Abstract
The cellular content of major cold shock protein (MCSP) mRNA transcribed from the tandem gene duplication cspA1/A2 and growth of Yersinia enterocolitica were compared when exponentially growing cultures of this bacterium were cold shocked from 30 to 20, 15, 10, 5, or 0°C, respectively. A clear correlation between the time point when exponential growth resumes after cold shock and the degradation of cspA1/A2 mRNA was found. A polynucleotide phosphorylase-deficient mutant was unable to degrade cspA1/A2 mRNA properly and showed a delay, as well as a lower rate, of growth after cold shock. For this mutant, a correlation between decreasing cspA1/A2 mRNA and restart of growth after cold shock was also observed. For both wild-type and mutant cells, no correlation of restart of growth with the cellular content of MCSPs was found. We suggest that, after synthesis of cold shock proteins and cold adaptation of the cells, MCSP mRNAs must be degraded; otherwise, they trap ribosomes, prevent translation of bulk mRNA, and thus inhibit growth of this bacterium at low temperatures.
Yersinia enterocolitica is a psychrotolerant pathogen carrying a tandem duplication of cspA (named cspA1/A2 [22]). The ability to rapidly produce high amounts of major cold shock proteins (MCSPs) (22) may contribute to the extensive psychrotolerance of this organism, some strains of which can grow at temperatures as low as −5°C (2). The regulation of MCSPs in bacteria has been studied with the mesophiles Escherichia coli and Bacillus subtilis for more than a decade (reviewed in references 13 and 25). However, extensive research still has not resolved why these proteins are so exactly up- and down-regulated upon cold shock and subsequent cold adaptation (6). MCSP mRNA is translated more efficiently than other mRNAs at low temperatures (6). The binding of the MCSPs to mRNA appears to be relatively nonspecific and based on the secondary structure of the mRNA, rather than on its sequence (18). Such a universal role might explain the need for high quantities of MCSPs upon cold shock and why there is a significant delay in the synthesis of other cold shock proteins (9, 20). It is not exactly known which signal triggers the start of exponential growth after a cold shock, but it has been suggested that a sufficiently high concentration of MCSPs is responsible (14, 19).
Strains, determination of cell numbers, and sampling.
In this study, Y. enterocolitica NCTC 10460 (hereafter called Y. enterocolitica) and Y. enterocolitica YM 205 (polynucleotide phosphorylase [PNPase]-deficient mutant; pnp::tnp Kanr Nalr) (12) and its parent Y. enterocolitica W 22703 (Nalr pYV−) were used. Batch cultures (350 ml) were grown at 30°C for about 5 h (approximately 10 generations) to an optical density at 600 nm of 0.5 on a shaker and cold shocked to 20, 15, 10, 5, or 0°C as described previously (22). Suitable dilutions were plated twice on Luria-Bertani plates to determine the viable cell numbers. Samples (10 ml) were taken immediately before (control [CT]) and after (2 min) cold shock and after different times, indicated in the figures, following cold shock. The cells were centrifuged (10,000 × g; 2 min at shock temperature), and the pellet was frozen in liquid N2.
Preparation of RNA and Northern and Southern blot analysis.
Total RNA was isolated with a guanidine-phenol buffer as described previously (15). Northern blotting with 20 μg of total RNA was carried out according to the procedure given in reference 21 with minor changes as described previously (22). The hybridization solution contained a 5′-digoxigenin-labeled oligonucleotide (MWG Biotech, Ebersberg, Germany), YeA1-DIG (5′-GCC ACA ATA CTG TTT TGC CAC AAT ATG T-3′), complementary to cspA1/A2 (22). For Southern blots, 10 μg of DNA was cut with different restriction enzymes according to the manufacturer's protocol. Hybridization was carried out as described previously (22).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and two-dimensional (2D) gel electrophoresis.
