Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic resulted in unprecedented emphasis on infection control procedures; however, it is unknown whether the pandemic altered Clostridioides difficile infection (CDI) prevalence. This study investigated CDI prevalence before and during the COVID-19 pandemic in a national sample of United States (US) hospitals.
Methods
This was a retrospective cohort study using the Premier Healthcare Database. Patients with laboratory-confirmed CDI from April 2019 through March 2020 (pre–COVID-19 period) and April 2020 through March 2021 (COVID-19 period) were included. CDI prevalence (CDI encounters per 10 000 total encounters) and inpatient outcomes (eg, mortality, hospital length of stay) were compared between pre–COVID-19 and COVID-19 periods using bivariable analyses or interrupted time series analysis.
Results
A total of 25 992 CDI encounters were included representing 22 130 unique CDI patients. CDI prevalence decreased from the pre–COVID-19 to COVID-19 period (12.2 per 10 000 vs 8.9 per 10 000, P < .0001), driven by a reduction in inpatient CDI prevalence (57.8 per 10 000 vs 49.4 per 10 000, P < .0001); however, the rate ratio did not significantly change over time (RR, 1.04 [95% confidence interval, .90–1.20]). From the pre–COVID-19 to COVID-19 period, CDI patients experienced higher inpatient mortality (5.5% vs 7.4%, P < .0001) and higher median encounter cost ($10 832 vs $12 862, P < .0001).
Conclusions
CDI prevalence decreased during the COVID-19 pandemic in a national US sample, though at a rate similar to prior to the pandemic. CDI patients had higher inpatient mortality and encounter costs during the pandemic.
Keywords: Clostridioides difficile, COVID-19, epidemiology, mortality
In a national sample of hospital systems, Clostridioides difficile infection (CDI) prevalence decreased during the COVID-19 pandemic in a national US sample; however, CDI patients had higher inpatient mortality and encounter costs during the pandemic.
Clostridioides difficile infection (CDI) continues to be a major public health concern in hospitals worldwide and increasingly in the outpatient setting. CDI affects approximately half a million Americans annually, resulting in 29 000 deaths [1]. This devastating intestinal infection disproportionately affects patients recently exposed to antibiotics or other microbiome-disrupting therapies, those with poor immune response (eg, older age, severe underlying conditions, immunosuppression), and those with recent healthcare exposures (eg, hospitalization, nursing home residence) [2]. Prevention of CDI involves a combination of limiting unnecessary antibiotic exposure through antimicrobial stewardship efforts, preventing C difficile acquisition and transmission through infection control measures, and identifying CDI early and accurately through optimal diagnostic approaches [3, 4].
Since early 2020, the coronavirus disease (COVID-19) pandemic has become the most urgent threat to human health and has directly impacted several factors that may have influenced CDI epidemiology since this time. First, the pandemic has prompted unprecedented adherence to infection control practices, including hand hygiene, social distancing, masking, patient isolation, and more rigorous cleaning practices, which may have limited C difficile transmission [5–7]. Conversely, hospitals may have suffered high patient census in general and critical care wards, inadequate staffing, shortages of personal protective equipment, and prioritization of COVID-19 over traditional healthcare-associated infections, especially early in the pandemic and during surges [8–10]. Additionally, while studies have demonstrated a slight decrease in outpatient antibiotic prescribing since the pandemic [11], inpatient prescribing has increased [12]. In a systematic review and meta-analysis by Langford et al [13], 62.4% of all COVID-19 patients received at least 1 antibiotic agent. Of those, fluoroquinolones (20.0%), macrolides (18.9%), β-lactam/β-lactamase inhibitors (15.0%), and cephalosporins (15.0%) were the most prescribed antibiotics. Additionally, 8.6% of COVID-19 patients were reported to have concomitant bacterial infection, suggesting that many of these antibiotics prescribed may not have been necessary. Last, CDI diagnostics may have been affected recently as well. Given that diarrhea is a common symptom of COVID-19, clinicians may lack clinical suspicion of CDI in these patients, which may delay timely diagnosis and treatment and affect patient outcomes.
Prior studies have noted varying impact of the COVID-19 pandemic on CDI epidemiology. These predominantly single-center studies have either noted increased, decreased, or stable CDI prevalence over time and fewer have evaluated changes in patient outcomes or treatment patterns since the beginning of the pandemic. Therefore, the primary objective of this study was to compare the prevalence of CDI among a national sample of United States (US) hospital systems prior to and during the COVID-19 pandemic. Secondary outcomes included comparisons of CDI severity, treatment patterns, and health outcomes (eg, inpatient mortality, encounter costs, and hospital length of stay [LOS]).
METHODS
Data Source
This was a national, retrospective cohort study using data from the Premier Healthcare Database from 1 April 2019 to 31 March 2021. The Premier Healthcare Database collects data from more than 1041 academic and nonacademic hospitals, representing approximately 25% of all hospitals in the US, and includes >230 million unique patient visits [14]. While the exact location of each hospital is unavailable, hospitals are categorized by “provider region” as Midwest, Northeast, South, or West [11]. The database provides information on inpatient and outpatient encounters, including patient and facility characteristics, medications administered, diagnoses and procedures during the encounter, and financial data (eg, overall and treatment-specific charges and costs). Additionally, the database uses a masked identifier that allows for tracking patients longitudinally across encounters. The Premier Healthcare Database is considered exempt from institutional review board oversight.
