Purpose of review
The treatment of sarcoidosis remains uncertain, despite 70 years of study. The conventional approach is to initiate corticosteroids in individuals who require treatment. The position of more aggressive regimes is unknown.
Recent findings
Recent recognition that many patients will require prolonged therapy, and the observation that corticosteroids lead to overt and insidious toxicities, have led to suggestions that steroid-sparing medications be used earlier in the management of sarcoidosis. Individuals with poor prognostic features, designated as ‘high-risk’ sarcoidosis may, especially benefit from a broader palette of therapeutic options in the initial treatment regimen. An even more aggressive approach, known as ‘top-down’ or ‘hit-hard and early’ therapy has emerged in the fields of gastroenterology and rheumatology in the past 15 years, on the premise that highly effective early control of inflammation leads to better outcomes. These regimens typically involve early initiation of biologic therapies.
Summary
For certain subpopulations of sarcoidosis patients, ‘top-down’ therapy could be helpful. Severe pulmonary sarcoidosis, neurosarcoidosis, cardiac sarcoidosis and multiorgan sarcoidosis are phenotypes that may be most relevant for revised therapeutic algorithms. Precision medicine approaches and randomized trials will be necessary to confirm a role for top-down therapy in the routine management of sarcoidosis.
Keywords: hit-hard, sarcoidosis, step-up, top-down, treatment
INTRODUCTION: CURRENT THERAPEUTIC APPROACH IN SARCOIDOSIS
Corticosteroids have been used for sarcoidosis for over 70 years. Even now, they remain the most commonly used medication for systemic management [1▪]. Corticosteroids are highly effective, inexpensive, easily titrated, work quickly, are perceived to require less monitoring than other agents and are familiar to patients as well as prescribers. They have the added advantage in some patients of increasing energy, sense of wellbeing and ameliorating nonspecific symptoms such as arthralgias, at least when first prescribed. In addition, corticosteroids have been studied in various sarcoidoses over the past 6 decades [2]. As such, corticosteroids are still deemed by expert opinion to be the first-line therapy for all forms of sarcoidosis that require systemic treatment [3▪▪]. It should be noted that these opinions are based not on controlled data but rather on the absence of head-to-head comparisons between corticosteroids and other agents.
Although sarcoidosis experts typically advocate for earlier inclusion of steroid-sparing agents in treatment algorithms [4▪], in practice steroid-sparing agents are prescribed for much less than half of treatment-requiring patients [5,6]. Long-term management typically entails higher prednisone doses than the commonly referenced prednisone goal of less than 10 mg/day; for example the mean daily dose in two randomized pulmonary sarcoidosis trials was approximately 13 mg/day [7]. In an unselected cohort from our institution, the median treatment duration among all treated patients was 8 months [7]. These data accord with a multicenter prospective observational study in the United States that demonstrated that the likelihood of requiring treatment after 2 years in patients requiring treatment is approximately 50% [8,9]. In corticosteroid-treated patients, relapses after tapering of corticosteroids have been noted in 20–74% [10–12]. For those individual requiring tumor necrosis factor inhibitors, the likelihood of very early relapse after stopping therapy is especially high [13]. Taken as a whole, the available data paint a picture of frequent treatment initiation with corticosteroids but a failure of clinicians to reduce the corticosteroid dose by sufficiently aggressive use of steroid-sparing medications. This practice gap is likely due to uneven implementation of recommended steroid-sparing strategies, patient preference, access to medication, lack of knowledge, or the absence of sufficiently effective steroid-sparing agents.
Box 1.
no caption available
RATIONALE FOR HIT HARD AND EARLY TREATMENT
Not always a benign disease
Sarcoidosis is an extremely heterogeneous disease – whereas many patients enjoy spontaneous resolution, a quantitatively important subset experience severe morbidity, loss of quality of life, or even death as a result. Most of these patients with poor outcomes exhibit chronic, nonself-limiting sarcoidosis, ongoing granulomatous inflammation and sometimes fibrosis. Uncontrolled inflammation is believed to be the instigator of fibrotic sarcoidosis, perhaps in the context of profibrotic genetic features and immune responses [14]. For pulmonary disease, the latency from presentation to the development of disability was over 100 months in one Japanese cohort, emphasizing the slow build-up of fibrosis and the need to view management decisions through a chronic treatment lens [15].