For SDS-PAGE, the cell pellets were boiled for 15 min in solubilization buffer (2% SDS, 10% glycerol, 62.5 mM Tris-HCl, bromphenol blue for color, pH 6.8). The protein concentration was determined with Coomassie brilliant blue G-250 dye reagent (Bio-Rad GmbH, Munich, Germany) according to the supplier. The SDS-PAGE was performed with a polyacrylamide gradient Excel Gel from 12 to 14% with a low-molecular-weight peptide standard (Amersham Pharmacia Biotech, Freiburg, Germany). Electrophoresis was performed on a Multiphor unit (Amersham Pharmacia Biotech) at 200 V for 40 min and 600 V for 4 h. The gels were silver stained (4) and evaluated with Image Master 1D Elite software (Amersham Pharmacia Biotech) after a scan with reflected light. For 2D gels, pellets were resuspended in solubilization buffer according to the procedure in reference 10 and lysed by a single passage through a French press (SLM Aminco Inc., Rochester, N.Y.) as described previously (22). 2D gel electrophoresis was performed as described previously (11, 22) using high-resolution immobilized pH gradients (pH 5 to 6) (23). Protein samples, with an identical load of total protein on each gel, were resolved by isoelectric focusing using Pharmacia's DryStrip Kit (Amersham Pharmacia Biotech), and the gels were silver stained (4). Two to seven gels per time point were evaluated with Image Master 2D Elite software after scanning in reflected light. The MCSP amount was referenced to six other proteins which remained unchanged over the entire sampling period.
MCSP mRNA and restart of growth after cold shock.
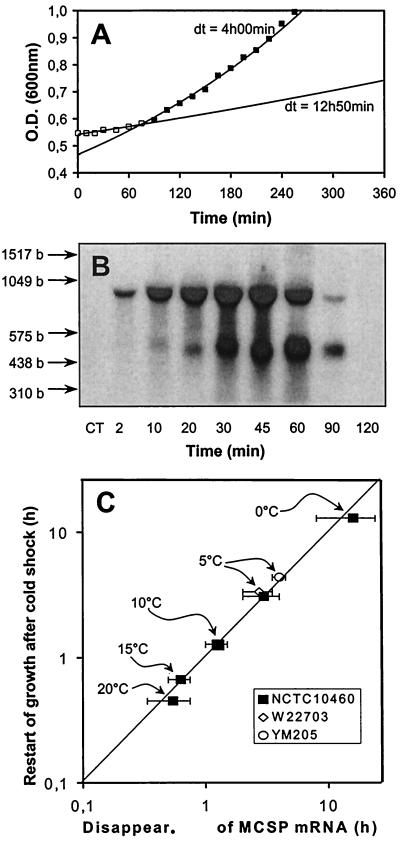
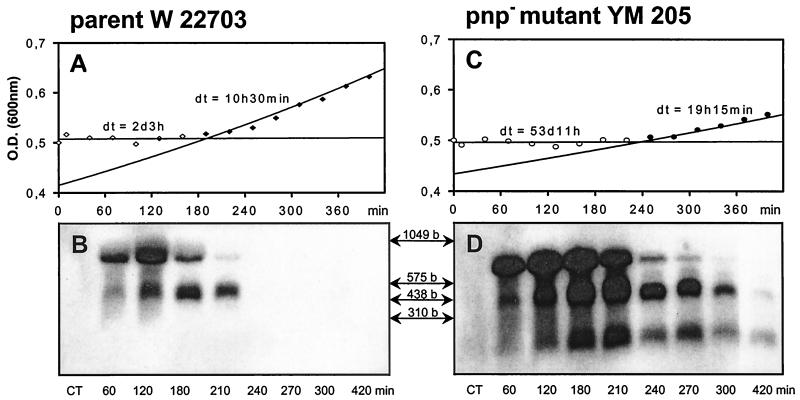
At 30°C, Y. enterocolitica has a doubling time of approximately 30 min (data not shown). A cold shock decelerates growth, but after a certain time, Y. enterocolitica resumes exponential growth (Fig. 1A). At each temperature tested, the time point of restart of growth (Fig. 1A) correlates very well with the time after which a significant decrease of cspA1/A2 mRNA is visible in the corresponding Northern blots (Fig. 1B), as summarized in Fig. 1C for different shock temperatures. Similarly, in E. coli degradation of cspA mRNA takes place between 75 and 120 min after a cold shock from 37 to 15°C (24), and after approximately 100 min, E. coli starts growing again. However, such a correlation may turn out to be coincidental. If growth is indeed inhibited by cspA mRNA, inhibition of mRNA degradation should inhibit restart of growth. The turnover of mRNA is mediated by nuclease cleavage. Disruption of pnp, the gene encoding the cold-inducible 3′-to-5′ mRNA exonuclease PNPase, has recently been shown to inhibit low-temperature growth of Y. enterocolitica, in particular because PNPase should replace RNase II at low temperatures (12). In order to test our hypothesis that restart