Patient Population and Study Definitions
Premier Inc built the original dataset; all patients with an inpatient or outpatient diagnosis for CDI within the study period were eligible for inclusion. CDI was first identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 008.45 and International Classification of Diseases, Tenth Revision codes A04.71 or A04.72. This sample was then limited to those patients who had any positive C difficile stool test (eg, toxin enzyme immunoassay, glutamate dehydrogenase antigen, nucleic acid amplification test). The study period was classified as either pre–COVID-19 (1 April 2019 to 31 March 2020) or COVID-19 (1 April 2020 to 31 March 2021). The index visit was defined as the first encounter to include laboratory-confirmed CDI within the study period.
Patient baseline characteristics during the index visit included age, sex, race, ethnicity, and payor type. Facility characteristics included US Census region, rural or urban location, teaching status, and bed size. Additional CDI-related characteristics included inpatient or outpatient encounter, diagnosis type (admitting, primary, secondary), admission type (emergency, urgent, elective), severity indicators, and treatment patterns. An inpatient encounter is defined as a single visit that results in a patient being admitted to the hospital. An outpatient encounter is defined as a single visit to an outpatient clinic or emergency department. Each encounter is recorded as an individual event regardless of whether multiple encounters occurred on the same day. Serum creatinine (SCr) and white blood cell (WBC) count values were stratified according to the Infectious Diseases Society of America/Society for Healthcare Epidemiology of America guideline severity criteria if they occurred anytime during the encounter: SCr ≥1.5 mg/dL and WBC count >15 × 103 cells/µL [4]. Patients with either severity criterion were classified as “severe CDI.” CDI therapies included administration of at least 1 dose of the following agents during the encounter: metronidazole, oral vancomycin, fidaxomicin, bezlotoxumab, and fecal microbiota transplantation (FMT). These treatments were identified using Premier's standard charge code for each therapy. Total encounter costs were extracted from the patient cost variable provided by Premier, which represents the total cost to the hospital treating the patient during the encounter (includes all supplies, labor, equipment, etc). Last, for hospitalized patients only, hospital LOS was captured as a continuous variable and inpatient mortality was identified based on a discharge disposition of “expired.” This represents all-cause mortality.
Data and Statistical Analyses
Analyses were conducted using JMP 16 (SAS Institute, Cary, North Carolina) or StataMP 16 (StataCorp, College Station, Texas) software. CDI prevalence was determined by dividing the number of laboratory-confirmed CDI encounters for a given time period by the number of total patient encounters for any hospital system that contributed laboratory data during that same time period multiplied by 10 000. All recorded encounters within a given time period would be included in the number of total patient encounters for an “all encounters” analysis. We also repeated this calculation using index encounters only as the numerator and total unique patients as the denominator multiplied by 10 000. Monthly and total encounter and unique patient denominators were provided by Premier Inc. CDI rates and patient baseline characteristics were compared between the pre–COVID-19 and COVID-19 periods for index visits only. CDI severity, treatment characteristics, costs, and health outcomes for index encounters were compared between pre–COVID-19 and COVID-19 periods overall and by encounter type (inpatient vs outpatient) using the χ2 test for nominal dependent variables and the Wilcoxon rank-sum test for continuous, nonnormally distributed dependent variables. Outcome subgroup analyses were conducted by region, teaching hospital status, and rural or urban hospital status. Additionally, to evaluate for changes in CDI rates over time, an interrupted time series analyses was conducted using a generalized linear model assumed to follow a Poisson distribution allowing for overdispersion. All tests were 2-sided and performed at the 5% level of significance.
RESULTS
Population Characteristics
A total of 24.7 million patient encounters were available for analysis over the study period. Total patient encounters remained relatively stable throughout the study period (approximately 1.1 million in April 2019 and March 2021) with the exception of early in the pandemic period when encounters were lower (average 0.8 million encounters from March to May 2020). Using administrative codes only, a total of 149 189 CDI encounters were identified. Of these, 25 992 encounters included laboratory-confirmed CDI, representing 22 139 unique CDI patients/index encounters. CDI patient characteristics during the index encounter are provided in Table 1. CDI patients were predominantly older (median age, 68 years), female (57.5%), and White (79.5%). Comparisons of characteristics between study periods demonstrated numerically small but statistically significant differences in race, ethnicity, payor type, region, admission type, CDI diagnosis type, CDI present on admission, teaching and urban hospital status, and hospital bed size due to the large sample size.
Table 1.
