were 1.9 ± 0.18 [n = 6] and 2.1 ± 0.16 [n = 3] corrected optical density units respectively, P = 0.15, t test).
FIG. 1.
Vacuolating activity and VacA production of the 60190 control strain and its N-terminal insertion mutant derivative, 60190 vacA::N(s2). (A) Difference in the N-terminal amino acid sequence of mature VacA between wild type 60190 and 60190 vacA::N(s2). The presence of the s2-specific N-terminal extension in VacA from strain 60190 vacA::N(s2) was confirmed for the first six residues by N-terminal peptide sequencing. (B) The effect on AGS cells following overnight incubation with 48-h broth culture supernatant of either the 60190 control strain (left) or 60190 vacA::N(s2) (right) diluted fivefold in RPMI 1640 medium (Gibco BRL) containing 10 mM ammonium chloride. The presence of cytoplasmic vacuolation was assessed visually by light microscopy at a ×50 magnification. (C) Immunoblot of 48-h broth culture supernatant of 60190 control (left lane) and 60190 vacA::N(s2) (right lane) detected with rabbit antisera raised against the recombinant VacA type m1 midregion. (D) Quantification of VacA in 48-h broth culture supernatants by ELISA. Mean VacA ELISA titers, corrected for bacterial density, of six 60190 vacA::N(s2) and three 60190 control strain supernatants are shown. OD, optical density.
In conclusion, we have shown that adding the hydrophilic, 12-amino-acid, N-terminal extension, which is specific to VacA type s2 strains, to the N terminus of type s1 VacA abolishes vacuolating cytotoxin activity. Loss of vacuolating activity is not explained by differences in VacA production or stability, because both are unaffected by the addition of the s2-specific region. Our results raise the interesting question of why vacA s2 strains of H. pylori (producing the blocked s2 form of toxin) survive and thrive in apparent competition with vacA s1 strains. This is particularly curious given the freely recombinational population structure of H. pylori (6), which should amplify any negative selection pressure. This and the fact that sequence homology among type s2 vacA alleles is as close as among type s1 alleles (3) lead us to speculate that the “blocked” s2 form of VacA has an important function for the bacterium, and we are investigating what this function may be. Our results are also of major medical importance, for they explain why H. pylori strains with the s2 form of vacA are nontoxigenic, cause little inflammation, and are rarely associated with peptic ulceration. These results also provide a rational basis for testing for vacA signal region type.
Acknowledgments
We acknowledge the technical assistance of Rachel Twells, Brian Dove, and Joanne Bower.
This work was funded by the Medical Research Council (United Kingdom).
REFERENCES
- 1.Atherton J C, Cao P, Peek R M, Tummuru M K R, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori: association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 2.Atherton J C, Peek R M, Jr, Tham K T, Cover T L, Blaser M J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 3.Atherton J C, Sharp P M, Cover T L, Gonzalez-Valencia G, Peek R M, Thompson S A, Hawkey C J, Blaser M J. Vacuolating cytotoxin (vacA) alleles of Helicobacter pylori comprise two geographically widespread types, m1 and m2, and have evolved through limited recombination. Curr Microbiol. 1999;39:211–218. doi: 10.1007/s002849900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cover T L, Tummuru M K R, Cao P, Thompson S A, Blaser M J. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994;269:10566–10573. [PubMed] [Google Scholar]
- 5.de Bernard M, Burroni D, Papini E, Rappuoli R, Telford J, Montecucco C. Identification of the Helicobacter pylori VacA toxin domain active in the cell cytosol. Infect Immun. 1998;66:6014–6016. doi: 10.1128/iai.66.12.6014-6016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suerbaum S, Maynard-Smith J, Bapumia K, Morelli G, Smith N H, Kunstmann E, Dyrek I, Achtman M. Free recombination within Helicobacter pylori. Proc Natl Acad Sci USA. 1998;95:12619–12624. doi: 10.1073/pnas.95.21.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye D, Willhite D C, Blanke S R. Identification of the minimal intracellular vacuolating domain of the Helicobacter pylori vacuolating toxin. J Biol Chem. 1999;274:9277–9282. doi: 10.1074/jbc.274.14.9277. [DOI] [PubMed] [Google Scholar]