Abstract
Objectives
In patients with RA treated with (ultra-)low-dose rituximab (RTX), we investigated the association of dosing and timing of RTX on seroconversion after a third coronavirus disease 2019 (COVID-19) vaccination and the persistence of humoral response after a two-dose vaccination.
Material and methods
In this monocentre observational study, patients from the COVAC cohort were included in the third vaccine analysis if humoral response was obtained 2–6 weeks after a third vaccination in previous non-responders and in the persistence analysis if a follow-up humoral response was obtained before a third vaccination in previous responders. Dichotomization between positive and negative response was based on the assay cut-off. The association between the latest RTX dose before first vaccination, timing between the latest RTX dose and vaccination and response was analysed with univariable logistic regression.
Results
Of the 196 patients in the cohort, 98 were included in the third vaccine analysis and 23 in the persistence analysis. Third vaccination response was 19/98 (19%) and was higher for 200 mg RTX users [5/13 (38%)] than for 500 and 1000 mg users [7/37 (19%) and 7/48 (15%), respectively]. Non-significant trends were seen for higher response with lower dosing [200 vs 1000 mg: odds ratio (OR) 3.66 (95% CI 0.93, 14.0)] and later timing [per month since infusion: OR 1.16 (95% CI 0.97, 1.35)]. Humoral response persisted in 96% (22/23) and 89% (8/9) of patients who received RTX between the two measurements.
Conclusions
Repeated vaccination as late as possible after the lowest RTX dose possible seems the best vaccination strategy. A once positive humoral response after COVID-19 vaccination persists irrespective of intercurrent RTX infusion.
Study registration. Netherlands Trial Registry (https://www.trialregister.nl/), NL9342.
Keywords: RA, rituximab, biologic therapies, COVID-19, vaccination
Rheumatology key messages.
Approximately 20% of rheumatoid arthritis patients treated with rituximab seroconverted after a third COVID-19 vaccination.
Seroconversion numbers were higher for patients treated with 200 mg of rituximab.
Persistence of humoral response was high, irrespective of intercurrent rituximab infusion.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has led to large numbers of COVID-19-related hospitalizations and deaths. Several vaccines against COVID-19 are available, which have been shown to induce humoral and cellular response and to reduce the risk and severity of COVID-19. RA patients treated with rituximab (RTX) have both an increased risk of COVID-19 hospitalization [1] and a reduced humoral response after two-dose vaccination [2] when compared with other DMARDs. Therefore, to optimize management of COVID-19 risk in these patients, it is important to identify strategies for increasing response in this population.
Previously we demonstrated that both the use of an ultra-low dose of 200 mg RTX and a longer time between the latest RTX dose and vaccination are associated with positive humoral response after two-dose vaccination, with the effect of timing also confirmed by a recent meta-analysis [3, 4]. Now that (at least) a third-dose vaccination has been advised for these patients, it is of interest whether these factors also positively influence humoral response after follow-up COVID-19 vaccines. Previous studies found seroconversion in ∼20% of patients, but mostly included patients treated with a registered dose RTX (≥1000 mg) [5–7].
Additionally, there are scarce data on humoral response persistence in RA patients treated with RTX. So far, persistence of humoral response after two-dose vaccination was investigated in one study, which found a persistence rate of 88% after 6 months in a population with a median dose of 1000 mg RTX [7].
Therefore, we aimed to investigate the association of dosing and timing of RTX on humoral response after three-dose vaccination in previous non-responder, and the persistence of an initial positive humoral response after two doses of COVID-19 vaccine in RA patients treated with (ultra-) low-dose RTX.