Patient and Visit Characteristics of Clostridioides difficile Infection Index Encounters
| Characteristic | Overall (N = 22 139) |
Pre–COVID-19 (n = 12 878) |
COVID-19 (n = 9261) |
P Value |
|---|---|---|---|---|
| Age, y, median (IQR) | 68 (56–78) | 67 (54–78) | 68 (57–78) | .3775 |
| Female sex | 57.5 | 59.2 | 57.7 | .3678 |
| Race | .0206 | |||
| White | 79.5 | 79.1 | 79.6 | |
| Black | 12.3 | 11.2 | 12.5 | |
| Asian | 1.7 | 1.3 | 1.8 | |
| Other | 4.8 | 6.6 | 4.2 | |
| Unknown | 1.8 | 1.8 | 1.8 | |
| Hispanic ethnicity | 5.9 | 7.8 | 6.4 | .0001 |
| Payor | .0007 | |||
| Medicare | 64.2 | 64.2 | 64.3 | |
| Medicaid | 11.5 | 11.4 | 11.7 | |
| Managed care | 14.3 | 14.7 | 13.8 | |
| Commercial | 3.7 | 3.8 | 3.7 | |
| Indigent/charity/self-pay | 3.4 | 3.5 | 3.2 | |
| Other | 2.9 | 2.5 | 3.4 | |
| US Census region | .0010 | |||
| Midwest | 22.3 | 22.0 | 22.6 | |
| Northeast | 12.6 | 12.4 | 12.8 | |
| South | 62.4 | 62.4 | 62.4 | |
| West | 2.8 | 3.1 | 2.3 | |
| Inpatient admission | 86.7 | 86.4 | 87.2 | .0846 |
| Admission type | <.0001 | |||
| Emergency | 80.1 | 70.2 | 81.0 | |
| Urgent | 9.8 | 12.4 | 9.4 | |
| Elective | 8.6 | 14.4 | 8.2 | |
| Trauma | 0.5 | 0.4 | 0.6 | |
| Unknown | 1.0 | 2.3 | 0.7 | |
| CDI diagnosis type | <.0001 | |||
| Admitting | 10.5 | 11.5 | 9.0 | |
| Primary | 23.0 | 24.3 | 21.1 | |
| Secondary | 66.6 | 64.2 | 69.9 | |
| CDI present on admission | 59.3 | 59.6 | 59.0 | <.0001 |
| Teaching hospital | 47.3 | 46.4 | 48.7 | .0009 |
| Urban hospital | 83.5 | 84.3 | 82.3 | .0001 |
| Hospital bed size | .0002 | |||
| 0–99 | 7.8 | 7.6 | 8.1 | |
| 100–199 | 14.7 | 15.1 | 14.2 | |
| 200–299 | 16.0 | 15.7 | 16.3 | |
| 300–399 | 18.9 | 19.5 | 18.1 | |
| 400–499 | 11.8 | 12.2 | 11.2 | |
| ≥500 | 30.9 | 29.9 | 32.2 |
Data are presented as % unless otherwise indicated.
Abbreviations: CDI, Clostridioides difficile infection; COVID-19, coronavirus disease 2019; IQR, interquartile range.
CDI Prevalence and Characteristics
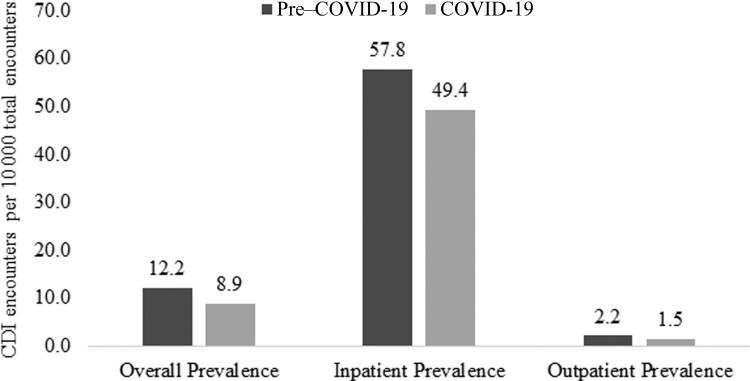
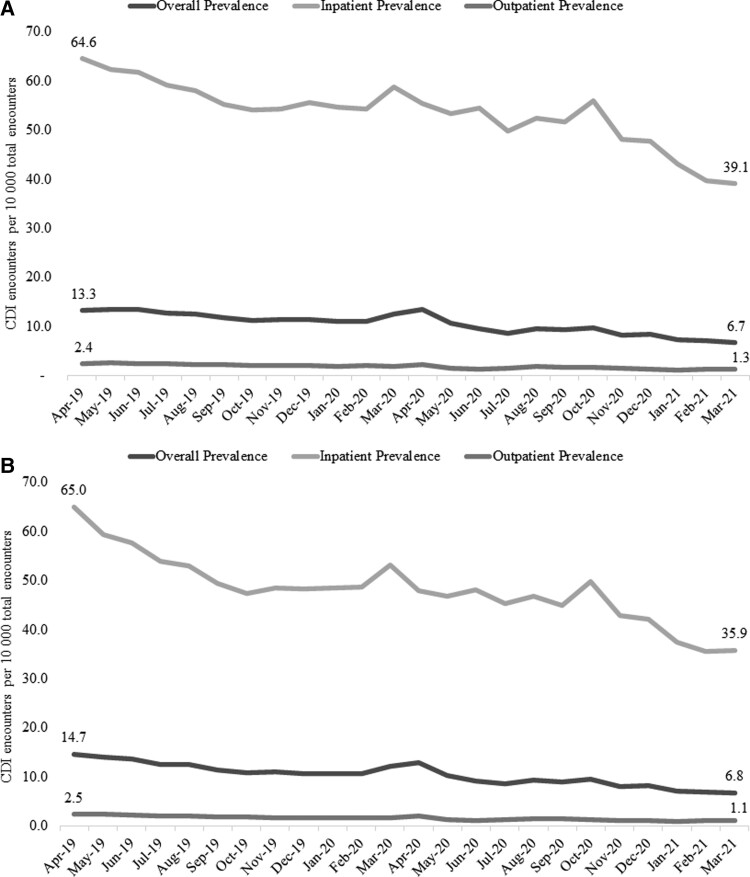
