Abstract
Background
The safety of a third dose of SARS-CoV-2 mRNA vaccination in patients with inflammatory bowel disease is unknown.
Methods
We compared symptoms following a third SARS-CoV-2 mRNA vaccine dose with symptoms after the second dose in IBD.
Results
The study group included 594 patients (70% female, 58% BNT162b2). Overall, 41% reported symptoms after a third dose. Symptom frequency and severity were lower after the third dose relative to the second dose for every organ system, except for gastrointestinal symptoms which were marginally worse.
Conclusion
The frequency and severity of symptoms after a third mRNA vaccine dose are generally similar or milder than after a second dose for most organ systems.
Key Messages.
What is already known?
Post-mRNA-vaccination symptoms are worse after a second dose relative to after a first dose in patients with IBD.
What is new here?
Symptoms after a third mRNA vaccine dose in patients with IBD are less frequent and generally milder than after a second dose and less frequent than symptoms reported in the general population.
How can this study help patient care?
Patients with IBD and their providers can be reassured that postvaccination symptoms after a third mRNA vaccine dose are generally mild and less frequent than after a second dose.
Introduction
Vaccine safety concerns are a major contributor to vaccine hesitancy.1 Symptoms after SARS-CoV-2 primary vaccination among patients with inflammatory bowel disease (IBD) are generally of similar frequency, severity, and duration to those reported in the general population.2–5 In the general population, the frequency of reactions after a third dose of mRNA vaccination in the general population was similar to the second dose.6 However, the symptom profile after a third mRNA vaccine dose in the predominantly immune-compromised IBD population is unknown. We aimed to assess symptomology after a third or booster dose of mRNA vaccination in adults with IBD.
Methods
Participants included those with IBD ages 13 and older enrolled in the prospective Coronavirus Risk Associations and Longitudinal Evaluation in IBD (CORALE-IBD) vaccine registry7 who received a third mRNA vaccine dose. Subjects were initially recruited for enrollment at the time of initial vaccination at Cedars-Sinai Medical Center, through referrals from 26 IBD practices/clinics in the United States and through a social media campaign. Subjects were queried regarding postvaccination symptoms 1 week after each vaccine dose. Symptoms were assessed across 11 organ systems, including injection site symptoms (eg, pain, erythema, swelling); fatigue or malaise; headache, dizziness or lightheadedness; fever or chills; rheumatologic (eg, muscle, joint, or nerve) symptoms; gastrointestinal (eg, nausea, vomiting, abdominal pain, diarrhea, or other) symptoms; sleep changes; swollen lymph nodes; skin/nail or facial changes; eye, ear, mouth, or throat changes; cough, chest, or breathing symptoms; and memory or mood changes. Symptoms were graded by severity as mild, moderate, or severe impact on activities of daily living or requiring hospitalization.2 The category of “severe+” included those with severe symptoms or hospitalization. We stratified patients by age as younger than 55 years or 55 years and older due to the known influence of age on adverse events. Categorical and continuous variables were summarized and compared across age strata (<55 and >55 years) using χ2 and Student t test, respectively. Pairwise χ2 (McNemar test) and t test were used when appropriate.
We also compared the frequency and severity of symptoms after dose 3 relative to those reported after dose 2, given that symptoms after the second dose of the primary series were more frequent and severe than after the first dose (R version 3.5.1). We additionally assessed severity after each vaccine dose (none or mild, moderate, and severe+) to clarify the frequency of severe symptoms after dose 3 relative to the severity of symptoms experienced after dose 2. The study protocol was approved by the Cedars-Sinai institutional review board. All study participants provided informed consent.
Results
The cohort included 524 participants (70% female, mean age 45 years) reporting a third dose of mRNA vaccination through October 11, 2021. The majority had Crohn’s disease (71%), with the remainder having ulcerative colitis or IBD-unclassified, and 89% were receiving biologic therapies. The majority of participants (58%) received primary vaccination with BNT562b2 (Pfizer), with the remainder receiving mRNA-1273 (Moderna), and only 3.5% of the overall cohort reported a previous COVID infection at the time of initial vaccination. Overall, 97% of subjects received a third dose with the same mRNA vaccine as in their initial series, with the remainder receiving the other mRNA vaccine type.