Patients and methods
Study design and participants
This is a follow-up study of the RTX-COVAC cohort in which we demonstrated that the humoral response after two-dose COVID-19 vaccination in RA patients treated with (ultra-) low-dose RTX is dependent on both dosage and timing [4]. In the current study, the first aim was to investigate the efficacy of a third vaccine and the second was to investigate the persistence of response after two-dose COVID-19 vaccination. Patients were included in the first analysis if they had a negative humoral response after two doses, had received a third COVID-19 vaccination and had a blood sample drawn 2–6 weeks thereafter (‘third dose sample’). Patients were included in the second analysis if they had a previous positive humoral response and had a blood sample drawn ≥6 weeks after a second severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination but before the third vaccination (‘persistence sample’). All participants provided written informed consent. This study was registered at the Netherlands Trial Registry (https://www.trialregister.nl/; NL9342). The follow-up study took place from June 2021 to January 2022 in the Sint Maartenskliniek, Nijmegen, the Netherlands and was approved by the local ethics committee (CMO Arnhem-Nijmegen, 2021-7406).
Procedures
All participants received their COVID-19 vaccinations through the Dutch national vaccine programme. For the first two vaccinations, patients in the cohort received either BNT162b2 (Comirnaty; Pfizer-BioNTech), ChAdOx1 nCoV-19 (Vaxzevria; AstraZeneca) or mRNA-1273 (Spikevax; Moderna). For the third vaccination, only the mRNA vaccines BNT162b2 and mRNA-1273 were approved for RTX patients in the Netherlands. Relevant demographics and RA disease characteristics were obtained at baseline. Also, we recorded relevant treatment characteristics, including concomitant conventional synthetic DMARD (csDMARD) use, prednisolone use, current biologic/targeted synthetic DMARD (b/tsDMARD) use, cumulative RTX dose and dosage and date of the latest RTX administration. Details on a previous COVID-19 infection (including the date of the positive test and dichotomized between before or after second vaccination) and COVID-19 vaccination dates were provided by the participant. The ‘persistence samples’ were evaluated using the Wantai SARS-CoV-2 antibody assay measuring the ratio of total immunoglobulin with a cut-off of positive (≥1.1) [8]. Most samples of the ‘third dose sample’ were evaluated with the prementioned assay, however, not all patients were able to visit our clinic during the established time frame. Therefore, humoral response measured with routinely used and validated assays at a local laboratory was also accepted and results were categorized as either positive or negative based on assay-specific cut-offs. Follow-up of patients ended after the last blood sample was drawn for the study.
Outcomes
The main outcomes of the study were to assess the proportion of patients with a seroconversion after a third vaccination and the association with dosing and timing of RTX and the proportion of patients with persistence of humoral response after the second vaccination.
Statistical analysis
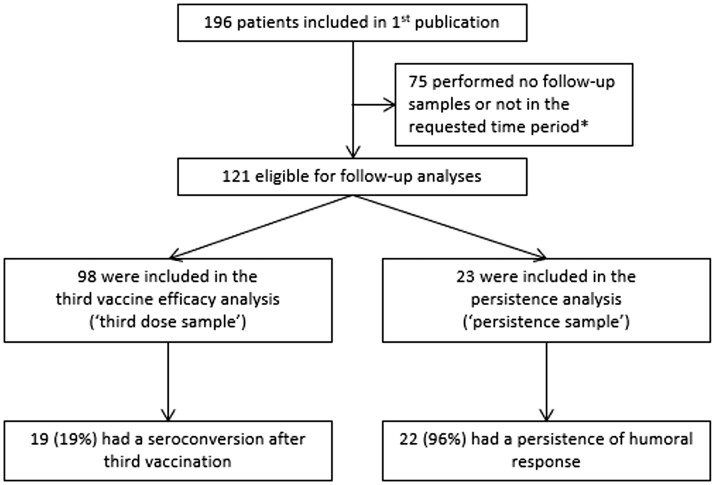
All eligible patients from the first study were included [4]. Three dosage groups (200 mg, 500 mg and 1000 mg) were defined based on the last received RTX dose before the first vaccination, similar to our first study. We used a dichotomous outcome to assess seroconversion, based on the cut-off of ≥1.1 of the total immunoglobulin index number for the Wantai assay; for other assays we used the dichotomous outcome as provided by the local laboratory [8]. Descriptive statistics were appropriately used to assess group characteristics. We used the ‘third dose sample’ (see Fig. 1) for the analysis of efficacy after a third vaccine. Fisher’s exact test was used to test the differences between the vaccine types for third vaccine efficacy. To assess the associations between dosing and timing on humoral response in the efficacy after third vaccine analysis, we used univariable logistic regression with humoral response 2–6 weeks after the third vaccination as the dependent variable and the latest RTX dose before baseline and time between the latest RTX dose and the first vaccination as the central determinant. The ‘persistence sample’ was used for the secondary analysis, investigating the persistence of humoral response. All data were entered in an electronic data capture database (Castor EDC, Amsterdam, Netherlands) and subsequently exported to Stata/IC (version 13, StataCorp, College Station, TX, USA) for statistical analyses.