CDI prevalence between time periods is depicted in Figure 1. CDI prevalence significantly decreased from the pre–COVID-19 to the COVID-19 period (12.2 per 10 000 vs 8.9 per 10 000, P < .0001). This was driven primarily by a reduction in inpatient CDI prevalence (57.8 per 10 000 vs 49.4 per 10 000, P < .0001), though outpatient CDI prevalence decreased as well (2.2 per 10 000 vs 1.5 per 10 000, P < .0001). These trends were similar when limiting to index visits alone: overall prevalence (12.1 per 10 000 vs 8.7 per 10 000), inpatient prevalence (52.8 per 10 000 vs 43.8 per 10 000), and outpatient prevalence (1.7 per 10 000 vs 1.1 per 10 000). Monthly CDI prevalence for all encounters and index encounters is presented in Figure 2. Overall, CDI prevalence for all encounters decreased over the study period from 13.3 per 10 000 in April 2019 to 6.7 per 10 000 in March 2021. This was mainly driven by decreasing trends in inpatient prevalence (64.6 per 10 000 vs 39.1 per 10 000), while outpatient prevalence decreased as well (2.4 per 10 000 vs 1.3 per 10 000). These trends were similar when limiting to index visits only: overall prevalence (14.7 per 10 000 vs 6.8 per 10 000), inpatient prevalence (65.0 per 10 000 vs 35.9 per 10 000), and outpatient prevalence (2.5 per 10 000 vs 1.1 per 10 000). Notably, interrupted time series analysis indicated no significant difference in the rate ratio (RR) from the pre–COVID-19 to COVID-19 period for all encounters (RR, 1.04 [95% confidence interval {CI}, .90–1.20]; P = .570) or inpatient encounters (RR, 1.08 [95% CI, .98–1.19]; P = .115).
Figure 1.
Clostridioides difficile infection (CDI) prevalence in the pre–coronavirus disease 2019 (COVID-19) and COVID-19 periods.
Figure 2.
Monthly Clostridioides difficile infection (CDI) prevalence over the study period. A, All CDI encounters. B, Index CDI encounters only.
During the index encounter, CDI was more commonly the primary diagnosis during the pre–COVID-19 period compared to the COVID-19 period (24.3% vs 21.1%, P < .0001), reflecting a shift to secondary diagnosis during the COVID-19 period (64.2% vs 69.9%, P < .0001) (Table 1).
For index encounters, the percentage of patients with severe CDI increased slightly from the pre–COVID-19 to COVID-19 period (41.3% vs 44.0%, P = .0003), driven primarily by an increase in the percentage of patients with WBC count >15 × 103 cells/µL (19.6% vs 22.0%, P = .0001) (Table 2). When stratified by visit type, only inpatients experienced a significant increase in the percentage of patients with severe CDI over time (43.2% vs 45.8%, P = .0007).
Table 2.