Compared with the general population, sarcoidosis leads to increased mortality [16–19]. A large population based study in Sweden, including over 8000 sarcoidosis patients revealed that those with sarcoidosis are at a higher risk of death compared with the overall population [16]. Moreover, patients with an indication for systemic treatment had a two-fold increased risk of death. A recent Danish study suggested that mortality rates in sarcoidosis patients are higher than controls for all age groups and sexes and are highest in patients treated with corticosteroids [20▪]. For pulmonary disease, death occurs mainly in those with fibrosis [18]. On the contrary, mortality rates in the United States and in Europe appear to be increasing [17,19]. In addition to pulmonary disease, cardiac involvement, neurologic involvement and multiorgan sarcoidosis are most closely associated with poor outcomes [15,21]. Disability and death were closely tied to multiple organ involvement at disease outset in a Japanese cohort, with three or more overtly involved organs at presentation delineating a substantial increased risk for poor outcome [15]. Other features can also be used to identify patients at higher risk, including older age at diagnosis, black race, lower socioeconomic status, extrapulmonary involvement, advanced cardio/pulmonary disease and pulmonary hypertension [22–25].
Role of immunomodulators and biologics in sarcoidosis
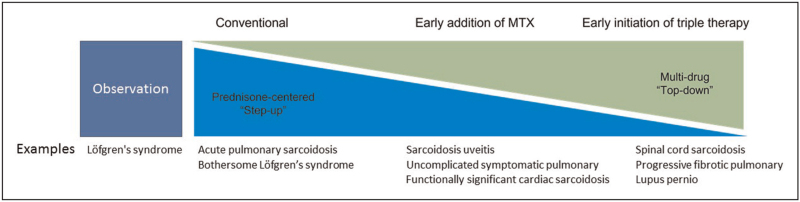
First, a decision about whether to treat sarcoidosis must be established [26▪]. In patients without organ damage or severely impaired quality of life the decision not to treat can be made [26▪,27▪▪]. Patients in definite need for treatment are those with severe organ dysfunction such as severe pulmonary sarcoidosis, significant hypercalcemia, clinically important cardiac sarcoidosis or clinically important neurosarcoidosis [2,22], Fig. 1. It is estimated that approximately half of all sarcoidosis patients need systemic treatment [9].
FIGURE 1.
Proposed view on treatment in sarcoidosis. Sarcoidosis treatment strategies vary from observation or watchful waiting in cases without organ damage or severe diminished quality of life, for example uncomplicated Lofgren's syndrome to strategies with more upfront use of steroid sparing agents or even biologics for example cardiac or neurosarcoidosis.
As mentioned previously, the initial need for treatment is a predictor for mortality and prolonged treatment duration [16]. However, the use of corticosteroids portends (often) unsuspected long-term consequences that can ultimately worsen outcomes. For example, initiation of corticosteroids has been associated with side effects such as diabetes, obesity, osteoporosis and infections [7]. A large Swedish register study found a hazard ratio of 2.4 of developing type 2 diabetes in corticosteroid-treated sarcoidosis patients versus controls [28]. Furthermore, the dose of prednisone has been directly correlated with the amount of weight gain in sarcoidosis patients, without an extra beneficial effect on lung function with higher doses [29]. The initiation of therapy has been associated with a higher risk for myocardial infarction, a potential consequence of steroid-related toxicities [30▪]. Even in the short term, patients receiving higher doses of steroids had a lower health related quality of life compared with patients on lower doses, when adjusting for disease severity [31]. Thus, there is a growing recognition of significant morbidity and mortality due to steroid use in sarcoidosis, and an appetite for earlier implementation of steroid sparing regimens [32]. This sentiment is reflected in the recent ERS Treatment Guidelines, which suggest consideration of a steroid-sparing medication at disease outset in cardiac sarcoidosis, but which stopped short of suggesting it for all treatment-requiring patients with cardiac sarcoidosis due to the lack of robust evidence [3▪▪].