of growth can occur only if the MCSP mRNA is sufficiently degraded, we included a PNPase-deficient mutant strain (YM 205) of Y. enterocolitica and its parent (W 22703) in this study. The parental strain W 22703 behaves like the wild-type NCTC 10460: both strains restart exponential growth at approximately 180 min after a cold shock from 30 to 5°C (Fig. 2A), and an mRNA degradation is visible around this time point (Fig. 2B). In contrast, the PNPase-deficient strain Y. enterocolitica YM 205 resumes exponential growth after approximately 240 min (Fig. 2C), and likewise, a substantial degradation of mRNA in the corresponding Northern blot is observable around this time point (Fig. 2D).
FIG. 1.
(A) Example of a growth curve from Y. enterocolitica NCTC 10460 after a cold shock from 30 to 10°C. The lag period after cold shock is indicated by open squares. Filled squares indicate restart of exponential growth. dt, doubling times. O.D., optical density. (B) Example of a Northern blot after cold shock from 30 to 10°C (corresponding to panel A). The blot was performed using a probe against cspA1 and shows a lower monocistronic and an upper bicistronic signal of the cspA1/A2 tandem. The CT was obtained from a sample without cold shock. The locations of markers are shown by arrows, and their lengths are given in bases (b). (C) Correlation of the time points when cold-shocked cultures of Y. enterocolitica restart exponential growth (y axis, calculated from growth data) and the time points when the cspA1/A2 mRNA disappears (x axis, derived from Northern blot data). The temperature of the cold shock is indicated. The error bars include the time points before and after degradation is clearly visible in the Northern blots. The indicated theoretical line would be obtained if there were a 100% correlation of both parameters. The data for the parent strain W 22703 and the PNPase-deficient strain YM 205 from Fig. 2 are included (open symbols).
FIG. 2.
Comparison of growth curves and cspA1/A2 mRNA content between a pnp mutant (Y. enterocolitica YM 205) (A and B) and its parental strain (Y. enterocolitica W 22703) (C and D) following a cold shock from 30 to 5°C. dt, doubling time. The lag periods after the cold shock (A and C) are indicated by open symbols; restart of exponential growth is indicated by filled symbols. The Northern blots (B and D) were obtained with a probe specific for cspA1 and show a lower monocistronic and an upper bicistronic signal of the cspA1/A2 tandem. In addition, the blot of the pnp mutant shows a band below the 310-base (b) marker band, which is probably a degradation product. The CTs are obtained from samples without cold shock. The locations of markers are shown by arrows, and their lengths are indicated. O.D., optical density.
Restart of growth after cold shock does not correlate with MCSP content.