Clostridioides difficile Infection Characteristics and Health Outcomes During Index Visit
| Characteristic | Overall | Pre–COVID-19 | COVID-19 | P Value |
|---|---|---|---|---|
| (N = 22 139) | (n = 12 878) | (n = 9261) | ||
| Overall population | ||||
| Severity indicators, %a | ||||
| SCr ≥1.5 mg/dL | 26.9 | 26.4 | 27.5 | .0670 |
| WBC count >15 × 103/µL | 20.6 | 19.6 | 22.0 | .0001 |
| Severe CDI | 42.4 | 41.3 | 44.0 | .0003 |
| CDI therapies, %b | ||||
| Metronidazole | 40.1 | 41.2 | 38.6 | .0001 |
| Oral vancomycin | 79.0 | 77.6 | 81.0 | <.0001 |
| Fidaxomicin | 5.7 | 6.2 | 5.1 | .0003 |
| Bezlotoxumab | <0.1 | <0.1 | <0.1 | .0610 |
| FMT | 0.2 | 0.3 | <0.1 | <.0001 |
| Cost (USD), median (IQR) | 11 661 (5531–27 298) | 10 834 (5231–2523) | 12 862 (6054–30 473) | <.0001 |
| Outpatients | (n = 2945) | (n = 1756) | (n = 1189) | |
| Severity indicators, %a | ||||
| SCr ≥1.5 mg/dL | 12.3 | 11.8 | 12.9 | .4653 |
| WBC count >15 × 103/µL | 12.6 | 12.3 | 13.0 | .6756 |
| Severe CDI | 24.4 | 23.5 | 25.7 | .2904 |
| CDI therapies, %b | ||||
| Metronidazole | 13.4 | 13.1 | 14.0 | .5011 |
| Oral vancomycin | 34.9 | 32.6 | 38.2 | .0020 |
| Fidaxomicin | 1.1 | 1.4 | 0.7 | .0661 |
| Bezlotoxumab | 0.0 | 0.0 | 0.0 | 1.0000 |
| FMT | <0.1 | <0.1 | 0.0 | .3091 |
| Cost (USD), median (IQR) | 733 (83–2800) | 637 (75–2462) | 875 (89–3309) | <.0001 |
| Inpatients | (n = 19 194) | (n = 11 122) | (n = 8072) | |
| Severity indicators, %a | ||||
| SCr ≥1.5 mg/dL | 28.3 | 27.7 | 29.0 | .0682 |
| WBC count > 15 × 103/µL | 21.5 | 20.5 | 22.9 | .0002 |
| Severe CDI | 44.3 | 43.2 | 45.8 | .0007 |
| CDI therapies, %b | ||||
| Metronidazole | 44.2 | 45.6 | 42.2 | <.0001 |
| Oral vancomycin | 85.8 | 84.7 | 87.3 | <.0001 |
| Fidaxomicin | 6.4 | 7.0 | 5.7 | .0005 |
| Bezlotoxumab | <0.1 | 0.1 | <0.1 | .0589 |
| FMT | 0.2 | 0.4 | <0.1 | <.0001 |
| Cost (USD), median (IQR) | 14 194 (7372–31 514) | 13 142 (6901–29 130) | 15 682 (8056–34 657) | <.0001 |
| Health outcomes | ||||
| Inpatient mortality, % | 6.3 | 5.5 | 7.4 | <.0001 |
| Length of stay, median (IQR) | 7 (4–13) | 7 (4–13) | 7 (4–14) | <.0001 |
Abbreviations: CDI, Clostridioides difficile infection; COVID-19, coronavirus disease 2019; FMT, fecal microbiota transplantation; IQR, interquartile range; SCr, serum creatinine; USD, United States dollars; WBC, white blood cell.
Not all patients had documented laboratory values; percentages were calculated using the patients with a documented laboratory value as the denominator.
Patients may have received >1 CDI therapy; rows may add to >100%.
CDI treatment patterns during the index visit shifted slightly over the time period as well, with greatest numeric change seen with the increased use of oral vancomycin (77.6% vs 81.0%, P < .0001) and declines in fidaxomicin use (6.2% vs 5.1%, P = .0003), metronidazole use (41.2% vs 38.6%, P = .0001), and FMT use (0.3% vs <0.1%, P < .0001) during the COVID-19 period (Table 2).
Patient Health Outcomes
Patient costs significantly increased by an average of approximately $2000 from the pre–COVID-19 to COVID-19 period (P < .0001), driven primarily by inpatient treatment costs (Table 2). Among inpatient encounters only, all-cause mortality increased from the pre–COVID-19 to COVID-19 period (5.5% vs 7.4%, P < .0001). while median hospital LOS was numerically similar (7 days). These outcomes were worse for those presenting with severe CDI compared to nonsevere CDI: median patient costs ($16 762 vs $10 642, P < .0001), median hospital LOS (8 days vs 6 days, P < .0001), and mortality (10.6% vs 2.8%, P < .0001). Outcomes were also worse overall in the West region (median LOS, 9 days; mortality 10.0%) and among teaching hospitals (median LOS, 7 days; mortality 6.6%).
DISCUSSION
In this nationally representative study, CDI prevalence decreased from the pre–COVID-19 to the COVID-19 period; however, the rate of decline did not significantly change over time. During the COVID-19 period, patients with a CDI diagnosis confirmed with laboratory results experienced higher mortality and costs. Additionally, we noted a change in CDI treatment patterns, especially the limited use of FMT during this time. This study is strengthened by its large, multicenter design and inclusion of longitudinal inpatient and outpatient encounters that enhance the generalizability to the US population.