Immunomodulatory drugs such as methotrexate, azathioprine and leflunomide can be used as steroid sparing options in sarcoidosis, with methotrexate being used most often [4▪,33,34]. Methotrexate was recently found to be effective in up to 80% of pulmonary sarcoidosis patients [35]. However, an upfront approach for methotrexate or other steroid sparing medications in sarcoidosis is not yet supported by rigorous evidence. A Delphi consensus study on sarcoidosis treatment revealed large variations in treatment regimens but emphasized the use of immunomodulators such as methotrexate in disease likely to require prolonged treatment, or as a steroid-sparing option in patients with high risk of steroid toxicity [4▪]. The Delphi study does not provide firm guidance on exactly when to initiate methotrexate (e.g., upfront as monotherapy, upfront together with steroids or after inability to taper steroids). However, it is notable that a patient reported outcome study in sarcoidosis patients reported side effects in 78% of prednisone treated patients versus 49% of methotrexate-treated patients [36].
Significantly, a recent cohort study compared pulmonary sarcoidosis patients treated with first-line methylprednisone or methotrexate [37]. Patients receiving methotrexate as first-line monotherapy had contraindications for corticosteroids. The data suggest that effectiveness in pulmonary sarcoidosis was noninferior in the methotrexate group, with indications of lower rates of treatment resistance and relapse. Similarly, a small cohort study in cardiac sarcoidosis showed that methotrexate and corticosteroid combination therapy had better results on left ventricular function than corticosteroids alone [38]. To date, there are no published data from randomized trials comparing up-front corticosteroid versus steroid-sparing monotherapy approaches.
Biologics such as tumor necrosis factor inhibitors (TNFi) drugs have mostly been reserved for severe refractory sarcoidosis cases, as outlined in the ERS Guideline [3▪▪]. The ERS guideline provides a conditional recommendation with low-quality evidence for their use [3▪▪]. High disease activity on 18-fluorodeoxyglucose (FDG)-PET correlates with effectiveness of TNFi [39], but routine PET has not been sufficiently established to use as a gating criterion for the decision to start TNFi. Although TNFi are considered off-label for sarcoidosis, a Delphi consensus study amongst global sarcoidosis experts revealed consensus for their use in common practice for severe cases [4▪]. Whether to initiate TNFi only after progressing through other agents first is controversial, as they are remarkably effective for certain indications that otherwise are extremely difficult to control, such as neurosarcoidosis and lupus pernio [40,41]. However, there are no randomized trials in sarcoidosis comparing immediate use of TNFi versus the conventional step-up approach. Potentially, earlier use could better preserve organ function and diminish morbidity. A large cohort study comparing treatment outcomes of infliximab and methotrexate in sarcoidosis patients revealed that infliximab was more likely to improve clinical status after 1 year than methotrexate [42▪]. A high variability in biologic use for sarcoidosis currently exists globally, but even within countries, potentially due to local preferences, physician experience and also reimbursement. Data From the American College of Rheumatology's Rheumatology Informatics System for Effectiveness Registry showed that even in practices with over 30 sarcoidosis patients, biologic use ranged from 16 to 67% [43▪].
In the current guidelines, biologics are reserved for cases proven refractory to first-line and second-line therapy which often leads to a potential suspension of months to years until patients receive biologic treatment. In case of high-risk sarcoidosis it is unknown whether this is harmful. Two cohort studies of infliximab and inflectra treated patients showed a decrease in lung function while on first-line or second-line treatment in the 6 months prior to biologic therapy [39,44]. For spinal sarcoidosis, delay of effective treatment has been associated with poorer outcomes [45], and the use of TNFi resulted in earlier disease control with better outcomes [46]. Similarly, delay in effective therapy for cardiac sarcoidosis has been associated with lower chance for restoration of intrinsic conduction and cardiac function [47,48]. Obviously, all of these observational studies are fraught with methodologic pitfalls, rendering inferences from them extremely tenuous. Nonetheless, they are all concordant with the hypothesis that earlier use of highly effective therapy may result in better outcomes. Examples of low-risk versus high-risk sarcoidosis are mentioned in Table 1.
