Abstract
Background
Effective shielding measures and virus mutations have progressively modified the disease between the waves, likewise healthcare systems have adapted to the outbreak. Our aim was to compare clinical outcomes for older people with COVID-19 in Wave 1 (W1) and Wave 2 (W2).
Methods
All data, including the Clinical Frailty Scale (CFS), were collected for COVID-19 consecutive patients, aged ≥65, from 13 hospitals, in W1 (February–June 2020) and W2 (October 2020–March 2021). The primary outcome was mortality (time to mortality and 28-day mortality). Data were analysed with multilevel Cox proportional hazards, linear and logistic regression models, adjusted for wave baseline demographic and clinical characteristics.
Results
Data from 611 people admitted in W2 were added to and compared with data collected during W1 (N = 1340). Patients admitted in W2 were of similar age, median (interquartile range), W2 = 79 (73–84); W1 = 80 (74–86); had a greater proportion of men (59.4% vs. 53.0%); had lower 28-day mortality (29.1% vs. 40.0%), compared to W1. For combined W1–W2 sample, W2 was independently associated with improved survival: time-to-mortality adjusted hazard ratio (aHR) = 0.78 [95% confidence interval (CI) 0.65–0.93], 28-day mortality adjusted odds ratio = 0.80 (95% CI 0.62–1.03). W2 was associated with increased length of hospital stay aHR = 0.69 (95% CI 0.59–0.81). Patients in W2 were less frail, CFS [adjusted mean difference (aMD) = −0.50, 95% CI −0.81, −0.18], as well as presented with lower C-reactive protein (aMD = −22.52, 95% CI −32.00, −13.04).
Conclusions
COVID-19 older adults in W2 were less likely to die than during W1. Patients presented to hospital during W2 were less frail and with lower disease severity and less likely to have renal decline.
Introduction
Since the novel coronavirus SARS-CoV-2 first appeared in late 2019, it has spread globally leading to 175 million confirmed cases, and around 3.79 million COVID-19-related deaths in the second week of June 2021.1 Although the number of deaths represents only a small proportion of all infections, vulnerable older people represent a high percentage of the fatalities.1–3
While it has been a struggle to find a specific treatment, there have been definitely significant differences between the waves of the pandemic. With the rapid progression of the outbreak, national lockdowns, shielding measures, easier access to the swab test and active case detection have been required in order to reduce the viral transmission and number of cases. As a result, the number of people in hospital with COVID-19 has been gradually declining after the first wave. The national healthcare systems have been organized according to the COVID-related burden with hospital ward adaptation. In addition, therapies such as respiratory support4 and systemic corticosteroids5 have improved the COVID-19 management and contributed to better clinical outcomes in hospitalized patients. However, the access to these therapies for older adults is less certain.
Considering all these factors, there is little evidence comparing the first to the second wave and focusing on the outcomes in older people when hospitalized for COVID-19.
The COPE (COVID-19 in Older People) study6 assessed outcomes in patients hospitalized with COVID-19, with a particular focus on older adults living with frailty. We demonstrated that pre-admission frailty, measured using the Clinical Frailty Scale (CFS), was associated with both mortality and length of hospital stay independent of age.7
During the autumn of 2020, the second wave of the pandemic began in Europe. The COPE study team6,7 set up the COPE 1.1 study. Research comparing outcomes between different waves is limited to retrospective analyses, including mainly younger patients, those admitted to intensive care units, or analysed using underpowered samples.8–10
The COPE 1.1 is a multicentre prospective cohort study providing real-world data from people admitted to hospital with COVID-19. We aimed to compare clinical and demographic features of older adults and identify differences in outcomes between the two waves of the pandemic, specifically mortality and length of stay.
Methods
Study design
We conducted a prospective cohort study, full study details can be found within the COPE protocol.6 The data of the second cohort of patients were gathered between 1 October 2020 and 8 March 2021 as an extension of the COPE study7 during the second wave of the COVID-19 pandemic in the UK and Italy.
