Abstract
Current severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccines, based on the ancestral Wuhan strain, were developed rapidly to meet the needs of a devastating global pandemic. People living with Human Immunodeficiency Virus (PLWH) have been designated as a priority group for SARS-CoV-2 vaccination in most regions and varying primary courses (two- or three-dose schedule) and additional boosters are recommended depending on current CD4+ T cell count and/or detectable HIV viraemia. From the current published data, licensed vaccines are safe for PLWH, and stimulate robust responses to vaccination in those well controlled on antiretroviral therapy and with high CD4+ T cell counts. Data on vaccine efficacy and immunogenicity remain, however, scarce in PLWH, especially in people with advanced disease. A greater concern is a potentially diminished immune response to the primary course and subsequent boosters, as well as an attenuated magnitude and durability of protective immune responses. A detailed understanding of the breadth and durability of humoral and T cell responses to vaccination, and the boosting effects of natural immunity to SARS-CoV-2, in more diverse populations of PLWH with a spectrum of HIV-related immunosuppression is therefore critical. This article summarizes focused studies of humoral and cellular responses to SARS-CoV-2 infection in PLWH and provides a comprehensive review of the emerging literature on SARS-CoV-2 vaccine responses. Emphasis is placed on the potential effect of HIV-related factors and presence of co-morbidities modulating responses to SARS-CoV-2 vaccination, and the remaining challenges informing the optimal vaccination strategy to elicit enduring responses against existing and emerging variants in PLWH.
Keywords: COVID-19, SARS-CoV-2, vaccination, HIV, immune responses
INTRODUCTION
Coronavirus Disease (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS CoV-2), emerged in the late 2019, and was declared a global pandemic by the World Health Organization (WHO) in March 2020. As of December 2021, >277 million cases and >5 million deaths had been reported, almost certainly a significant under-estimation of the true numbers, and have led to significant pressures and disruption of local, national and international healthcare systems [1]. It has been estimated that People living with Human Immunodeficiency Virus (PLWH) represent ∼1% of total hospitalized cases [2]. However, the actual prevalence of SARS-CoV-2 infection could be higher in low- and middle-income countries where access to diagnosis is limited, and HIV burden is much higher. With nearly 40 million PLWH and 12.6 million people not under suppressive antiretroviral therapy (ART) [3], the dynamics of co-existing SARS-CoV-2 infection require a syndemic understanding of health and disease.
Unlike HIV infection, which in the absence of ART is invariably fatal, the course of COVID-19 disease is highly variable. The majority of cases are either asymptomatic or mildly symptomatic with cough, upper respiratory symptoms, myalgia and headache, but some progress to a potentially fatal condition of acute respiratory distress syndrome, septic shock and multiorgan failure [4–6]. There is an exponential increase in mortality with increasing age [7] and there is a clear correlation between risk of severe disease and comorbidities including hypertension, diabetes, cardiovascular and respiratory disease [8, 9]. PLWH have a higher burden of these disease risk factors than the general population. Furthermore, PLWH are an ageing population, with nearly half of the PLWH in the USA being >50 years of age, which is set to increase [10, 11].
Immunosuppressed patients, including people with haematological malignancies [12], solid organ transplant recipients [13] and those on chronic oral glucocorticoids for rheumatic conditions [14] have also been identified as being at high risk for severe COVID-19 disease. Similarly, PLWH have been included among those deemed vulnerable to worse outcomes from SARS-CoV-2 infection [15]. Large cohort studies from the UK, South Africa, the USA and data reported to the WHO from across the world have identified a higher risk of death and hospitalization from COVID-19 disease in PLWH [16–19]. There is also evidence for a more severe course of COVID-19 disease in people with cellular immune deficiency and a lower CD4+ T cell count/low CD4+ T cell nadir [20–22]. As a result, SARS-CoV-2 vaccination is recommended by national and international HIV societies for PLWH [15, 23, 24]. An informal poll of more than 100 countries from all regions, performed by the WHO, showed that at least 40 countries have an immunization policy that prioritizes vaccinations for PLWH [25]. In general, PLWH and especially those with a CD4+ T cell count <350 cells/µl or ongoing viraemia, are advised to have three doses of vaccine as part of their primary vaccination course [23, 24]. Given that sub-optimal responses to several other vaccines have been reported in PLWH [26], this raises concerns about the potential efficacy of SARS-Cov-2 vaccines in this potentially more vulnerable population. Additional vaccine doses are expected to increase responses in this group, reflected in recommendations by most Western countries, the USA and the UK, advising a first booster (fourth dose) and second booster (fifth dose). These guidelines are regularly updated in line with the evolving pandemic response [27].
Here, we review the complex interplay between HIV and SARS-CoV-2 infection in adults and summarize the knowns and many unknowns of COVID-19 vaccine responses in the setting of HIV infection.
IMMUNE CORRELATES OF PROTECTION AGAINST SARS-COV-2 INFECTION
Increased understanding of protective immune responses against SARS-CoV-2 infection and disease progression has provided valuable insights for the development and evaluation of vaccines. The humoral immune response to natural infection and vaccination against SARS-CoV-2 has received a lot of attention. Following infection with SARS-CoV-2, a specific humoral response is initiated [28]. Importantly, IgG antibodies which bind to spike protein, particularly the receptor-binding domain (RBD), are more likely to be neutralizing and these have been linked to viral clearance in patients who have recovered from SARS-CoV-2 infection [29]. Non-human primate (NHP) models illustrated protection from reinfection and total protection provided by passive transfer of neutralizing antibodies [30–32]. Indeed, studies in humans have shown that higher anti-spike IgG and neutralizing antibody titres generated following natural infection or vaccination are associated with a lower risk of reinfection [33], symptomatic disease [34] and a positive correlation between clinical severity and SARS-CoV-2 specific antibodies [35]. There is evidence that the timing of IgG anti-spike response may be a critical determinant in survival; Lucas et al. showed that deceased patients mounted a robust, specific response, with neutralizing antibodies. However, it was a delay in seroconversion that resulted in poor viral control in these patients [36]. Many studies have also evaluated the impact and timing of serum IgM- and IgA-specific antibodies [37] which have been related to serological diagnosis and prognosis prediction rather than protective effects [38–40]. Although these specific antibody responses can be detected within 2 weeks of initial infection [41, 42], it has been well-documented that humoral immune responses to coronaviruses are variable and short-lived; levels decay post-infection and vaccination after approximately the first month, with a half-life of ∼2 months [43, 44]. The level of neutralizing antibodies required for continuing protection following natural infection or vaccination has not yet been determined; this is further complicated by the emergence of variants of concern (VOC) which have mutations/deletions to the spike protein, particularly in the RBD, which can impact neutralizing sensitivity [45]. This is an important consideration as all of the currently licensed SARS-CoV-2 vaccines are based on the original Wuhan strain [46–48]. Khoury et al. developed a predictive model that suggests there is a proportionate response of neutralization titres whereby the lower the initial response to wild-type virus, the greater the impact on vaccine response to other strains [49]. Several studies have shown continued protection against variants following vaccination persisting for ∼6 months with implications for the timing of boosters [50–52]. Although antibody responses wane, class-switched spike-specific and RBD-specific memory B cells can provide a long-lived memory pool that can react rapidly upon reinfection or vaccine boosting [53]. Spike-specific memory B cells have been shown to persist for 6 months to a year following infection [54, 55], with evidence of higher levels of somatic hypermutation, higher binding affinity and neutralizing capacity [56, 57]. Memory B cell responses may even be of higher quality following mild compared to severe SARS-CoV-2 infection, producing more robust responses [58], even when neutralizing antibodies were undetectable. However, recall responses of RBD-specific memory B cells have been shown to decline with age [53, 59].
Increasing evidence supports a protective role versus pathogenic role of T cell immunity against SARS-CoV-2 infection [60]. Although the characterization of virus-specific T cell responses is more technically challenging, an early development of a cytotoxic CD8+ T cell response is associated with significantly milder disease [61–64] and accelerated viral clearance [65–68]. Further indirect evidence of the importance of T cell responses comes from studies of infection in patients with inherited immune defects of antibody responses and from patients receiving B cell depleting therapies in whom robust CD8+ T cell responses contributed to increased survival [69–72]. SARS-CoV-2-specific T cell responses are detected to a range of structural (NP, M, ORF3a, spike) and non-structural (ORF7/8, NSP7, NSP13) proteins following SARS-CoV-2 infection [65, 67, 73–76]. Despite these positive correlations, the exact role of T cell responses, and which epitopes will be the most protective, remain unclear. Following natural infection, the memory phase is dominated by more CD4+/helper T cells with follicular helper T cells (Tfh) correlating with humoral immunity [77, 78]. Experience from SARS-CoV-1 and MERS also suggests that T cell immunity against SARS-CoV-2 may be more enduring [67] and reassuringly largely retained against the highly transmissible Omicron viral variant [79, 80]. Burgeoning evidence also supports a potential role of pre-existing T cell responses in preventing initial infection [81]. Several studies have shown mainly CD4+ T cell responses in up to 50% of samples from blood donors prior to when SARS-CoV-2 appeared in the human population [67, 73, 82–85]. The majority of these T cell responses are to non-spike peptides, including polymerase-specific T cells that were found to expand in abortive SARS-CoV-2 infection [81], but some responses to spike were also reported [73]. It has been proposed that this cross-reactivity is due to previous infection with common cold coronaviruses, which were circulating in the human population prior to 2019 [86], such as human coronavirus HCoV-229E, HCoV-NL63, HCoV-HKU1 and OC43 [87–90]. Kundu et al. examined the role of pre-existing cross-reactive T cell responses in protecting SARS-CoV-2 naïve household contacts of patients infected with SARS-CoV-2. In this study, 52 confirmed exposed contacts were investigated, and T cell responses were assessed in both polymerase chain reaction (PCR)-positive (n = 26) and PCR-negative (n = 26) contacts. The authors found that the initial frequency of human coronaviruses primed cross-reactive T cells, which secrete interleukin-2 (IL-2), are key to protection in contacts who remained PCR-negative [91]. These findings have implications for future vaccine targets, strongly suggesting that the inclusion of non-spike proteins may be essential to increase the breadth of responses, including novel variants in the future.