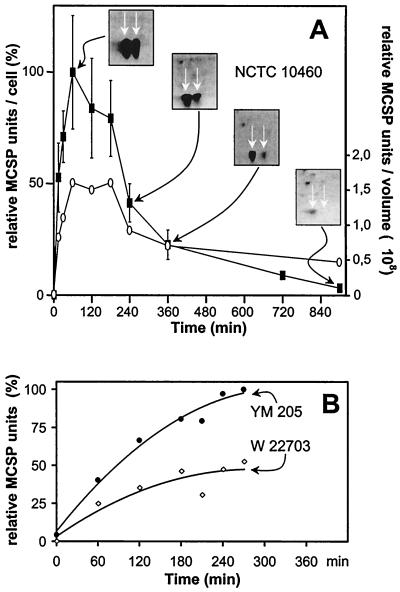
2D gels at different time points after a cold shock from 30 to 10°C for strain NCTC 10460 show that the MCSP production in Y. enterocolitica starts as early as 10 min, reaches a climax around 60 min, and decreases slightly up to 150 min (Fig. 3A, closed squares). Although the MCSPs increase rapidly and are present at a high level after only 40 min, the cells do not resume exponential growth prior to approximately 80 min (Fig. 1A). A slow decrease of MCSPs can be observed after 150 min, and the MCSPs are detectable at least up to 900 min (Fig. 3A).
FIG. 3.
(A) Closed squares, relative amount of MCSPs following a cold shock from 30 to 10°C, as determined by 2D analysis. Inserts are examples of partial 2D gels. The two spots indicated by arrows include the MCSPs. Spot 1 contains CspA1 and CspB, and spot 2 contains CspA2 (22). Mean values and error bars are calculated from two to seven experiments. The maximum level of relative MCSP units per cell achieved in this experiment was set as 100%. Open circles, total MCSP amount per volume; the relative amount of MCSP was multiplied with the cell number (from Fig. 1A) to give a total MCSP amount of 1 culture volume [(MCSP units/cell) × (cells/milliliter)]. A horizontal course shows that the MCSPs are not degraded but are diluted due to cellular growth. A declining curve shows that MCSPs are additionally degraded. (B) The relative MCSP content determined by SDS-PAGE for the parental strain W 22703 (open diamonds) and the PNPase-deficient mutant strain YM 205 (closed circles) of Y. enterocolitica is shown. The maximum MCSP level achieved with the pnp mutant strain was set as 100%. The protein levels were measured only up to 270 min, and they decreased after this time, as shown by another experiment (data not shown).
To examine whether the observed decrease of MCSPs is caused by dilution, because of cell division, or because of degradation, the MCSP amount of a given culture volume was determined [(MCSP units/cell) × (cells/milliliter)] (Fig. 3A, open circles). A horizontal graph would indicate that the decrease is due to dilution by growth. A sloping graph would indicate that the decrease, in addition to dilution by cell division, is due to degradation of the proteins. Between 60 and 180 min, the MCSP content of cells is reduced by dilution, because the cells start division after 80 min. After 180 min, the MCSPs also undergo degradation. Similarly, in E. coli the production of the MCSPs already reaches a climax around 120 to 150 min after a cold shock from 37 down to 10°C (9, 20), but growth is arrested until 240 min (20).
These data are in agreement with the well-known fact that MCSPs are necessary to overcome the impediment by the cold shock (25). However, a high MCSP content in the cell seems not to be sufficient to trigger restart of exponential growth after a cold shock. This was confirmed using the PNPase-deficient mutant strain. The amount of MCSPs in the PNPase-deficient mutant Y. enterocolitica YM 205 and its parent W 22703 was examined by SDS-PAGE after a cold shock from 30 to 5°C. Interestingly, MCSPs are synthesized in higher amounts in the pnp mutant after cold shock, and the final level is approximately twice that of the parent (Fig. 3B). This is in agreement with the higher concentration of cspA1/A2 mRNA in the mutant. Clearly, the mutant restarts growth after cspA1/A2 mRNA has dropped to a sufficiently low level at approximately 240 min and not when the MCSPs have increased to a (for the parental strain) sufficiently high level, which is already reached after 60 min (Fig. 3B).