Authority in the UK to conduct the study was granted by the Health Research Authority (20/HRA/1898), and in Italy by the Ethics Committee of Hospital Policlinico Modena (Reference 369/2020/OSS/AOUMO). This article follows the STROBE statement for reporting of cohort studies.11
Setting
We utilized the same network of clinical teams from 12 UK sites and 1 Italian site (www.opsoc.eu) that participated in the COPE study.6,7
The UK hospitals that participated in data collection are: Aberdeen Royal Infirmary, Glasgow Royal Infirmary, Inverclyde Royal Hospital, Maidstone Hospital, Nevill Hall Hospital in Abergavenny, Royal Alexandra Hospital in Paisley, Royal Gwent Hospital in Newport, Southmead Hospital in Bristol, Salford Royal Hospital, University Hospital of Wales in Cardiff, Ysbyty Gwynedd in Bangor and Ysbyty Ystrad Fawr in Caerphilly. The Italian centre is the University Hospital Policlinico in Modena.
Participants
Consecutive patients admitted to hospital between 27 February and 10 June 2020 (Wave 1), and between 1 October 2020 and 8 March 2021 (Wave 2), aged 65 years or older with a diagnosis of COVID-19 were included. Patients with ≥65 years hospitalized for other reason who acquired SARS-CoV-2 infection in hospital were also included. Diagnostic criteria were laboratory confirmed SARS-CoV-2 positive swabs, and a clinical diagnosis (made by the site clinical team and based on signs, symptoms and/or radiology) consistent with COVID-19. No exclusion criteria were applied. Data regarding demographics and comorbidities were systematically collected on admission. The diagnosis of a comorbidity was confirmed by patient’s medication list or medical record. Clinical teams at each site screened inpatient admission lists for eligibility.
Sample size justification
Prior to this study mortality was known during Wave 1 of 40%, and it was estimated to be 30% in Wave 2 [hazard ratio (HR) of 0.70]. In order to detect this difference with 90% power and 5% significance 1.000 participants would be needed, with at least 500 during Wave 2.
Variables and outcomes
Covariates collated included: age; sex; admission C-reactive protein as a marker of disease severity (CRP, ≥40 mg/dl)12; admission estimated glomerular filtration rate (eGFR, <60 ml/min/1.73 m2); smoking status (never, previous or current); frailty and current diagnosis of: hypertension, coronary artery disease (CAD) and diabetes mellitus. Dexamethasone usage and remdesivir (Wave 2 only). Frailty was measured by the researcher using the CFS 1–913,14 estimated 2 weeks prior to admission.
The primary outcome was in-hospital mortality (time to mortality and 28-day mortality). The secondary outcome were: (i) characteristics differences in patient cohorts between the two waves; (ii) length of hospital stay (time from admission to discharge); and (iii) potential predictors of death. Outcomes were assessed up to last data entry using systems of prospective follow-up and electronic health records.
Data analysis
Data from Wave 1 and Wave 2 were compared in terms of baseline demographic and clinical variables and outcomes.
Main outcomes
Time-to-event outcomes (mortality and time to discharge) were analysed using multilevel multivariable Cox proportional hazards (PH) regression models, and Day 28 mortality was analysed with a multilevel logistic regression.
Each Cox PH model fitted site as a random effect to account for heterogeneity between each hospital. Crude and adjusted HRs (aHRs) are presented with associated 95% confidence intervals (CIs). Day 28 mortality was analysed using multilevel logistic regression models fitting hospital as a random intercept effect, estimating crude and adjusted odds ratios (aORs) with associated 95% CIs. All models were adjusted for Wave (1 or 2), healthcare setting (Italy or UK), age (65–74; 75–84; 85–94; 95+), sex (female/male), smoking status (current, former, never), elevated CRP (≥40 mg/dl), diabetes (yes/no), CAD (yes/no), hypertension (no, yes, yes and on treatment), reduced renal function (<60 ml/min per 1.73 m2) and frailty (CFS 1–3; CFS 4; CFS 5–6; CFS 7–8). Due to the small number of patients with a terminal illness (CFS 9), these were excluded from the analyses. The sample size calculation was originally estimated in the COPE protocol.6 Time-to-event models were visualized using Kaplan–Meier survival curves. Analyses were carried out in Stata SE version 16. Kaplan–Meier plots were visualized in R.
Secondary analysis of the participant characteristics between Wave 1 vs. 2
To assess if mortality differed by wave due to participant characteristics we examined the difference between the first and second wave. Fitting each participant characteristic as the dependent variable the crude and adjusted effect of wave was estimated using mixed-effects linear regression models (for age, CRP, eGFR and CFS), and mixed effects logistic regressions [for healthcare (UK vs. Italy), sex, smoking (current vs. never/ex-smokers), hypertension, diabetes and CAD]. All models were fitted with a random effect to account for hospital. All multivariable analyses were adjusted using the same covariates from the main outcome analyses.