A limitation of many studies is that analysis of cellular responses has focused on peripheral blood. It is likely that key T cell responses are being underestimated in the lungs and several studies have shown an increase in T cells in bronchoalveolar lavage (BAL) samples from patients with moderate COVID-19 disease compared to patients with severe disease [64, 92, 93]. It is of note that mucosal immune responses are induced during natural infection [94, 95] but there is little evidence to suggest that current vaccines induce mucosal responses [96, 97] without prior SARS-CoV-2 infection [98]. This is an important area that needs further investigation.
IMMUNOLOGICAL INTERPLAY BETWEEN HIV AND SARS-COV-2
The immunological landscape of HIV infection and implications for vaccine efficacy
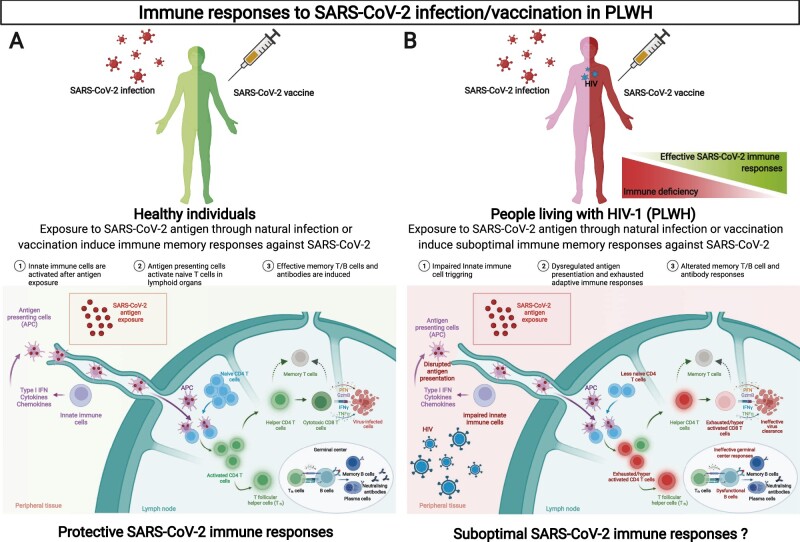
HIV infection induces profound disruption of both the innate and adaptive immune systems (Figure 1). Primary infection induces systemic immune activation and inflammation accompanied by depletion of the T cell compartment, especially in the gut [99, 100]. If left untreated, ongoing viral replication and chronic inflammation leads to the destruction of CD4+ T cells and a persistent expansion of circulating CD8+ T cell numbers. This resulting inversed CD4/CD8 ratio has been associated with frailty and premature ageing of the immune system leading to higher non-AIDS-related morbidity and mortality rates [10, 101–104]. There is an associated reduction in T cell proliferative capacity and cytotoxic potential, which eventually leads to exhaustion [105]. Altered innate immune cell function, such as dysregulation of dendritic cells (DCs), and aberrant responses may also contribute to chronic immune activation and exhaustion [106]. B-cells also develop features of exhaustion relatively early during HIV infection [107]. Abnormal polyclonal activation and poor effector function result in a lack of specific antibody responses, which has been well described [108, 109]. The introduction of ART leads to viral suppression, improved CD4+ T cell counts, and partially restored proportions of B-cell subpopulations [107, 110]. The earlier ART is started, the lower the levels of immune activation and inflammation [111], but despite treatment, chronic activation persists and antigen-specific B and T cell responses, including Tfh cell function, are still impaired [112]. PLWH, despite effective virological suppression, continue to have higher levels of inflammatory mediators, such as IL-6, TNF-α, sCD163, sCD14 and CRP in peripheral blood linked to adverse clinical outcomes [113, 114]. As a result, PLWH are 25 times more likely to suffer from pneumonia and other respiratory diseases, some cancers and infections, such as influenza and tuberculosis, than HIV-negative individuals [115–119]. This raised concerns early in the pandemic that PLWH had a higher risk of infection or a more severe disease course if infected with SARS-CoV-2, despite many PLWH receiving ART, as with other respiratory diseases [120]. Indeed, a more severe disease outcome and increased risk of death have been seen in PLWH, especially when viraemia is not well-controlled or CD4+ T cell count has not been reconstituted sufficiently [20].
Figure 1:
Immune responses to SARS-CoV-2 infection/vaccination in PLWH.
These immunological changes and persistent immune dysfunction in PLWH also have implications for vaccination success (Figure 1). PLWH have lower responses to several vaccines including influenza [121, 122], tetanus, diphtheria [123], yellow fever [124] and poliomyelitis [125]. Vaccine responses are better where the CD4+ T cell count has been reconstituted following the commencement of ART [121]. In addition, the total duration of seroprotection is shorter than in otherwise healthy persons for most licensed vaccines [26]. As treatment options have improved, the life expectancy for PLWH have increased. Additional health concerns such as obesity, hypertension and cardiovascular disease, which contribute further to chronic inflammation and reduce vaccine efficacy, have increased [126]. This mirrors the general trajectory of these conditions in the population. Furthermore, ageing is independently associated with senescence of both the innate and adaptive immune systems [127], leading to innate immune cell dysfunction and a reduction in the humoral and cellular responses to several viral and bacterial vaccinations [126]. This age-related loss of immune function, which may be accelerated in PLWH, in addition to changes to the T cell compartment and reduction in the naïve T cell pool, could decrease immune responses to vaccination [112, 121, 122, 128, 129]. Along these lines, the immunogenicity to mRNA [130, 131] and Adenovirus vector [132] SARS-CoV-2 vaccines have been shown to be diminished in healthy subjects over the age of 55 years compared to those under 55 [133, 134]. Elderly individuals also show evidence of reduction in somatic hypermutation of class-switched cells and lower cellular responses following BNT162b2 vaccination [135]. Interestingly, responses were improved following the administration of booster doses [130–132], highlighting that an ageing immune system is a key consideration for the efficacy of currently licensed SARS-CoV-2 vaccines, warranting specific measures to boost responses, especially considering circulating VOCs.
When debating additional factors influencing immune responses to vaccination in PLWH, it is essential to account for the effect of chronic co-infections (e.g. viral Hepatitis B and C). These commonly occur in PLWH and have overtaken other opportunistic infections as the leading cause of death in PLWH [129] and have been linked to a reduction in vaccine efficacy [10, 126, 136, 137]. Co-infection with cytomegalovirus (CMV) is particularly prevalent in PLWH [138]. This contributes to a persistent immune activation state, described herein, through modification of the gut microbiota and microbial translocation, directing responses against itself, and by induction of immune senescence. These factors lead to a decrease in vaccine responses. SARS-CoV-2 vaccination success is also improved when patient CD4+ T cell counts are >350 cells/µl, prior to immunization (Table 1). Similarly, in the case of Hepatitis B vaccination, the CD4/CD8 ratio has proved an accurate predictor of vaccine success [154]. This is not surprising given that a low CD4/CD8 ratio is a marker of immune senescence [155] and therefore may be an important stratification tool to consider as part of vaccination policies for PLWH.
Table 1:
Summary of SARS-CoV-2 vaccine trial data for PLWH
| Vaccine, dose, country and author | Trial design | Participant characteristics | CD4+ T cell count/HIV control (PLWH) | Prior SARS-CoV-2 infection | Immunological readout | Impact for PLWH |
|---|---|---|---|---|---|---|
|
Phase 2/3 |
|
|
Not part of study criteria |
|
|
|
Randomized, double-blind, placebo-controlled, phase 1B/2A trial |
|
|
|
|
|
|
Participants from the SISONKE South African clinical |
|
|
Actively enrolled unvaccinated and vaccinated participants with prior SARS-COV-2 infection | Neutralization vs. Delta variant only |
|
| Pilot study |
|
|
No evidence of prior SARS-CoV-2 infection (determined by lack of detectable nucleocapsid antibodies) |
|
|
|
|
Prospective open study |
|
|
Not part of study criteria |
|
|
|
Open-label, non-randomized prospective clinical trial |
|
|
Individuals with prior SARS-CoV-2 infection were excluded | Anti-spike IgG (ELISA) |
|
|
Cohort observational study |
|
|
Not included in study design |
|
|
|
Cohort observational study 3–4 weeks post-vaccination |
|
|
11 participants had seroconverted before vaccination and were excluded from study | Anti-RBD IgG (ELISA) |
|
|
Prospective single centre cohort |
|
|
9 PLWH and 2 healthy controls had prior infection with SARS-CoV-2 (Confirmed by antibodies to nucleocapsid) |
|
|
|
Prospective observational cohort | 14 PLWH only (13 male and 1 female), Median age 62 years (IQR 56–70) |
|
Not included in study | Binding IgG antibodies (RBD) (ELISA) | 2 doses of mRNA vaccine resulted in high binding antibody titres in PLWH with well-controlled HIV on ART, regardless of CD4+ T cell counts |
|
Non-interventional, retrospective study |
|
|
Participants with Prior SARS-COV-2 infection were excluded from the study |
|
|
|
Non-interventional trial |
|
Median CD4+ T cell count 710 |
|
|
|
|
Prospective |
|
|
Not included in study protocol |
|
|
|
Interventional Study |
|
CD4+ and CD8+ T-cell count levels were enumerated by flow cytometry after vaccination but numbers prior to vaccination not available | Excluded participants with prior history of exposure or infection with SAR-CoV-2 |
|
|
|
Open-label two-arm non-randomized study |
|
All HIV-positive participants required to have a CD4+ T cell count of >200 at baseline (mean CD4+ T cell count 659) and 4 weeks after vaccination (mean CD4+ T cell count 476.9) | Participants with prior infection with SAR-CoV-2 were excluded |
|
Inactivated whole virus vaccine is safe and capable of inducing neutralizing antibody responses in PLWH receiving ART and with a CD4+ T cell count of >200 CD3+, CD4+, CD8+ T Cell counts of PLWH decreased following vaccination but did not lead to clinical adverse events |
IMMUNE RESPONSES TO NATURAL SARS-COV-2 INFECTION IN PLWH
Insights from studies examining the quantity and quality of immune responses in people who have recovered from natural infection with SARS-CoV-2 can help inform the optimization of vaccines. Arguably any underlying differences in cellular compositions (both innate and adaptive immune phenotypes), in addition to uncontrolled viraemia and persistent inflammation in PLWH, could lead to poorly co-ordinated immune responses, affecting the trajectory of COVID-19 disease (Figure 1). Dysregulated immune cell co-ordination has been shown to attenuate protective immune responses in elderly individuals [61], which could be highly pertinent in PLWH with additional co-morbidities. To date, there are limited data on natural immunity following SARS-CoV-2 infection in PLWH from studies which are conducted in high-income countries, and in populations largely controlled on ART.
