Abstract
Background
Thromboembolic events are common complications of COVID-19. Clinical study results on safety and efficacy of anticoagulation in COVID-19 are controversial.
Material and methods
This report updates our systematic review and random-effects meta-analysis on randomized controlled trials (RCTs) comparing standard prophylactic anticoagulation and intermediate or therapeutic anticoagulation in COVID-19 patients. We searched eligible studies for the update up to 4 February 2022 by weekly monitoring of RCTs in the Cochrane COVID-19 Study Register. Certainty of evidence was assessed using GRADE (Grading of Recommendations Assessment, Development and Evaluation).
Results
For this update we included five new trials; a total of 13 RCTs with 7364 patients. Certainty of evidence was very low to low. We are uncertain whether low-dose prophylactic anticoagulation is favoured over placebo or no anticoagulation in the outpatient- or post-discharge-setting. In hospitalized patients with moderate and severe COVID-19, intermediate-dose anticoagulation may have little or no effect on thrombotic events or death (RR 1.03, 95 % CI 0.86–1.24), but may increase severe bleeding non-significantly (RR 1.48, 95 % CI 0.53–4.15). Therapeutic-dose anticoagulation may decrease thrombotic events or deaths in hospitalized patients with moderate COVID-19 (RR 0.64, 95 % CI 0.38–1.07; fixed-effect model RR 0.72, 95 % CI 0.57–0.91), but may have little or no effect in patients with severe disease (RR 0.98, 95 % CI 0.86–1.12). With therapeutic-dose anticoagulation, the risk of major bleeding may increase regardless of COVID-19 severity (RR 1.78, 95 % CI 1.15–2.74).
Conclusions
Hospitalized, moderately ill COVID-19 patients may benefit from therapeutic-dose anticoagulation, while critically ill patients may not. Risk of major bleeding must be considered.
Keywords: Systematic review, Anticoagulant therapy, COVID-19, Thrombosis, Bleeding
1. Introduction
In its severe form, COVID-19, the clinical manifestation of SARS-CoV-2 infection, is characterized by lung failure and high rates of thromboembolic complications [1]. Given the procoagulant status and increased thrombotic risk of COVID-19 patients, modified empirical dosage regimens have been used in many places to treat COVID-19 patients and the different modes of anticoagulation were consecutively subject of several clinical trials. However, the question whether early prophylactic anticoagulation in outpatient settings or intensified prophylactic intermediate-dose or therapeutic anticoagulation in inpatient settings can reduce the risk of disease progression without increasing the risk of adverse events remains unanswered [2]. At the same time, it is still unclear to what extent there is an increased risk of thromboembolism even after hospital discharge, and whether this can be prevented with appropriate anticoagulation [3], [4]. We therefore conducted a systematic review with meta-analysis of the available randomized controlled trials (RCTs) to determine the safety and efficacy of anticoagulation at any dosage with standard low-dose prophylactic anticoagulation or no prophylaxis in COVID-19 patients regardless of disease severity and treatment setting. The present meta-analyses informed the German AWMF-S2e guideline for outpatient COVID-19 patients and the AWMF-S3 guideline for the inpatient therapy of COVID-19 patients [5], [6].
2. Materials and methods
This report is an update of a systematic review published in December 2021 [7]. The systematic review protocol was registered with PROSPERO on January 21, 2021 (CRD42021229228). The question is continuously updated in the sense of a ‘living systematic review’. A detailed German version of the updated systematic review is accepted for publication at a German journal. This English version addresses the worldwide community of critical care specialists, primary care specialists and other healthcare personnel dealing with COVID-19 patients.
2.1. Inclusion criteria for studies in this systematic review
We included RCTs that compared prophylactic anticoagulation at any dosage with anticoagulation at a different dosage or with no anticoagulation in outpatients, hospitalized, or post-discharge patients with confirmed SARS-CoV-2 infection. All studies were considered for inclusion in the analysis, regardless of the severity of the disease, age, gender, and ethnicity of the study participants. COVID-19 severity of study participants was, if possible, classified according to the definition of the WHO ‘clinical progression scale’ (WHO 0 to 10): outpatient, mildly ill COVID-19 patients (WHO 1–3); inpatient, moderately ill COVID-19 patients (WHO 4–5) and intensive care, seriously ill COVID-19 patients (WHO 6–9) [8]. Separate meta-analyses were performed and reported for patients in outpatient, inpatient and post-discharge settings as well as according to the included severity of COVID-19 disease. There were no restrictions on the type of pharmacological anticoagulation used. All heparinoids, vitamin K antagonists and direct anticoagulants (factor Xa inhibitors and direct thrombin inhibitors) were considered regardless of dosage and administration regimen. Dosage regimens of anticoagulants have been divided into low-dose, intermediate-dose or therapeutic anticoagulation according to the definition of the studies and the general drug recommendations summarized in Table S1 [9]. Standard anticoagulation in the control arm in COVID-19 inpatients included both low-dose anticoagulation with low molecular weight heparin (LMWH) or unfractionated heparin (UFH), as well as intermediate-dose anticoagulation regimens. The latter were amended in the course of the pandemic by adjustments to national therapy guidelines (i.e. in the United Kingdom) and formed part of the standard treatment [10]. We therefore expanded our definition of standard prophylactic anticoagulation to include low-dose and intermediate-dose anticoagulation regimens and created the following comparisons for meta-analyses:
-
•
Therapeutic anticoagulation versus standard prophylactic anticoagulation (low-dose or intermediate-dose anticoagulation)
-
•
Intermediate-dose anticoagulation versus standard prophylactic anticoagulation (low-dose anticoagulation)
-
•
Standard prophylactic anticoagulation (low-dose) versus no prophylaxis or placebo
The evaluation of the efficacy of anticoagulation in hospitalized COVID-19 patients was carried out by recording mortality, worsening or improvement in clinical status, thrombotic events with and without death and quality of life (day 28 or longest follow-up). In COVID-19 outpatients, the combined outcome of hospitalization or death was supplemented. For this review update, no new data for the outcomes on clinical worsening or improvement of COVID-19 inpatients were available. The outcomes were therefore not included in this update. The safety of interventions was assessed by recording serious adverse events (SAEs), adverse events (AEs) and severe bleeding (according to ISTH criteria [11]) during the study period. In post-discharge patients, safety-relevant outcomes were supplemented by clinically relevant but non-severe bleedings and other bleeding according to the ISTH criteria [11].