Results
The study involved a total of 1951 patients aged 65 years and over (Wave 1, N = 1340; Wave 2, N = 611). People admitted during the second wave were of a similar age; median [interquartile range (IQR)] age in Wave 1 was 80 (74–86) and 79 (73–84) in Wave 2. Seven hundred and ten (53.0%) patients in Wave 1 were male, compared to 363 (59.4%) in Wave 2 (table 1). There were 1722 (88.3%) patients included from the UK (1251 in Wave 1 and 471 in Wave 2) and 229 (11.7%) from Italy (89 in Wave 1 and 140 in Wave 2). People admitted to hospital in Wave 2 were less likely to be living with frailty. In Wave 1, 66.5% of patients (N = 892) were frail (CFS 5 and greater), compared to 51.4% (N = 314) in Wave 2 (table 1). Fifty-five cases of missing smoking status were imputed as never smokers. Similarly, 64 cases of missing eGFR were imputed as normal (≥60 ml/min/1.73 m2). In Wave 1, only 23 (1.7%) of patients were taking dexamethasone compared to 339 (55.5%) in Wave 2. Remdesivir data were not collected in Wave 1, 87 (14.2%) of patients received it in the second wave. The prevalence of comorbidities was similar in the two waves. Disease severity assessed by elevated CRP appeared lower during Wave 2 (CRP ≥40, 59.7% vs. 68.8%) (table 1).
Table 1.
Sample characteristics by wavea
| Wave 1 (N = 1340) | Wave 2 (N = 611) | Total (N = 1951) | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| 28-Day mortality | |||
| Alive | 737 (55.0) | 425 (69.6) | 1162 (59.6) |
| Dead | 536 (40.0) | 178 (29.1) | 714 (36.6) |
| Missing | 81 | 8 | 89 |
| Healthcare | |||
| Healthcare | 1251 (93.4) | 471 (77.1) | 1722 (88.3) |
| UK | 89 (6.6) | 140 (22.9) | 229 (11.7) |
| Age | |||
| 65–74 | 378 (28.2) | 180 (29.5) | 558 (28.6) |
| 75–84 | 539 (40.2) | 285 (46.6) | 824 (42.2) |
| 85–94 | 384 (28.7) | 135 (22.1) | 519 (26.6) |
| 95+ | 39 (2.9) | 11 (1.8) | 50 (2.6) |
| Sex | |||
| Female | 629 (46.9) | 248 (40.6) | 877 (45.0) |
| Male | 710 (53.0) | 363 (59.4) | 1073 (55.0) |
| Missing | 1 | 0 | 1 |
| Smoking | |||
| Never smokers | 645 (48.1) | 264 (43.2) | 909 (46.6) |
| Ex-smokers | 568 (42.4) | 304 (49.8) | 872 (44.7) |
| Current smokers | 76 (5.7) | 39 (6.4) | 115 (5.9) |
| Missing | 51 | 4 | 55 |
| Diabetes | |||
| No | 952 (71.0) | 432 (70.7) | 1384 (70.9) |
| Yes | 384 (28.7) | 179 (29.3) | 563 (28.9) |
| Missing | 4 | 0 | 4 |
| Hypertension | |||
| No | 588 (43.9) | 260 (42.6) | 848 (43.5) |
| Yes | 200 (14.9) | 136 (22.3) | 336 (17.2) |
| Yes and on treatment | 552 (41.2) | 215 (35.2) | 767 (39.3) |
| CAD | |||
| No | 982 (73.3) | 455 (74.5) | 1437 (73.7) |
| Yes | 355 (26.5) | 155 (25.4) | 510 (26.1) |
| Missing | 3 | 1 | 4 |
| CRP | |||
| <40 | 418 (31.2) | 246 (40.3) | 664 (34.0) |
| ≥40 | 922 (68.8) | 365 (59.7) | 1287 (66.0) |
| eGFR | |||
| ≥60 | 707 (52.8) | 321 (52.5) | 1028 (52.7) |
| <60 | 575 (42.9) | 284 (46.5) | 859 (44.0) |
| Missing | 58 | 6 | 64 |
| CFS | |||
| CFS 1–3 | 256 (19.1) | 164 (26.8) | 420 (21.5) |
| CFS 4 | 172 (12.8) | 131 (21.4) | 303 (15.5) |
| CFS 5–6 | 487 (36.3) | 224 (36.7) | 711 (36.4) |
| CFS 7–8 | 377 (28.1) | 87 (14.2) | 464 (23.8) |
| CFS 9 | 28 (2.1) | 3 (0.5) | 31 (1.6) |
| Missing | 20 | 2 | 22 |
Excluded due to being alive and in hospital with less than 28 days of follow-up.