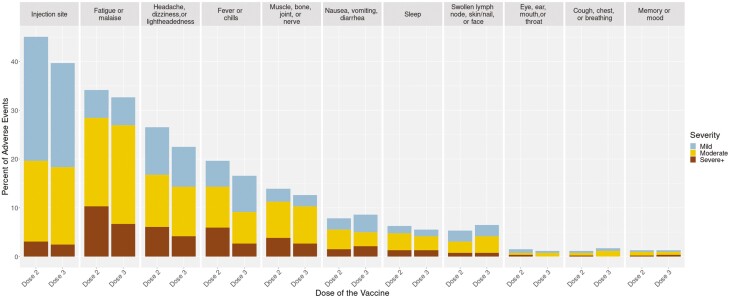
In total, 41% of patients reported symptoms after a third dose, with symptoms generally more frequent and more severe among participants younger than 55 years (Table 1). The most frequent postvaccination symptom was injection site pain (39%). Commonly reported systemic symptoms were fatigue or malaise (34%), headache (23%), and muscle, bone, and joint symptoms (13%). These symptoms were all less frequently reported after dose 3 than after dose 2 (Figure 1). Gastrointestinal symptoms were reported by 8.8%, which was slightly more frequent than after dose 2 (7.8%). Among those with postvaccination symptoms, the proportion with severe+ symptoms after dose 3 was lower than dose 2 for fatigue/malaise, headache, dizziness and lightheadedness, fever or chills, and rheumatologic symptoms—but was slightly higher than dose 2 for gastrointestinal symptoms (Figure 1).
Table 1.
Frequency and severity of postvaccination symptoms by age.
| Variable | Total | Age < 55 | Age>=55 | P |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| N | 524(100) | 387(100) | 137(100) | |
| Age | 45.3(14.44) | 38.07(8.24) | 65.72(6.33) | 2.09E-267 |
| Female | 370(70.75) | 293(75.91) | 77(56.2) | 7.17E-05 |
| Crohn’s disease | 370(70.61) | 274(70.8) | 96(70.07) | 0.872 |
| White | 491(93.88) | 360(93.26) | 131(95.62) | 0.422 |
| Primary vaccine: BNT162b2 (Pfizer/BioNtech) | 306(58.4) | 231(59.69) | 75(54.74) | 0.313 |
| Prior COVID | 18(3.45) | 15(3.9) | 3(2.19) | 0.347 |
| On biologic therapy at time of vaccination | 467(89.12) | 347(89.66) | 120(87.59) | 0.503 |
| Duration between doses 2 and 3 (days, SD)) | 166.00(32.36) | 164.19(34.11) | 170.80(26.76) | 0.456 |
| Adverse Events | ||||
| Local pain | 206(39.3) | 167(43.15) | 39(28.46) | 2.49E-03 |
| Local redness | 58(11.07) | 47(12.14) | 11(8.03) | 0.187 |
| Local swelling | 58(11.07) | 49(12.66) | 9(6.57) | 0.051 |
| Fever or chills | 87(16.6) | 72(18.6) | 15(10.95) | 0.038 |
| Fatigue or malaise | 176(33.59) | 142(36.69) | 34(24.82) | 0.011 |
| Headache | 119(22.71) | 97(25.06) | 22(16.06) | 0.031 |
| Eye, ear, mouth or throat symptoms | 11(2.1) | 11(2.84) | 0(0.00) | 0.046 |
| Lymph node, skin or facial symptoms | 36(6.87) | 31(8.01) | 5(3.65) | 0.083 |
| Cough, chest or breathing symptoms | 9(1.72) | 7(1.81) | 2(1.46) | 0.787 |
| Digestive symptoms | 46(8.78) | 40(10.34) | 6(4.38) | 0.034 |
| Urinary or genital | 0(0.00) | 0(0.00) | 0(0.00) | 1.000 |
| Muscle, bone or joint | 66(12.6) | 54(13.95) | 12(8.76) | 0.115 |
| Memory or mood | 7(1.34) | 6(1.55) | 1(0.73) | 0.472 |
| Sleep symptoms | 31(5.92) | 28(7.24) | 3(2.19) | 0.031 |
| Overall Severity | ||||
| None | 307(58.59) | 213(55.04) | 94(68.61) | 0.026 |
| Mild | 30(5.73) | 25(6.46) | 5(3.65) | |
| Moderate | 121(23.09) | 93(24.03) | 28(20.44) | |
| Severe+ | 66(12.6) | 56(14.47) | 10(7.3) | |
Figure 1.
