Purpose of review
PET has emerged as method to determine the location and extent of disease activity in sarcoidosis. As most clinicians do not routinely utilize PET in the management of sarcoidosis, an understanding of the imaging technique is needed to comprehend the impact that PET abnormalities have on diagnosis, prognosis, and treatment.
Recent findings
Although PET can detect inflammation because of sarcoidosis throughout the body, it is most often utilized for the diagnosis of cardiac sarcoidosis for which it may provide information about prognosis and adverse events. Whenever PET is combined with cardiac magnetic resonance (CMR), clinicians may be able to increase the diagnostic yield of imaging. Furthermore, PET abnormalities have the potential to be utilized in the reduction or augmentation of therapy based on an individual's response to treatment. Although various biomarkers are used to monitor disease activity in sarcoidosis, an established and reproducible relationship between PET and biomarkers does not exist.
Summary
PET has the potential to improve the diagnosis of sarcoidosis and alter treatment decisions but prospective trials are needed to define the role of PET while also standardizing the performance and interpretation of the imaging modality.
Keywords: cardiac sarcoidosis, PET/computed tomography, PET, sarcoidosis
INTRODUCTION
The hallmark of sarcoidosis is granulomatous inflammation because of a dysregulated response of the immune system [1▪]. Once initiated, the resultant organ damage is dependent upon the intensity and duration of the persistent granulomatous inflammation. Given that the lungs are affected in about 90% of individuals, the pulmonary system garners much attention but sarcoidosis is a multisystem disease [2,3]. With the potential to damage any organ system, identifying and localizing active inflammation in different systems can direct management. PET has emerged as a tool to detect inflammation in patients with sarcoidosis that may not be easily recognized by physical examination or methods of assessment currently used in clinical practice [4]. In this current opinion, we hope to present the role of PET in the diagnosis and treatment of sarcoidosis, the advantages and drawbacks of PET, and outline areas of further research.
Box 1.
no caption available
INTRODUCTION TO PET IMAGING, TRACERS AND PREPARATION
PET is an imaging modality that utilizes radiotracers to visualize areas of the body with increased metabolic activity. The radiotracer is composed of a positron emitting isotope that is complexed to a specific target protein in the body. PET is combined with computed tomography (PET/CT) to produce a three-dimensional mapping of the organs. Although a full review of the list of radiotracers used in PET imaging is beyond the scope of this opinion, there are a few relevant to the field of sarcoidosis that warrant brief discussion. Fluorodeoxyglucose (FDG) and gallium are two such radiotracers, which have different target proteins. Macrophages and CD4 T-lymphocytes express facilitative glucose transporters on the cell membrane that allow substrates, such as FDG to enter the cells that contribute to the granulomatous inflammation of sarcoidosis [5]. Review of 13 studies that utilized FDG-PET or FDG-PET/CT in biopsy-proven sarcoidosis demonstrated a sensitivity that ranged from 89 to 100% for detecting active inflammation from sarcoidosis [6]. The second radiotracer, gallium, has multiple isotopes. In a prospective observational study of 39 patients with a diagnosis of sarcoidosis, PET with 68Ga identified active disease in 92% of symptomatic but only 16% of asymptomatic patients [7]. To complete imaging, gallium must be injected 48–72 h prior to image acquisition, which is inconvenient for patients and requires greater exposure to radiation. Additionally, the interobserver variability during interpretation leads to varied sensitivity (14–90%) and specificity depending on the imaging findings [8,9]. Although clinicians do not often choose a certain radiotracer, it is worth mentioning that FDG-PET has been shown to be superior at detecting both pulmonary and extrapulmonary activity of sarcoidosis [10]. Clinicians should appreciate that based on expert opinion, the European Association of Nuclear Medicine (EANM) and the Society of Nuclear Medicine and Molecular Imaging (SNMMI) endorse FDG PET/CT as a major indication for the diagnosis of sarcoidosis [11].
The most important use of PET in sarcoidosis is for management of cardiac disease. A major barrier for PET/CT to be effective in identifying disease is that normal myocardium takes up FDG as well. The SNMMI recommends high-fat (>35 g) and low-carbohydrate (<3 g) meals the day before the study with fasting for 4–12 h prior to PET/CT to achieve myocardial suppression of FDG uptake [12]. A 24 h diet restriction has shown mixed results with anywhere from 24.5 to 41.7% of individuals having an indeterminate PET/CT result [13▪,14▪▪]. When a high-fat, high-protein, and low-carbohydrate diet was extended to 72 h prior to the PET/CT scan, the indeterminate rate was 3.6% [15]. A ketogenic diet for 72 h combined with a 3-day overnight fast prior to PET/CT resulted in more complete myocardial suppression, less indeterminate scans, and higher agreement between PET/CT reviewers [14▪▪]. Intravenous lipid emulsion (100 ml) 3 h prior to PET/CT led to higher rates of complete myocardial suppression of FDG uptake as compared with controls [16▪▪]. The combination of intravenous heparin (50 IU/kg), low-carbohydrate diet, and 12-h fast demonstrated a higher rate of complete myocardial suppression compared with the same diet combination with lower doses of intravenous heparin (15 IU/kg) [17▪▪]. Although myocardial suppression is followed to ensure high yield of PET for cardiac sarcoidosis, the exact protocol varies without one universal standard.