Primary outcome (time to mortality, and Day 28 mortality)
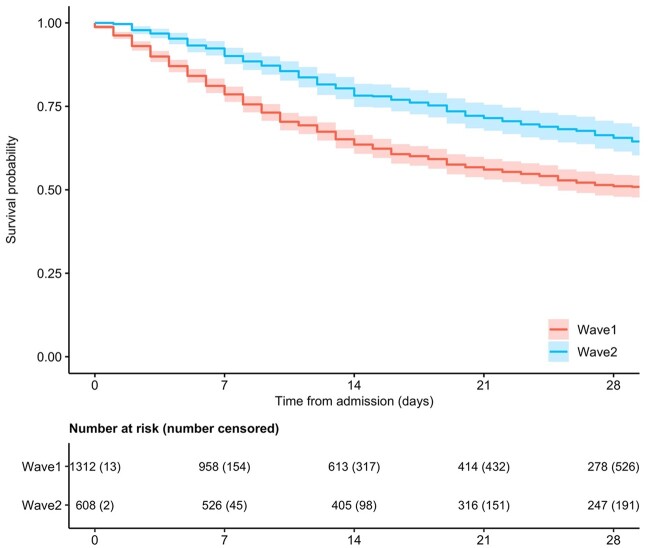
The 28-day mortality rate in Wave 1 was 40.0% (N = 536), and 29.1% (N = 178) in Wave 2. Median (IQR) time from admission to mortality was 12 (6–25) days in Wave 1 and 22 (11–50) days in Wave 2 (table 1, figure 1, and table 2). In the multivariable analysis, Wave 2 was independently associated with reduced mortality (aHR 0.78, 95% CI 0.65–0.93) (table 3). In addition, crude analysis revealed that older age, increasing frailty, male sex, elevated CRP, and reduced renal function were associated with increased mortality (table 3).
Figure 1.
Comparison of survival Wave 1 vs. Wave 2
Table 2.
Sample characteristics by in-hospital mortality
| Alive (N = 1141) | Dead (N = 810) | Total (N = 1951) | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Healthcare | |||
| UK | 1011 (58.7) | 711 (41.3) | 1722 (88.3) |
| Italy | 130 (56.8) | 99 (43.2) | 229 (11.7) |
| Wave | |||
| 1 | 760 (56.7) | 580 (43.3) | 1340 (68.7) |
| 2 | 381 (62.4) | 230 (37.6) | 611 (31.3) |
| Age | |||
| 65–74 | 378 (67.7) | 180 (32.3) | 558 (28.6) |
| 75–84 | 472 (57.3) | 352 (42.7) | 824 (42.2) |
| 85–94 | 268 (51.6) | 251 (48.4) | 519 (26.6) |
| 95+ | 23 (46.0) | 27 (54.0) | 50 (2.6) |
| Sex | |||
| Female | 544 (62.0) | 333 (38.0) | 877 (45.0) |
| Male | 596 (55.5) | 477 (44.5) | 1073 (55.0) |
| Missing | 1 | 0 | 1 |
| Smoking | |||
| Never smokers | 549 (60.4) | 360 (39.6) | 909 (46.6) |
| Ex-smokers | 485 (55.6) | 387 (44.4) | 872 (44.7) |
| Current smokers | 73 (63.5) | 42 (36.5) | 115 (5.9) |
| Missing | 34 | 21 | 55 |
| Diabetes | |||
| No | 821 (59.3) | 563 (40.7) | 1384 (70.9) |
| Yes | 318 (56.5) | 245 (43.5) | 563 (28.9) |
| Missing | 2 | 2 | 4 |
| Hypertension | |||
| No | 492 (58.0) | 356 (42.0) | 848 (43.5) |
| Yes | 200 (59.5) | 136 (40.5) | 336 (17.2) |
| Yes and on treatment | 449 (58.5) | 318 (41.5) | 767 (39.3) |
| CAD | |||
| No | 861 (59.9) | 576 (40.1) | 1437 (73.7) |
| Yes | 279 (54.7) | 231 (45.3) | 510 (26.1) |
| Missing | 1 | 3 | 4 |
| CRP | |||
| <40 | 460 (69.3) | 204 (30.7) | 664 (34.0) |
| ≥40 | 681 (52.9) | 606 (47.1) | 1287 (66.0) |
| eGFR | |||
| ≥60 | 650 (63.2) | 378 (36.8) | 1028 (52.7) |
| <60 | 452 (52.6) | 407 (47.4) | 859 (44.0) |
| Missing | 39 | 25 | 64 |
| CFS | |||
| CFS 1–3 | 299 (71.2) | 121 (28.8) | 420 (21.5) |
| CFS 4 | 183 (60.4) | 120 (39.6) | 303 (15.5) |
| CFS 5–6 | 411 (57.8) | 300 (42.2) | 711 (36.4) |
| CFS 7–8 | 223 (48.1) | 241 (51.9) | 464 (23.8) |
| CFS 9 | 9 (29.0) | 22 (71.0) | 31 (1.6) |
| Missing | 16 | 6 | 22 |
Table 3.