Given that antibody responses are thought to be an important immune correlate of protection, SARS-CoV-2 IgG levels and neutralizing antibody activity have been compared in PLWH and HIV-negative individuals following natural infection. In a matched case-control observational study involving 955 PLWH and 1062 people without HIV, the SARS-CoV-2 IgG seroprevalence was 3.7% and 7.4%, respectively. Notably, lower anti-RBD IgG and pseudovirus neutralizing antibody titres, with similar avidity, were observed in the HIV-positive group compared with HIV-negative individuals with evidence of past infection [156]. This is in contrast to smaller studies that did not show any difference in IgG concentrations or neutralization potency against SARS-CoV-2 infection in PLWH. Of note, the latter studies included patients who had well-controlled HIV on ART, which may have been a confounding factor [157–159]. Indeed, a correlation between higher CD4+ T cell count and higher neutralization titres in COVID-19 infection has been described in PLWH [141, 160–162]. At present, an in-depth assessment of B-cell-specific memory responses is lacking in the setting of HIV infection.
The role of T cells in SARS-CoV-2/HIV co-infection is still being deciphered. Unpicking the increased risk due to HIV infection rather than the high risk of co-morbidities is challenging. It remains unclear whether HIV-associated immune dysfunction and inflammation are linked to severe COVID-19 disease outcomes [163, 164] or whether paradoxically a low CD4+ T cell count ameliorate disease severity [134]. A recent study by Sharov et al. compared the T cell profile and cytokine dynamics of patients with COVID-19 disease with and without HIV infection [161]. Of the 367 patients with HIV, 171 were not on ART due to medication shortages during the pandemic. While a similar T cell response was seen in HIV seronegative and HIV-positive patients receiving ART, patients with uncontrolled HIV infection had an attenuated T cell response. A decline in CD4/CD8 ratio was associated with a poorer disease outcome. As expected, T cells displayed a higher rate of T cell exhaustion in HIV infection, characterized by an increased expression of PD-1 and TIM-3. This was more pronounced in the presence of HIV viraemia, suggesting a synergistic effect of HIV/SARS-CoV-2 co-infection on T cell dysfunction. PLWH in the absence of ART had decreased serum concentrations of IL-2, TNF-α and IFN-γ and higher levels of the immunosuppressive cytokines IL-10 and TGF-β [161]. Findings by Alrubayyi and colleagues showed that PLWH, with well-controlled HIV, in the convalescent phase of predominately mild COVID-19 disease, showed equivalent magnitude of SARS-CoV-2 specific T cell responses compared to HIV-negative individuals, targeting both structural and non-structural proteins [133]. SARS-Cov-2-specific T cell responses were dominated by CD4+ T cells. Remarkably, a positive association was noted between naïve CD4+ T cells, the CD4:CD8 ratio and the magnitude of T cell responses against SARS-CoV-2 in PLWH. These findings suggest that in addition to viraemic HIV infection, inadequate reconstitution of the T cell compartment and fewer pre-existing naïve T cells could hinder the development of memory responses to SARS-CoV-2 infection [157]. Whether dysregulated priming, impairment of Tfh cells and other biological factors not captured in the published studies contribute to poorly co-ordinated humoral and cellular responses remains to be determined.
Remarkably, an increasing number of cases of prolonged COVID-19 infection and/or asymptomatic shedding are being reported in people with advanced immunosuppression [160, 165, 166]. Whilst this underscores the importance of a functional immune response in viral clearance [65] it also has implications for SARS-CoV-2 viral evolution. Prolonged infections provide an opportunity for SARS-CoV-2 to evolve a multitude of mutations, as SARS-CoV-2 mutates at a relatively slow rate compared to other RNA viruses, due the presence of a proofreading mechanism [167]. This was recently demonstrated in an HIV-positive woman with unsuppressed HIV and persistent shedding of SARS-CoV-2 for 210 days, during which time SARS-CoV-2 accumulated 30 mutations, some associated with vaccine escape [166, 168].
Additionally, evidence is emerging that PLWH may be more likely to develop post-acute sequalae or ‘long-covid’ [169, 170] However, an accurate picture of the burden of long-covid in this population remains to be determined, including whether immune cell perturbations described in HIV infection may predispose to long-standing symptoms.
Despite the significant gaps in our knowledge and lack of granular data on immune responses in PLWH with different levels of HIV-related immunosuppression, these findings highlight the need for early access to effective ART and support vaccine prioritization in PLWH. Larger studies are needed, particularly for sub-populations of PWLH (e.g. those with low CD4+ T cell counts) or those with identified high-risk co-morbidities, especially in high HIV burden areas to help inform vaccine recommendations and therapeutics.
SARS-COV2 VACCINE TRIAL DATA IN PLWH
The Spike glycoprotein has been an excellent target for SARS-CoV-2 vaccines, which have been developed at an impressive speed, as a result of a collective effort by regulatory agencies, pharmaceutical companies and the scientific community [171]. Currently licensed vaccines include mRNA vaccines (mRNA-1273 and BNT162b2) [46, 48], non-replicating adenoviral vectors (ChAdOx1 nCoV-19 and Ad26.COV2.S), viral proteins with an adjuvant (NVX-CoV2373) [172] and inactivated SARS-CoV-2 virus (BIBP-CorV) [153]. Several large Phase 2/3 trials of SARS-CoV-2 vaccines have shown them to be safe and highly effective in the general population. However, after two doses effectiveness reaches 65–90% against infection or mild disease, and 90–100% against severe disease prior to the emergence of VOCs [46–48, 173]. Although individuals with stable treated HIV infection were not excluded in some from the larger Phase 2/3 trials, they made up a small proportion of participants (∼196 for the BNT162b2 mRNA vaccine [48], 176 for the mRNA-1273 mRNA vaccine [46] and 107 PLWH for the ChAdOx1 viral vectored vaccine [47]). Not all the data on PLWH has been presented to date and the small numbers make interpretation on vaccine efficacy difficult. The Ad26.COV2.S trial has included by far the largest number of PLWH (467 people well-controlled with a CD4+ T cell count >300 received a single dose and 498 received a placebo). Two people from the vaccine group and four people from the placebo group developed moderate to severe COVID-19 disease 28 days post-vaccination [174]. It may be that certain vaccine platforms will not be as effective in PLWH or other immunocompromised individuals. Some concerns about the efficacy of NVX-CoV2373 sub-unit vaccine in PLWH have been raised. In one of the pivotal Phase 2a–b trials conducted in South Africa, overall vaccine efficacy dropped from 60.1% to 49.4% when PLWH were included. It is of note that this study was not powered to specifically describe efficacy in the participants with HIV but highlighted the need to specifically assess vaccine efficacy in PLWH [172]. Importantly in this study, 92.7% of sequence cases of SARS-CoV-2 infection accounted for the B.1.351 variant [172]. When assessing vaccine efficacy in PLWH, in addition to numbers included, it is important to consider definitions of efficacy and the epidemiological setting. To date, there are no head-to-head comparisons between vaccines and, as such, whether a certain vaccine platform is more effective in PLWH remains unknown. Future planned studies are planned to address remaining concerns/uncertainties for COVID-19 vaccines in PLWH (NCT04533399; NCT04754698). The main findings of COVID-19 vaccine studies in PLWH are summarized in Table 1.
VACCINE SAFETY FOR PLWH
Whilst safety concerns surrounding the licensed SAR-CoV-2 vaccines have been publicly voiced and in turn addressed by the scientific community, there have been no additional concerns regarding safety of SARS-CoV-2 vaccinations in PLWH. The most commonly reported side effects include mild local and systemic reactions, and these have been shown to occur equally in PLWH and the general population [175]. There have been some reports of HIV viral blips following mRNA vaccinations. Levy et al. highlighted three cases who have low-level viraemia (<100 copies/ml) and a separate case report described a patient who had a viral load of 1760 copies/ml [144, 176] following vaccination. All of these cases had nadir CD4+ T cell counts of <200 cells/ml and/or very high viral loads at diagnosis. However, Levy and colleagues concluded that SARS-CoV-2 vaccination is safe and efficacious in PLWH, with stable CD4+ T cell counts and well-controlled viraemia. Viral blips have been noted with other vaccines, including influenza and hepatitis B, typically 7–14 days following vaccination [177] but these are transient and may be attributed to a reactivation of the latent reservoir. The interplay of SARS-CoV-2 vaccines, the immune system, and latent HIV infection is yet to be thoroughly understood. However, these observations suggest that viral load monitoring post-vaccination may be useful in future studies, particularly for those with low CD4+ T cell counts. It should be highlighted that the benefit of receiving vaccination outweighs the risk.; this key finding is highlighted by the vaccine trials summarized in Table 1.