ROLE OF PET IN THE DETECTION OF CARDIAC SARCOIDOSIS
Detection and management of cardiac sarcoidosis, a high-risk phenotype of sarcoidosis, remains enigmatic. Advanced cardiac imaging, such as PET and cardiac magnetic resonance (CMR), has a major role in the management of cardiac sarcoidosis [3]. Although sarcoidosis can affect any component of the cardiac system, FDG uptake can localize the inflammation to chambers of the heart. Pathologic uptake of FDG has been shown to occur in the left ventricle (LV) in approximately 24% of individuals undergoing PET/CT for concern of cardiac sarcoidosis [18▪▪]. The basal region is the most commonly involved area (91%), followed by the middle region (35.8%) and the apical region (11.9%) of the LV [19▪▪] (Fig. 1). The rate of abnormal metabolic activity in the right ventricle is not as clearly described but was noted in 9% individuals who underwent PET/CT [18▪▪]. Right ventricular disease and biatrial disease activity appears to be more common in individuals being evaluated for cardiac sarcoidosis as compared with those without a known history of sarcoidosis [20].
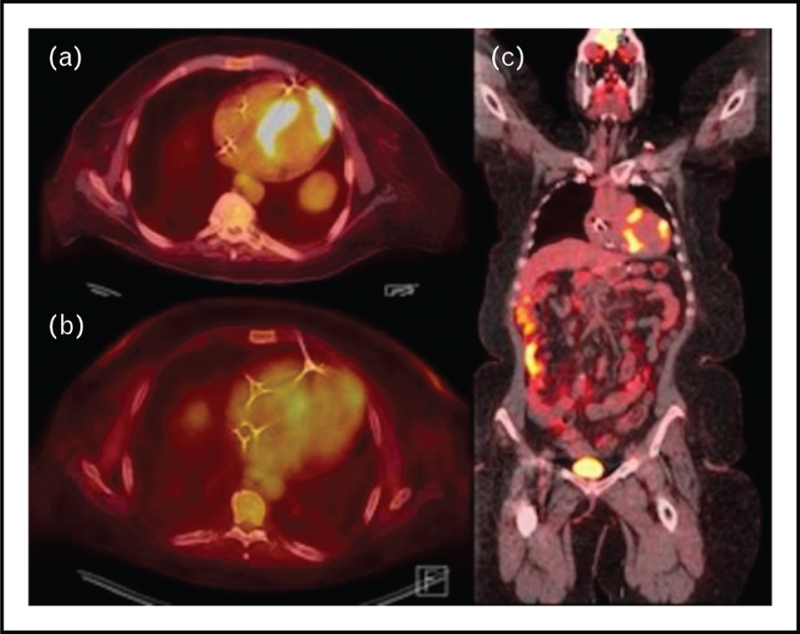
FIGURE 1.
Axial and coronal views of PET scan in patient with cardiac sarcoidosis. Methods: this is an original PET image from the authors’. Results: panel a shows disease activity in the left apical (SUV of 22.6) and septum (SUV of 21.6). Panel b shows a reduction in SUV after treatment (SUV 4.2 and 5.7 respectively). Panel c shows apical and septal disease in the coronal view.
Finding an association between abnormal PET and prognosis may allow clinicians to alter treatment prior to the onset of major adverse cardiac events. Metabolic abnormalities on PET had an association with the adverse events of death and ventricular tachycardia. The associations were strongest when the FDG uptake was paired with a perfusion abnormality by rubidium perfusion but focal right ventricular uptake was independently associated with death or ventricular tachycardia [21]. To further support the observation that the location of FDG uptake may provide clues to which individuals are prone to adverse events, a review of 137 patients undergoing PET/CT for concern for cardiac sarcoidosis, demonstrated that right ventricular abnormality on PET/CT was associated with a significantly higher rate of cardiovascular events whereas pathologic LV FDG uptake was not [18▪▪].
Quantitative analysis of standardized uptake values (SUV) act as an indicator of disease activity but EANM/SNMMI guidelines state that SUV has not been validated in cardiac sarcoidosis [11]. Although SUV has not been vigorously studied, a threshold of 4.0 has been shown to have a sensitivity of 97.3% and a specificity of 83.6% [22]. SUV may be helpful in not only diagnosing cardiac sarcoidosis but also predicting, which patients are at risk of developing cardiac complications from sarcoidosis. In a review of 67 patients undergoing PET/CT, visually identified diseased segments had a maximum SUV (SUVmax) of 5.4 and a mean SUV of 4.9. SUVmax, mean SUV, and LV ejection fraction on gated rest PET perfusion scan were shown to be associated with the number of cardiac events (ventricular tachycardia, automatic implantable cardioverter-defibrillator placement, complete heart block, pacemaker placement, atrial fibrillation, and New York Heart Association class III/IV heart failure, cardiac-related hospitalization) to occur after performance of the scan [19▪▪]. This is supported by a recent study that showed patients who developed a reduced LV ejection fraction, ventricular tachycardia or experienced death had a statistically significant higher SUVmax [18▪▪]. Overall, the role of PET is promising for management and prognostication of cardiac sarcoidosis and further studies will hopefully improve the evidence base.