Severity of symptoms by system for dose 3 relative to dose 2.
We evaluated the severity of symptoms after dose 3 by the severity of symptoms after dose 2 (Supplemental Table 1). Overall, the majority (75%) experienced none or mild symptom severity for both doses. Severe+ symptoms were comparable at dose 2 and 3 (17% and 14%, respectively). Of those with severe+ symptoms after dose 2, 34% experienced severe+ symptoms after dose 3 (odds ratio [OR], 5.15; P < .001). In comparison, about 22% experienced severe+ symptoms after dose 3 but did not report severe+ symptoms after dose 2.
Discussion
We demonstrate several key findings with respect to symptoms after a third mRNA vaccine among patients with IBD. First, postvaccination symptom frequency and severity are significantly greater among those younger than 55 years, similar to findings after a second vaccine dose and similar to findings reported in the pivotal BNT162b2 vaccine trials.3 Second, symptoms after dose 3 were generally less frequent and less severe for most organ systems, with the notable exception of gastrointestinal symptoms which were slightly more common and severe after dose 3 relative to dose 2. Third, the frequency of severe+ symptoms were comparable after dose 2 and dose 3. However, because about 1 in 5 experience severe+ symptoms after dose 3 even without previous severe+ symptoms, patients should consider vaccination with a third dose at a time when short-lived severe symptoms can be best tolerated and addressed. We found that the frequency of adverse events after a third mRNA vaccine dose in the IBD population was generally lower than rates reported in the general population, where approximately 75% reported a local or systemic reaction within 7 days of vaccination.6 Our results are consistent with the lower frequencies of postvaccination reactions reported in various immune-compromising conditions.8 Our finding that gastrointestinal symptoms after a third dose were slightly higher than after a second dose raises the question about whether these represented postvaccination reactions or coincidental exacerbation of IBD. Previous publications are reassuring that IBD disease exacerbation does not occur more frequently after COVID vaccination.5,9
Limitations of this study include recall bias and a lack of racial and ethnic diversity. To minimize recall bias, we sent our survey at 1 week after vaccination, similar to the period of solicitation of adverse events in the pivotal BNT162b and mRNA-1273 clinical trials.3 However, symptoms manifesting after 1 week, such as menstrual irregularities, might therefore not have been captured. A further limitation is our female predominance of study participants which is skewed relative to the 1:1 female:male ratio in the IBD population.
Our findings can reassure the IBD patients and providers that the likelihood and distribution of symptoms after a third mRNA vaccine dose are generally similar to those after a second dose and that fewer people experienced postvaccination symptoms after dose 3 than after dose 2 for most organ systems. Postvaccination symptoms appear to occur less frequently in IBD than in the general population, although further evaluation of postvaccination gastrointestinal symptoms among those with IBD is warranted.
Supplementary Material
Acknowledgments
Authors thank all patient participants of the CORALE-IBD Vaccine study.
Contributor Information
Dalin Li, Inflammatory Bowel and Immunobiology Research Institute, Karsh Division of Digestive and Liver Diseases, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Philip Debbas, Inflammatory Bowel and Immunobiology Research Institute, Karsh Division of Digestive and Liver Diseases, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Angela Mujukian, Inflammatory Bowel and Immunobiology Research Institute, Karsh Division of Digestive and Liver Diseases, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Susan Cheng, Smidt Heart Institute, Department of Medicine, Cedars-Sinai, Los Angeles, CA, USA.