SARS-COV-2 VACCINE IMMUNOGENICITY IN PLWH
mRNA vaccines
Immune responses in PLWH following vaccination with mRNA-based vaccines have been studied more extensively. Two prospective cohort trials [146, 147] and one non-interventional study [150] which compared humoral responses in PLWH and people without HIV found that while the responses to the priming dose of mRNA vaccine were lower in PLWH, following the second dose humoral responses these were comparable to that observed in HIV negative participants. Several small studies have demonstrated excellent seroconversion rates (as measured by detection of spike-RBD specific IgG) with positive responses in 97–98% of PLWH following two vaccines. Notably, these findings were observed in the context of well-controlled HIV [144, 145, 149] with comparable neutralizing antibody titres to HIV-negative people [148]. The requirement for at least two doses of mRNA vaccines was further highlighted by Woldemeskel et al. demonstrating equivalent SARS-CoV-2 spike binding antibody titres and cellular responses (assessed by T cell IFN-γ production) irrespective of HIV status. Additionally, there was no significant difference in BNT162b2-elicited SARS-CoV-2 binding antibody levels to the Beta, Alpha and Gamma variants. Despite this, the numbers in this study are small and its findings need to be interpreted with caution [142, 143].
mRNA vaccine immunogenicity is less well-described in PLWH with ongoing immunosuppression and viraemia, who are a particularly vulnerable group that is poorly represented in vaccine trials. In a single case report, lack of seroconversion and no detectable cellular responses were observed following two doses of BNT162b2 in a patient who was vaccinated prior to ART initiation (CD4+ T cell count of 20 cells/µl) [178]. This is consistent with lower seroconversion rates in people with underlying malignancies and transplant recipients [179]. Emerging evidence presented at recent international meetings, indicates that lower CD4+ T cell counts <250 cells/µl, viraemia and/or previous AIDS associate with significantly weaker spike antibody responses, weaker cellular responses and a higher risk of waning neutralizing activity after a median of 5 months in PLWH. This identifies them as more vulnerable to reduced vaccine efficacy [178, 180–183]. PLWH with a CD4+ T cell count <250 cells/µl were found to have a reduced neutralizing ability against the Beta and the Delta variant. No data against Omicron are currently available [183]. As expected, prior SARS-CoV-2 infection predicted higher spike antibodies, as observed for the general population [182]. In an Italian study, a third dose mRNA booster of either BNT162B2 or mRNA-1273 > 28 days following a complete mRNA vaccination course was found to strongly boost humoral responses in PLWH with advanced disease (CD4+ T cell count <200 cells/µl and/or previous AIDS). This was irrespective of the patients’ CD4+ T cell count at the time of boosting and supports the use of an additional vaccine dose in this patient group [184].
ADENOVIRUS VECTORED VACCINES
The Adenovirus vector-based vaccine ChAdOx1 nCoV-19 has also been shown to induce equivalent humoral responses in PLWH and HIV-negative volunteers. Three published studies compared spike-specific IgG responses and neutralizing antibody profiles of HIV-negative individuals to PLWH with well-controlled HIV and CD4+ T cell counts >350 cells/µl. No significant differences were found based on HIV status [139, 140, 185]. Encouragingly, Madhi et al. demonstrated that 50% of PLWH had cross-reactive binding antibodies to the Beta variant and wild-type [140]. High responders retained this neutralization capacity against the Beta variant [139, 140, 185]. Additionally, T cell responses, determined by ELISpot were comparable to the HIV-negative group [140]. Data on the durability of these responses have been recently published showing no significant differences in ChadOx1 nCov19 vaccine-mediated responses, according to HIV status, in 54 PLWH CD4+ T cells >350 cells/µl and 50 HIV-negative age and sex-matched controls. Waning but detectable humoral and T cell immune responses against the wild type and VOCs (Alpha, Beta, Gamma and Delta) were observed 6 months after vaccination [139, 186]. Interestingly in this study, prior exposure to circulating β coronaviruses (HKU1 and OC43) was associated with detectable proliferative SARS-CoV-2 T cell responses at baseline, which were further augmented post-vaccination. This suggests that pre-existing cross-reactive responses could modulate post-vaccine responses in PLWH [186].
Khan et al. reported similar neutralization responses in PLWH and HIV-negative individuals who had been vaccinated with a different adenovirus-based vaccine (Ad26.COV2.S) and subsequently became infected with the Delta variant [141]. Whereas PLWH had previously been infected with SARS-CoV-2 and then vaccinated, a 9-fold higher Delta variant neutralization was seen compared to the vaccinated-only group, indicating that vaccination boosted the neutralization response reflecting the same phenomena in the general population [140, 141, 148].
How these data extrapolate to PLWH with lower CD4+ T cell counts and/or ongoing viraemia is not known and additional research is required to address the immunogenicity and durability of adenovirus vectored vaccines in this sub-group of PLWH.
HETEROGENOUS VACCINATION SCHEDULES AND BREAKTHROUGH INFECTION STUDIES
Optimizing the immunogenicity of vaccines is critical to either stimulate waning immunity or to increase the breadth of immunity. This is either as part of a primary course or against SARS-CoV-2 protein lineage variants, where reduced efficacy has been reported. Data in HIV infection are scarce regarding the optimal vaccination schedule, including the time interval between prime and boost. In the UK a third dose is given as part of the primary immunization course in advanced HIV infection (at least 8 weeks after the last dose) and subsequent booster doses are recommended after the last vaccine dose for all PLWH. In individuals who completed the ChAdOx1 nCoV-19 vaccine schedule, an mRNA booster vaccination is preferentially advised. Thus far, heterogenous vaccination approaches have shown superior immunogenicity outcomes, quantified by both humoral and cellular responses to the wild-type virus and its variants [187]. Both animal studies and emerging evidence in humans, suggest that adenovirus-vectored prime followed by an mRNA boost, at an interval of 6–12 weeks, provides enhanced humoral and cellular responses compared to homologous vaccination [187–192]. In a non-interventional retrospective study, including 665 PLHW in Germany, Noe et al. described the anti-SARS-CoV-2 antibody response following standard vaccination (heterologous and homologous) schedules [21]. They found that mRNA vaccination schedules, being female and having a higher CD4+ T cell count were associated with a higher concentration of antibodies in PLWH. There was a markedly lower response in PLWH with a CD4+ T cell count <200 cells/µl, however, as with other studies, the numbers were small. Further studies would be required to confirm if these reduced responses do result in a higher risk of infection and more severe disease. Questions on the optimization of current vaccine schedules and flexibility in using different COVID-19 vaccines were addressed in the Com-CoV2 study in HIV-negative adults aged 50 years and over. These adults were immunized with either: a single dose of ChAdOx1 nCoV-19 or BNT162B2, or heterologous dosing with mRNA-1273 but not NVX-CoV2373. This resulted in increased reactogenicity compared with homologous schedules [193]. Further work is required to address the effects of this mix and match approach prospectively in PLWH with differing levels of immunosuppression and/or natural exposure to SARS-CoV-2 and circulating variants as the epidemic evolves. It is likely that these approaches will add resilience to circulating variants by inducing stimulation of complementary immune pathways, leading to more effective and durable B cell and T cell responses.
To date, few studies have analysed the rates of breakthrough infections in PLWH. Data from Israel has estimated that ∼40% of breakthrough infections occur in immunocompromised individuals [179]. Two large longitudinal cohorts in the USA have estimated a similar number of breakthrough SARS-CoV-2 infections in vaccinated PLWH compared to people without HIV which included 8536 [194] and 31 840 PWH [195]. Both studies found a 33–41% higher risk of breakthrough infection in PLWH, which persisted after regression analysis for covariates such as age, race/ethnicity and sex at birth. Conversely, booster recipients had a reduced risk of infection compared to those who were not boosted, as well as a reduced risk of severe COVID-19 disease outcomes. This indicates that boosters are important tools of protection for PLWH. Interestingly, in contrast to vaccination studies described herein, Coburn and colleagues did not find any correlation between CD4+ T cell count and/or HIV viraemia to be associated with breakthrough risk [195]. However, it should be noted that data on breakthrough infections is limited by diagnostic testing practices and access to healthcare. As with many of the studies included in this review, the duration of ART and the level of suppression required are not consistent between studies and therefore, it is more difficult to untangle the specific effects of these variables and how they may impact vaccine responses.
As for the general population, it is expected that additional vaccine doses will offer some degree of protection against omicron and severe disease requiring hospitalization. Early data from Israel in people aged 60 or older showed that a fourth dose mRNA vaccine against omicron reduces the risk of infection and disease severity. At present HIV-specific data following a fourth (and/or additional vaccine doses) are lacking [196].
LIMITATIONS OF SARS-COV-2 VACCINES STUDIES IN PLWH
There is currently a lack of standardized assays for determining vaccine efficacy and correlation of protection for humoral or cellular immune responses. The gold standard for vaccine efficacy is neutralizing antibody responses but there are a number of different assays utilized in studies. These include live-virus neutralization [197], pseudotype virus neutralization [197, 198] and surrogate neutralization assays [199]. A consensus on the ideal neutralization assay has not yet been reached as pseudovirus-based assays are not routinely utilized in clinical care. Live-virus neutralization assays are labour intensive and can only be performed by specialist high-containment laboratories with highly trained staff [200]. To address this, several groups have attempted to produce standards, which could be used for comparison of data between labs [35, 201]. This is critical to fully comprehend vaccine responses in PLWH as aggregation of data collected from diverse neutralization, RBD and ELISA assays, and clinical trial designs are required to make statistically significant conclusions. Moreover, the selection of appropriate assays is complicated by the potential for false positives due to interference with anti-retrovirals (e.g. reverse transcriptase and integrase inhibitors), especially in cell-based assays and lentiviral-vector pseudotype virus assays [202]. Additionally, the inclusion of some patients with prior SARS-CoV-2 infection makes interpretation of vaccine response more complex, especially as studies in HIV-negative people have shown that previous infection with SARS-CoV-2 enhances T cell and antibody responses post-vaccination [131, 203, 204].