Combining PET with cardiac magnetic resonance
A systematic review investigated the role of PET or PET/CT in the diagnosis of cardiac sarcoidosis . After evaluating 17 studies, a pooled sensitivity and specificity of 0.84 and 0.83 was noted [23]. It remains that PET is an imperfect tool for the detection of cardiac sarcoidosis . Evaluation of 118 consecutive patients referred for PET/CT with rubidium perfusion scan because of concern for cardiac sarcoidosis demonstrated that 60% of patients had abnormal imaging findings [21]. By combining PET with CMR, it may be possible to increase the diagnostic yield. The role of combined PET after CMR was evaluated in 107 consecutive patients referred for evaluation of known or suspected cardiac sarcoidosis. Fifty three percent of patients were found to have probable or highly probable cardiac sarcoidosis by CMR (>50% likelihood of having cardiac sarcoidosis). When PET was integrated with CMR, 32 (29.9%) of participants were reclassified to a higher likelihood classification. Twelve patients were reclassified as highly probable cardiac sarcoidosis (>90% likelihood), five of which experienced adverse events on follow-up evaluation [24]. Whenever CMR and PET abnormalities are both present, mortality and adverse events were significantly increased [25]. Clinicians must exercise caution when comparing PET and CMR findings as the two are not always consistent. Only 11% of patients were found to have concordant positive findings consistent with cardiac sarcoidosis on both CMR and PET [26]. PET and CMR may provide important different information in these patients. Abnormal enhancement on CMR could indicate inflammation or scarring whereas abnormal PET uptake is indicative of inflammatory activity. Whenever combined, this information can be useful in the management of patients with cardiac sarcoidosis (anti-inflammatory therapy, device placement, etc.).
ROLE OF PET IN ASSESSING PULMONARY SARCOIDOSIS
Although chest radiograph is the first imaging test used for the evaluation of sarcoidosis, staging by the Scadding criteria is subjective and does not correlate to activity on PET/CT [27]. A more detailed imaging technique is high-resolution CT (HRCT) of the chest. Parenchymal consolidations, lymphadenopathy, intraparenchymal nodules, septal and nonseptal lines, and pleural thickening on HRCT have been found to be associated with increased FDG uptake [28] (Fig. 2). Most individuals with fibrosis had persistent abnormalities on PET beyond the expected uptake of FDG in fibrotic areas that is because of increased glucose metabolism of fibroblasts. Despite this finding, a threshold SUV that distinguishes active disease from fibrosis is yet to be determined to the authors’ knowledge. Few studies have compared the value of HRCT versus PET/CT for the diagnosis of sarcoidosis. Although HRCT and PET/CT can detect abnormalities at similar rates, concordant findings for disease activity were present in only 46% of reviewed imaging [29]. The benefit of PET over HRCT is the ability of PET to identify ongoing inflammation from ‘burnt-out’ sarcoidosis and can assist in management planning.
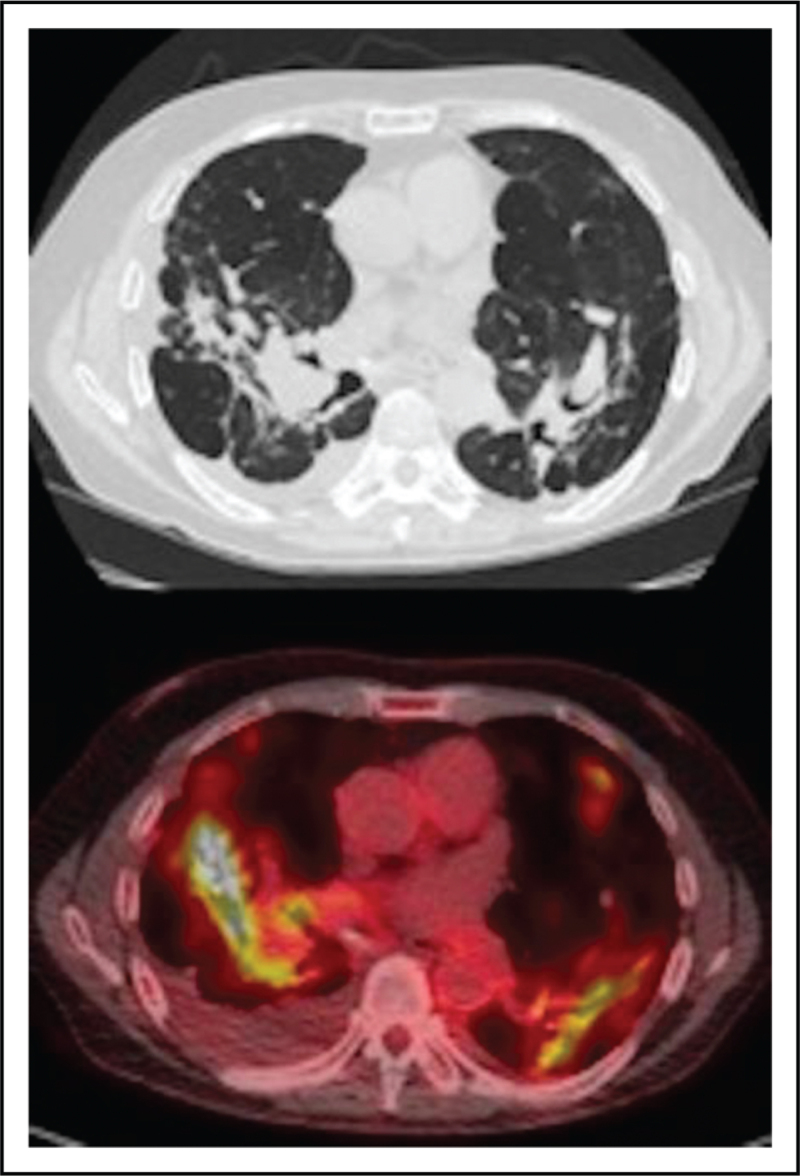
FIGURE 2.