Table 1.
Examples of sarcoidosis manifestations and risk stratification
| Low-risk sarcoidosis | Intermediate-risk sarcoidosis | High-risk sarcoidosis • Factors warranting intensive treatment |
| Lofgrens syndrome | ||
| Nodal sarcoidosis | ||
| Scar sarcoidosis | ||
| (Ankle) arthritis | Osseous sarcoidosis | |
| Uncomplicated or local cutaneous involvement | Lupus Pernio (known to warrant long-term treatment) | |
| Uncomplicated uveitis | Chronic or severe uveitis • Loss of vision | |
| Mild hypercalcemia hypercalciuria with nefrocalcinosis | Severe hypercalcemia (known to warrant long-term treatment) | |
| Possible cardiac sarcoidosis | Probable or definite cardiac sarcoidosis • Large extent of involvement on CMR or FDG-PET • Rhythm or function abnormalities | |
| Parenchymal sarcoidosis without impaired PFT | Progressive fibrosing pulmonary sarcoidosis • Impaired pulmonary function • High inflammatory burden and signs of fibrosis on FDG-PET/CT | |
| Central nervous system sarcoidosis • Spinal cord involvement • Hydrocephalus | ||
| Hepatic sarcoidosis |
Simplified stratification of sarcoidosis examples into risk categories. Risk of organ damage or mortality are taken into account. Low-risk sarcoidosis can be managed by watchful waiting and screening for organ involvement. Intermediate-risk sarcoidosis should be managed case-by-case, some patients warrant systemic treatment following current guidelines with a step-up regime. High-risk sarcoidosis warrants systemic treatment and could potentially benefit from a more aggressive ‘top-down’ or ‘hit-hard’ regimen with a more upfront use of steroid sparing agents or in some cases even biologicals. CMR Cardiac Magnetic Resonance Imaging; FDG, fluorodeoxyglucose; PFT, pulmonary function test.
Hit-hard and early, evidence in rheumatology and inflammatory bowel disease
In Crohn's disease, a granulomatous inflammatory bowel disease (IBD), the use of top-down versus step-up treatment has been a subject of investigation in the last decade with promising results for a combination therapy of immunomodulators and biologicals for severe disease [49]. A large meta-analysis favored biologic and azathioprine combination therapy as induction therapy above immunosuppressants alone [50]. Furthermore, in Crohn's disease earlier introduction (within 3 months of diagnosis) of TNFi tended to correlate with the slower progression of long-term bowel damage [51]. The British Society of Gastroenterology consensus guidelines recommend escalation to an alternative agent in refractory patients after 8 weeks of therapy, and suggest even earlier use of biologic agents in patients with poor prognostic features [52]. Importantly, the British Guideline also strongly recommends against monotherapy with corticosteroids for maintenance of remission, due in part to therapeutic toxicity [52].
Similar to IBD, current management philosophy in rheumatoid arthritis also emphasizes earlier disease control. The European League Against Rheumatism endorses the immediate initiation of methotrexate or another immunomodulator (with or without corticosteroids) to diminish the chance of permanent joint damage at the time of diagnosis [53]. After 3 months an improvement should be seen, otherwise a biologic should be introduced. When after 6 months the established treatment target is not reached, a biologic (such as TNFi) should be introduced [53]. This principle of hit-hard and early with methotrexate up-front and tight control with biologics after a maximum of 3–6 months from diagnosis when needed could potentially be extrapolated to severe ‘High risk’ sarcoidosis patients. Of course, sarcoidosis patients can also experience spontaneous remission, so this strategy requires careful periodic reassessment and will benefit from precision medicine approaches to prognosis and treatment.