Why should MCSP mRNA inhibit growth?
Jiang et al. (16) showed that E. coli is unable to grow when a 5′ fragment of cspA is transcribed from a plasmid. The ribosomes are trapped with the truncated cspA mRNA and are no longer able to initiate and translate bulk mRNA. An explanation for these results may be provided by the particular sequence of the MCSP mRNAs, because cspA mRNA is able to initiate and to be translated at the ribosome even after a cold shock or other translational blocks (6), but the mechanism is, unfortunately, still poorly understood (3, 7). However, bulk mRNA not only is misfolded at low temperatures and causes ribosomal stalling but also lacks the particular features of the MCSP mRNAs, i.e., the unusually long 5′ untranslated region. Therefore, its translation is inhibited (8, 14, 18), and cspA mRNA may outcompete the bulk mRNA at the ribosome. This will stop growth due to the inability to produce many proteins required for cell doubling. In addition, CspA binds to its own mRNA (1, 5, 17). This could mean that not only the ribosomes but also the MCSPs are partly trapped by the massive amount of cspA mRNA and therefore are not available to unfold bulk mRNA.
Taken together, the above findings indicate that the bacteria must degrade MCSP mRNA to restart growth after cold shock. If they are hindered in reducing the MCSP mRNA, as is the case for the PNPase-deficient mutant, they cannot grow, even if there are sufficiently high levels of MCSPs.
Acknowledgments
We thank Roos Goverde and Jos Huis in't Veld (both of the University of Utrecht, Utrecht, The Netherlands) for providing us with the PNPase-deficient mutant strain Y. enterocolitica YM 205 and its parent Y. enterocolitica W 22703.
REFERENCES
- 1.Bae W, Jones P G, Inouye M. CspA, the major cold shock protein of Escherichia coli, negatively regulates its own gene expression. J Bacteriol. 1997;179:7081–7088. doi: 10.1128/jb.179.22.7081-7088.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergann T, Kleemann J, Sohr D. Model investigations into psychrotrophic growth of Yersinia enterocolitica. J Vet Med Ser B. 1995;42:523–531. [PubMed] [Google Scholar]
- 3.Bläsi U, O'Connor M, Squires C L, Dahlberg A E. Misled by sequence complementarity: does the DB-anti-DB interaction withstand scientific scrutiny? Mol Microbiol. 1999;33:439–441. doi: 10.1046/j.1365-2958.1999.01488.x. [DOI] [PubMed] [Google Scholar]
- 4.Blum H, Beier H, Gross H J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- 5.Brandi A, Pietroni P, Gualerzi C O, Pon C L. Post-transcriptional regulation of CspA expression in Escherichia coli. Mol Microbiol. 1996;19:231–240. doi: 10.1046/j.1365-2958.1996.362897.x. [DOI] [PubMed] [Google Scholar]
- 6.Etchegaray J P, Inouye M. CspA, CspB, and CspG, major cold shock proteins of Escherichia coli, are induced at low temperature under conditions that completely block protein synthesis. J Bacteriol. 1999;181:1827–1830. doi: 10.1128/jb.181.6.1827-1830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etchegaray J P, Inouye M. DB or not DB in translation? Mol Microbiol. 1999;33:438–439. doi: 10.1046/j.1365-2958.1999.01487.x. [DOI] [PubMed] [Google Scholar]
- 8.Farewell A, Neidhardt F C. Effect of temperature on in vivo protein synthetic capacity in Escherichia coli. J Bacteriol. 1998;180:4704–4710. doi: 10.1128/jb.180.17.4704-4710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein J, Pollitt N S, Inouye M. Major cold shock protein of Escherichia coli. Proc Natl Acad Sci USA. 1990;87:283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Görg A. Two-dimensional electrophoresis. Nature. 1991;349:545–546. [Google Scholar]