Pulmonary parenchymal changes because of sarcoidosis on computed tomography and corresponding PET findings. Methods: this is an original PET image from the authors. Results: the top image was obtained from computed tomography and shows parenchymal architectural changes while the bottom image shows increase FDG uptake by PET in the corresponding location.
ROLE OF PET IN EXTRATHORACIC SARCOIDOSIS
Although PET has been primarily used to identify cardiac sarcoidosis , it is a whole-body imaging technique and can identify other organ involvement in sarcoidosis (Fig. 3). The role of PET in the diagnosis of extrathoracic sarcoidosis is described by small retrospective evaluations, case series, and case reports. Of the 165 PET/CT scans that were negative for cardiac sarcoidosis, 34% were found to have extrathoracic disease. The extrathoracic lesions were in the bone (9%), liver (12%), spleen (11%), extrathoracic lymph nodes (21%), and cutaneous locations (3%) [30]. These findings are incidental but are important for assessment of disease burden, correlation of symptoms, signs, and laboratory abnormalities for individualized management of the patient.
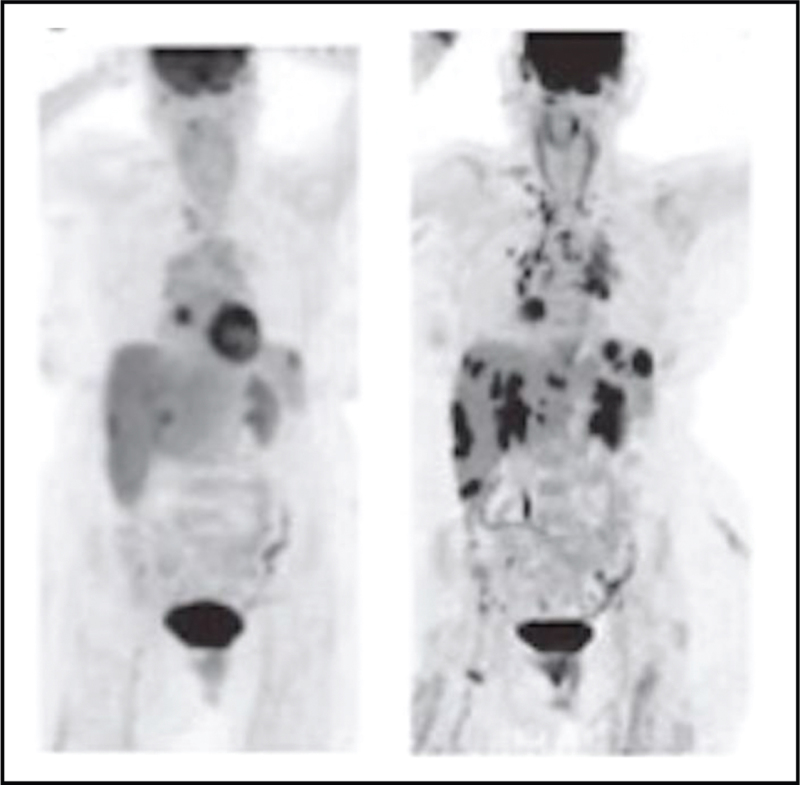
FIGURE 3.
FDG-PET findings in multisystem sarcoidosis. Methods: this is an original PET image from the authors. Results: the image on the left demonstrated FDG uptake in the heart while the image on the right reveals multisystem disease involving the liver, spleen, right acetabular bone, and lymph nodes in the low cervical, supraclavicular, mediastinal, and hilar regions. Both images are from the same individual at different rime intervals of the disease course.