2.2. Search methods
A systematic search of the Cochrane COVID-19 Study Register (consisting of MEDLINE, Embase, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, medRxiv and the Cochrane Central Register of Controlled Trials), Web of Science (Emerging Citation Index and Science Citation Index), the COVID-19 Global literature on coronavirus disease Database of the WHO and ResearchSquare, took place up to and including 24 September 2021. The subsequent period up to and including 4 February 2022 was covered by weekly RCT monitoring in the Cochrane COVID-19 Study Registry. The search strategies are reported in the supplement.
Two review authors independently examined titles and abstracts of all entries and the full texts of potentially relevant studies for their inclusion in this review. The study selection process is reported in a flowchart and follows PRISMA guidelines [12].
2.3. Data collection and analysis
Two review authors extracted data independently using a custom data extraction sheet according to Cochrane guidelines [13].
The Risk of Bias 2 (RoB 2) tool was used to assess the bias risk of study results that contributed information to the specified outcomes [14]. The RoB 2 ratings per study outcome were independently evaluated by two review authors according to Cochrane's recommendations and for the following domains [15]: selection bias, performance bias, detection bias, attrition bias, and selective reporting bias. For each domain and all domains together (overall bias risk), the bias risk of a study result was classified as low, some concerns or high.
For dichotomous outcomes, the number of events and the total number of participants in both the intervention and control groups were recorded. The relative risk (RR) with 95 % confidence interval (CI) was used as an effect measure.
Meta-analyses were carried out using the Mantel Haenszel method under a random effects model [16]. Random effects meta-analyses were performed with RevMan Web 3.11.1 and R (package “meta”, version 5.2–0) [17], [18]. Fixed-effects meta-analyses and exclusion of studies with high risk of bias were carried out as sensitivity analyses. For hospitalized individuals with moderate or severe COVID-19, subgroup analyses were performed according to the disease severity at baseline (moderate (WHO 4 to 5) versus severe COVID-19 disease (WHO 6 to 9) as defined by the WHO clinical progression scale [8]). Studies that only provided data for a mixed population of moderately and severely ill participants were included in the subgroup “moderate to severe COVID-19 (WHO 4 to 9)”. Statistical heterogeneity was defined as P < 0.1 for the Chi2 test for heterogeneity or an I2 statistic ≥50 %.
Quality of the evidence was assessed according to the GRADE methodology (Grading of Recommendations, Assessment, Development and Evaluations) [19]. GRADE assesses the trustworthiness (certainty) of the evidence in four levels: very low, low, moderate, and high. Certainty of evidence was downgraded by one or two levels for risk of bias, imprecision, inconsistency (heterogeneity), indirectness (indirect study results) and publication bias.
3. Results
The search strategy identified a total of 1153 entries in registers and databases, two more entries were identified from other sources. After removal of duplicates, 1076 titles and abstracts were viewed, of which 942 were deemed irrelevant. The screening of 134 full texts resulted in 20 studies with a total of 32 entries that were excluded and 59 included studies with 102 entries. Of these, 50 studies (66 entries) had not yet been completed at the time of the search. Nine studies (36 entries) were included in this systematic review from the search. Monitoring of the RCT database from 25 September 2021 onwards provided a further 14 entries up to and including 4 February 2022, four of which belonged to studies not yet completed or published in full text (Letter). Four studies (10 entries) were included. The search is summarized in a PRISMA flowchart (Fig. S1).
3.1. Characteristics of included studies
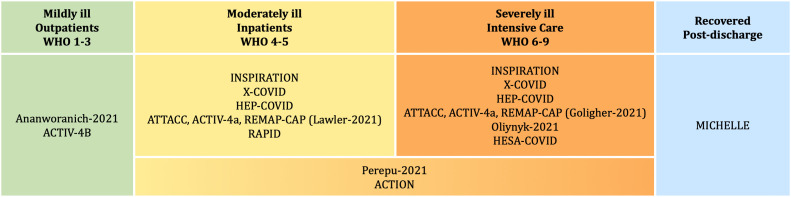
Thirteen RCTs with a total of 7364 randomized patients, investigating anticoagulation in COVID-19 patients were included in this review (Fig. 1 , Table 1 ). ACTIV-4B and Ananworanich-2021 studied outpatients with symptomatic COVID-19 disease (WHO 2–3) [20], [21]. The ATTACC, ACTIV-4a, REMAP-CAP platform study (Lawler-2021) and the RAPID study predominantly examined moderately ill COVID-19 patients (WHO 4–5) [22], [23]. Three studies, the HESA-COVID study [24], the platform study ATTACC, ACTIV-4a, REMAP-CAP (Golligher-2021) [25] and Oliynyk-2021 [26] recruited patients with severe COVID-19 disease (WHO ≥6) [24], [25], [26]. A total of five studies examined a mixed study population (WHO 4–9) [27], [28], [29], [30], [31], [32]. For three of them, HEP-COVID, INSPIRATION and X-COVID, data could be evaluated separately according to disease severity; for Perepu-2021 and ACTION splitting into moderate and severe COVID-19 at baseline was not possible. MICHELLE reported data for post-discharge patients who had been hospitalized due to COVID-19 infection [33].
Fig. 1.
Classification of included studies according to COVID-19 severity of participants at randomization (WHO clinical progression scale, WHO 0 to 10).
Table 1.
Characteristics of included studies.