There are very few studies that focus on the cellular response to SARS-CoV-2 vaccination in PLWH, which may be in part due to technical difficulties in carrying out cellular-based assays. The assumption that the degree of humoral response is paralleled by the cellular immune response may not hold true for PLHW given the distinct T cell dysregulation that occurs. This might be particularly relevant for PLWH with depleted CD4+ T cells, who appear to be at higher risk of severe COVID-19, and reduced responsiveness to vaccine. As with neutralization data, the numbers of PLHW included in published studies are small. Hence, they are unable to adequately adjust for many confounding variables that may affect vaccine responses. In addition, data for SARS-CoV-2 vaccine response for PLWH over the age of 55 are scarce and the combined effect of ageing, chronic illness and HIV infection on vaccines responses is yet to be fully understood, and may in part, account for the findings of higher risk breakthrough infections described in PLWH.
CONCLUDING REMARKS AND REMAINING CHALLENGES
PLWH have been dealing with a great deal of uncertainty throughout the pandemic, particularly as evidence regarding risk of disease severity has been conflicting, and data on vaccine efficacy remain limited. Studies of seroconversion rates, and neutralization titres post-SARS-CoV-2 vaccination in PLWH, are reassuring for those who have stable HIV on ART and preserved immune function. These findings further highlight the critical role of CD4+ T cells as facilitators of effective humoral responses and offer insights into the complementary role of T cell-specific responses in mediating protection, which may be hindered in people with incomplete immune reconstitution and/or a diminished repertoire of naïve T cells. However, as there is not a consensus on what constitutes protective immunity, it is hard to define protective efficacy in immunocompromised individuals. In particular further work is required to disentangle the importance of T cell immunity in vaccine-mediated protection against SARS-CoV-2 and circulating variants. What is becoming apparent is that PLWH should follow current recommended vaccination schedules and boosters as they become available. This is given that SARS-CoV-2 vaccination is safe and efficacious; overall vaccine effectiveness was 65% (95% CI 56–72%, P < 0.001) among vaccinated compared to unvaccinated PLWH [175]. However, these data need to be continuously evaluated in the context of the evolving pandemic, prevalence of circulating variants, different vaccination schedules and number of doses.
Male adults living in Europe, the United States, and South Africa are the most represented participants to date, which poorly reflects the global prevalence of PLWH. Although the primary aim is to start PLWH on ART immediately, this is not always possible in resource-limited settings. The pandemic has further highlighted disparities in access to ART and global disparities in vaccine coverage, which may leave PLWH potentially vulnerable [161, 205]. There is evidence of worse COVID-19 disease outcomes in patients with coinfections, such as Mycobacterium tuberculosis (TB) [16, 17]. The intersecting SARS-CoV-2, HIV, and TB epidemics pose additional concerns, particularly as T cell immunity and SARS-CoV-2–specific CD4+ T cells are reduced and display lower polyfunctional capacity in the setting of co-infection [162].
A potential confounding factor in the evaluation of vaccine efficacy in PLWH is the use of ART as some, i.e. lopinavir–ritonavir, have anti-coronavirus activity in vitro [206]. Although the role of ART in preventing complications of COVID-19 has been postulated, it is unlikely that the plasma concentrations of ART are enough to inhibit SARS-CoV-2 replication [207]. Lopinavir–ritonavir has not been shown to reduce inpatient mortality or hospitalization length in patients with COVID-19 and is not currently a recommended therapy [208].
Importantly, it is also becoming increasingly apparent that PLWH represent a diverse population in terms of their immune phenotype and levels of immunosuppression. Specific subgroups could therefore benefit from distinct immunization strategies, such as an adapted vaccine schedule and additional doses to increase protection against severe disease. For instance, altered dose regimens, repeat vaccine series or use of adjuvants may be needed as an additional strategy to improve immunological responses in PLWH with evidence of immunodeficiency or additional co-morbidities, as shown for other vaccines [209, 210]. Assessment of total CD4+ T cell, CD4:CD8 ratios and levels of viraemia should be considered in determining vaccine scheduling and efficacy, with the caveat that it will not capture the full immune profile. Although correlates of protection are currently unknown, spike-antibody ELISA assays are accessible assays and have been shown to correlate with neutralizing antibody responses [29] with the caveat that these responses are reduced against circulating VOCs [211, 212]. Post-vaccination testing for spike antibody could be considered, however, to identify subpopulations of immunocompromised people who may not mount an immune response and therefore require additional protection. Future research should aim to assess the magnitude and the durability of SARS-CoV-2 vaccine-induced antibody and T cell responses in PLWH with particular focus on those with uncontrolled viral infection and/or who have low CD4+ T cell counts to inform the best strategy for boosting. Greater attention needs to be paid to the combined effect of ageing, co-morbidities, and HIV infection as part of the research agenda. Finally, a consensus of assays used for assessment of vaccine responses and a threshold of protection for humoral and cellular responses would greatly benefit assessment of required responses in PLWH.
Contributor Information
Claire Mullender, Centre for Clinical Research in Infection and Sexual Health, Institute for Global Health, University College London Institute for Global Health, London, UK.
Kelly A S da Costa, Division of Infection and Immunity, University College London, London, UK.
Aljawharah Alrubayyi, Division of Infection and Immunity, University College London, London, UK; Nuffield Department of Clinical Medicine, University of Oxford, Oxford, UK.
Sarah L Pett, Centre for Clinical Research in Infection and Sexual Health, Institute for Global Health, University College London Institute for Global Health, London, UK; Medical Research Council Clinical Trials Unit, Institute of Clinical Trials and Methodology, London, UK.
Dimitra Peppa, Division of Infection and Immunity, University College London, London, UK.
Data Availability
All data are contained within the manuscript.
FUNDING
D.P. and K.A.S.d.C. Receive funding from NIH grant R01AI155182. S.L.P. has grant funding in support of trials unrelated to the work from Janssen-Cilag, Gilead Sciences, EDCTP, ViiV Healthcare. She receives salary support from the MRC grants MC_UU_00004/03 and MC_UU_00004/04.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1. Dong E, Du H, Gardner L.. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ambrosioni J, Blanco JL, Reyes-Urueña JM. et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV 2021;8:e294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(UNAIDS) UJPoHA. UNAIDS DATA2020. https://www.unaids.org/en/resources/documents/2020/unaids-data (February 2022, date last accessed).
- 4. Li C, Zhu Y, Qi C. et al. Estimating the prevalence of asymptomatic COVID-19 cases and their contribution in transmission - using Henan Province, China, as an example. Front Med 2021;8:591372. https://doi.org/10.3389/fmed.2021.591372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ho FK, Petermann-Rocha F, Gray SR. et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS One 2020;15:e0241824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng Z, Peng F, Xu B. et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 2020;81:e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richardson S, Hirsch JS, Narasimhan M. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guaraldi G, Orlando G, Zona S. et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011;53:1120–6. [DOI] [PubMed] [Google Scholar]
- 11. CDC. HIV among People Aged 50 and Over | Age | HIV by Group | HIV/AIDS | CDC.https://www.cdc.gov/hiv/group/age/olderamericans/index.html (February 2022, date last accessed).
- 12. Kim JLKH, Kim GE, Kim S. et al. Clinical characteristics and mortality of patients with hematologic malignancies and COVID-19: a systematic review. Eur Rev Med Pharmacol Sci 2020;24:11926–33. [DOI] [PubMed] [Google Scholar]
- 13. Kates OS, Haydel BM, Florman SS. et al. Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study. Clin Infect Dis 2021;73:e4090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gianfrancesco M, Hyrich KL, Al-Adely S. et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organisation (WHO). WHO-2019-nCoV-Vaccines-SAGE-Prioritization-2022.1. https://www.who.int/publications/i/item/who-sage-roadmap-for-prioritizing-uses-of-covid-19-vaccines (March 2022, date last accessed).
- 16. Baskaran V, Lawrence H, Lansbury LE. et al. Co-infection in critically ill patients with COVID-19: an observational cohort study from England. J Med Microbiol 2021;70:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boulle A, Davies M-A, Hussey H. et al. Risk factors for coronavirus disease 2019 (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis 2021;73:e2005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang X, Sun J, Patel RC. et al. Associations between HIV infection and clinical spectrum of COVID-19: a population level analysis based on US National COVID Cohort Collaborative (N3C) data. Lancet HIV 2021;8:e690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bertagnolio S, Thwin SS, Silva R. et al. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: analysis of data from the WHO Global Clinical Platform of COVID-19. Lancet HIV 2022;9:e486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dandachi D, Geiger G, Montgomery MW. et al. Characteristics, comorbidities, and outcomes in a multicenter registry of patients with human immunodeficiency virus and coronavirus disease 2019. Clin Infect Dis 2021;73:e1964–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noe S, Ochana N, Wiese C. et al. Humoral response to SARS-CoV-2 vaccines in people living with HIV. Infection 20213:617–623. https://doi.org/10.1007/s15010-021-01721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoffmann C, Casado JL, Härter G. et al. Immune deficiency is a risk factor for severe COVID‐19 in people living with HIV. HIV Med 2021;22:372–8. [DOI] [PubMed] [Google Scholar]
- 23.(BHIVA) BHA. BHIVA Statement on JCVI Recommendations for a Third COVID-19 Vaccine Dose. https://www.bhiva.org/BHIVA-statement-on-JCVI-recommendations-for-a-third-COVID-19-vaccine-dose (March 2022, date last accessed).
- 24. Centre for Disease Control (CDC) CfDCap. COVID-19 Vaccines for Moderately or Severely Immunocompromised People. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html (March 2022, date last accessed).