Several prior studies have evaluated the impact of the COVID-19 pandemic on CDI prevalence. Most have demonstrated a decline in CDI prevalence similar to our study [15–19], but some others have noted increased [20, 21] or stable prevalence/incidence [22, 23]. The studies with differing results diverge in design from the current study in a variety of ways including location of study, number of patients, types of patients, and time period examined. Furthermore, all of these studies were single-center studies with much smaller sample sizes. For example, Sandhu et al examined CDI rates in a metropolitan medical center in Detroit, Michigan, and found an increase from January–February 2020 to March–April 2020 (3.3 vs 3.6 per 10 000 patient-days) [20]. Similarly, another study demonstrating an increase in CDI trends by Baccolini et al studied only ICU patients at a single center in Rome, Italy, and recorded very few CDI patients in their cohorts (2019 cohort: 0/14 patients vs 2020 cohort: 2/45 patients) [21]. Last, studies by Luo et al and Hawes et al noted stable trends in CDI incidence, and both observed a decrease in diagnostic testing either due to masked symptoms by gastrointestinal symptoms caused by COVID-19 or a less perceived need to test after precautions were enforced [22, 23]. One of the largest published studies using data from the National Healthcare Safety Network noted a slight decline in the CDI standardized infection ratio from 2019 to 2020, which then remained stable throughout 2020 [24]. Similarly, Bentivegna et al found that the prevalence of hospital-acquired CDI was significantly lower during the COVID-19 pandemic compared to 2017 (odds ratio [OR], 2.98; P = .002), 2018 (OR, 2.27; P = .023), and 2019 (OR, 2.07; P = .047) [17]. This prior study and the current study support that CDI prevalence has decreased in recent years, though the rate of decline may not have been significantly affected by the pandemic. Interestingly, we did note 2 minor spikes in CDI rates during the COVID-19 period (April 2020 and October 2020) that may coincide with major COVID-19 waves in the US.
The continued decline in CDI prevalence during the pandemic may be due to several factors. First, the pandemic has prompted better adherence to infection control practices (eg, improved hand hygiene, donning of personal protective equipment, patient isolation) in hospitals and new practices in the outpatient setting (eg, masking, social distancing), which may have limited C difficile transmission. In addition, CDI rates have been declining in general in recent years due to enhanced antimicrobial and diagnostic stewardship efforts. Utilizing the CDC's Emerging Infections Program, a study by Guh et al reported that CDI incidence decreased from 2011 to 2017 for all CDI (154.9–143.6 per 100 000 population) and for healthcare-associated and community-associated CDI; however, after adjusting for nucleic acid amplification testing use to the 2011 rate (55%), overall and healthcare-associated CDI estimated incidence decreased while community-associated CDI incidence did not significantly change [1]. While this study cannot directly assess the cause of the decline in infection rates both before and during the pandemic, it is possible that diagnostic stewardship efforts utilized before COVID-19, such as 2-step testing, were extended into the COVID-19 period to reduce inappropriate testing and overdiagnosis. Next, recent studies have shown that hospitalizations for a variety of non–COVID-19 acute conditions have decreased despite there being a risk for the patient to avoid adverse outcomes, potentially due to patients’ desire to avoid the contagion by waiting to seek out care [25]. A study by Splinter et al [26] found that 20% of their patient population avoided healthcare during the COVID-19 pandemic despite a third of those patients reporting having had symptoms severe enough to warrant urgent care. In addition, CDI testing may have been impacted by the pandemic. It is estimated that 1 in 5 COVID-19 patients experiences gastrointestinal symptoms [27], which may impact clinical suspicion of CDI in these patients. Additionally, some single-center studies have reported a decline in CDI stool testing during the pandemic [19, 23]. This may be due to transition of care for some patients to the outpatient setting or possible concern about the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the stool [28]. Ultimately, it may be that these factors outweighed the impact of increased hospitalizations, critical illness, and antibiotic use during the pandemic. Interestingly, other healthcare-associated infections, including central line–associated bloodstream infections, catheter-associated urinary tract infection, ventilator-associated events, and methicillin-resistant Staphylococcus aureus bacteremia have significantly increased during the pandemic [24].
While this study noted a decline in CDI prevalence during the pandemic, more patients diagnosed with CDI during the pandemic experienced severe CDI and mortality. This could have led to higher treatment costs in the COVID-19 period as well, as severe CDI was associated with higher patient costs. Few prior studies have evaluated changes in these outcomes. One study by Granata et al [29] highlighted significant complications among patients with concomitant CDI and COVID-19 compared to COVID-19 alone. CDI patients experienced more complications at discharge (eg, pressure ulcers, chronic heart decompensation), longer hospital LOS (35 vs 19 days, P = .0007), increased values for CDI severity indicators (ie, WBC, SCr), and higher mortality (28.9% vs 21.9%, P = .1). These poorer outcomes disproportionately affected older, frail patients with previous antibiotic and healthcare exposures. Another case report noted severe CDI as a potential late complication of COVID-19 [30]. Although we were unable to determine coinfection with COVID-19 among our CDI cohort, presumably coinfection would likely have impacted CDI severity and patient outcomes during the pandemic. This is also consistent with the shift to more secondary CDI diagnosis compared to admitting or primary CDI diagnoses during the pandemic in our study. Consequently, it is possible that only patients with severe cases would have been admitted to healthcare facilities and potentially make up a larger percentage of admitting and primary CDI diagnosis types than in the pre–COVID-19 period.