| Study reference | Study design | Randomized patients (n) | Patient status | Intervention | Comparator | Outcomes |
|---|---|---|---|---|---|---|
| Ananworanich-2021 [20] | RCT, double-blind, 14 centers in the U.S. Recruitment: 08/2020–02/2021 Up to 10 days after positive SARS-CoV-2 test |
497 | Outpatient WHO 2–3 At least 1 risk factor for severe course Thrombosis risk factorsA: NA COVID-19 risk factorsB: 3 |
Low doseC: Rivaroxaban 10 mg OD for 21 days |
Placebo | 35-Day mortality, hospitalization within 28 days, severe adverse events within 35 days, adverse events within 35 days, severe bleeding within 35 days |
| ACTIV 4B, Connors-2021 [21] | RCT, double-blind, 4 study arms, 52 centers in the U.S. Recruitment: 09/2020–06/2021 Start of therapy: NA |
657 | Outpatient WHO 2–3 Thrombosis risk factorsA: NA COVID-19 risk factorsB: 3 |
Low doseC: Apixaban 2.5 mg BID for 45 days |
Placebo | 45-Day mortality, hospitalization due to cardiovascular events or death within 45 days, thrombotic events within 45 days, severe bleeding within 45 days |
| INSPIRATION, Sadeghipour-2021 [27], [28] | RCT, open-label, 10 Centers in Iran Recruitment: 07/2020–11/2020 Start of therapy: NA |
600 | Hospitalized + Intensive Care Unit WHO 5–9, with 45 % WHO 5 Stratified results according to WHO 4–5 and WHO 6–9 Thrombosis risk factorsA: 1 COVID-19 risk factorsB: 3 |
Intermediate doseC: Enoxaparin 1 mg/kg OD weight and CrCI adjusted, for 30 days |
Low doseC: enoxaparin 40 mg OD; Weight and CrCI adjusted |
30-Day mortality, 90-day mortality, venous thrombotic events, venous thrombotic events or death, major bleeding |
| Perepu-2021 [32] | RCT open-label, multicentric: 3 centers in the USA Recruitment: 04/2020–01/2021 Start of therapy: NA |
173 | Hospitalized + Intensive Care Unit and/or mod. ISTH Overt DIC Score ≥ 3, WHO 5–9 * no information on respiratory status reported Thrombosis risk factorsA: 2 COVID-19 risk factorsB: 4 |
Intermediate doseC: Enoxaparin 1 mg/kg OD weight and CrCI adjusted until discharge from hospital |
Low doseC: Enoxaparin 40 mg OD weight and CrCI adjusted, until discharge |
30-Day mortality, venous thrombotic events, major bleeding |
| X-COVID, Morici-2021 [30] | RCT open-label, multicentric: 9 centers in Italy Recruitment: 04/2020–04/2021 Start of therapy: within 6–7 days after admission |
186 | Hospitalized + Intensive Care Unit WHO 4–7 with 61 % WHO 4–5 Stratified results according to WHO 4–5 and WHO 6–7 Thrombosis risk factorsA: NA COVID-19 risk factorsB: 2 |
Intermediate doseC: Enoxaparin 40 mg BID until discharge from the hospital |
Low doseC: Enoxaparin 40 mg OD until discharge |
30-Day mortality, venous thrombotic events, venous thrombotic events or deaths, major bleeding |
| HESACOVID, Lemos-2020 [24] | RCT open-label, unicentric: Brazil Recruitment: 05/2020–05/2021 Start of therapy: NA |
20 | Hospitalized + Intensive Care Unit + ↑ D-Dimer WHO ≥7 Thrombosis risk factorsA: 1 COVID-19 risk factorsB: 4 |
Therapeutic doseC: Enoxaparin 1 mg/kg BID for at least 96 h and up to 14 days |
Low doseC: Enoxaparin 40 mg OD, weight and CrCI adjusted |
28-Day mortality, hospital mortality, thrombotic events |
| ACTION, Lopes-2021 [31] | RCT open-label, multicentric: 31 centers in Brazil Recruitment: 06/2020–02/2021 Start of therapy: Up to 14 days after symptom onset |
614 | Hospitalized + intensive care unit + ↑ D-dimer, WHO 4–9, with 85 % WHO 4–5 Thrombosis risk factorsA:1 COVID-19 risk factorsB: 4 |
Therapeutic doseC: Rivaroxaban 20 mg OD (280 patients, 90 %) for 30 days |
Low doseC: Enoxaparin 40 mg OD, weight and CrCI adjusted, continued until discharge |
30-Day mortality, survival until discharge from hospital (30 days), thrombotic events, thrombotic events or deaths, major bleeding |
| ATTACC, ACTIV-4a, REMAP-CAP, Lawler-2021 [22] | RCT open-label, Platform study: 121 centers in 9 countries Recruitment: 04/2020–01/2022 Start of therapy: within 72 h after admission |
2244 | Hospitalized WHO 4–5, of which 5 % WHO 6–7 Thrombosis risk factorsA: 1 COVID-19 risk factorsB: 3 |
Therapeutic doseC: Enoxaparin 1 mg/kg sc minus 10 % BID, weight and CrCl adjusted (79.6 %) |
Low/intermediate doseC: 78.7 %: enoxaparin, 9.6 %: dalteparin; Low dose: 71.7 %, Intermediate dose: 26.5 % |
Mortality in hospital, clinical improvement: discharge without organ support, thrombotic event, thrombotic event or death, major bleeding |
| ATTAC, ACTIV-4a, REMAP-CAP, Goligher-2021 [25] | RCT open-label, Platform study: 121 centers in 9 countries Recruitment: 04/2020–01/2022 Start of therapy: Randomization within 72 h after hospital admission |
1207 | Intensive care unit WHO 6–9, 1.5 % WHO 4–5 Thrombosis risk factorsA: 1 COVID-19 risk factorsB: 3 |
Therapeutic doseC: Enoxaparin 1 mg/kg minus 10 % BID, weight and CrCl adjusted (77.6 %) |
Low/intermediate doseC: 52.1 %: enoxaparin, 32.8 %: dalteparin; Low dose: 40.4 %, Intermediate dose: 51.7 % |
Mortality in hospital, thrombotic events, thrombotic events or deaths, major bleeding |
| RAPID, Sholzberg-2021 [23] | RCT open-label, multicentric: 28 centers in 6 countries Recruitment: 05/2020–04/2021 Start of therapy 24 h/48 h after randomization |
465 | Hospitalized + ↑ D-Dimer, WHO 4–5, with 6 % WHO 6 Thrombosis risk factorsA: 1 COVID-19 risk factorsB: 3 |
Therapeutic doseC: Enoxaparin 1 mg/kg BID; Weight and CrCI adjusted |
Low doseC: Enoxaparin 40 mg OD, weight and CrCl adjusted |
28-day mortality, thrombotic events, major bleeding |
| HEP-COVID, Spyropoulos-2021 [29] | RCT open-label, multicentric: 12 centers in the USA Recruitment: 05/2020–05/2021 Start of therapy: Screening within 72 h from hospital admission |
257 | Hospitalized + ↑ D-Dimer or ISTH SIC Score ≥ 4, WHO 5–7, with 77 % WHO 5 Stratified results according to WHO 5 and WHO 6–7 Thrombosis risk factorsA: 1 COVID-19 risk factorsB: 4 |
Therapeutic doseC: Enoxaparin 1 mg/kg BID or 40 mg OD/BID weight and CrCI adjusted, until discharge |