Cox-regression, time to mortality
| HR (95% CI) | P | aHR | P | |
|---|---|---|---|---|
| Italy | 0.64 (0.23–1.81) | 0.405 | 0.85 (0.32–2.23) | 0.740 |
| Wave 2 | 0.75 (0.63–0.90) | 0.001 | 0.78 (0.65–0.93) | 0.007 |
| Age (65–74) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | ||
| 75–84 | 1.47 (1.22–1.78) | P < 0.001 | 1.38 (1.14–1.68) | 0.001 |
| 85–94 | 1.70 (1.39–2.09) | P < 0.001 | 1.51 (1.21–1.88) | P < 0.001 |
| 95 and over | 2.41 (1.58–3.68) | P < 0.001 | 2.30 (1.49–3.56) | P < 0.001 |
| Male | 1.12 (0.97–1.30) | 0.119 | 1.20 (1.03–1.39) | 0.022 |
| Smoking (never) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | ||
| Ex-smoker | 1.22 (1.06–1.42) | 0.008 | 1.14 (0.98–1.33) | 0.086 |
| Current smoker | 0.96 (0.69–1.35) | 0.832 | 0.95 (0.67–1.34) | 0.766 |
| CRP ≥40 | 1.84 (1.56–2.17) | P < 0.001 | 1.82 (1.54–2.15) | P < 0.001 |
| Diabetes | 1.08 (0.92–1.26) | 0.353 | 1.03 (0.87–1.21) | 0.749 |
| CAD | 1.13 (0.96–1.33) | 0.136 | 1.02 (0.86–1.21) | 0.797 |
| Hypertension (no) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | ||
| Yes | 0.95 (0.78–1.17) | 0.660 | 0.96 (0.78–1.19) | 0.726 |
| On treatment | 0.90 (0.76–1.05) | 0.182 | 0.89 (0.75–1.05) | 0.167 |
| eGFR <60 | 1.41 (1.22–1.62) | P < 0.001 | 1.30 (1.12–1.50) | P < 0.001 |
| CFS 1–3 | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | ||
| CFS 4 | 1.36 (1.06–1.76) | 0.018 | 1.26 (0.97–1.64) | 0.079 |
| CFS 5–6 | 1.49 (1.20–1.86) | P < 0.001 | 1.34 (1.06–1.69) | 0.015 |
| CFS 7–8 | 2.01 (1.60–2.54) | P < 0.001 | 1.78 (1.39–2.27) | P < 0.001 |
Note: aHR adjusted for healthcare, wave, age, sex, smoking status, CRP, diabetes, CAD, hypertension, eGFR and CFS.
Similar associations were found for 28-day mortality. Wave 2 was marginally associated with reduced mortality (aOR 0.80, 95% CI 0.62–1.03) (Supplementary table S2). Older age, living with frailty, being male, increased CRP and decreased renal function were associated with increased mortality (Supplementary table S2).
Secondary outcome (time to discharge)
Wave 2 was associated with a longer length of hospital stay (aHR 0.69, 95% CI 0.59–0.81) (Supplementary table S3). Additionally, increasing frailty (compared to CFS 1–3: CFS 5–6, aHR 0.78, 95% CI 0.65–0.94; CFS 7–8, aHR 0.76, 95% CI 0.61–0.94), and age (85–94 compared to 65–74, aHR 0.76, 95% CI 0.62–0.94) were associated with longer length of stay.