- 25.World Health Organisation. WHO SAGE Roadmap for prioritizing uses of COVID-19 vaccines. https://www.who.int/publications/i/item/who-sage-roadmap-for-prioritizing-uses-of-covid-19-vaccines (March 2022, date last accessed).
- 26. Kerneis S, Launay O, Turbelin C. et al. Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis. Clin Infect Dis 2014;58:1130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.British HIV Association. BHIVA Statement on JCVI Recommendations for COVID Vaccine Spring 2022 Booster Dose. https://www.bhiva.org/BHIVA-community-statement-on-JCVI-recommendations-for-COVID-vaccine-spring-2022-booster-dose (March 2022, date last accessed).
- 28. Yang Y, Du L.. SARS-CoV-2 spike protein: a key target for eliciting persistent neutralizing antibodies. Signal Transduct Target Ther 2021;6:95. https://doi.org/10.1038/s41392-021-00523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Y, Zuiani A, Fischinger S. et al. Quick COVID-19 healers sustain anti-SARS-CoV-2 antibody production. Cell 2020;183:1496–507.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McMahan K, Yu J, Mercado NB. et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021;590:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu J, Tostanoski LH, Peter L. et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020;369:806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deng W, Bao L, Liu J. et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 2020;369:818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hall VJ, Foulkes S, Charlett A. et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet 2021;397:1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feng S, Phillips DJ, White T. et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021;27:2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castillo-Olivares J, Wells DA, Ferrari M. et al. Analysis of serological biomarkers of SARS-CoV-2 infection in convalescent samples from severe, moderate and mild COVID-19 cases. Front Immunol 2021;12:748291. https://doi.org/10.3389/fimmu.2021.748291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lucas C, Klein J, Sundaram M. et al. Kinetics of antibody responses dictate COVID-19 outcome. medRxiv: the preprint server for health sciences2020:2020.12.18.20248331.
- 37. Zheng J, Deng Y, Zhao Z. et al. Characterization of SARS-CoV-2-specific humoral immunity and its potential applications and therapeutic prospects. Cell Mol Immunol 2022;19:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Zhang L, Sang L. et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest 2020;130:5235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siracusano G, Brombin C, Pastori C. et al. Profiling antibody response patterns in COVID-19: spike S1-reactive IgA signature in the evolution of SARS-CoV-2 infection. Front Immunol 2021;12:772239. https://doi.org/10.3389/fimmu.2021.772239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao J, Yuan Q, Wang H. et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis 2020;71:2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lou B, Li T-D, Zheng S-F. et al. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur Respir J 2020;56:2000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lynch KL, Whitman JD, Lacanienta NP. et al. Magnitude and kinetics of anti–severe acute respiratory syndrome coronavirus 2 antibody responses and their relationship to disease severity. Clin Infect Dis 2021;72:301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seow J, Graham C, Merrick B. et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol 2020;5:1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Y, Li J, Li H. et al. Persistence of SARS-CoV-2-specific antibodies in COVID-19 patients. Int Immunopharmacol 2021;90:107271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harvey WT, Carabelli AM, Jackson B. et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 2021;19:409–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baden LR, El Sahly HM, Essink B. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Voysey M, Clemens SAC, Madhi SA. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Polack FP, Thomas SJ, Kitchin N. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khoury DS, Cromer D, Reynaldi A. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205–11. [DOI] [PubMed] [Google Scholar]
- 50. Feikin D R, Higdon MM, Abu-Raddad Laith J. et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022;399:924–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Munro APS, Janani L, Cornelius V. et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021;398:2258–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cromer D, Steain M, Reynaldi A. et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe 2022;3:e52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jeffery-Smith A, Burton AR, Lens S. et al. SARS-CoV-2–specific memory B cells can persist in the elderly who have lost detectable neutralizing antibodies. J Clin Invest 2022;132:e152042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Z, Muecksch F, Schaefer-Babajew D. et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021;595:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sokal A, Chappert P, Barba-Spaeth G. et al. Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell 2021;184:1201–13.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sakharkar M, Rappazzo CG, Wieland-Alter WF. et al. Prolonged evolution of the human B cell response to SARS-CoV-2 infection. Sci Immunol 2021;6:eabg6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Muecksch F, Weisblum Y, Barnes CO. et al. Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity 2021;54:1853–68.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reyes RA, Clarke K, Gonzales SJ. et al. SARS-CoV-2 spike-specific memory B cells express higher levels of T-bet and FcRL5 after non-severe COVID-19 as compared to severe disease. PLoS One 2021;16:e0261656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bergamaschi L, Mescia F, Turner L. et al. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity 2021;54:1257–75.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol 2022;23:186–93. [DOI] [PubMed] [Google Scholar]
- 61. Rydyznski Moderbacher C, Ramirez SI, Dan JM. et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020;183:996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sekine T, Perez-Potti A, Rivera-Ballesteros O. et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020;183:158–68.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhou R, To KK-W, Wong Y-C. et al. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity 2020;53:864–77.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liao M, Liu Y, Yuan J. et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020;26:842–4. [DOI] [PubMed] [Google Scholar]
- 65. Tan AT, Linster M, Tan CW. et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep 2021;34:108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Notarbartolo S, Ranzani V, Bandera A. et al. Integrated longitudinal immunophenotypic, transcriptional, and repertoire analyses delineate immune responses in patients with COVID-19. Sci Immunol 2021;6:eabg5021. [DOI] [PubMed] [Google Scholar]
- 67. Le Bert N, Tan AT, Kunasegaran K. et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020;584:457–62. [DOI] [PubMed] [Google Scholar]
- 68. Oberhardt V, Luxenburger H, Kemming J. et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature 2021;597:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Soresina A, Moratto D, Chiarini M. et al. Two X‐linked agammaglobulinemia patients develop pneumonia as COVID‐19 manifestation but recover. Pediatr Allergy Immunol 2020;31:565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Safavi F, Nourbakhsh B, Azimi AR.. B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult Scler Relat Disord 2020;43:102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Montero-Escribano P, Matías-Guiu J, Gómez-Iglesias P. et al. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid, Spain. Mult Scler Relat Disord 2020;42:102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bange EM, Han NA, Wileyto P. et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med 2021;27:1280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grifoni A, Weiskopf D, Ramirez SI. et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020;181:1489–501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grifoni A, Sidney J, Vita R. et al. SARS-CoV-2 human T cell epitopes: adaptive immune response against COVID-19. Cell Host Microbe 2021;29:1076–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Peng Y, Felce SL, Dong D. et al. An immunodominant NP105–113-B07:02 cytotoxic T cell response controls viral replication and is associated with less severe COVID-19 disease. Nat Immunol 2022;23:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Peng Y, Mentzer AJ, Liu G. et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol 2020;21:1336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Juno JA, Tan H-X, Lee WS. et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat Med 2020;26:1428–34. [DOI] [PubMed] [Google Scholar]
- 78. Stephenson E, Reynolds G, Botting RA. et al. Single-cell multi-omics analysis of the immune response in COVID-19. Nat Med 2021;27:904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tarke A, Coelho CH, Zhang Z. et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022;185:847–59.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu J, Chandrashekar A, Sellers D. et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 omicron. Nature 2022;603:493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Swadling L, Diniz MO, Schmidt NM. et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 2022;601:110–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schulien I, Kemming J, Oberhardt V. et al. Characterization of pre-existing and induced SARS-CoV-2-specific CD8+ T cells. Nat Med 2021;27:78–85. [DOI] [PubMed] [Google Scholar]
- 83. Mateus J, Grifoni A, Tarke A. et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020;370:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Weiskopf D, Schmitz KS, Raadsen MP. et al. Phenotype and kinetics of SARS-CoV-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol 2020;5:2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Braun J, Loyal L, Frentsch M. et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020;587:270–4. [DOI] [PubMed] [Google Scholar]
- 86. Cassaniti I, Percivalle E, Bergami F. et al. SARS-CoV-2 specific T-cell immunity in COVID-19 convalescent patients and unexposed controls measured by ex vivo ELISpot assay. Clin Microbiol Infect 2021;27:1029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Killerby ME, Biggs HM, Haynes A. et al. Human coronavirus circulation in the United States 2014-2017. J Clin Virol 2018;101:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gorse GJ, Patel GB, Vitale JN. et al. Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clin Vaccine Immunol 2010;17:1875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Walsh EE, Shin JH, Falsey AR.. Clinical impact of human coronaviruses 229E and OC43 infection in diverse adult populations. J Infect Dis 2013;208:1634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Guo L, Wang Y, Kang L. et al. Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study. Emerg Microbes Infect 2021;10:664–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kundu R, Narean JS, Wang L. et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat Commun 2022;13:80. https://doi.org/10.1038/s41467-021-27674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Oja AE, Saris A, Ghandour CA. et al. Divergent SARS‐CoV‐2‐specific T‐ and B‐cell responses in severe but not mild COVID‐19 patients. Eur J Immunol 2020;50:1998–2012. [DOI] [PubMed] [Google Scholar]