Low doseC: Enoxaparin 40 mg OD/BID weight and CrCI adjusted until discharge |
30-day mortality, thrombotic events, thrombotic events or deaths, major bleeding |
| Oliynyk-2021 [26] | RCT double-blind, unicentric: Ukraine Recruitment: 07/2020–03/2021 Start of treatment: NO |
126 | Intensive care unit + CAC, not intubated WHO 6 Thrombosis risk factorsA: 3 COVID-19 risk factorsB: 2 |
Therapeutic doseC: Enoxaparin: 100 Anti-Xa IU/kg BID or UFH: Initial: 80 U/kg/h i.v.; danac 18 U/kg/h until normalization of D-dimer |
Low doseC: Enoxaparin 50 Anti-Xa IU/kg QD for 28 days |
28-day mortality |
| MICHELLE, Ramacciotti-2022 [33] | RCT open-label, multi-centric: 14 centers in Brazil Recruitment: 10/2020–06/2021 Start of therapy: 24 h after hospital discharge |
318 | Post discharge + IMPROVE-Score ≥ 4 or 2–3 + ↑ D-Dimer Thrombosis risk factorsA: 1 COVID-19 risk factorsB: 3 |
Low doseC: Rivaroxaban 10 mg OD for 35 days |
No anticoagulation | Any thrombotic event and cardiovascular death, any symptomatic venous thrombotic event or all causes, major bleeding, clinically relevant non-severe bleeding, other bleeding |
RCT, randomized controlled trial; ^ D-Dimer, D-Dimer elevation; OD, once daily; BID, twice daily; UFH, unfractionated heparin; CrCl, creatinine clearance, CAC, COVID-19 associated coagulopathy.
Thrombosis risk factors: D-dimer elevation, genetic predisposition, clinical signs of DVT/LAE, LAE/DVT more likely than other diagnoses, tachycardia (heart rate > 100/min), surgery/immobilization (at least 3 days) within the last month, Previous LAE or DVT, Hemoptysis, Malignancy (under therapy, palliative therapy or diagnosis younger than 6 months), Wells Score.
COVID-19 risk factors [34]: Age > 50, Male, Smoker, BMI > 30, Pregnancy, Trisomy 21, Cardiovascular Disease (High Blood Pressure, Coronary Heart Disease), Chronic Lung Disease (COPD, Asthma), Chronic Kidney or Liver Disease, Neurological or Psychiatric Diseases, Diabetes Mellitus, Immunodeficiency, Cancer.
According to study definition.
Seven studies used markers of hypercoagulability and coagulopathy as inclusion criteria [23], [24], [26], [29], [31], [32], [33]. Three studies reported on thrombosis risk factors of the enrolled patients in the tables describing the study collective [22], [25], [28], three studies did not provide information [20], [21], [30].
MICHELLE, Ananworanich-2021 and ACTIV-4B investigated low-dose oral anticoagulation (rivaroxaban 10 mg once daily and apixaban 2.5 mg twice daily) compared to no anticoagulation [33] and placebo [20], [21] respectively. ACTIV-4B also investigated a therapeutic dosage, which was not included in our meta-analysis [21]. Three studies, INSPIRATION, Perepu-2021 and X-COVID, examined intermediate-dose anticoagulation (enoxaparin 1 mg/kg OD or 40 mg BID) compared to standard prophylactic anticoagulation [27], [28], [30], [32]. All other studies looked at therapeutic anticoagulation versus standard prophylactic anticoagulation [22], [23], [24], [25], [26], [29], [31]. Five studies defined standard prophylactic anticoagulation as low-dose anticoagulation [2], [23], [24], [26], [29], [31] while new national treatment guidelines in the UK resulted in 26.5 % and 51.7 % of participants in the control groups of the large platform studies ATTAC, ACTIV-4a and REMAP-CAP receiving intermediate-dose anticoagulation [2], [22], [25]. The type and dosage of intervention and control treatment of the respective studies are listed in Table 1.
All studies reported relevant outcomes for this systematic review. The time period for outcomes collection was 28–30 days in the majority of studies. MICHELLE examined a period of 35 days [33] ACTIV-4B 45 days [21] and INSPIRATION reported on the 90-day mortality [27]. No study reported data on quality of life.
3.2. Risk of bias
In total, the thirteen studies contributed 48 study results to 23 outcomes reported here, seven outcomes for the comparison “COVID-19 outpatients: standard prophylactic anticoagulation versus placebo”, five for the comparison “COVID-19 inpatients: intermediate-dose anticoagulation versus standard prophylactic anticoagulation”, five for the comparison “COVID-19 inpatients: therapeutic anticoagulation versus standard prophylactic anticoagulation” and six for the comparison “post-discharge COVID-19 patients: standard prophylactic anticoagulation versus no prophylaxis.” One third of the 48 study results (33.3 %) were rated as “overall low risk of bias”, 54.2 % as “some concerns about the overall bias risk” and 12.5 % as “overall high risk of bias”.
COVID-19 outpatients: standard prophylactic anticoagulation versus placebo
Ananworanich-2021 [20] and ACTIV-4B [21] were included in the comparison of standard prophylactic anticoagulation with low-dose anticoagulation versus placebo in the outpatient setting (meta-analyses in Table S2). We are uncertain whether standard prophylactic anticoagulation compared to placebo treatment increases or decreases all-cause mortality (RR 0.33, 95 % CI 0.01–8.07, 778 patients, 2 studies), hospitalization rate or death (RR 0.43, 95 % CI 0.11–1.64, 444 patients, 1 study), hospitalization rate due to cardiopulmonary events or death (RR 0.62, 95 % CI 0.21–1.86, 329 patients, 1 study), any thrombotic events (RR 0.33, 95 % CI 0.01–8.07, 329 patients, 1 study) or serious adverse events (RR 0.30, 95 % CI 0.06–1.43, 449 patients, 1 study). Standard prophylactic anticoagulation may increase improvement of clinical status assessed as the rate of asymptomatic participants slightly compared to placebo (RR 1.16, 95 % CI 0.97–1.38, 444 participants, 1 study). Standard prophylactic anticoagulation may have little or no difference on the risk of adverse events compared to placebo (RR 1.02, 95 % CI 0.67–1.56, 449 patients, 1 study). Evidence certainty for adverse events and clinical improvement was considered low. For all other outcomes, evidence certainty was rated as very low (Table S2).