GAP OF EVIDENCE IN THE CURRENT LITERATURE
Sarcoidosis is already an orphan disease, with a treatment-requiring proportion of approximately 50%. The category of patients truly refractory to standard first/second-line treatment is even smaller, but ‘refractory’ is an arbitrary term that may overestimate how many patients can be managed with tolerable or minimally toxic doses of corticosteroids. It is estimated that 10–30% of patients develop progressive pulmonary disease [54]. Due to small numbers of patients with severe disease, most available evidence, outside corticosteroids, comes from small randomized controlled trials (RCTs), retrospective cohort studies and expert opinion. Although some pharmaceutical RCTs have been performed in sarcoidosis in the last 2 decades, these have mostly been investigating new drugs or sarcoidosis as a new target disease. Due to the absence of regulatory approval and difficulty with access, these medications are at this moment not replacing long-standing therapies. There is also an evidence gap in high-quality RCTs for effective conventional therapies due to the lack of interest in funding such trials for generic medications. At the moment of writing this article, clinicaltrials.gov mentions worldwide only nine treatment related trials in any form of sarcoidosis currently recruiting patients (including one in ocular, one in hepatic sarcoidosis and two treatment trials in sarcoidosis-associated pulmonary hypertension).
A highlight related to this particular article is that two extant trials are currently investigating the role of methotrexate as a substitute for initial treatment of sarcoidosis. The PREDMETH trial is a multicenter RCT currently enrolling patients with pulmonary sarcoidosis and impaired lung function in the Netherlands [55▪]. This study intents to investigate the efficacy and tolerability of methotrexate as first-line therapy with the hypothesis that methotrexate is just as effective as prednisone treatment, but with fewer side effects. Another currently enrolling treatment-related RCT is CHASM-CS-RCT [56▪]. This multicenter RCT is designed to evaluate the optimal first-line treatment strategy for patients with active cardiac sarcoidosis. The hypothesis is that a low-dose prednisone/methotrexate combination will not have inferior efficacy to first-line prednisone treatment but will have a reduced burden of side effects resulting in a better quality of life.
Besides RCTs, the use of (global) registries with well phenotyped patients is a critical first step to systematically study high-risk or severe sarcoidosis and the optimal treatment regiments. The marginal benefit of registries compared with RCTs is the option to include a more heterogeneous (and more representative) sarcoidosis population, gain large numbers and also gain insight in the use of off-label biologics.
CONCLUSION
With the recent publication of the ERS and BTS guidelines on sarcoidosis management the gap of knowledge and paucity of rigorous evidence in this field becomes strikingly clear. However, these guidelines do provide consistency of direction for physicians treating sarcoidosis patients. A step-up approach with initial corticosteroid monotherapy is favored by the ERS/BTS guideline, although some other reviews or expert opinions leave room for initiation of upfront second-line treatment (e.g. methotrexate) together with steroids in severely endangered or ‘High risk’ sarcoidosis patients.
Patient selection will always pose a significant challenge, because not all patients need systemic treatment and all available treatment options have potential side effects. The optimal treatment strategy for these severe, sometimes even life threatening, sarcoidosis cases is unknown. These cases include severe pulmonary, cardiac and neurosarcoidosis. Potentially, extrapolating literature from other disciplines, a ‘hit-hard and early’ approach could benefit severe life or organ threatening sarcoidosis for which a prolonged treatment regimen is anticipated. Evidence from ongoing and future trials in sarcoidosis treatment are needed.
Acknowledgements
None.
Financial support and sponsorship
The current work was not supported or sponsored.
Conflicts of interest
A.D.M.V. received consulting fees from Boehringer Ingelheim. D.A.C. received grant support from Boehringer-Ingelheim, Mallinkrodt, AI therapeutics, aTyr Pharma and Janssen. Personal consulting fees, steering committee or travel fees from aTyr Pharma, Krevant, Roche, Boehringer-Ingelheim, Fibrogen and Pliant.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Innabi A, Alzghoul BN, Kalra S, et al. Sarcoidosis among US Hispanics in a Nationwide Registry. Respir Med 2021; 190:106682. [DOI] [PubMed] [Google Scholar]; The first study investigating sarcoidosis in Hispanic US citizens showing differences compared with nonhispanics.