During the pandemic, this study found notable changes in CDI treatment patterns. For example, oral vancomycin use increased during the pandemic, while fidaxomicin, metronidazole, and FMT use decreased. These changes do not reflect newer recommendations made by the updated CDI clinical practice guidelines [4], and the use of front-line agents (eg, fidaxomicin, bezlotoxumab) remains low. Perhaps the most striking treatment pattern was the decline in FMT use during the pandemic. Studies have demonstrated the presence of SARS-CoV-2 in the stool and the potential to transmit COVID-19 through FMT [25]. This led to an update by the US Food and Drug Administration to recommend using only stool donated prior to 1 December 2019 [28, 31]. This also prompted temporary inaccessibility of FMT product from US-based stool banks (eg, OpenBiome), highlighting the need for more rigorous screening of donor stool and US Food and Drug Administration–approved, commercially available microbiota replacement therapies.
This study has potential limitations. First, this study utilized a retrospective design and data collected from electronic medical records. This design may be subject to errors in documentation of patient and treatment characteristics. Administrative coding was used to initially identify CDI patients, then diagnosis was confirmed with a positive stool test. It is possible that some CDI cases were missed using this method (eg, lack of a CDI administrative code). This study may also be subject to ascertainment bias whereby fewer C difficile laboratory tests may have been conducted during the COVID-19 period. Nucleic acid amplification testing alone, which was allowed in this study, may have potentially misclassified some C difficile colonizers as CDI. Furthermore, metronidazole use for CDI may be overestimated since metronidazole may be used for non-CDI indications. We were unable to define community or hospital-onset CDI because the specific surveillance definitions in Premier lack exact timing of diagnosis. CDI diagnosis type (eg, admitting, primary, secondary) was used as a surrogate marker to estimate these surveillance definitions. COVID-19 administrative codes and instruction on their use were made available on 1 April 2020 by the CDC. Given that these codes were unavailable during part of the study period with potentially varying uptake by healthcare systems, it was not possible to quantify coinfection with COVID-19. Additionally, the Premier Inc COVID-19 Special Release as well as data beyond that originally acquired (through March 2021) was unavailable without purchase, further making it difficult to determine whether there was a causative association between CDI prevalence and health outcomes and our ability to validate these findings with more recent data. Next, specific information regarding infection control practices and population-level characteristics (eg, antibiotic use, other CDI risk factors) was unavailable; thus, we were unable to determine causative or risk factors associated with changes in CDI prevalence or outcomes over time. Last, only healthcare systems that contributed data to Premier Inc were included for analysis and results might not be generalizable to noncontributing hospitals, including Veterans Affairs facilities.
CONCLUSIONS
Overall, CDI prevalence decreased during the COVID-19 pandemic in a national US sample of hospitals; however, this decrease was similar to declining rates prior to the pandemic and does not provide direct evidence that the pandemic directly influenced CDI prevalence. CDI patients also experienced higher inpatient mortality and costs during the pandemic. Further studies are needed to identify the specific factors that contributed to changes in CDI prevalence and health outcomes, such that targeted interventions can be maintained or created in the future to prevent and treat CDI effectively.
Contributor Information
Kelly R Reveles, College of Pharmacy, The University of Texas at Austin, Austin, Texas, USA; Pharmacotherapy Education and Research Center, University of Texas Health San Antonio, San Antonio, Texas, USA.
Alexa L Frei, College of Pharmacy, The University of Texas at Austin, Austin, Texas, USA; Pharmacotherapy Education and Research Center, University of Texas Health San Antonio, San Antonio, Texas, USA; College of Agriculture and Life Sciences, Texas A&M University, College Station, Texas, USA.
Kelsey A Strey, College of Pharmacy, The University of Texas at Austin, Austin, Texas, USA; Pharmacotherapy Education and Research Center, University of Texas Health San Antonio, San Antonio, Texas, USA.
Eric H Young, College of Pharmacy, The University of Texas at Austin, Austin, Texas, USA; Pharmacotherapy Education and Research Center, University of Texas Health San Antonio, San Antonio, Texas, USA.
Notes
Acknowledgments. The authors acknowledge the staff at Premier Inc for providing the data and technical support for this project.
Patient consent. The Premier Healthcare Database is considered exempt from institutional review board oversight based on US Title 45 Code of Federal Regulations, Part 46 for the use of existing deidentified data that cannot be directly linked to individuals. Given the deidentified nature of the data, the need for informed consent is also waived.