Secondary analysis of the participant characteristics between Wave 1 vs. 2
Additional analyses suggested that patients in Wave 2 were less frail (adjusted mean difference, aMD = −0.50, 95% CI −0.81, −0.18), and presented with a reduced disease severity expressed by lower CRP (aMD = −22.52, 95% CI −32.00, −13.04). There was no difference found in age between Waves 1 and 2 (Supplementary table S1).
Discussion
We included 1951 participants in this study and found that the mortality rate for hospitalized older patients was lower in the second wave, when compared with the first wave of COVID-19. Our data also showed that people admitted to hospital during the second wave were noticeably less frail and presented with a lower disease severity as expressed by lower CRP.
An enlarging evidence base and increasing experience from frontline clinicians should have improved patient management since the first wave of the pandemic. Our findings demonstrated a reduction in hospital mortality during the second wave that can be explained by healthier patients being admitted who were less frail and had lower disease severity. It is likely that a better use of respiratory support (high flow nasal cannula oxygen therapy and non-invasive ventilation) and critical care4 may have partially contributed to the better outcomes we found.
Additionally, since the first wave, there has been an increase in the use of systemic corticosteroids in COVID-19 patients.5 In the second wave, 55% of participants were taking dexamethasone, compared to 1.7% and 15% of our participants were taking remdesivir. Further, it is possible that other factors such as severity of infection (viral load and virus variants) and host response15 may have contributed to the reduced mortality in our population. There is some evidence confirming that in-hospital mortality from COVID-19 declined after the first wave. As an example, a prospective study of hospitalized patients of all ages in Spain found that mortality rate decreased in the second wave of outbreak with less patients treated with intubation.9 Finally, it is also possible that easier access to testing for SARS-CoV-2 along with advice about seeking medical help might have resulted in earlier presentation to hospital, resulting in earlier treatments.16
We also found that the time until hospital discharge was longer during the second wave, compared to the first. There are several potential reasons for this. Firstly, increased survival will result in more people being discharged from hospital, some of which may have stayed for a longer period of time. Hospital care was also more organized and prepared for the second wave, leading to a lower pressure on hospital beds and the urgent need for discharge. Further to this, all hospital patients, including those requiring external help at home, were required to test negative for COVID-19, to prevent onwards transmission and people living in residential care facilities were not able to be discharged if there was an ongoing outbreak at their care facility.
We found that the prevalence of frailty in our population aged over 65 years was 51.4%, markedly lower than we previously reported in the original COPE-Wave 1 study (66.9%).7,17 Frailty is known to contribute to mortality in COVID-19.7,18,19 Therefore, it is not surprising that the mortality rate in the second wave was lower. As the pandemic has progressed, awareness of the impact of the disease severity in people living with frailty has increased.20,21 SARS-CoV-2 infection has been under-diagnosed at the beginning and COVID-19 outbreaks have been common and severe in long-term care facilities.22 It is therefore possible that many older people living with frailty and with diagnosis of COVID-19 died in the first wave or were not admitted to hospital and remained in their place of residence in the second wave. This fact is highlighted by the numbers of people admitted with CFS of 7–8 between the two waves (28.1% vs. 14.2%).
While community-based care of people with COVID-19 has increased and improved since the first wave, our study demonstrates a reduction in admission in the group of frail people, who are known to be susceptible to COVID-19.
We previously demonstrated that a raised CRP can predict mortality, supporting the role of this acute-phase protein as a prognostic marker in COVID-19.12,23,24 We also found that levels of CRP were lower in Wave 2 than Wave 1 which is indicative of lower disease severity of the viral disease showing a lower inflammatory response in Wave 2. We also observed a reduced renal decline in Wave 2. Acute deterioration in chronic kidney disease due to systemic infection by COVID-19 as potential underlying trigger has been frequently observed at hospital presentation.25,26
Our findings should be interpreted in the light of a number of limitations. First, data for our Wave 2 study were collected from a subset of hospitals in the Wave 1 study.7 Second, we only included patients who were admitted to hospital and we only analysed mortality during hospital stay. This has implications in assessing the overall mortality rate for COVID-19 and the need to ensure that both inpatient and community mortality are considered. Moreover, the study also did not include patients who died in emergency departments (before hospital admission).