Figure 1.
Study flow chart. *For third vaccine analysis: non-response after two vaccines and blood sample taken 2–6 weeks after the third vaccination. For persistence analysis: previous response after two vaccines and follow-up blood sample taken after second COVID-19 vaccination but before the third vaccination
Results
Patients
A total of 98 patients provided a third dose sample for third vaccine analysis and 23 provided a persistence sample for persistence analysis (see Fig. 1). The baseline characteristics of the patients included in this study are displayed in Supplementary Table S1, available at Rheumatology online.
Factors associated with seroconversion after a third vaccine dose
Third vaccinations took place between 5 October 2021 and 9 January 2022. The RTX dose at baseline did not differ between before and after the respective vaccinations in 89% of patients. The median time between the second and third vaccination was 145 days [interquartile range (IQR) 130–160]. Samples for third vaccine efficacy were drawn in 98 patients 2–6 weeks after the third vaccination and took place between 28 October 2021 and 9 February 2022. Of the 98 patients, 13 (13%) had received 200 mg as the latest RTX dose before first vaccination, 37 (38%) received 500 mg and 48 (49%) received 1000 mg (Table 1). Nineteen patients (19%) reached a positive response after the third vaccination, of which two had a COVID-19 infection between the second and third vaccination. Response rates were numerically higher for patients who received the AstraZeneca vaccine as the first two COVID-19 vaccines [5/19 (26%)] vs the Pfizer and Moderna vaccines [combined 10/79 (13%), P = 0.16]. Between 200 mg and 1000 mg as the latest RTX dose at the first vaccination, the percentage of humoral response after the third vaccination was higher for the 200 mg group [5/13 (38%)] vs the 1000 mg group [7/48 (15%)], although not significantly [odds ratio (OR) 3.66 (95% CI 0.93, 14), P = 0.06]. Between 500 mg and 1000 mg, response rates were similar: 19% (7/37) vs 15% (7/48), respectively [OR 1.37 (95% CI 0.43, 4.3), P = 0.59]. These values were similar when analysing with the latest RTX dose before the third vaccination. The median time between the third vaccination and the latest RTX dose was 138 days (IQR 111–156) for responders and 119 days (IQR 91–147) for non-responders, resulting in a non-significant association between humoral response and timing [OR 1.16 (95% CI 0.97, 1.35) per month increased time, P = 0.10].
Table 1.
Third vaccine efficacy
| RTX dosea, mg | Positive response, n (%) | Negative response, n (%) | Total, n |
|---|---|---|---|
| 200 | 5 (38) | 8 (62) | 13 |
| 500 | 7 (19) | 30 (81) | 37 |
| 1000 | 7 (15) | 41 (85) | 48 |
| Total, n | 19 | 79 | 98 |
Latest RTX dose before the first COVID-19 vaccination.