- 93. Gelarden I, Nguyen J, Gao J. et al. Comprehensive evaluation of bronchoalveolar lavage from patients with severe COVID-19 and correlation with clinical outcomes. Hum Pathol 2021;113:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Smith N, Goncalves P, Charbit B. et al. Distinct systemic and mucosal immune responses during acute SARS-CoV-2 infection. Nat Immunol 2021;22:1428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wright PF, Prevost-Reilly AC, Natarajan H. et al. Longitudinal systemic and mucosal immune responses to SARS-CoV-2 infection. J Infect Dis 2022:jiac065. https://doi.org/10.1093/infdis/jiac065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chan RWY, Liu S, Cheung JY. et al. The mucosal and serological immune responses to the novel coronavirus (SARS-CoV-2) vaccines. Front Immunol 2021;12:744887. https://doi.org/10.3389/fimmu.2021.744887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Azzi L, Dalla Gasperina D, Veronesi G. et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. eBioMedicine 2022;75:103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sano K, Bhavsar D, Singh G. et al . Efficient Mucosal Antibody Response to SARS-CoV-2 Vaccination Is Induced in Previously Infected Individuals. medRxiv;2021. https://doi.org/10.1101/2021.12.06.21267352. [Google Scholar]
- 99. Asowata OE, Singh A, Ngoepe A. et al. Irreversible depletion of intestinal CD4+ T cells is associated with T cell activation during chronic HIV infection. JCI Insight 2021;6:e146162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Guadalupe M, Reay E, Sankaran S. et al. Severe CD4 + T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 2003;77:11708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hunt PW, Lee SA, Siedner MJ.. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J Infect Dis 2016;214(suppl 2):S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Smith R, de Boer R, Brul S. et al. Premature and accelerated aging: HIV or HAART? Front Genet 2013;3:328. https://doi.org/10.3389/fgene.2012.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Deeks SG, Phillips AN.. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 2009;338:a3172. [DOI] [PubMed] [Google Scholar]
- 104. Erlandson KM, Karris MY. HIV and aging: reconsidering the approach to management of comorbidities. Infect Dis Clin North Am 2019;33:769–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fenwick C, Joo V, Jacquier P. et al. T‐cell exhaustion in HIV infection. Immunol Rev 2019;292:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sabado RL, O'Brien M, Subedi A. et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood 2010;116:3839–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bussmann BM, Reiche S, Bieniek B. et al. Loss of HIV-specific memory B-cells as a potential mechanism for the dysfunction of the humoral immune response against HIV. Virology 2010;397:7–13. [DOI] [PubMed] [Google Scholar]
- 108. Kardava L, Moir S, Wang W. et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Invest 2011;121:2614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Moir S, Fauci AS.. B-cell responses to HIV infection. Immunol Rev 2017;275:33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bart P-A, Rizzardi GP, Tambussi G. et al. Immunological and virological responses in HIV-1-infected adults at early stage of established infection treated with highly active antiretroviral therapy. AIDS 2000;14:769–786. https://doi.org/10.1016/j.idc.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 111. Group TISS. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pallikkuth S, de Armas L, Rinaldi S. et al. T follicular helper cells and B cell dysfunction in aging and HIV-1 infection. Front Immunol 2017;8:1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Deeks SG, Tracy R, Douek DC.. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013;39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Margolick JB, Bream JH, Martínez-Maza O. et al. Frailty and circulating markers of inflammation in HIV+ and HIV− men in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr 2017;74:407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Triplette M, Justice A, Attia EF. et al. Markers of chronic obstructive pulmonary disease are associated with mortality in people living with HIV. AIDS 2018;32:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Costiniuk CT, Jenabian M-A.. The lungs as anatomical reservoirs of HIV infection. Rev Med Virol 2014;24:35–54. [DOI] [PubMed] [Google Scholar]
- 117. Shiels MS, Cole SR, Kirk GD. et al. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr 2009;52:611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kirk GD, Merlo C, O'Driscoll P. et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis 2007;45:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Alexandrova Y, Costiniuk CT, Jenabian M-A. Pulmonary immune dysregulation and viral persistence during HIV infection. Front Immunol 2022;12:808722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Brown J, Pickett E, Smith C. et al. The effect of HIV status on the frequency and severity of acute respiratory illness. PLoS One 2020;15:e0232977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pallikkuth S, De Armas LR, Pahwa R. et al. Impact of aging and HIV infection on serologic response to seasonal influenza vaccination. AIDS 2018;32:1085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. George VK, Pallikkuth S, Parmigiani A. et al. HIV infection worsens age-associated defects in antibody responses to influenza vaccine. J Infect Dis 2015;211:1959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bonetti TC, Succi RCM, Weckx LY. et al. Tetanus and diphtheria antibodies and response to a booster dose in Brazilian HIV-1-infected women. Vaccine 2004;22:3707–12. [DOI] [PubMed] [Google Scholar]
- 124. Avelino-Silva VI, Miyaji KT, Hunt PW. et al. CD4/CD8 ratio and KT ratio predict yellow fever vaccine immunogenicity in HIV-infected patients. PLoS Negl Trop Dis 2016;10:e0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kroon FP, Van Dissel JT, Labadie J. et al. Antibody response to diphtheria, tetanus, and poliomyelitis vaccines in relation to the number of CD4+ T lymphocytes in adults infected with human immunodeficiency virus. Clin Infect Dis 1995;21:1197–203. [DOI] [PubMed] [Google Scholar]
- 126. Rees-Spear C, McCoy LE.. Vaccine responses in ageing and chronic viral infection. Oxf Open Immunol 2021;2:iqab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Goronzy JJ, Weyand CM.. Understanding immunosenescence to improve responses to vaccines. Nat Immunol 2013;14:428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Rinaldi S, Pallikkuth S, George VK. et al. Paradoxical aging in HIV: immune senescence of B cells is most prominent in young age. Aging 2017;9:1307–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chauvin M, Sauce D.. Mechanisms of immune aging in HIV. Clin Sci 2022;136:61–80. [DOI] [PubMed] [Google Scholar]
- 130. Walsh EE, Frenck RW, Falsey AR. et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020;383:2439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Torres I, Albert E, Giménez E. et al. B- and T-cell immune responses elicited by the Comirnaty® COVID-19 vaccine in nursing-home residents. Clin Microbiol Infect 2021;27:1672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhu F-C, Guan X-H, Li Y-H. et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020;396:479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Li J, Hui A, Zhang X. et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo-controlled, double-blind phase 1 study. Nat Med 2021;27:1062–70. [DOI] [PubMed] [Google Scholar]
- 134. Anderson EJ, Rouphael NG, Widge AT. et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020;383:2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Collier DA, Ferreira IATM, Kotagiri P. et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021;596:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. De Francesco D, Wit FW, Bürkle A. et al. Do people living with HIV experience greater age advancement than their HIV-negative counterparts? AIDS 2019;33:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Royston L, Isnard S, Lin J. et al. Cytomegalovirus as an uninvited guest in the response to vaccines in people living with HIV. Viruses 2021;13:1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Perello R, Vergara A, Monclus E. et al. Cytomegalovirus infection in HIV-infected patients in the era of combination antiretroviral therapy. BMC Infect Dis 2019;19:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Frater J, Ewer KJ, Ogbe A. et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021;8:e474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Madhi SA, Koen AL, Izu A. et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021;8:e568–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Khan K, Lustig G, Bernstein M. et al. Immunogenicity of SARS-CoV-2 infection and Ad26.CoV2.S vaccination in people living with HIV. Clin Infect Dis 2021. https://doi.org/10.1093/cid/ciab1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Woldemeskel BA, Karaba AH, Garliss CC. et al. The BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with human immunodeficiency virus (HIV). Clin Infect Dis 2022;74:1268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Woldemeskel BA, Garliss CC, Blankson JN.. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J Clin Investig 2021;131:e149335. https://doi.org/10.1172/JCI149335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Levy I, Wieder-Finesod A, Litchevsky V. et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect 2021;27:1851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bergman P, Blennow O, Hansson L. et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. eBioMedicine 2021;74:103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Jedicke N, Stankov MV, Cossmann A. et al. Humoral immune response following prime and boost BNT162b2 vaccination in people living with HIV on antiretroviral therapy. HIV Med 2021:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Nault L, Marchitto L, Goyette G. et al . Covid-19 Vaccine Immunogenicity in People Living with HIV-1. Vaccine 2022;40:3633–3637. https://doi.org/10.1016/j.vaccine.2022.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lombardi A, Butta GM, Donnici L. et al. Anti-spike antibodies and neutralising antibody activity in people living with HIV vaccinated with COVID-19 mRNA-1273 vaccine: a prospective single-centre cohort study. Lancet Reg Health 2022;13:100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ruddy JA, Boyarsky BJ, Bailey JR. et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS 2021;35:2399–2401. https://doi.org/10.1097/QAD.0000000000003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Brumme ZL, Mwimanzi F, Lapointe HR. et al. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. medRxiv: the preprint server for health sciences: Cold Spring Harbor Laboratory, 2021, 2021.10.03.21264320.