- 2.Culver DA, Judson MA. New advances in the management of pulmonary sarcoidosis. BMJ 2019; 367:l5553. [DOI] [PubMed] [Google Scholar]
- 3▪▪.Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J 2021; 58:2004079. [DOI] [PubMed] [Google Scholar]; Very comprehensive review using the GRADE system to extensively describe what is known and unknown in sarcoidosis treatment.
- 4▪.Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev 2020; 29:190146. [DOI] [PMC free article] [PubMed] [Google Scholar]; A semi recent article on treatment prereferences of worldwide sarcoidosis specialists based on the Delphi consensus method, this gives insight in real-life treatment regimes used by sarcoidosis specialists.
- 5.Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis based on healthcare use. Ann Am Thorac Soc 2016; 13:1244–1252. [DOI] [PubMed] [Google Scholar]
- 6.Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis 2012; 29:119–127. [PubMed] [Google Scholar]
- 7.Khan NA, Donatelli CV, Tonelli AR, et al. Toxicity risk from glucocorticoids in sarcoidosis patients. Respir Med 2017; 132:9–14. [DOI] [PubMed] [Google Scholar]
- 8.Judson MA, Baughman RP, Thompson BW, et al. Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis Vasc Diffuse Lung Dis 2003; 20:204–211. [PubMed] [Google Scholar]
- 9.Baughman RP, Judson MA, Teirstein A, et al. Presenting characteristics as predictors of duration of treatment in sarcoidosis. QJM 2006; 99:307–315. [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb JE, Israel HL, Steiner RM, et al. Outcome in sarcoidosis. The relationship of relapse to corticosteroid therapy. Chest 1997; 111:623–631. [DOI] [PubMed] [Google Scholar]
- 11.Rizzato G, Montemurro L, Colombo P. The late follow-up of chronic sarcoid patients previously treated with corticosteroids. Sarcoidosis Vasc Diffuse Lung Dis 1998; 15:52–58. [PubMed] [Google Scholar]
- 12.Hunninghake GW, Gilbert S, Pueringer R, et al. Outcome of the treatment for sarcoidosis. Am J Respir Crit Care Med 1994; 149 (4 Pt 1):893–898. [DOI] [PubMed] [Google Scholar]
- 13.Vorselaars AD, Verwoerd A, van Moorsel CH, et al. Prediction of relapse after discontinuation of infliximab therapy in severe sarcoidosis. Eur Respir J 2014; 43:602–609. [DOI] [PubMed] [Google Scholar]
- 14.Bonham CA, Strek ME, Patterson KC. From granuloma to fibrosis: sarcoidosis associated pulmonary fibrosis. Curr Opin Pulm Med 2016; 22:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takada K, Ina Y, Noda M, et al. The clinical course and prognosis of patients with severe, moderate or mild sarcoidosis. J Clin Epidemiol 1993; 46:359–366. [DOI] [PubMed] [Google Scholar]
- 16.Rossides M, Kullberg S, Askling J, et al. Sarcoidosis mortality in Sweden: a population-based cohort study. Eur Respir J 2018; 51:1701815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swigris JJ, Olson AL, Huie TJ, et al. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med 2011; 183:1524–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nardi A, Brillet PY, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J 2011; 38:1368–1373. [DOI] [PubMed] [Google Scholar]
- 19.Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J 2016; 48:1700–1709. [DOI] [PubMed] [Google Scholar]
- 20▪.Sikjaer MG, Hilberg O, Ibsen R, Lokke A. Sarcoidosis-related mortality and the impact of corticosteroid treatment: a population-based cohort study. Respirology 2022; 27:217–225. [DOI] [PubMed] [Google Scholar]; Recent cohort study showing a high mortality rate in sarcoidosis patients compared with healthy controls.