3.3. COVID-19 inpatients: intermediate-dose anticoagulation versus standard prophylactic anticoagulation (low-dose anticoagulation)
Intermediate-dose anticoagulation may have little or no effect on mortality after 30 days (RR 1.02, 95 % CI 0.75–1.40, 913 participants, 3 studies, moderate-certainty evidence, Table 2 and fig. S2) and after 90 days (RR 1.07, 95 % CI 0.89–1.28, 590 participants, 1 study, low-certainty evidence, Table 2), and the occurrence of venous thrombotic events or deaths (RR 1.02, 95 % CI 0.85–1.23, 764 participants, 2 studies, low-certainty evidence, Table 2 and Fig. S2). Intermediate-dose anticoagulation resulted in a non-significant increase in the risk of major bleeding compared to standard prophylactic anticoagulation (RR 1.43, 95 % CI 0.54–3.74, 913 participants, 3 studies, low-certainty evidence, Table 2). Certainty of evidence was downgraded once for the outcome 30-day mortality due to imprecision, and twice for all other outcomes due to risk of bias and imprecision.
Table 2.
Meta-analysis for intermediate-dose anticoagulation versus standard prophylactic anticoagulation in COVID-19 inpatients including absolute effect estimates, risk of bias assessment, and certainty of evidence.
| Outcome | Study population⁎ | Relative effect (RR, M-H, Random, 95 % CI) | Relative effect (RR, M-H, Fixed, 95 % CI) | Absolute effect per 1000 (95 % CI) |
Heterogeneity |
Risk of bias⁎⁎ (overall) |
Certainty of evidence |
|
|---|---|---|---|---|---|---|---|---|
| Standard prophylactic anticoagulation | Intermediate anticoagulation | |||||||
| 30-Day mortality | Pooled effect, mixed study population (WHO 4–9), 913 participants, 3 studies [27], [28], [30], [32] | 1.02 [0.75–1.40] | 1.00 [0.84–1.20] | 296 per 1000 | 302 per 1000 | Chi2 = 6.20, df = 4 (P = 0.18); I2 = 35 % |
Some concerns | Moderate due to imprecision (−1) |
| Difference 6 more (95 % CI 74 fewer – 118 more) |
||||||||
| 90-Day mortality | Mixed study population (WHO 4–9), 590 participants, 1 study [27], [28] | 1.07 [0.89–1.28] | 1.07 [0.89–1.28] | 418 per 1000 | 447 per 1000 | NA | Some concerns | Low due to risk of bias (−1) and imprecision (1-) |
| Difference 29 more (95 % CI 46 fewer – 117 more |
||||||||
| Venous thrombotic events or death (30 days) | Pooled effect, mixed study population (WHO 4–9), 764 participants, 2 studies [27], [28], [30] | 1.02 [0.85–1.23] | 1.02[0.85–1.22] | 346 per 1000 | 353 per 1000 | Chi2 = 0.26, df = 2 (P = 0.88); I2 = 0 % |
High | Low due to risk of bias (−1) and imprecision (−1) |
| Difference 7 more 95 % CI 52 fewer – 80 more |
||||||||
| Major bleeding (30 days) | Pooled effect, mixed study population (WHO 4–9), 913 participants, 3 studies [27], [28], [30], [32] | 1.43 [0.54–3.74] | 1.44 [0.55–3.73] | 15 per 1000 | 21 per 1000 | Chi2 = 0.32, df = 3 (P = 0.96); I2 = 0 % |
Some concerns | Low due to risk of bias (−1) and imprecision (−1) |
| Difference 6 more 95 % CI 7 fewer - 41 more |
||||||||
RR, relative risk;M-H, Mantel-Haenszel; CI, confidence interval.
Patient status according to WHO clinical progression scale.
Overall bias risk of studies with events in at least one study arm.
3.4. COVID-19 inpatients: therapeutic dose anticoagulation versus standard prophylactic anticoagulation (low-dose or intermediate-dose anticoagulation)
Seven studies [22], [23], [24], [25], [26], [29], [31] involving 4933 hospitalized patients with moderate to severe COVID-19 were included in the comparison of therapeutic-dose anticoagulation versus standard prophylactic anticoagulation with low-dose or intermediate-dose anticoagulation (Table 3 ). In the pooled meta-analysis with moderately and severely ill COVID-19 patients, therapeutic-dose anticoagulation may have little or no effect on reduction of all-cause mortality after 28 days (RR 0.69, 95 % CI 0.42–1.14, 1478 participants, 5 studies, low-certainty evidence, Table 3 and fig. S3), with high heterogeneity regarding individual study results (P = 0.02; I2 = 63 %). Subgroup analysis by COVID-19 severity showed a significant subgroup difference (P = 0.02). In moderately ill COVID-19 patients, therapeutic-dose anticoagulation may reduce mortality (RR 0.39, 95 % CI 0.16–0.96, 635 participants, 2 studies, low-certainty evidence, Table 3 and Fig. S3). The meta-analysis of in-hospital mortality showed no difference in therapeutic-dose anticoagulation compared to standard prophylactic anticoagulation (RR 0.97, 95 % CI 0.79–1.19, 3344 participants, 3 studies, low-certainty evidence, Table 3 and Fig. S3) with low heterogeneity regarding study results with moderate and severe COVID-19 participants (P = 0.25; I2 = 28 %). In the pooled meta-analysis, therapeutic-dose anticoagulation may have little or no effect on the occurrence of thrombotic events or deaths within 28 days (RR 0.86, 95 % CI 0.71–1.06, 4184 participants, 4 studies, low-certainty evidence, Table 3 and Fig. S3) with high heterogeneity between the individual study results (P = 0.07; I2 = 54 %). Subgroup analysis by COVID-19 severity showed no significant subgroup difference (P = 0.27). However, in patients with moderate COVID-19, therapeutic-dose anticoagulation may decrease the risk of thrombotic events or death compared to standard prophylactic anticoagulation when using the fixed-effect model in a sensitivity analysis (RR 0.72, 95 % CI 0.57–0.91, 2396 participants, 2 studies, low-certainty evidence, Table 3 and Fig. S3). In participants with severe COVID-19, therapeutic-dose anticoagulation may have little or no effect on this outcome compared to standard thromboprophylaxis (RR 0.98, 95 % CI 0.86–1.12, 1174 participants, 2 studies, low-certainty evidence, Table 3 and Fig. S3). Therapeutic-dose anticoagulation may increase the risk of major bleeding compared to standard prophylactic anticoagulation, regardless of disease severity (RR 1.78, 95 % CI 1.15–2.74, 4650 participants, 5 studies, low-certainty evidence, Table 3). Certainty of evidence was assessed as low for all outcomes and downgraded due to risk of bias, indirectness, imprecision, or inconsistency (Table 3). The indirectness was assumed based on two studies with major weight in the analyses that used a mixture of low- and intermediate-dose anticoagulation in the control arm [22], [25].