This study has several clinical implications which impact on public health and future research. First, the study provided real-world data from large cohort of older patients with COVID-19 in hospital settings in the UK and Italy, this adds value to the wider generalizability of the study findings. The population during the second wave was representative of other cohorts, as demonstrated by the demographic data and prevalence of comorbidities that are in line with other studies and comparable to other COVID populations.9,20,21
Second, the study highlights the importance of assessing the level of frailty in patients presented to emergency departments with suspected SARS-CoV-2 infection, confirming that frailty has important implications for therapy and prognosis. Chronological age may not be sufficient for describing the concept of wider vulnerability in older populations. Future research might explore the relationship between the degree of pre-admission frailty and care, such as use of mechanical ventilation (intubation) and systemic corticosteroids in older patients.
Third, our study reported a reduced proportion of frail people admitted to hospital; however, patients living with frailty should continue to present to hospital for care and should be investigated and offered the best available care for COVID-19. Further, it is essential to look for differences between outcomes of COVID-19 between primary and secondary care to ensure the people living with frailty are being managed in the correct healthcare setting.
Conclusions
This study demonstrated a lower mortality rate between the first two waves of the COVID-19 pandemic. The population in the second wave was significantly less frail, and presented with a lower disease severity.
Supplementary data
Supplementary data are available at EURPUB online.
Supplementary Material
Acknowledgments
COPE Study Collaborators: Hospital Policlinico in Modena, University of Modena and Reggio Emilia, Italy: Prof Enrico Clini. North Bristol NHS Trust (Southmead): Frances Rickard, James Hesford and Emma Mitchell. Glasgow Royal Infirmary (Department of Medicine for the Elderly): Kerr Hartrop and Caitlin Murphy. University of Glasgow: Ken Aggrey, Jimmy Bilan and Thomas Quinn. King’s College London: Joanna Kelly and Caroline Murphy. Royal Alexandra Hospital in Paisley: Susan Moug and Fanella-Barlow-Pay. Salford Royal Hospital: Amarah Khan, Maria Fernanda Ramon Espinoza, Thomas Kneen, Hala Allafi, Anna Dafnis, Maria Narro Vidal, Angeline Price and Lyndsay Pearce. Aberdeen Royal Infirmary, Scotland: Alice Einarsson, Eilidh Bruce and Kirsty Mccrorie.
Funding
This study received no specific funding. The work was partially supported through the NIHR Maudsley Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust in partnership with King’s College London (B.C.).
Conflicts of interest: None declared.
Contributor Information
Alessia Verduri, Respiratory Unit, Hospital Policlinico Modena, University of Modena and Reggio Emilia, Modena, Italy.
Roxanna Short, Forensic and Neurodevelopmental Sciences, King’s College London, London, UK.
Ben Carter, Department of Biostatistics and Health Informatics, King’s College London, London, UK.
Philip Braude, Department of Surgery and Care of the Elderly, Southmead Hospital, North Bristol NHS Trust, Bristol, UK.
Arturo Vilches-Moraga, Ageing and Complex Medicine Department, Salford Royal NHS Trust, Manchester, UK.
Terence J Quinn, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Jemima Collins, Cardiff and Vale UHB, Heath Park, Cardiff, UK.
Jane Lumsden, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Kathryn McCarthy, Department of Surgery and Care of the Elderly, Southmead Hospital, North Bristol NHS Trust, Bristol, UK.
Louis Evans, Ysbyty Gwynedd Hospital, Betsi Cadwaladr University Health Board, Bangor, UK.
Phyo K Myint, Institute of Applied Health Science, University of Aberdeen, Aberdeen, UK.
Jonathan Hewitt, Cardiff and Vale UHB, Heath Park, Cardiff, UK.
COPE Study Team:
Enrico Clini, Frances Rickard, James Hesford, Emma Mitchell, Kerr Hartrop, Caitlin Murphy, Ken Aggrey, Jimmy Bilan, Thomas Quinn, Joanna Kelly, Caroline Murphy, Susan Moug, Fanella- Barlow-Pay, Amarah Khan, Maria Fernanda Ramon Espinoza, Thomas Kneen, Hala Allafi, Anna Dafnis, Maria Narro Vidal, Angeline Price, Lyndsay Pearce, Alice Einarsson, and Eilidh BruceKirsty Mccrorie
Data Availability
All data sharing and collaboration requests should be directed to the corresponding author. The data underlying this article are available in the article and in its online supplementary material.
Key points.
Real-world data on the comparison of COVID-19 in-hospital mortality in older adults between Wave 1 and Wave 2 of the pandemic were analysed.