- 21.Sauer WH, Stern BJ, Baughman RP, et al. High-risk sarcoidosis. Current concepts and research imperatives. Ann Am Thorac Soc 2017; 14: (Suppl_6): S437–S444. [DOI] [PubMed] [Google Scholar]
- 22.Drent M, Crouser ED, Grunewald J. Challenges of sarcoidosis and its management. N Engl J Med 2021; 385:1018–1032. [DOI] [PubMed] [Google Scholar]
- 23.Kirkil G, Lower EE, Baughman RP. Predictors of mortality in pulmonary sarcoidosis. Chest 2018; 153:105–113. [DOI] [PubMed] [Google Scholar]
- 24.Harper LJ, Gerke AK, Wang XF, et al. Income and other contributors to poor outcomes in US sarcoidosis patients. Am J Respir Crit Care Med 2020; 201:955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest 2015; 147:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Culver DA, Wells AU. Bonella F, Culver DA, Israel-Biet D. When to treat sarcoidosis. European respiratory monograph. Sarcoidosis: European Respiratory Society; 2022. 316–327. [Google Scholar]; Expert-based review on decisions regarding treatment in sarcoidosis.
- 27▪▪.Thillai M, Atkins CP, Crawshaw A, et al. BTS clinical statement on pulmonary sarcoidosis. Thorax 2021; 76:4–20. [DOI] [PubMed] [Google Scholar]; A concise and highly clinical applicable guideline focussing on pulmonary sarcoidosis.
- 28.Entrop JP, Kullberg S, Grunewald J, et al. Type 2 diabetes risk in sarcoidosis patients untreated and treated with corticosteroids. ERJ Open Res 2021; 7:00028–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broos CE, Poell LHC, Looman CWN, et al. No evidence found for an association between prednisone dose and FVC change in newly-treated pulmonary sarcoidosis. Respir Med 2018; 138S:S31–S37. [DOI] [PubMed] [Google Scholar]
- 30▪.Rossides M, Kullberg S, Grunewald J, et al. Risk of acute myocardial infarction in sarcoidosis: a population-based cohort study from Sweden. Respir Med 2021; 188:106624. [DOI] [PubMed] [Google Scholar]; The study reveiled a high prevalence of myocardial infarction in sarcoidosis patients.
- 31.Cox CE, Donohue JF, Brown CD, et al. Health-related quality of life of persons with sarcoidosis. Chest 2004; 125:997–1004. [DOI] [PubMed] [Google Scholar]
- 32.Sweiss N, Yeager H. Sarcoidosis requiring systemic treatment: why not a steroid-sparing regimen up-front? Sarcoidosis Vasc Diffuse Lung Dis 2010; 27:3–4. [PubMed] [Google Scholar]
- 33.Vorselaars AD, Wuyts WA, Vorselaars VM, et al. Methotrexate versus azathioprine in second line therapy of sarcoidosis. Chest 2013; 144:805–812. [DOI] [PubMed] [Google Scholar]
- 34.Rossides M, Kullberg S, Di Giuseppe D, et al. Infection risk in sarcoidosis patients treated with methotrexate compared to azathioprine: a retrospective ‘target trial’ emulated with Swedish real-world data. Respirology 2021; 26:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang C, Zhang Q, Wang N, et al. Effectiveness and tolerability of methotrexate in pulmonary sarcoidosis: a single center real-world study. Sarcoidosis Vasc Diffuse Lung Dis 2019; 36:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahlmann V, Moor CC, Veltkamp M, Wijsenbeek MS. Patient reported side-effects of prednisone and methotrexate in a real-world sarcoidosis population. Chron Respir Dis 2021; 18:14799731211031935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gavrysyuk V, Merenkova I, Dziublyk Y, et al. Efficacy and tolerability of methotrexate and methylprednisolone in a comparative assessment of the primary and long-term outcomes in patients with pulmonary sarcoidosis. Diagnostics (Basel) 2021; 11:1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagai S, Yokomatsu T, Tanizawa K, et al. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med 2014; 53:427–433. [DOI] [PubMed] [Google Scholar]
- 39.Vorselaars AD, Crommelin HA, Deneer VH, et al. Effectiveness of infliximab in refractory FDG PET-positive sarcoidosis. Eur Respir J 2015; 46:175–185. [DOI] [PubMed] [Google Scholar]
- 40.Sodhi M, Pearson K, White ES, Culver DA. Infliximab therapy rescues cyclophosphamide failure in severe central nervous system sarcoidosis. Respir Med 2009; 103:268–273. [DOI] [PubMed] [Google Scholar]
- 41.Stagaki E, Mountford WK, Lackland DT, Judson MA. The treatment of lupus pernio: the results of 116 treatment courses in 54 patients. Chest 2008; 135:468–476. [DOI] [PubMed] [Google Scholar]
- 42▪.Baughman RP, Cremers JP, Harmon M, et al. Methotrexate in sarcoidosis: hematologic and hepatic toxicity encountered in a large cohort over a six year period. Sarcoidosis Vasc Diffuse Lung Dis 2020; 37:e2020001. [DOI] [PMC free article] [PubMed] [Google Scholar]; A cohort study comparing treatment outcomes of infliximab and methotrexate in sarcoidosis patients, showing superior results from infliximab.