- 11.Görg A, Weiss W. Two dimensional electrophoresis of proteins using immobilized pH gradients. In: Celis J, editor. Cell biology: a laboratory handbook. 2nd ed. Vol. 4. New York, N.Y: Academic Press, Inc.; 1998. pp. 386–397. [Google Scholar]
- 12.Goverde R L, Huis in't Veld J H, Kusters J G, Mooi F R. The psychrotrophic bacterium Yersinia enterocolitica requires expression of pnp, the gene for polynucleotide phosphorylase, for growth at low temperature (5 degrees C) Mol Microbiol. 1998;28:555–569. doi: 10.1046/j.1365-2958.1998.00816.x. [DOI] [PubMed] [Google Scholar]
- 13.Graumann P, Marahiel M A. Some like it cold: response of microorganisms to cold shock. Arch Microbiol. 1996;166:293–300. doi: 10.1007/s002030050386. [DOI] [PubMed] [Google Scholar]
- 14.Graumann P, Wendrich T M, Weber M H W, Schröder K, Marahiel M A. A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol Microbiol. 1997;25:741–756. doi: 10.1046/j.1365-2958.1997.5121878.x. [DOI] [PubMed] [Google Scholar]
- 15.Grsic S, Sauerteig S, Neuhaus K, Albrecht M, Rossiter J, Ludwig-Müller J. Physiological analysis of transgenic Arabidopsis thaliana plants expressing one nitrilase isoform in sense or antisense direction. J Plant Physiol. 1998;153:446–456. [Google Scholar]
- 16.Jiang W, Fang L, Inouye M. Complete growth inhibition of Escherichia coli by ribosome trapping with truncated cspA mRNA at low temperature. Genes Cells. 1996;1:965–976. doi: 10.1046/j.1365-2443.1996.d01-219.x. [DOI] [PubMed] [Google Scholar]
- 17.Jiang W, Fang L, Inouye M. The role of the 5′-end untranslated region of the mRNA for CspA, the major cold shock protein of Escherichia coli, in cold shock adaptation. J Bacteriol. 1996;178:4919–4925. doi: 10.1128/jb.178.16.4919-4925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang W, Hou Y, Inouye M. CspA, the major cold shock protein of Escherichia coli, is an RNA chaperone. J Biol Chem. 1997;272:196–202. doi: 10.1074/jbc.272.1.196. [DOI] [PubMed] [Google Scholar]
- 19.Jones P G, Inouye M. The cold shock response—a hot topic. Mol Microbiol. 1994;11:811–818. doi: 10.1111/j.1365-2958.1994.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones P G, van Bogelen R A, Neidhardt F C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987;169:2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Löw R, Rausch T. Sensitive, nonradioactive northern blots using alkaline transfer of total RNA and PCR-amplified biotinylated probes. BioTechniques. 1994;17:1026–1028. [PubMed] [Google Scholar]
- 22.Neuhaus K, Francis K P, Rapposch S, Görg A, Scherer S. Pathogenic Yersinia species carry a novel, cold-inducible major cold shock protein tandem gene duplication producing both bicistronic and monocistronic mRNA. J Bacteriol. 1999;181:6449–6455. doi: 10.1128/jb.181.20.6449-6455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Righetti P G. Immobilized pH gradients. Theory and methodology. Amsterdam, The Netherlands: Elsevier; 1990. [Google Scholar]
- 24.Tanabe H, Goldstein J, Yang M, Inouye M. Identification of the promoter region of the Escherichia coli major cold shock gene, cspA. J Bacteriol. 1992;174:3867–3873. doi: 10.1128/jb.174.12.3867-3873.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamanaka K, Fang L, Inouye M. The CspA family in Escherichia coli: multiple gene duplication for stress adaptation. Mol Microbiol. 1998;27:247–255. doi: 10.1046/j.1365-2958.1998.00683.x. [DOI] [PubMed] [Google Scholar]