Jonathan Braun, Inflammatory Bowel and Immunobiology Research Institute, Karsh Division of Digestive and Liver Diseases, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Dermot P B McGovern, Inflammatory Bowel and Immunobiology Research Institute, Karsh Division of Digestive and Liver Diseases, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Gil Y Melmed, Inflammatory Bowel and Immunobiology Research Institute, Karsh Division of Digestive and Liver Diseases, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
CORALE-IBD Study Group:
Andrea Banty, Edward Feldman, Christina Ha, Susie Lee, Shervin Rabizadeh, Theodore Stein, Theodore Solomon, Gaurav Syal, Stephan Targan, Eric Vasiliauskas, David Ziring, Niru Bonthala, Gregory S Botwin, Melissa Hampton, Emebet Mengesha, Elizabeth Khanishian, Valeriya Pozdnyakova, Phillip Gu, Shane White, Cindy Zamudio, Daniel Gonzalez, Alyssa Parry, Mary Hanna, Justin Chan, Joe Ebinger, Sandy Joung, Min Wu, Amy Hoang, Timothy Wynter, Nancy Sun, Jane C Figueiredo, Akil Merchant, Noah Merin, Karen L Reckamp, Keren Appel, Rashmi Kumar, Brigid Boland, Aline Charabaty, Michael Chiorean, Erica Cohen, Ann Flynn, John Valentine, Adam C Ehrlich, David Fudman, Arash Horizon, Dmitry Karayev, Benjamin Kretzmann, Jason Hou, Caroline Hwang, Mark Lazarev, Donald Lum, Rebecca Fausel, Swapna Reddy, Ryan McConnell, Mark C Mattar, Mark Metwally, Arthur Ostrov, Nimisha Parekh, Laura Raffals, David T Rubin, Sarah Sheibani, Corey A Siegel, Douglas Wolf, Ziad Younes, and Oriana Damas
Author Contributions
Guarantor of the article: G.M.
Study concept and design: G.M., D.L., G.B., J.B., D.M.
Acquisition of data: G.M., D.L., G.B., D.M.
Analysis and interpretation of data: all authors
Drafting of the manuscript: G.M.
Critical revision of the manuscript for important intellectual content: all authors
Statistical analysis: D.L.
Obtained funding: G.M., J.B., D.M.
Study supervision: G.M., J.B., D.M.
Funding
This study was supported by the The Leona M. and Harry B. Helmsley Charitable Trust, the Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, and the National Institute of Diabetes and Digestive and Kidney Disease Grants P01DK046763 and U01DK062413. This study has been additionally supported by the Cedars-Sinai Precision Health Initiative and the Erika J. Glazer Family Foundation.
Conflicts of Interest
G.Y.M. has consulted for AbbVie, Arena Pharmaceuticals, Boehringer-Ingelheim, Bristol-Myers Squibb/Celgene, Entasis, Janssen, Medtronic, Pfizer, Samsung Bioepis, Shionogi, Takeda, and Techlab and has received research funding from Pfizer for an unrelated investigator-initiated study. J.B. has received research funding from Janssen. J.C.P., J.L.S., and E.C.F. work for Abbott Diagnostics, a company that performed the serological assays on the biospecimens that were collected for this study. D.P.B.M.: Bridge Biotherapeutics, Gilead, Palatin, Pfizer, Promteheus Biosciences, Prometheus Laboratories, Takeda. M.C.: Abbvie, Arena, Bristol-Meyers Squibb, Janssen, Medtronic, Pfizer, and Takeda. E.C.: Abbvie and Pfizer. D.F.: Pfizer. C.H.: Abbvie, Janssen and Pfizer. D.L.: Abbvie, Janssen and Takeda. R.M.: Abbvie, Bristol Myers Squibb, Pfizer, and Prometheus Bioscience. N.P.: Pfizer. DW: Abbvie, Arena, Bristol Meyers Squibb, Corevitas, Janssen, Lilly, Pfizer, and Takeda. B.M.: Abbvie, Bristol Myers Squibb, Janssen, Pfizer and Takeda. S.G.: Abbvie, Janssen, and Takeda. C.H.: Abbvie, Bristol Meyers Squibb, Genentech, InbDex Pharmaceuticals, Janssen, Lilly, and Pfizer. G.S.: research funding for unrelated investigator study from Pfizer. S.T.: Prometheus Bioscience.
The remaining authors have no competing interests.