Humoral response persistence
Samples for humoral response persistence were drawn between 30 June and 4 November 2021, with a median time after the second vaccination of 83 days (IQR 66–122). Detectable response persisted in 96% (22/23; Table 2). Nine patients with a previous positive response had received an RTX dose between the samples, of which four had a dose of 1000 mg (44%), three had 500 mg (33%) and two had 200 mg (22%). Response persisted in 8/9 patients who retrieved intercurrent RTX (89%), except for one patient who received 500 mg.
Table 2.
Humoral response persistence
| RTX dosea, mg | Positive response, n (%) | Negative response, n (%) | Total, n |
|---|---|---|---|
| 200 | 5 (100) | 0 (0) | 5 |
| 500 | 5 (83) | 1 (17) | 6 |
| 1000 | 12 (100) | 0 (0) | 12 |
| Total, n | 22 | 1 | 23 |
Latest RTX dose before the first COVID-19 vaccination.
Discussion
Our main results illustrate that humoral response after a third vaccination occurs in a relevant proportion of patients who did not respond to earlier vaccination. Also, with a similar OR as in our first study [4]—although not significantly so due to a smaller study population—humoral response was associated with 200 mg RTX and a longer time between RTX infusion and vaccination. Additionally, we have shown that the persistence of humoral response is very high even in the context of intercurrent RTX infusions.
The association between 200 mg RTX and positive response after two-dose and three-dose vaccination could be explained by faster B cell repletion. Previous studies showed that B cell repopulation is associated with humoral response [3, 9] and that B cell numbers are non-significantly higher at 6 months after a dose of 200 mg RTX compared with 1000 mg [10]. Unfortunately, B cell counts were not performed in our current study. We also found a non-significant higher response rate after a third vaccination for patients receiving the AstraZeneca vaccine for the first two vaccinations compared with the Pfizer or Moderna vaccines. This may be explained by the beneficial effect of a heterologous booster [11], as only mRNA vaccines were approved for a third vaccination.
A limitation of this study is the smaller sample size compared with the first study, possibly leading to reduced power. Also, T cell measurements were not performed, which may lead to an underrepresentation of responders in our study, as T cell responses are present in the majority of RTX patients after two-dose vaccination [12]. To extend this, the optimal outcome would of course be the occurrence of COVID-19, but this would require a longer follow-up, more patients and is dependent on COVID-19 incidence in the population. Of note, this study did not include patients with other diseases in which RTX is used, therefore extrapolation of our recommendations to treatment with RTX in general may be difficult.
Based on the results of our study, repeated vaccination as late as possible after the lowest RTX dose possible seems the best vaccination strategy. Once seroconversion is achieved, humoral response persists despite RTX continuation.
Supplementary Material
Acknowledgements
We thank Kasper Jolink and the staff of the rheumatology outpatient clinic of the Sint Maartenskliniek for performing additional blood sampling for this study and Paul Daemen for performing the assays. C.J.T.v.d.T., D.F.T.C., B.J.F.v.d.B., N.d.B. and A.A.d.B. designed the study. C.J.T.v.d.T., D.F.T.C. and A.A.d.B. informed and included patients. J.R. supervised and interpreted the antibody measurements. C.J.T.v.d.T. had access to all the data and performed the statistical analyses. C.J.T.v.d.T., D.F.T.C. and A.A.d.B. drafted the manuscript. All authors critically revised the final version of the manuscript. The study was approved by the Ethics Committee of the Radboudumc CMO Arnhem-Nijmegen (protocol number 2021-7406) and the National Ethics Committee of the Netherlands CCMO (protocol number NL76709.091.21). The study was conducted in accordance with the Declaration of Helsinki and International Council for Harmonization Good Clinical Practice guidelines.
Contributor Information
Céleste J T van der Togt, Department of Rheumatology, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands; Department of Rheumatology, Sint Maartenskliniek, Ubbergen, The Netherlands.
David F Ten Cate, Department of Rheumatology, Sint Maartenskliniek, Ubbergen, The Netherlands.