CORRELATION OF PET WITH BIOMARKERS
Ideally, there would exist a single serological, physiological or imaging marker, which has sufficient sensitivity and specificity for diagnosis, prognosis, severity, and medication response. However, the exact pathophysiology of sarcoidosis has been elusive with no single pathognomonic finding that can pinpoint the diagnosis or prognosis. Nevertheless, serological and imaging findings are correlated, which suggest that measuring various serological biomarkers can complement PET [31]. A prospective study, which enrolled 30 patients with untreated sarcoidosis measured various biomarkers including soluble interleukin-2 receptor (sIL-2R), C-reactive protein (CRP), ACE, and urine calcium in addition to splenic FDG uptake on PET and found that there was a significant linear correlation between sIL-2R and SUVmax on PET [32]. It has also been shown that a reduction in absolute peripheral CD4+ T-lymphocyte count is associated with increased PET activity and possibly serves as a proxy for inflammatory activity [33▪]. A less commonly known laboratory marker, serum chitotriosidase, was found to be significantly correlated with PET activity whereas no correlation was found with ACE [34].
Being able to correlate PET results with data from pulmonary function test (PFT) could allow clinicians to monitor progression of disease. PFT results do not allow clinicians to differentiate between irreversible parenchymal damage or reversible inflammation. Multiple measurements are often needed to assess trends to ensure that declines are related to disease activity rather than changes in technique or effort. Individuals with parenchymal lung disease on PET will likely have a decline in gas exchange if the disease is not treated. And intuitively, when individuals with parenchymal disease receive therapy, vital capacity, forced expiratory volume in 1 s (FEV1), and the diffusion capacity of carbon monoxide (DLCO) improve significantly [35]. In patients undergoing treatment with infliximab, the change in the SUVmax after starting therapy has been shown to correlate with the change in forced vital capacity (FVC) and FEV1 but not with DLCO [36].
Additional studies are needed to provide evidence on the utility of biomarkers before performing or supplementing PET to determine the impact of biomarkers on the management of sarcoidosis.
ROLE OF PET IN TREATMENT DECISIONS
Determining when and how to treat a patient with sarcoidosis is challenging and depends on a combination of impairment of patient's quality of life and the potential for organ damage. PET may potentially be used to initiate treatment, monitor response, and modify treatment (Fig. 4). Positive findings on PET/CT have been shown to lead to increasing doses of corticosteroids and the initiation of methotrexate [37]. SUVmax has been shown to decrease once therapy is enacted, which also coincided with statistically significant improvement in self-reported symptoms [38]. Findings observed on PET have long-term implications, as the treatment for sarcoidosis involves medications, which can cause immunosuppression, metabolic, and other side effects. Tailoring doses to the appropriate level allow for effective treatment while minimizing these unnecessary consequences. A subset of patients who have reduced FDG uptake on follow-up PET can have immunosuppression reduced or even discontinued [39].
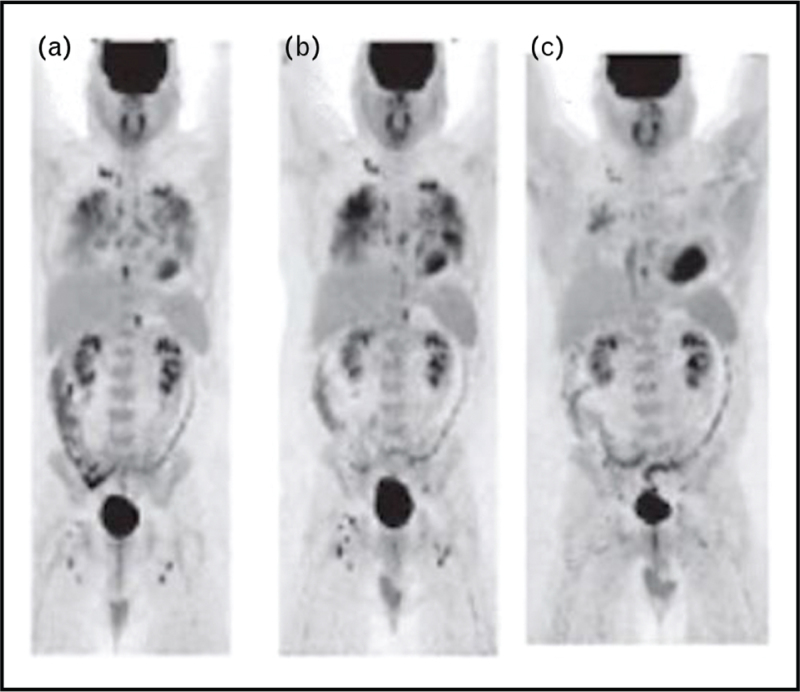
FIGURE 4.
Consecutive FDG-PET scans in patient with multisystem sarcoidosis. Methods: this is an original PET image from the authors’. Results: this figure shows three consecutive FDG-PET performed in a patient with progressively more aggressive therapy for sarcoidosis.