The Clinical Frailty Scale was used for a clinical assessment of frailty.
A reduced COVID-19 in-hospital mortality in older adults was demonstrated and a less frail population admitted to hospital was reported in Wave 2.
This evidence supports that patients living with frailty need to be treated with the same level of investigation as non-frail patients and further research on the mortality rate within this population should be precisely estimated.
These findings confirm that frailty has important implications for COVID-19 management and prognosis.
References
- 1. World Health Organisation COVID-19. Weekly Epidemiological Update on COVID-19. 2021. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update–15-june-2021 (15 June 2021, date last accessed).
- 2.Gov.UK Coronavirus (COVID-19) in the UK. Available at: https://coronavirus.data.gov.uk/ (15 June 2021, date last accessed).
- 3.ECDC COVID-19 situation update for the EU/EEA. Available at: https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea (15 June 2021, date last accessed).
- 4. Attaway AH, Scheraga RG, Bhimraj A, et al. Severe COVID-19 pneumonia: pathogenesis and clinical management. BMJ 2021;372:n436. [DOI] [PubMed] [Google Scholar]
- 5. The Recovery Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID-19. N Eng J Med 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Price A, Barlow-Pay F, Duffy S, et al. ; COPE Study Collaborators. Study protocol for the COPE study: COVID-19 in older people: the influence of frailty and multimorbidity on survival. A multicentre, European observational study. BMJ Open 2020;10:e040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hewitt J, Carter B, Vilches-Moraga A, et al. ; COPE Study Collaborators. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health 2020;5:e444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borghesi A, Golemi S, Carapella N, et al. Lombardy, Northern Italy: COVID-19 second wave less severe and deadly than the first? A preliminary investigation. Infect Dis (Lond) 2021;53:370–5. [DOI] [PubMed] [Google Scholar]
- 9. Iftimie S, López-Azcona AF, Vallverdú I, et al. First and second waves of Coronavirus disease-19: a comparative study in hospitalized patients in Reus, Spain. PLoS One 2021;16:e0248029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Contou D, Fraisse M, Pajot O, et al. Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: no prognostic improvement during the second wave? Crit Care 2021;25:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 12. Stringer D, Braude P, Myint PK, et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int J Epidemiol 2021;50:420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rockwood K, Theou O. Using the Clinical Frailty Scale in allocating scarce health care resources. Can Geriatr J 2020;23:210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fokas AS, Kastis GA. SARS-CoV-2: the second wave in Europe. J Med Internet Res 2021;23:e22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bruce E, Carter B, Quinn TJ, et al. Multiple house occupancy is associated with mortality in hospitalised patients with COVID-19. Eur J Public Health 2022;32:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collins JT, Short R, Carter B, et al. The Clinical Frailty Scale: estimating the prevalence of frailty in older patients hospitalized with COVID-19. The COPE study. Geriatrics 2020;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Y, Luo K, Jiang Y, et al. The impact of frailty on COVID-19 outcomes: a systematic review and meta-analysis of 16 cohort studies. J Nutr Health Aging 2021;25:702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pranata R, Henrina J, Lim MA, et al. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis. Arch Gerontol Geriatr 2021;93:104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blomaard LC, van der Linden CMJ, van der Bol JM, et al. Frailty is associated with in-hospital mortality in older hospitalized COVID-19 patients in the Netherlands: the COVID-OLD study. Age Ageing 2021;50:631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geriatric Medicine Research Collaborative. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing 2021;50:617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hashan MR, Smoll N, King C, et al. Epidemiology and clinical features of COVID-19 outbreaks in aged care facilities: a systematic review and meta-analysis. EClinicalMedicine 2021;33:100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis 2020;96:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: a prospective cohort study. BMJ 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020;97:829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data sharing and collaboration requests should be directed to the corresponding author. The data underlying this article are available in the article and in its online supplementary material.
Key points.
Real-world data on the comparison of COVID-19 in-hospital mortality in older adults between Wave 1 and Wave 2 of the pandemic were analysed.
The Clinical Frailty Scale was used for a clinical assessment of frailty.
A reduced COVID-19 in-hospital mortality in older adults was demonstrated and a less frail population admitted to hospital was reported in Wave 2.
This evidence supports that patients living with frailty need to be treated with the same level of investigation as non-frail patients and further research on the mortality rate within this population should be precisely estimated.
These findings confirm that frailty has important implications for COVID-19 management and prognosis.