Bart J F van den Bemt, Department of Pharmacy, Sint Maartenskliniek, Ubbergen, The Netherlands; Department of Clinical Pharmacy, Radboudumc, Nijmegen, The Netherlands.
Janette Rahamat-Langendoen, Department of Viroscience, Erasmus MC, Rotterdam, The Netherlands.
Nathan den Broeder, Department of Rheumatology, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands; Department of Rheumatology, Sint Maartenskliniek, Ubbergen, The Netherlands.
Alfons A den Broeder, Department of Rheumatology, Sint Maartenskliniek, Ubbergen, The Netherlands; Department of Rheumatology, Radboudumc, Nijmegen, The Netherlands.
Supplementary data
Supplementary data are available at Rheumatology online.
Data availability statement
Data are available upon request from A.A.d.B., clinical research lead (a.denbroeder@maartenskliniek.nl). Researchers who are interested in doing additional analyses using these data can contact the corresponding author. Data can only be used for scientific research without conflicts of interest.
Funding
No funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: A.A.d.B. reports grants from AbbVie, Pfizer, Eli Lilly, Novartis and Sanofi to the institution, outside of the submitted work. The other authors declare no competing interests.
References
- 1. Avouac J, Drumez E, Hachulla E. et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol 2021;3:e419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Furer V, Eviatar T, Zisman D. et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. [DOI] [PubMed] [Google Scholar]
- 3. Schietzel S, Anderegg M, Limacher A. et al. Humoral and cellular immune responses on SARS-CoV-2 vaccines in patients with anti-CD20 therapies: a systematic review and meta-analysis of 1342 patients. RMD Open 2022;8:e002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Togt CJT, Ten Cate DF, den Broeder N. et al. Humoral response to coronavirus disease-19 vaccines is dependent on dosage and timing of rituximab in patients with rheumatoid arthritis. Rheumatology (Oxford) 2022;61(SI2):SI175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonelli M, Mrak D, Tobudic S. et al. Additional heterologous versus homologous booster vaccination in immunosuppressed patients without SARS-CoV-2 antibody seroconversion after primary mRNA vaccination: a randomised controlled trial. Ann Rheum Dis 2022;81:687–94. [DOI] [PubMed] [Google Scholar]
- 6. Jyssum I, Kared H, Tran TT. et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol 2022;4:e177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sidler D, Born A, Schietzel S. et al. Trajectories of humoral and cellular immunity and responses to a third dose of mRNA vaccines against SARS-CoV-2 in patients with a history of anti-CD20 therapy. RMD Open 2022;8:e002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beijing Wantai Biological Pharmacy Enterprise. Wantai SARS-CoV-2 Ab ELISA. https://www.fda.gov/media/140929/download (7 October 2021, date last accessed).
- 9. Avouac J, Miceli-Richard C, Combier A. et al. Risk factors of impaired humoral response to COVID-19 vaccination in rituximab treated patients. Rheumatology (Oxford) 2022;61(SI2):SI163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wientjes MHM, Gijzen TMG, den Broeder N. et al. Drug levels, anti-drug antibodies and B-cell counts were not predictive of response in rheumatoid arthritis patients on low dose rituximab. Rheumatology (Oxford) 2022;doi: 10.1093/rheumatology/keac024. [DOI] [PubMed] [Google Scholar]
- 11. Liu X, Munro APS, Feng S. et al. Persistence of immunogenicity after seven COVID-19 vaccines given as third dose boosters following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK: three month analyses of the COV-BOOST trial. J Infect 2022;84:795–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prendecki M, Clarke C, Edwards H. et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis 2021;80:1322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from A.A.d.B., clinical research lead (a.denbroeder@maartenskliniek.nl). Researchers who are interested in doing additional analyses using these data can contact the corresponding author. Data can only be used for scientific research without conflicts of interest.