Table 3.
Meta-analyses of therapeutic-dose anticoagulation versus standard prophylactic anticoagulation in COVID-19 inpatients including absolute effect estimates, risk of bias assessment, and certainty of evidence.
| Outcome | Study population⁎ | Relative effect (RR, M-H, Random, 95 % CI) | Relative effect (RR, M-H, Fixed, 95 % CI) | Absolute effect estimation per 1000 (95 % CI) |
Heterogeneity |
Risk of bias⁎⁎ (overall) |
Certainty of evidence |
|
|---|---|---|---|---|---|---|---|---|
| Standard prophylactic anticoagulation | Therapeutic anticoagulation | |||||||
| 28-Day mortality | Moderately ill population (WHO 4–5), 635 participants, 2 studies [23], [29] | 0.39 [0.16–0.96] | 0,39 [0.21–0.73] | 105 per 1000 | 41 per 1000 | Chi2 = 1.92, df = 1 (P = 0.17); I2 = 48 % | Low | Low due to imprecision (−1) and inconsistency (−1) |
| Difference 64 fewer 95 % CI 88 less–4 less) | ||||||||
| Severely ill population (WHO 6–9), 229 participants, 3 studies [24], [26], [29] | 0.73 [0.49–1.09] | 0.71 [0.48–1.06] | 356 per 1000 | 260 per 1000 | Chi2 = 1.46, df = 2 (P = 0.48); I2 = 0 % | Some concerns | Low due to risk of bias (−1) and imprecision (−1) | |
| Difference 96 fewer 96 % CI 182 less–32 more | ||||||||
| Mixed population (WHO 4–9), 614 participants, 1 study (31) | 1.49 [0.90–2.46] | 1.49 [0.90–2.46] | 76 per 1000 | 113 per 1000 | NA | Low | Low due to imprecision (−2) | |
| Difference 37 more 96 % CI 8 less–35 more | ||||||||
| Pooled effect, mixed population (WHO 4–9),1478 participants, 5 studies [23], [24], [26], [29], [31] | 0.69 [0.42–1.14] | 0.79 [0.60–1.04] | 124 per 1000 | 86 per 1000 | Chi2 = 13.53, df = 5 (P = 0.02); I2 = 63 % | Some concerns | Low due to imprecision (−1) and inconsistency (−1) | |
| Difference 38 fewer 95 % CI 72 less–17 more | ||||||||
| All-cause mortality in hospital | Pooled effect, mixed population (WHO 4–9), 3344 participants, 3 studies [22], [24], [25] | 0.97 [0.79–1.19] | 0.99 [0.86–1.13] | 180 per 1000 | 175 per 1000 | Chi2 = 2.78, df = 2 (P = 0.25); I2 = 28 % | Some concerns | Low due to bias risk (−1) and indirectness (−1) |
| Difference 5 fewer 95 % CI 38 less – 34 more | ||||||||
| Thrombotic events or death (28 days) | Moderately diseased population (WHO 4–5), 2396 participants, 2 studies [22], [29] | 0.64 [0.38–1.07] | 0.72 [0.57–0.91] | 123 per 1000 | 77 per 1000 | Chi2 = 2.90, df = 1 (P = 0.09); I2 = 66 % | Some concerns | Low due to bias risk (−1) and indirectness (−1) |
| Difference 44 fewer 95 % CI 76 less – 9 more | ||||||||
| Severely ill population (WHO 6–9), 1174 participants, 2 studies [25], [29] | 0.98 [0.86–1.12] | 0.98 [0.86–1.12] | 423 per 1000 | 415 per 1000 | Chi2 = 0.09, df = 1 (P = 0.77); I2 = 0 % | Some concerns | Low due to bias risk (−1) and indirectness (−1) | |
| Difference 8 fewer 95 % CI 59 less–51 more | ||||||||
| Mixed population WHO 4–9), 614 participants, 1 study (31) | 1.03 [0.70–1.50] | 1.03 [0.70–1.50] | 145 per 1000 | 149 per 1000 | NA | Some concerns | Low due to risk of bias (−1) and imprecision (−1) | |
| Difference 4 more 96 % CI 43 less–73 more | ||||||||
| Pooled effect, mixed population (WHO 4–9), 4184 participants, 4 studies [22], [25], [29], [31] | 0.86 [0.71–1.06] | 0.90 [0.8–1.01] | 214 per 1000 | 184 per 1000 | Chi2 = 8.61, df = 4 (P = 0.07); I2 = 54 % | Some concerns | Low due to risk of bias (−1) and indirectness/inconsistency (−1) | |
| Difference 30 fewer 95 % CI 62 less–13 more | ||||||||
| Major bleeding (28 days) |
Pooled effect, mixed population (WHO 4–9), 4650 participants, 5 studies [22], [23], [25], [29], [31] | 1.78 [1.15–2.74] | 1.82 [1.19–2.78] | 14 per 1000 | 25 per 1000 | Chi2 = 3.95, df = 5 (P = 0.56); I2 = 0 % | Some concerns | Low due to bias risk (−1) and indirectness (−1) |
| Difference 11 more 95 % CI 2 more–24 more | ||||||||
RR relative risk; M-H, Mantel-Haenszel; CI, confidence interval.
Patient status according to WHO clinical progression scale.
Overall bias risk of studies with events in at least one study arm.