- 151. Zou S, Wu M, Ming F. et al. Immune response and safety to inactivated COVID19 vaccine: a comparison between people living with HIV and HIV-naive individuals. AIDS Res Ther 2022;19:33. https://doi.org/10.1186/s12981-022-00459-y. [DOI] [PMC free article] [PubMed]
- 152. Lv Z, Li Q, Feng Z. et al. Inactivated SARS-CoV-2 vaccines elicit immunogenicity and T-cell responses in people living with HIV. Int Immunopharmacol 2022;102:108383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Feng Y, Zhang Y, He Z. et al. Immunogenicity of an inactivated SARS-CoV-2 vaccine in people living with HIV-1: a non-randomized cohort study. eClinicalMedicine 2022;43:101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Fuster F, Vargas JI, Jensen D. et al. CD4/CD8 ratio as a predictor of the response to HBV vaccination in HIV-positive patients: a prospective cohort study. Vaccine 2016;34:1889–95. [DOI] [PubMed] [Google Scholar]
- 155. Hadrup SR, Strindhall J, Køllgaard T. et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol 2006;176:2645–53. [DOI] [PubMed] [Google Scholar]
- 156. Spinelli MA, Lynch KL, Yun C. et al. SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: a matched case-control observational study. Lancet HIV 2021;8:e334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Alrubayyi A, Gea-Mallorquí E, Touizer E. et al. Characterization of humoral and SARS-CoV-2 specific T cell responses in people living with HIV. Nat Commun 2021;12:5839. https://doi.org/10.1038/s41467-021-26137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Snyman J, Sanders EJ, Ndung'u T. COVID-19 in Africa: preexisting immunity and HIV. AIDS 2021;35:2391–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Pallikkuth S, Sharkey M, Beauchamps L. et al. Persistence of SARS-CoV-2 –specific AB reseponse in HIV+ individuals on ART. In: Conference on Retroviruses and Opportunistic Infections: Topics in Antiviral Medicine, 2021;29:88. [Google Scholar]
- 160. Riddell AC, Kele B, Harris K. et al. Generation of novel SARS-CoV-2 variants on B.1.1.7 lineage in three patients with advanced HIV disease. Clin Infect Dis 2022;ciac409. https://doi.org/10.1093/cid/ciac409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sharov KS. HIV/SARS-CoV-2 co-infection: T cell profile, cytokine dynamics and role of exhausted lymphocytes. Int J Infect Dis 2021;102:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Riou C, Du Bruyn E, Stek C. et al. Relationship of SARS-CoV-2–specific CD4 response to COVID-19 severity and impact of HIV-1 and tuberculosis coinfection. J Clin Investig 2021;131:e149125. https://doi.org/10.1172/JCI149125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bhaskaran K, Rentsch CT, Mackenna B. et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021;8:e24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Etienne N, Karmochkine M, Slama L. et al. HIV infection and COVID-19: risk factors for severe disease. AIDS 2020;34:1771–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Maponga TG, Jeffries M, Tegally H. et al. Persistent SARS-CoV-2 infection with accumulation of mutations in a patient with poorly controlled HIV infection. MedRxiv2021. [DOI] [PMC free article] [PubMed]
- 166. Cele S, Karim F, Lustig G. et al. SARS-CoV-2 prolonged infection during advanced HIV disease evolves extensive immune escape. Cell Host Microbe 2022;30:154–62.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Robson F, Khan KS, Le TK. et al. Coronavirus RNA proofreading: molecular basis and therapeutic targeting. Mol Cell 2020;79:710–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Karim F, Moosa MY, Gosnell B. et al . Persistent SARS-CoV-2 Infection and Intra-Host Evolution in Association with Advanced HIV Infection. Dec 2021;09:21263564. https://doi.org/10.1101/2021.09.14.21263564..
- 169. Peluso MJ, Spinelli MA, Deveau T-M. et al . Post-Acute Sequelae And Adaptive Immune Responses in People Living with HIV Recovering from SARS-CoV-2 Infection. Cold Spring Harbor Laboratory, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Pujari S, Gaikwad S, Chitalikar A. et al. Long‐coronavirus disease among people living with HIV in western India: an observational study. Immu Inflamma Dis 2021;9:1037–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Luxi N, Giovanazzi A, Capuano A. et al. COVID-19 vaccination in pregnancy, paediatrics, immunocompromised patients, and persons with history of allergy or prior SARS-CoV-2 infection: overview of current recommendations and pre- and post-marketing evidence for vaccine efficacy and safety. Drug Saf 2021;44:1247–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Shinde V, Bhikha S, Hoosain Z. et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 Variant. N Engl J Med 2021;384:1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Sadoff J, Gray G, Vandebosch A. et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021;384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. World Health Organisation (WHO). Background Document on the Janssen Ad26.COV2.S (COVID-19) Vaccine: Background Document to the WHO Interim Recommendations for Use of Ad26.COV2.S (COVID-19) Vaccine. https://apps.who.int/iris/handle/10665/340180 (March 2022, date last accessed).
- 175. Tamuzi JL, Muyaya LM, Mitra A. et al. Systematic Review and Meta-Analysis of COVID-19 Vaccines Safety, Tolerability, and Efficacy among HIV-Infected Patients. MedrXiv; 2022. https://doi.org/10.1101/2022.01.11.22269049. [Google Scholar]
- 176. Bozzi G, Lombardi A, Ludovisi S. et al. Transient increase in plasma HIV RNA after COVID-19 vaccination with mRNA-1272. Int J Infect Dis 2021;113:125–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Yek C, Gianella S, Plana M. et al. Standard vaccines increase HIV-1 transcription during antiretroviral therapy. AIDS 2016;30:2289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Antinori A, Cicalini S, Meschi S. et al. Immunogenicity of mRNA vaccination against SARS-CoV-2 in persons living with HIV (PLWHs) with low CD4 count or previous AIDS. In: 18th European AIDS Conference London, 2021.
- 179. Whitaker HJ, Tsang RSM, Byford R. et al. Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response among individuals in clinical risk groups. J Infect 2022;84:675–683. https://doi.org/10.1016/j.jinf.2021.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hensley KS, Geurts van Kessel CH. et al. SARS-CoV-2 vaccine immunogenicity in PLWH in the Netherlands. Poster CROI,2022
- 181. Cicalini S, Vergori A, Cozzi-Lepri A. et al. Durability of SARS-CoV-2 mRNA vaccine immune response in PLWH with advanced disease. Poster CROI, 2022
- 182. Ferrari L PL, Piermatteo LCaldara F, Andreassi E. et al. How HIV modulates the safety and immunogenicity of the BNT162b2 COVID-19 vaccine. Poster CROI, 2022.
- 183. Pourcher V BL, Belin LSoulie C, Michelle Rosenzwajg M. et al. High seroconversion rate and delta neutralization in PLWHIV vaccinated with BNT162b2. Poster CROI, 2022. [DOI] [PubMed]
- 184. Vergori A CS, Cozzi-Lepri A, Matusali G. et al. Immunogenicity to COVID-19 mRNA vaccine booster shot in PLWH by current CD4+ count. In: Poster abstract 293 BHIVA Spring Conference2022.
- 185. Ogbe A T, Pace M, Bittaye M. et al. ChAdOx1 nCoV-19 vaccination in PWH: immune responses to SARS-CoV-2, VOCs, and HCoVs. Poster CROI, 2022.
- 186. Ogbe A, Pace M, Bittaye M. et al. Durability of ChAdOx1 nCov-19 vaccination in people living with HIV. JCI Insight 2022;8:e157031. https://doi.org/10.1172/jci.insight.157031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Deming ME, Lyke KE.. A ‘mix and match’ approach to SARS-CoV-2 vaccination. Nat Med 2021;27:1510–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Spencer AJ, McKay PF, Belij-Rammerstorfer S. et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat Commun 2021;12:2893. https://doi.org/10.1038/s41467-021-23173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Barros-Martins J, Hammerschmidt SI, Cossmann A. et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med 2021;27:1525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Atmar RL, Lyke KE, Deming ME. et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med 2022;386:1046–1057. https://doi.org/10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Schmidt T, Klemis V, Schub D. et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med 2021;27:1530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Borobia AM, Carcas AJ, Pérez-Olmeda M. et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021;398:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Stuart ASV, Shaw RH, Liu X. et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet 2022;399:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sun J, Zheng Q, Madhira V. et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med 2022;182:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Coburn SB, Humes E, Lang R. et al . COVID-19 Infections Post-Vaccination by HIV Status in the United States. medRxiv [Preprint]. 2021;12:21267182. https://doi.org/10.1101/2021.12.02.21267182. [Google Scholar]
- 196. Garcia-Beltran WF, St Denis KJ, Hoelzemer A. et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022;185:457–66.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Hyseni I, Molesti E, Benincasa L. et al. Characterisation of SARS-CoV-2 lentiviral pseudotypes and correlation between pseudotype-based neutralisation assays and live virus-based micro neutralisation assays. Viruses 2020;12:1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Cantoni D, Mayora-Neto M, Temperton N.. The role of pseudotype neutralization assays in understanding SARS CoV-2. Oxf Open Immunol 2021;2:iqab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Valcourt EJ, Manguiat K, Robinson A. et al. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Diagn Microbiol Infect Dis 2021;99:115294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Lake DF, Roeder AJ, Kaleta E. et al. Development of a rapid point-of-care test that measures neutralizing antibodies to SARS-CoV-2. J Clin Virol 2021;145:105024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Guan L, Yu Y, Wu X. et al. The first Chinese national standards for SARS-CoV-2 neutralizing antibody. Vaccine 2021;39:3724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Huang D, Tran JT, Peng L. et al. A rapid assay for SARS-CoV-2 neutralizing antibodies that is insensitive to antiretroviral drugs. J Immunol 2021;207:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Angyal A, Longet S, Moore SC. et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe 2022;3:e21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Keeton R, Richardson SI, Moyo-Gwete T. et al. Prior infection with SARS-CoV-2 boosts and broadens Ad26.COV2.S immunogenicity in a variant-dependent manner. Cell Host Microbe 2021;29:1611–19.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Velavan TP, Meyer CG, Esen M. et al. COVID-19 and syndemic challenges in ‘Battling the Big Three’: HIV, TB and malaria. Int J Infect Dis 2021;106:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zumla A, Chan JFW, Azhar EI. et al. Coronaviruses — drug discovery and therapeutic options. Nat Rev Drug Discov 2016;15:327–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Schoergenhofer C, Jilma B, Stimpfl T. et al. Pharmacokinetics of lopinavir and ritonavir in patients hospitalized with coronavirus disease 2019 (COVID-19). Ann Intern Med 2020;173:670–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Consortium WST. Repurposed antiviral drugs for Covid-19 — interim WHO solidarity trial results. N Engl J Med 2021;384:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Mohareb AM, Kim AY.. Hepatitis B vaccination in people living with HIV—if at first you don’t succeed, try again. JAMA Netw Open 2021;4:e2121281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Lacey CJ. HPV vaccination in HIV infection. Papillomavirus Res 2019;8:100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Cantoni D, Mayora-Neto M, Nadesalingam A. et al. Neutralisation hierarchy of SARS-CoV-2 variants of concern using standardised, quantitative neutralisation assays reveals a correlation with disease severity; towards deciphering protective antibody thresholds. Front Immunol 2022;13:773982. https://doi.org/10.3389/fimmu.2022.773982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Cheng SMS, Mok CKP, Leung YWY. et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med 2022;28:486–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the manuscript.