Financial support. This work was supported by institutional funds from The University of Texas at Austin.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Guh AY, Mu Y, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med 2020; 382:1320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis 2008; 46(Suppl 1):S12–8. [DOI] [PubMed] [Google Scholar]
- 3. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 2021; 73:e1029–44. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Yang J, Qiao F, et al. Compared hand hygiene compliance among healthcare providers before and after the COVID-19 pandemic: a rapid review and meta-analysis. Am J Infect Control 2022; 50:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kea B, Johnson A, Lin A, et al. An international survey of healthcare workers use of personal protective equipment during the early stages of the COVID-19 pandemic. J Am Coll Emerg Physicians Open 2021; 2:e12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Masters NB, Shih SF, Bukoff A, et al. Social distancing in response to the novel coronavirus (COVID-19) in the United States. PLoS One 2020; 15:e0239025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doidge JC, Gould DW, Ferrando-Vivas P, et al. Trends in intensive care for patients with COVID-19 in England, Wales, and Northern Ireland. Am J Respir Crit Care Med 2021; 203:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Veneti L, Seppala E, Larsdatter Storm M, et al. Increased risk of hospitalisation and intensive care admission associated with reported cases of SARS-CoV-2 variants B.1.1.7 and B.1.351 in Norway, December 2020–May 2021. PLoS One 2021; 16:e0258513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGarry BE, Grabowski DC, Barnett ML. Severe staffing and personal protective equipment shortages faced by nursing homes during the COVID-19 pandemic. Health Aff (Millwood) 2020; 39:1812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. King LM, Lovegrove MC, Shehab N, et al. Trends in US outpatient antibiotic prescriptions during the coronavirus disease 2019 pandemic. Clin Infect Dis 2021; 73:e652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abelenda-Alonso G, Padulles A, Rombauts A, et al. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect Control Hosp Epidemiol 2020; 41:1371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langford BJ, So M, Raybardhan S, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect 2021; 27:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Premier Inc . Premier Healthcare Database: data that informs and performs. 2020. https://products.premierinc.com/downloads/PremierHealthcareDatabaseWhitepaper.pdf. Accessed 9 February 2022.
- 15. Baker MA, Sands KE, Huang SS, et al. The impact of COVID-19 on healthcare-associated infections. Clin Infect Dis 2021; 74:1748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ochoa-Hein E, Rajme-Lopez S, Rodriguez-Aldama JC, et al. Substantial reduction of healthcare facility-onset Clostridioides difficile infection (HO-CDI) rates after conversion of a hospital for exclusive treatment of COVID-19 patients. Am J Infect Control 2021; 49:966–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bentivegna E, Alessio G, Spuntarelli V, et al. Impact of COVID-19 prevention measures on risk of health care–associated Clostridium difficile infection. Am J Infect Control 2021; 49:640–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allegretti JR, Nije C, McClure E, et al. Prevalence and impact of Clostridioides difficile infection among hospitalized patients with coronavirus disease 2019. JGH Open 2021; 5:622–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ponce-Alonso M, de la Fuente J S, Rincon-Carlavilla A, et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on nosocomial Clostridioides difficile infection. Infect Control Hosp Epidemiol 2021; 42:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sandhu A, Tillotson G, Polistico J, et al. Clostridioides difficile in COVID-19 patients, Detroit, Michigan, USA, March–April 2020. Emerg Infect Dis 2020; 26:2272–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baccolini V, Migliara G, Isonne C, et al. The impact of the COVID-19 pandemic on healthcare-associated infections in intensive care unit patients: a retrospective cohort study. Antimicrob Resist Infect Control 2021; 10:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luo Y, Grinspan LT, Fu Y, et al. Hospital-onset Clostridioides difficile infections during the COVID-19 pandemic. Infect Control Hosp Epidemiol 2021; 42:1165–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hawes AM, Desai A, Patel PK. Did Clostridioides difficile testing and infection rates change during the COVID-19 pandemic? Anaerobe 2021; 70:102384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weiner-Lastinger LM, Pattabiraman V, Konnor RY, et al. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: a summary of data reported to the National Healthcare Safety Network. Infect Control Hosp Epidemiol 2022; 43:12–25. [DOI] [PubMed] [Google Scholar]
- 25. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood) 2020; 39:2010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Splinter MJ, Velek P, Ikram MK, et al. Prevalence and determinants of healthcare avoidance during the COVID-19 pandemic: a population-based cross-sectional study. PLoS Med 2021; 18:e1003854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tariq R, Saha S, Furqan F, Hassett L, Pardi D, Khanna S. Prevalence and mortality of COVID-19 patients with gastrointestinal symptoms: a systematic review and meta-analysis. Mayo Clin Proc 2020; 95:1632–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol 2020; 92:833–40. [DOI] [PubMed] [Google Scholar]
- 29. Granata G, Bartoloni A, Codeluppi M, et al. The burden of Clostridioides difficile infection during the COVID-19 pandemic: a retrospective case-control study in Italian hospitals (CloVid). J Clin Med 2020; 9:3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paramo-Zunzunegui J, Ortega-Fernandez I, Calvo-Espino P, et al. Severe Clostridium difficile colitis as potential late complication associated with COVID-19. Ann R Coll Surg Engl 2020; 102:e176–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. US Food and Drug Administration . Fecal microbiota for transplantation: safety alert—risk of serious adverse events likely due to transmission of pathogenic organisms. 2020. https://www.fda.gov/safety/medical-product-safety-information/fecal-microbiota-transplantation-safety-alert-risk-serious-adverse-events-likely-due-transmission. Accessed 9 February 2022.