Pulmonary fibrosis is an independent predictor of mortality in patients with sarcoidosis [40▪▪]. In select patients, treatment of pulmonary disease because of sarcoidosis can lead to an improvement in lung function [41]. Pulmonary fibrosis, the result of persistent inflammation from sarcoidosis, represents inactive tissue but there can be co-existent active inflammatory activity in these area [27,28]. This is extremely useful in cases of advanced pulmonary sarcoidosis where identification of inflammatory activity leads to alteration of management (and possibly prognosis by preventing further fibrosis from ongoing inflammation) [28].
Data on the appropriate time interval for follow-up assessment and the role of PET-guided therapy is scarce. This latter is of particular importance in cardiac sarcoidosis , for which PET is the major method of assessing treatment response [42].
LIMITATIONS AND FUTURE DIRECTIONS
Although PET has a satisfactory sensitivity and specificity for identifying inflammation because of sarcoidosis, the current state of the literature is composed mostly of retrospective investigations. Prospective trials are needed to strengthen the role of PET in management of sarcoidosis. These trials should investigate a connection between PET findings and practical clinical outcomes. These studies have the potential to set a standard for preparation and interpretation of PET as well which is currently lacking. The importance of abnormal PET results in context of an asymptomatic patient needs more evidence base to support or withhold treatment. These results would make further studies, collaboration, and patient management more efficient.
CONCLUSION
At present, the primary role of PET is to diagnose cardiac sarcoidosis but PET may potentially be used to identify multisystem disease. PET can provide clinicians with valuable information regarding prognosis of cardiac sarcoidosis. With more research, PET may be utilized to monitor therapy efficacy. More prospective trials are necessary to determine how to optimize and when to apply PET.
Acknowledgements
The acknowledgements are limited to the three authors of this opinion.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Contributor Information
Robert J. Vender, Email: rjv5063@gmail.com.
Hamad Aldahham, Email: Aldahham@Temple.edu.
Rohit Gupta, Email: Rohit.Gupta@tuhs.temple.edu.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Lee S, Birnie D, Dwivedi G. Current perspectives on the immunopathogenesis of sarcoidosis. Respir Med 2020; 173:106161. [DOI] [PubMed] [Google Scholar]; This review discussed the role of the innate and adaptive immune system in generating the inflammation from sarcoidosis. It also discussed the current literature in regards to possible causes including auto-immunity.
- 2.Hunninghake GW, Costabel U, Ando M, et al. Statement on sarcoidosis. An J Respir Crit Care Med 2012; 160:736–755. [Google Scholar]
- 3.Crouser ED, Maier LA, Baughman RP, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society Clinical Practice guideline 2020; 201:E26–E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams H, Keijsers RG, Korenromp IHE, Grutters JC. FDG PET for gauging of sarcoid disease activity. Semin Respir Crit Care Med 2014; 35:352–361. [DOI] [PubMed] [Google Scholar]
- 5.Fu Y, Maianu L, Melbert BR, Garvey WT. Facilitative glucose transporter gene expression in human lymphocytes, monocytes, and macrophages: a role for GLUT isoforms 1, 3, and 5 in the immune response and foam cell formation. Blood Cells Mol Dis 2004; 32:182–190. [DOI] [PubMed] [Google Scholar]
- 6.Keijsers RGM, Grutters JC. In which patients with sarcoidosis is FDG PET/CT indicated? J Clin Med 2020; 9:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, Singh AD, Sharma SK, et al. Gallium-68 DOTA-NOC PET/CT as an alternate predictor of disease activity in sarcoidosis. Nucl Med Commun 2018; 39:768–778. [DOI] [PubMed] [Google Scholar]
- 8. Specificity and sensitivity of distinctive chest radiographic and/or 67Ga images in the noninvasive diagnosis of sarcoidosis - Document - Gale Academic OneFile [internet]. Available at: https://go-gale-com.libproxy.temple.edu/ps/i.do?p=AONE&u=temple_main&id=GALE%7CA13635157&v=2.1&it=r [Accessed 6 March 2022] [Google Scholar]
- 9.Klech H, Kohn H, Kummer F, Mostbeck A. Assessment of activity in sarcoidosis: sensitivity and specificity of 67gallium scintigraphy, serum ACE levels, chest roentgenography, and blood lymphocyte subpopulations. Chest 1982; 82:732–738. [DOI] [PubMed] [Google Scholar]
- 10.Nishiyama Y, Yamamoto Y, Fukunaga K, et al. Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med 2006; 47:1571–1576. [PubMed] [Google Scholar]
- 11.Jamar F, Buscombe J, Chiti A, et al. EANM/SNMMI guideline for 18 F-FDG use in inflammation and infection. J Nucl Med 2013; 54:647–658. [DOI] [PubMed] [Google Scholar]
- 12.Chareonthaitawee P, Beanlands RS, Chen W, et al. Joint SNMMI-ASNC expert consensus document on the role of 18F-FDG PET/CT in cardiac sarcoidosis detection and therapy monitoring. J Nucl Card 2017; 24:1741–1758. [DOI] [PubMed] [Google Scholar]
- 13▪.Lu Y, Sweiss NJ, Macapinlac HA. What is the optimal method on myocardial suppression in FDG PET/CT evaluation of cardiac sarcoidosis? Clin Nucl Med 2021; 46:904–905. [DOI] [PubMed] [Google Scholar]; This opinion reviewed different dietary modification undertaken prior to PET. The guidelines produced by the SNMMI/ASNC expert consensus produce variable rates of complete myocardial suppression. The group demonstrated lower rate of indeterminate PET results when a high-fat, high-protein, very low-carbohydrate diet was extended to 72 h prior to PET.