- 43▪.Hammam N, Evans M, Morgan E, et al. Treatment of sarcoidosis in US rheumatology practices: data from the American College of Rheumatology's Rheumatology Informatics System for Effectiveness (RISE) Registry. Arthritis Care Res 2022; 74:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large rheumatology database showing a high diversity in the use of biologicals in sarcoidosis.
- 44.Schimmelpennink MC, Vorselaars ADM, van Beek FT, et al. Efficacy and safety of infliximab biosimilar Inflectra® in severe sarcoidosis. Respir Med 2018; 138S:S7–S13. [DOI] [PubMed] [Google Scholar]
- 45.Sohn M, Culver DA, Judson MA, et al. Spinal cord neurosarcoidosis. Am J Med Sci 2013; 347:195–198. [DOI] [PubMed] [Google Scholar]
- 46.Gavoille A, Desbois AC, Joubert B, et al. Prognostic factors and treatment efficacy in spinal cord sarcoidosis: an observational cohort with long-term follow-up. Neurology 2022; 98:e1479–e1488. [DOI] [PubMed] [Google Scholar]
- 47.Kaida T, Inomata T, Minami Y, et al. Importance of early diagnosis of cardiac sarcoidosis in patients with complete atrioventricular block. Int Heart J 2018; 59:772–778. [DOI] [PubMed] [Google Scholar]
- 48.Padala SK, Peaslee S, Sidhu MS, et al. Impact of early initiation of corticosteroid therapy on cardiac function and rhythm in patients with cardiac sarcoidosis. Int J Cardiol 2017; 227:565–570. [DOI] [PubMed] [Google Scholar]
- 49.D’Haens GR. Top-down therapy for Crohn's disease: rationale and evidence. Acta Clin Belg 2009; 64:540–546. [DOI] [PubMed] [Google Scholar]
- 50.Hazlewood GS, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: a network meta-analysis. Gastroenterology 2015; 148:344–354.e5. quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 51.Panchal H, Wagner M, Chatterji M, et al. Earlier anti-tumor necrosis factor therapy of Crohn's disease correlates with slower progression of bowel damage. Dig Dis Sci 2019; 64:3274–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019; 68: (Suppl 3): s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79:685–699. [DOI] [PubMed] [Google Scholar]
- 54.Belperio JA, Shaikh F, Abtin FG, et al. Diagnosis and treatment of pulmonary sarcoidosis: a review. JAMA 2022; 327:856–867. [DOI] [PubMed] [Google Scholar]
- 55▪.Kahlmann V, Janssen Bonas M, Moor CC, et al. Design of a randomized controlled trial to evaluate effectiveness of methotrexate versus prednisone as first-line treatment for pulmonary sarcoidosis: the PREDMETH study. BMC Pulm Med 2020; 20:271. [DOI] [PMC free article] [PubMed] [Google Scholar]; Outline of an randomized controlled trial (RCT) which is going to be of importance to the pulmonary sarcoidosis field.
- 56▪.Birnie D, Beanlands RSB, Nery P, et al. Cardiac sarcoidosis multicenter randomized controlled trial (CHASM CS- RCT). Am Heart J 2020; 220:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]; Outline of an RCT of which the results are going to be significant to the cardiac sarcoidosis field.