3.5. Post-discharge COVID-19 patients: standard prophylactic anticoagulation versus no prophylaxis
Only data from one study on post-discharge anticoagulation were available (Table S3) [33]. We are uncertain whether post-discharge low-dose anticoagulation increases or decreases mortality after 35 days (RR 0.25, 95 % CI 0.03–2.21, 318 patients, 1 study), symptomatic venous thrombotic events or death (RR 0.44, 95 % CI 0.14–1.41, 318 patients, 1 study), serious bleeding (318 patients, 1 study, no events), clinically relevant non-serious bleeding (RR 1.00, 95 % CI 0.14–7.01, 318 patients, 1 study) and other bleedings (RR 2.00, 95 % CI 0.18–21.84, 318 patients, 1 study) compared to no prophylaxis. Anticoagulation reduced the occurrence of venous thrombotic events or cardiovascular deaths compared to control intervention (RR 0.33, 95 % CI 0.12–0.90, 318 patients, 1 study). Because this single significant outcome was not prospectively defined in the study protocol, this effect estimate is subject to a high risk of bias due to the suspicion of selective outcome reporting. Evidence certainty was rated as very low for all outcomes (Table S3).
4. Discussion
In this updated systematic review, the high heterogeneity regarding the anticoagulation schemes continues to make the interpretation of the meta-analyses difficult and reduces the certainty of evidence. The insufficient standardized definition of COVID-19 severity in individual studies however could be improved by newly identified studies and requests for additional stratified data from the study authors. So far, little is known about the extent to which individual virus variants and the vaccination status affect the risk of thrombosis. Measured by the lower rate of severe courses with the current Omicron variant [35] and an assumed increasing thrombotic risk proportional to the disease severity, one could assume that the predominant variant at the time of study has also influenced the presented findings and may explain the heterogeneous results between studies. Unfortunately, no information (e.g. genome sequencing) was available for the included patients to allow further exploration of these assumptions.
To the best of our knowledge, there is currently no comparable and more up-to-date systematic review with meta-analysis which includes more RCTs on this topic available. Despite being published recently, the latest update of the Cochrane Review entitled ‘Anticoagulants for people hospitalised with COVID-19’ from March 2022 only includes four RCTs based on a search from April 2021 and is therefore lacking important study results [36]. Given the fast pace of the pandemic, we believe it is more than ever important to base clinical practice on up-to-date evidence.
Based on the data available so far, intermediate-dose anticoagulation cannot be expected to have an effect on all-cause mortality after 30 and 90 days or on the risk of thrombotic events or death. However, even intermediate-dose anticoagulation may increase the risk of major bleeding based on a clinically relevant, non-significant effect estimate.
Therapeutic-dose anticoagulation may have a benefit in terms of mortality after 28 days in the subgroup of patients with moderate COVID-19, based on data from the RAPID and HEP-COVID study of 635 participants. While based on the large ATTACC, ACTIV-4a, REMAP-CAP platform study with 2226 moderately ill participants, little or no effect on the risk of in-hospital mortality can be expected. The certainty of evidence for these contradictory results is low and further studies may change the interpretation. Therapeutic-dose anticoagulation may reduce the rate of thrombotic events or deaths in patients with moderate COVID-19 but has had no effect in patients with severe COVID-19. However, this effect was only statistically significant when using the fixed-effect model. Therapeutic-dose anticoagulation can increase the rate of severe bleeding events compared to standard prophylactic anticoagulation. Thus, moderately ill COVID-19 patients without increased bleeding risks may be considered for therapeutic anticoagulation, the increased risk of bleeding should however be taken into account in decision-making and anticoagulated COVID-19 patients should be carefully monitored for bleeding events [5], [6]. Severely ill COVID-19 patients may not benefit from therapeutic anticoagulation while at the same time the risk of severe bleeding can be increased. Without further specific indication, therapeutic anticoagulation can therefore not be recommended for critically ill COVID-19 patients [5], [6].
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
The Federal Ministry of Education and Research, Germany, NaFoUniMedCovid19 (funding number: 01KX2021); part of the project “CEOsys” supported this work (funding ended 31 December 2021). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
CRediT authorship contribution statement
Stefanie Reis: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Maria Popp: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – review & editing. Selina Schießer: Writing – review & editing. Maria-Inti Metzendorf: Conceptualization, Writing – review & editing. Peter Kranke: Conceptualization, Resources, Writing – review & editing. Patrick Meybohm: Conceptualization, Resources, Writing – review & editing. Stephanie Weibel: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the Federal Ministry of Education and Research, Germany, NaFoUniMedCovid19 (funding number: 01KX2021); part of the project “CEOsys” for supporting this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2022.09.001.