- 14▪▪.Özütemiz C, Koksel Y, Froelich JW, et al. Comparison of the effect of three different dietary modifications on myocardial suppression in 18 F-FDG PET/CT evaluation of patients for suspected cardiac sarcoidosis (diet myocardial supp PET/CT car sarcoid). J Nucl Med 2021; 62:1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]; This retrospective, single institution review demonstrated that extension of a 24 h ketogenic diet was extended to 72 h, the indeterminate rate of PET/CT decreased to 2% (P < 0.001) and the rate of complete myocardial suppression increased. This resulted in fewer false-positive results.
- 15.Lu Y, Grant C, Xie K, Sweiss NJ. Suppression of myocardial 18F-FDG uptake through prolonged high-fat, high-protein, and very-low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. Clin Nucl Med 2017; 42:88–94. [DOI] [PubMed] [Google Scholar]
- 16▪▪.Dietz M, Paulmier B, Berthier F, et al. An intravenous 100-ml lipid emulsion infusion dramatically improves myocardial glucose metabolism extinction in cardiac FDG PET clinical practice. Clin Nucl Med 2021; 46:E317–E324. [DOI] [PubMed] [Google Scholar]; This study demonstrated that higher rates of competed myocardial suppression can be achieved when intravenous lipid emulsion (100 ml) is administered in addition to a high-fat, low-carbohydrate diet. With the addition of intravenous lipid emulsion 3 h prior to PET/CT, the rate of complete myocardial suppression increased from 63 to 89% (P = 0.021).
- 17▪▪.Scholtens AM, van den Berk AM, van der Sluis NL, et al. Suppression of myocardial glucose metabolism in FDG PET/CT: impact of dose variation in heparin bolus preadministration. Eur J Nucl Med Mol Imaging 2020; 47:2698–2702. [DOI] [PubMed] [Google Scholar]; This study evaluated the rate of myocardial suppression among four different pre-PET dietary preparations. This demonstrated that the combination of low-carbohydrate diet, 12 h fast and intravenous heparin (50 IU/kg) produced higher rates of complete myocardial suppression when compared with lower doses of intravenous heparin (15 IU/kg).
- 18▪▪.Tuominen H, Haarala A, Tikkakoski A, et al. FDG-PET in possible cardiac sarcoidosis: right ventricular uptake and high total cardiac metabolic activity predict cardiovascular events. J Nuclear Cardiol 2021; 28:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]; This retrospective, single institution review described the rate of LV and right ventricular FDG uptake (24 vs. 9%). This study also demonstrated that individuals with a higher SUVmax had higher rates of LV dysfunction, ventricular tachycardia, and death (P < 0.001).
- 19▪▪.Flores RJ, Flaherty KR, Jin Z, Bokhari S. The prognostic value of quantitating and localizing F-18 FDG uptake in cardiac sarcoidosis. J Nucl Cardiol 2020; 27:2003–2010. [DOI] [PubMed] [Google Scholar]; This retrospective, single institution analysis of PET performed for the concern of cardiac sarcoidosis described the location of FDG uptake within the LV. It also demonstrated that SUVmax (OR 1.068, P = 0.002), SUVmean, (OR 1.059, P = 0.023), and LV EF (OR 0.731, P < 0.001) on resting perfusion scan were associated with the adverse cardiovascular events.