Contributing Authors
Coronavirus Risk Associations and Longitudinal Evaluation-Inflammatory Bowel Disease (CORALE-IBD) Study Group:
1Andrea Banty, 1Edward Feldman, 1Christina Ha, 1Susie Lee, 1Shervin Rabizadeh, 1Theodore Stein, 1Theodore Solomon, 1Gaurav Syal, 1Stephan Targan, 1Eric Vasiliauskas, 1David Ziring, 1Niru Bonthala, 1Gregory S. Botwin, 1Melissa Hampton, 1Emebet Mengesha, 1Elizabeth Khanishian, 1Valeriya Pozdnyakova, 1Phillip Gu, 1Shane White, 1Cindy Zamudio, 1Daniel Gonzalez, 1Alyssa Parry, 1Mary Hanna, 1Justin Chan,
2Joe Ebinger, 2Sandy Joung, 2Min Wu, 2Amy Hoang, 2Timothy Wynter, 2Nancy Sun, 3Jane C. Figueiredo, 3Akil Merchant, 3Noah Merin, 3Karen L. Reckamp, 4Keren Appel, 5Rashmi Kumar, 6Brigid Boland, 7Aline Charabaty, 8Michael Chiorean, 9Erica Cohen, 10Ann Flynn, 10John Valentine, 11Adam C. Ehrlich, 12David Fudman, 13Arash Horizon, 13Dmitry Karayev, 13Benjamin Kretzmann, 14Jason Hou, 15Caroline Hwang, 16Mark Lazarev, 17Donald Lum, 17Rebecca Fausel, 17Swapna Reddy, 18Ryan McConnell, 19Mark C. Mattar, 20Mark Metwally, 20Arthur Ostrov, 21Nimisha Parekh, 22Laura Raffals, 23David T. Rubin, 24Sarah Sheibani, 25Corey A. Siegel, 26Douglas Wolf, 27Ziad Younes, 28Oriana Damas
1Inflammatory Bowel and Immunobiology Research Institute, Karsh Division of Digestive and Liver Diseases, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA
2Smidt Heart Institute, Department of Medicine, Cedars-Sinai, Los Angeles, CA, USA
3Samual Oschin Comprehensive Cancer Institute, Cedars-Sinai, Los Angeles, CA, USA
4Children’s Hospital, Orange County, CA, USA
5Banner University Medical Center, Phoenix, AZ, USA
6University of California, San Diego, CA, USA
7Sibley Memorial Hospital, Johns Hopkins, Washington, DC, USA
8Swedish Medical Center, Seattle, WA, USA
9Capital Digestive Care, Chevy Chase, MD, USA
10University of Utah, Salt Lake City, UT, USA
11Temple University, Philadelphia, PA, USA
12UT Southwestern, Dallas, TX, USA
13Center for Rheumatology, Los Angeles, CA, USA
14Baylor College of Medicine, Houston, TX, USA
15Hoag Hospital, Newport Beach, CA, USA
16Johns Hopkins Hospital, Baltimore, MD, USA
17The Oregon Clinic, Portland, OR, USA
18Sutter Health, Palo Alto, CA, USA
19Medstar-Georgetown, Washington, DC, USA
20Saratoga-Schenectady Gastroenterology, Saratoga Springs, NY, USA
21University of California, Irvine, CA, USA
22The Mayo Clinic, Rochester, MN, USA
23University of Chicago, Chicago, IL, USA
24Keck Medicine of University of Southern California, Los Angeles, CA, USA
25Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA
26Atlanta Gastroenterology Associates, Atlanta, GA, USA
27Gastro One, Germantown, TN, USA
28University of Miami, Miami, FL, USA
References
- 1. Dalal RS, McClure E, Marcus J, et al. COVID-19 vaccination intent and perceptions among patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2021;19(8):1730-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Botwin GJ, Li D, Figueiredo J, et al. Adverse events following SARS-CoV-2 mRNA vaccination: among patients with inflammatory bowel disease. Am J Gastroenterol. 2021;116(8):1746-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weaver KN, Zhang X, Dai X, et al. Impact of SARS-CoV-2 vaccination on inflammatory bowel disease activity and development of vaccine-related adverse events: results from Prevent-Covid. Inflamm Bowel Dis. 2021. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hause AM, Baggs J, Gee J, et al. Safety monitoring of an additional dose of COVID-19 vaccine - United States, August 12-September 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(39):1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Melmed GY, Botwin GJ, Sobhani K, et al. Antibody responses after SARS-CoV-2 mRNA vaccination in adults with inflammatory bowel disease. Ann Intern Med. 2021;174(12):1768-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wieske L, Kummer LYL, van Dam KPJ, et al. Risk factors associated with short-term adverse events after SARS-CoV-2 vaccination in patients with immune-mediated inflammatory diseases. BMC Med. 2022;20(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lev-Tzion R, Focht G, Lujan R, et al. COVID-19 vaccine is effective in inflammatory bowel disease patients and is not associated with disease exacerbation. Clin Gastroenterol Hepatol. 2022;20(6):e1263-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.