Appendix A. Supplementary data
Supplementary material including meta-analyses and search strategies
References
- 1.Synowiec A., Szczepanski A., Barreto-Duran E., Lie L.K., Pyrc K. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a systemic infection. Clin. Microbiol. Rev. 2021;34(2) doi: 10.1128/CMR.00133-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Ani F., Chehade S., Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb. Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patell R., Bogue T., Koshy A., Bindal P., Merrill M., Aird W.C., et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136(11):1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelen M.M., Vandenbriele C., Balthazar T., Claeys E., Gunst J., Guler I., et al. Venous thromboembolism in patients discharged after COVID-19 hospitalization. Semin. Thromb. Hemost. 2021;47(4):362–371. doi: 10.1055/s-0041-1727284. [DOI] [PubMed] [Google Scholar]
- 5.Kluge S., Janssens U., Welte T., Weber-Carstens S., Schälte G., Spinner C.D. 2022. S3-Leitlinie - Empfehlungen zur stationären Therapie von Patienten mit COVID-19. AWMF Online. 28.02.2022. [DOI] [PubMed] [Google Scholar]
- 6.Blankenfeld H., Kaduszkiewicz H., Kochen M.M., J P . AWMF online; 2022. S2e-Leitlinie: SARS-CoV-2/Covid-19- Informationen & Praxishilfen für niedergelassene Hausärztinnen und Hausärzte. 04.02.2022. [Google Scholar]
- 7.Reis S., Popp M., Schmid B., Stegemann M., Metzendorf M.I., Kranke P., et al. Safety and efficacy of intermediate- and therapeutic-dose anticoagulation for hospitalised patients with COVID-19: a systematic review and meta-analysis. J. Clin. Med. 2022;11(1):57. doi: 10.3390/jcm11010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall J.C., Murthy S., Diaz J., Cheng A., Denholm J., Hodgson C., et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020;20(8):E192–E197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rote-Liste-Service-GmbH Rote Liste Fachinfo Service Online. https://www.fachinfo.de/ Available from:
- 10.National Institute for Health and Care Excellence . NICE Guideline. Vol. 186. NICE; November 20, 2020. COVID-19 rapid guideline: reducing the risk of venous thromboembolism in over 16s with COVID-19; p. 2020. [PubMed] [Google Scholar]
- 11.Schulman S., Kearon C., Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li T., Higgins J.P., Deeks J.J. In: Cochrane Handbook for Systematic Reviews of Interventions Version 61 (Updated September 2020) Cochrane. Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., editors. 2020. Chapter 5: collecting data. [Internet]. Available from: training.cochrane.org/handbook. [Google Scholar]
- 14.Sterne J.A.C., Savovic J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 15.Higgins J.P., Savović J., Page M.J., Elbers R.G., SJ J.R. Cochrane Handbook for Systematic Reviews of Interventions Version 61 (updated September 2020) Cochrane. 2020. Chapter 8: assessing risk of bias in a randomized trial. [Internet]. Available from: training.cochrane.org/handbook. [Google Scholar]
- 16.Deeks J.J., Higgins J.P., Altman D.G. Cochrane Handbook for Systematic Reviews of Interventions Version 61 (Updated September 2020) Cochrane. 2020. Chapter 10: analysing data and undertaking meta-analyses. [Internet]. Available from: training.cochrane.org/handbook. [Google Scholar]
- 17.The-Cochrane-Collaboration . 2021. The Cochrane Collaboration Review Manager Web (RevManWeb). Version 3.11.1. 28.02.2022. [Google Scholar]
- 18.Balduzzi S., Rucker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid. Based Ment. Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schünemann H.J., Higgins J.P.T., Vist G.E., Glasziou P., Akl E.A., Skoetz N. Cochrane Handbook for Systematic Reviews of Interventions Version 62 (Updated February 2021) 2021. Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence.www.training.cochrane.org/handbook [Internet]. Available from: [Google Scholar]
- 20.Ananworanich J., Mogg R., Dunne M.W., Bassyouni M., David C.V., Gonzalez E., et al. Randomized study of rivaroxaban vs. placebo on disease progression and symptoms resolution in high-risk adults with mild COVID-19. Clin. Infect. Dis. 2021;75:e473–e481. doi: 10.1093/cid/ciab813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connors J.M., Brooks M.M., Sciurba F.C., Krishnan J.A., Bledsoe J.R., Kindzelski A., et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B Randomized Clinical Trial. JAMA. 2021;326(17):1703–1712. doi: 10.1001/jama.2021.17272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawler P.R., Goligher E.C., Berger J.S., Neal M.D., McVerry B.J., Nicolau J.C., et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N. Engl. J. Med. 2021;385(9):790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sholzberg M., Tang G.H., Rahhal H., Kreuziger L.B., Ni Ainle F., AlHamzah M. medRxiv; 2021. Heparin for Moderately Ill Patients with Covid-19. 2021.07.08.21259351. [Google Scholar]
- 24.Lemos A.C.B., do Espirito Santo D.A., Salvetti M.C., Gilio R.N., Agra L.B., Pazin-Filho A. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID) Thromb. Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goligher E.C., Bradbury C.A., McVerry B.J., Lawler P.R., Berger J.S., Gong M.N., et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N. Engl. J. Med. 2021;385(9):777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliynyk O., Barg W., Slifirczyk A., Oliynyk Y., Dubrov S., Gurianov V., et al. Comparison of the effect of unfractionated heparin and enoxaparin sodium at different doses on the course of COVID-19-associated coagulopathy. Life-Basel. 2021;11(10):1032. doi: 10.3390/life11101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bikdeli B., Talasaz A.H., Rashidi F., Bakhshandeh H., Rafiee F., Rezaeifar P., et al. Intermediate-dose versus standard-dose prophylactic anticoagulation in patients with COVID-19 admitted to the intensive care unit: 90-day results from the INSPIRATION randomized trial. Thromb. Haemost. 2022;122(1):131–141. doi: 10.1055/a-1485-2372. [DOI] [PubMed] [Google Scholar]
- 28.Sadeghipour P., Talasaz A.H., Rashidi F., Sharif-Kashani B., Beigmohammadi M.T., Farrokhpour M., et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION Randomized Clinical Trial. JAMA. 2021;325(16):1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spyropoulos A.C., Goldin M., Giannis D., Diab W., Wang J., Khanijo S., et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern. Med. 2021;181(12):1612–1620. doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morici N., Podda G., Birocchi S., Bonacchini L., Merli M., Trezzi M., et al. Enoxaparin for thromboprophylaxis in hospitalized COVID-19 patients: The X-COVID-19 Randomized Trial. Eur. J. Clin. Investig. 2021;52 doi: 10.1111/eci.13735. [DOI] [PubMed] [Google Scholar]
- 31.Lopes R.D., de Barros E.S.P.G.M., Furtado R.H.M., Macedo A.V.S., Bronhara B., Damiani L.P., et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perepu U.S., Chambers I., Wahab A., Ten Eyck P., Wu C., Dayal S., et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. J. Thromb. Haemost. 2021;19(9):2225–2234. doi: 10.1111/jth.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramacciotti E., Barile Agati L., Calderaro D., Aguiar V.C.R., Spyropoulos A.C., de Oliveira C.C.C., et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet. 2022;399(10319):50–59. doi: 10.1016/S0140-6736(21)02392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robert-Koch-Institut Epidemiologischer Steckbrief zu SARS-CoV-2 und COVID-19. 26.11.2021. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Steckbrief.html;jsessionid=DE7D5E3CF13721ACDA04F4C11A9A0D7D.internet101?nn=13490888 [07.02.2022]. Available from:
- 35.Madhi S.A., Ihekweazu C., Rees H., Pollard A.J. Decoupling of omicron variant infections and severe COVID-19. Lancet. 2022;399(10329):1047–1048. doi: 10.1016/S0140-6736(22)00109-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flumignan R.L., Civile V.T., Tinoco J.D.S., Pascoal P.I., Areias L.L., Matar C.F., et al. Anticoagulants for people hospitalised with COVID-19. Cochrane Database Syst. Rev. 2022;3 doi: 10.1002/14651858.CD013739.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material including meta-analyses and search strategies
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.