- 20.Juneau D, Nery P, Russo J, et al. How common is isolated cardiac sarcoidosis? Extra-cardiac and cardiac findings on clinical examination and whole-body 18F-fluorodeoxyglucose positron emission tomography. Int J Cardiol 2018; 253:189–193. [DOI] [PubMed] [Google Scholar]
- 21.Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014; 63:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokoyama R, Miyagawa M, Okayama H, et al. Quantitative analysis of myocardial 18F-fluorodeoxyglucose uptake by PET/CT for detection of cardiac sarcoidosis. Int J Cardiol 2015; 195:180–187. [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Pak K, Kim K, Kim J. Diagnostic performance of F-18 FDG PET for detection of cardiac sarcoidosis: a systematic review and meta-analysis. J Nucl Cardiol 2020; 27:2103–2115. [DOI] [PubMed] [Google Scholar]
- 24.Vita T, Okada DR, Veillet-Chowdhury M, et al. Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging 2018; 11:e007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wicks EC, Menezes LJ, Barnes A, et al. Diagnostic accuracy and prognostic value of simultaneous hybrid 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in cardiac sarcoidosis. Eur Heart J – Cardiovasc Imaging 2018; 19:757–767. [DOI] [PubMed] [Google Scholar]
- 26.Sgard B, Brillet PY, Bouvry D, et al. Evaluation of FDG PET combined with cardiac MRI for the diagnosis and therapeutic monitoring of cardiac sarcoidosis. Clin Radiol 2019; 74:81.e9–81.e18. [DOI] [PubMed] [Google Scholar]
- 27.Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med 2015; 36:603–619. [DOI] [PubMed] [Google Scholar]
- 28.Mostard RLM, Verschakelen JA, Van Kroonenburgh MJPG, et al. Severity of pulmonary involvement and 18 F-FDG PET activity in sarcoidosis. Respir Med 2013; 107:439–447. [DOI] [PubMed] [Google Scholar]
- 29.Ambrosini V, Zompatori M, Fasano L, et al. 18F-FDG PET/CT for the assessment of disease extension and activity in patients with sarcoidosis: results of a preliminary prospective study. Clin Nucl Med 2013; 38:e171–e177. [DOI] [PubMed] [Google Scholar]
- 30.Patel DC, Gunasekaran SS, Goettl C, et al. FDG PET-CT findings of extra-thoracic sarcoid are associated with cardiac sarcoid: a rationale for using FGD PET-CT for cardiac sarcoid evaluation. J Nucl Cardiol 2019; 26:486–492. [DOI] [PubMed] [Google Scholar]
- 31.Enyedi A, Csongrádi A, Altorjay IT, et al. Combined application of angiotensin converting enzyme and chitotriosidase analysis improves the laboratory diagnosis of sarcoidosis. Clin Chim Acta 2020; 500:155–162. [DOI] [PubMed] [Google Scholar]
- 32.Kalkanis A, Kalkanis D, Drougas D, et al. Correlation of spleen metabolism assessed by 18F-FDG PET with serum interleukin-2 receptor levels and other biomarkers in patients with untreated sarcoidosis. Nucl Med Commun 2016; 37:273–277. [DOI] [PubMed] [Google Scholar]
- 33▪.Vagts C, Ascoli C, Fraidenburg DR, et al. Unsupervised clustering reveals sarcoidosis phenotypes marked by a reduction in lymphocytes relate to increased inflammatory activity on 18FDG-PET/CT. Front Med (Lausanne) 2021; 8:595077. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is a retrospective, single institution review that demonstrated that a reduced absolute CD4+ T-lymphocyte count was associated with increased FDG uptake. With future research that confirms the reproducibility of this result, CD4+ count may serve as a marker for disease activity in sarcoidosis.
- 34.Popevi S, umarac Z, Jovanovi D, et al. Verifying sarcoidosis activity: chitotriosidase versus ACE in sarcoidosis- a case-control study. J Med Biochem 2016; 35:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keijsers RG, Verzijlbergen FJ, Van Den Bosch JM, et al. 18F-FDG PET as a predictor of pulmonary function in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2011; 28:123–129. [PubMed] [Google Scholar]
- 36.Schimmelpennink MC, Vorselaars ADM, Veltkamp M, Keijsers RGM. F-FDG PET/CT: SUVmax versus total lung glycolysis. EJNMMI Res 2019; 9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobic-Saranovic D, Grozdic I, Videnovic-Ivanov J, et al. The utility of 18F-FDG PET/CT for diagnosis and adjustment of therapy in patients with active chronic sarcoidosis. J Nucl Med 2012; 53:1543–1549. [DOI] [PubMed] [Google Scholar]
- 38.Sobic-Saranovic DP, Grozdic IT, Videnovic-Ivanov J, et al. Responsiveness of FDG PET/CT to treatment of patients with active chronic sarcoidosis. Clin Nucl Med 2013; 38:516–521. [DOI] [PubMed] [Google Scholar]
- 39.Ahmadian A, Pawar S, Govender P, et al. The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J Nucl Cardiol 2017; 24:413–424. [DOI] [PubMed] [Google Scholar]
- 40▪▪.Jeny F, Uzunhan Y, Lacroix M, et al. Predictors of mortality in fibrosing pulmonary sarcoidosis. Respir Med 2020; 169:105997. [DOI] [PubMed] [Google Scholar]; This retrospective analysis demonstrated that composite physiologic index (CPI), extent of lung fibrosis, pulmonary hypertension on echocardiography, Walsh's algorithm, and geographic region were independent predictors of mortality in patient with sarcoidosis.
- 41.Broos CE, Wapenaar M, Looman CWN, et al. Daily home spirometry to detect early steroid treatment effects in newly treated pulmonary sarcoidosis. Eur Resp J 2018; 51:1702089. [DOI] [PubMed] [Google Scholar]
- 42.Osborne MT, Hulten EA, Singh A, et al. Reduction in 18F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol 2014; 21:166–174. [DOI] [PubMed] [Google Scholar]